Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints
Abstract
1. Introduction
2. Experimental Details
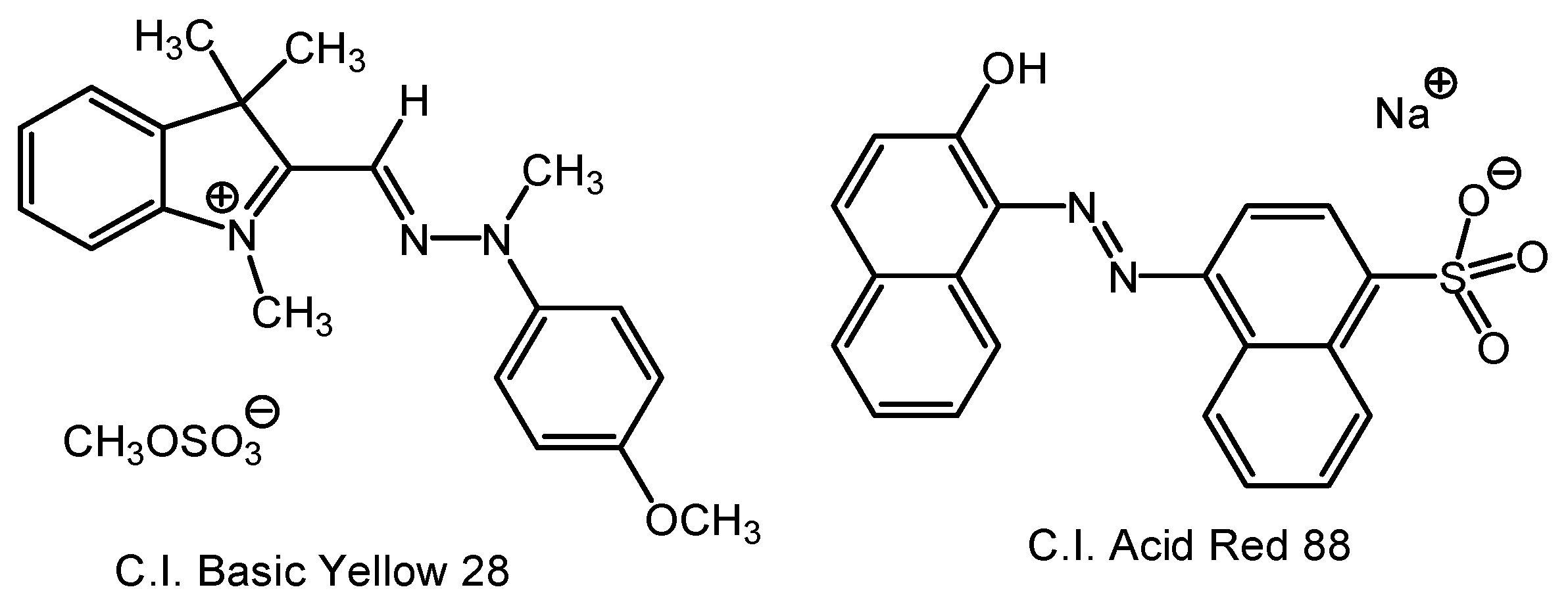
2.1. Materials and Substrates
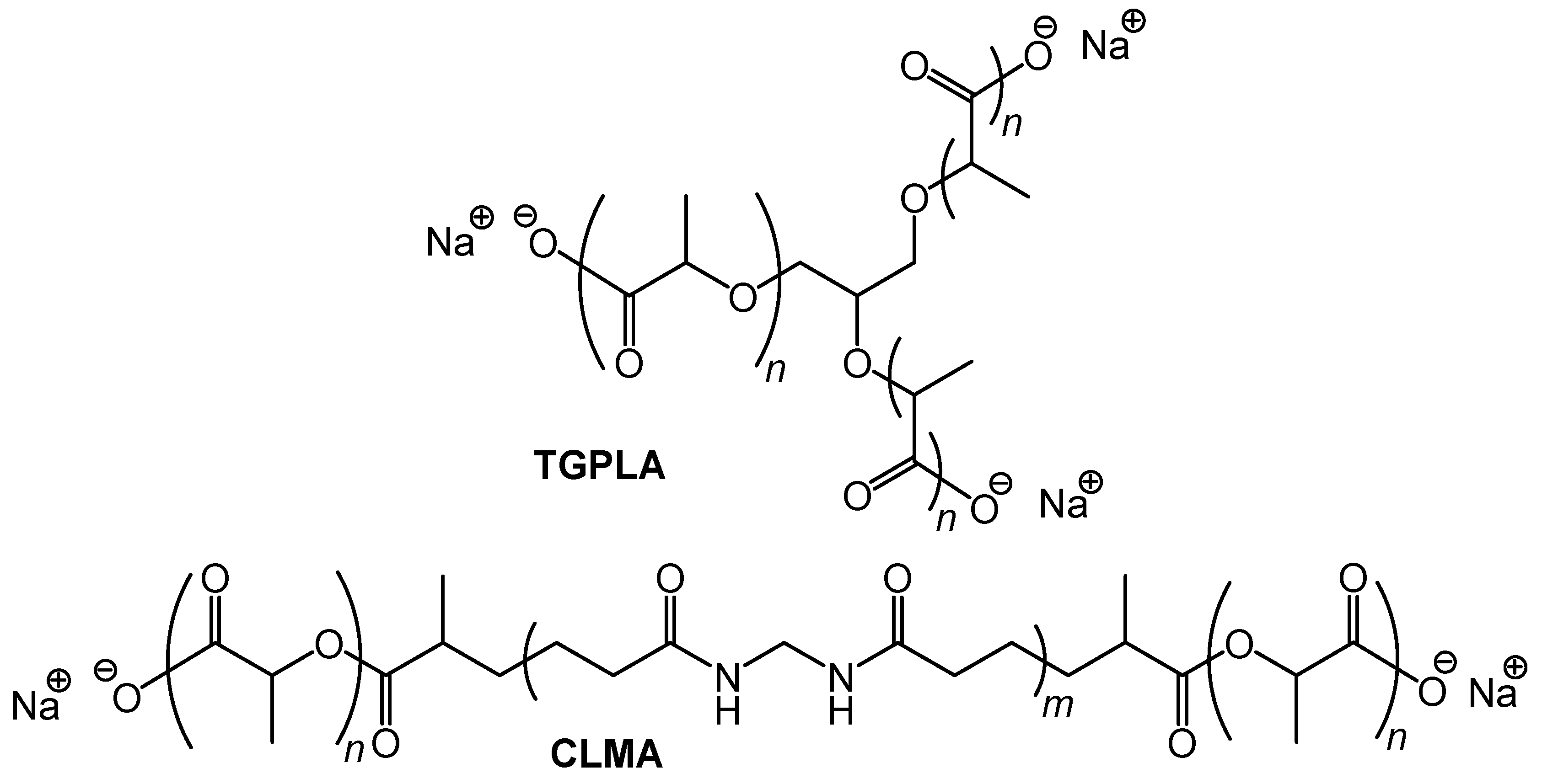
2.2. Preparation of Thickening Agents
2.3. Preparation of Metal Oxide-Based Nanoparticles
2.3.1. Synthesis of ZnO Nanoparticles
2.3.2. Synthesis of TiO2 Nanoparticles
2.3.3. Synthesis of MgO nanoparticles
2.4. Pre- and Post-Treatment of Wool and Acrylic Fabrics by Nanoparticles
2.5. Screen-Printing Technique
2.6. Analysis and Measurements
2.6.1. Morphology and Elemental Composition
2.6.2. Color Strength and Colorfastness Properties
2.6.3. Antimicrobial Activity
2.6.4. Self-Cleaning Activity
2.6.5. Ultraviolet Protection
3. Results and Discussion
3.1. Preparation of Multifunctional Fabrics
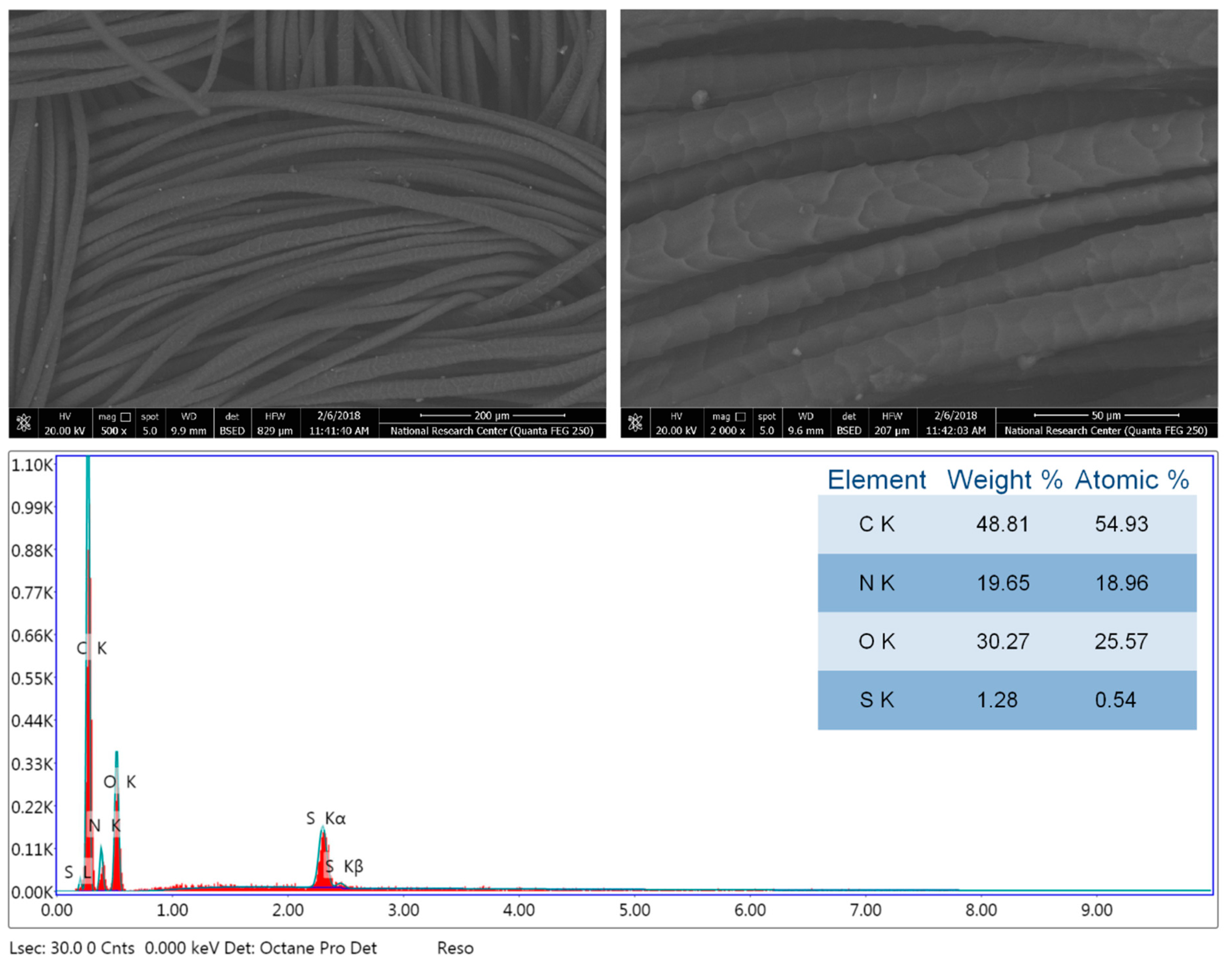
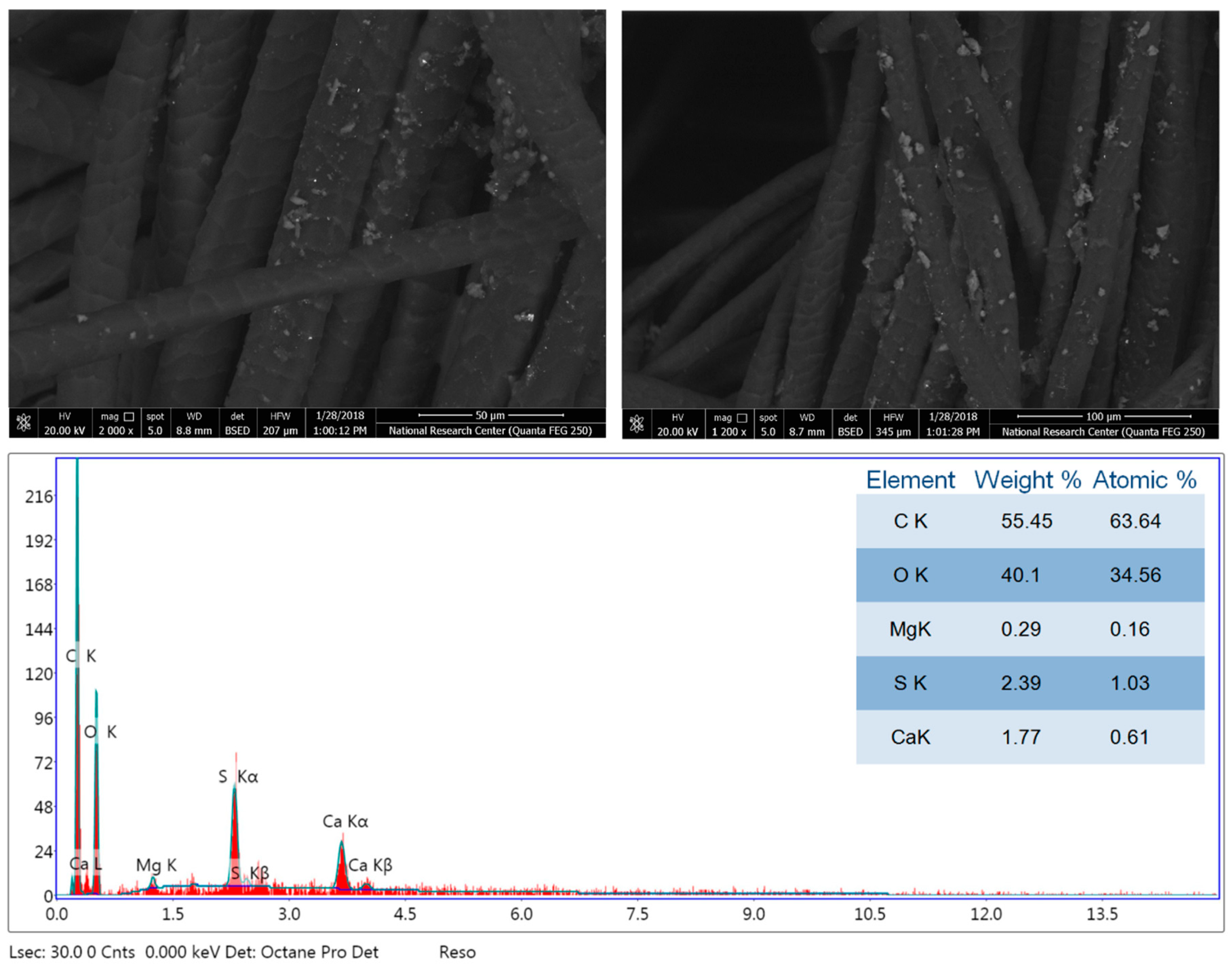
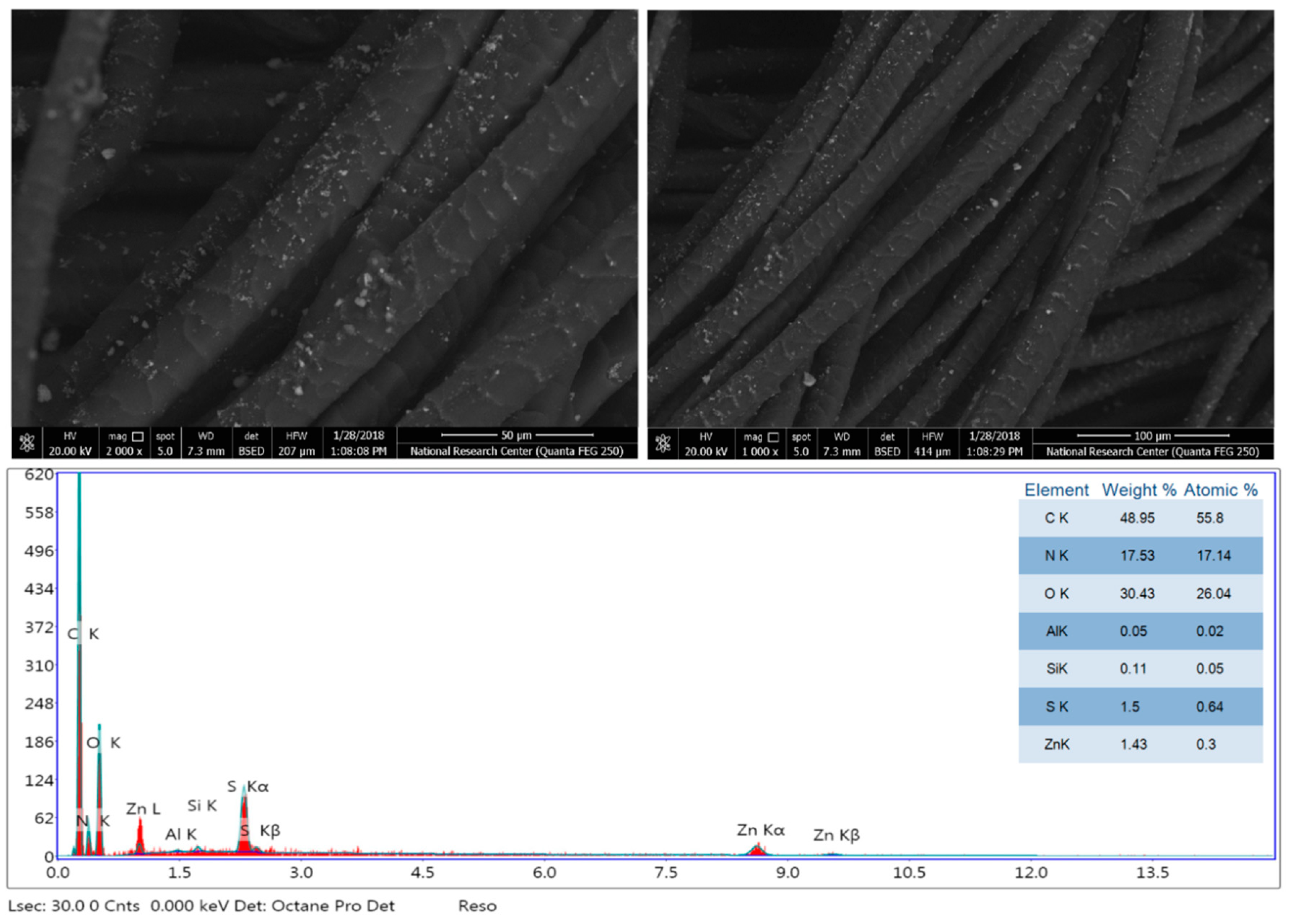
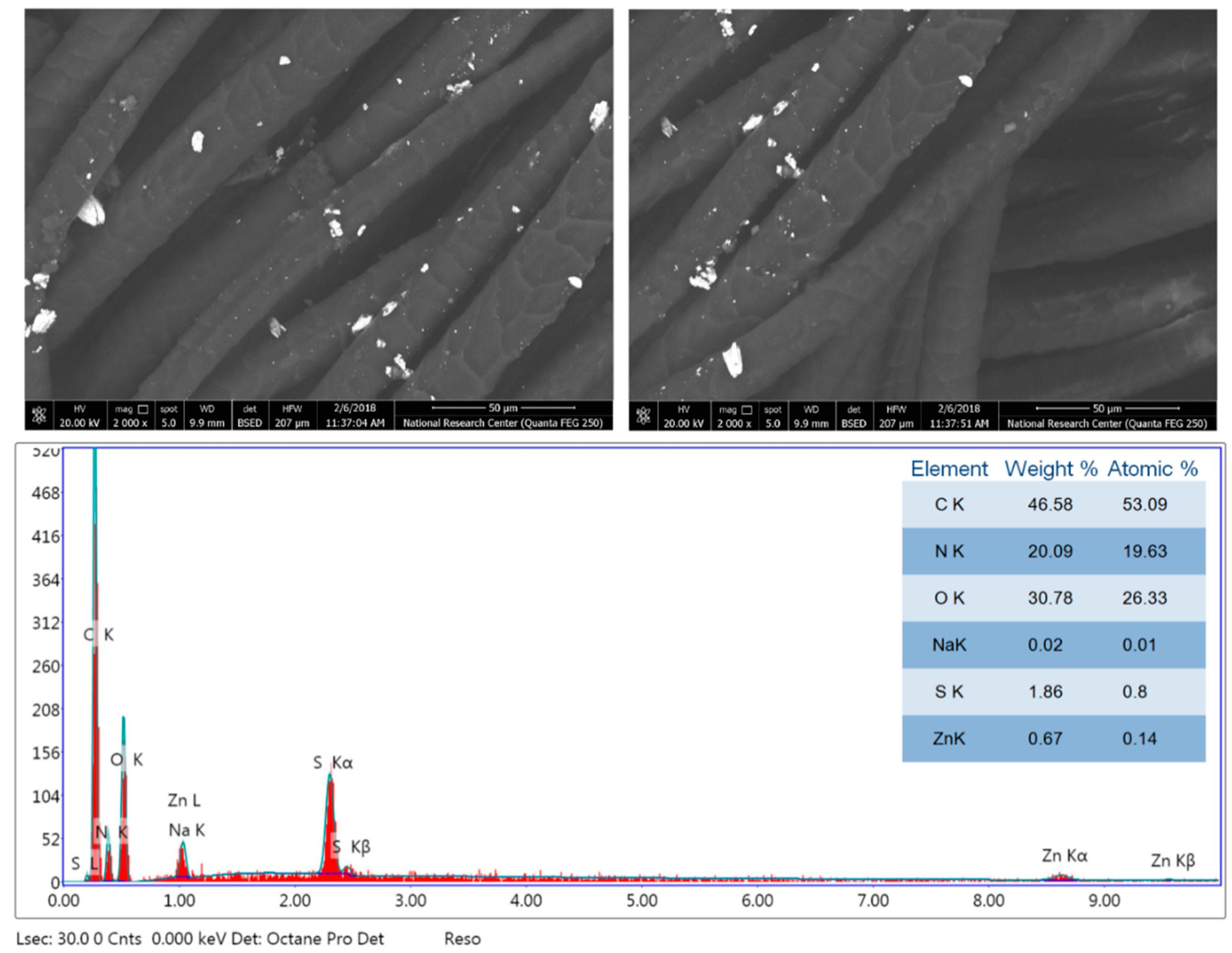
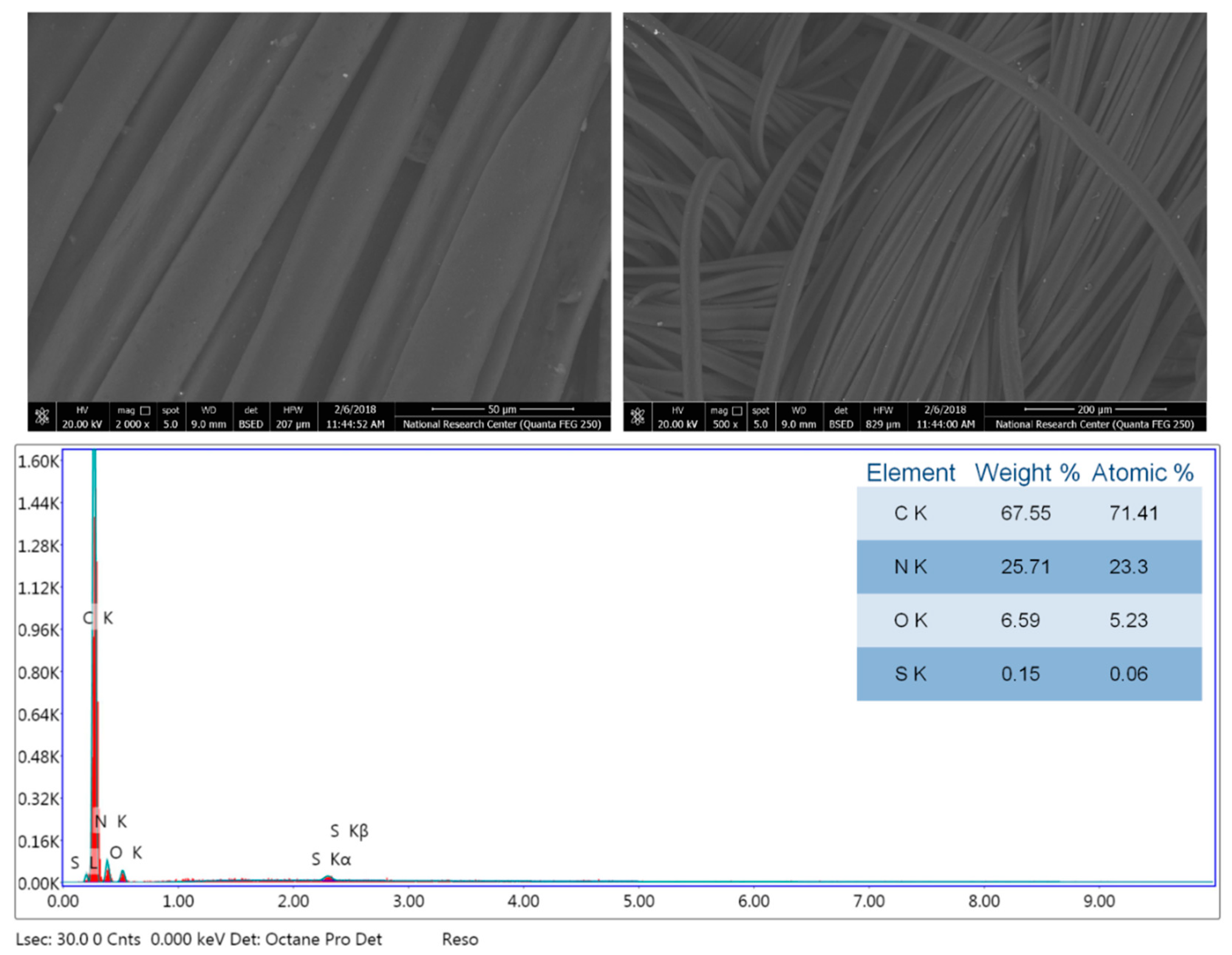
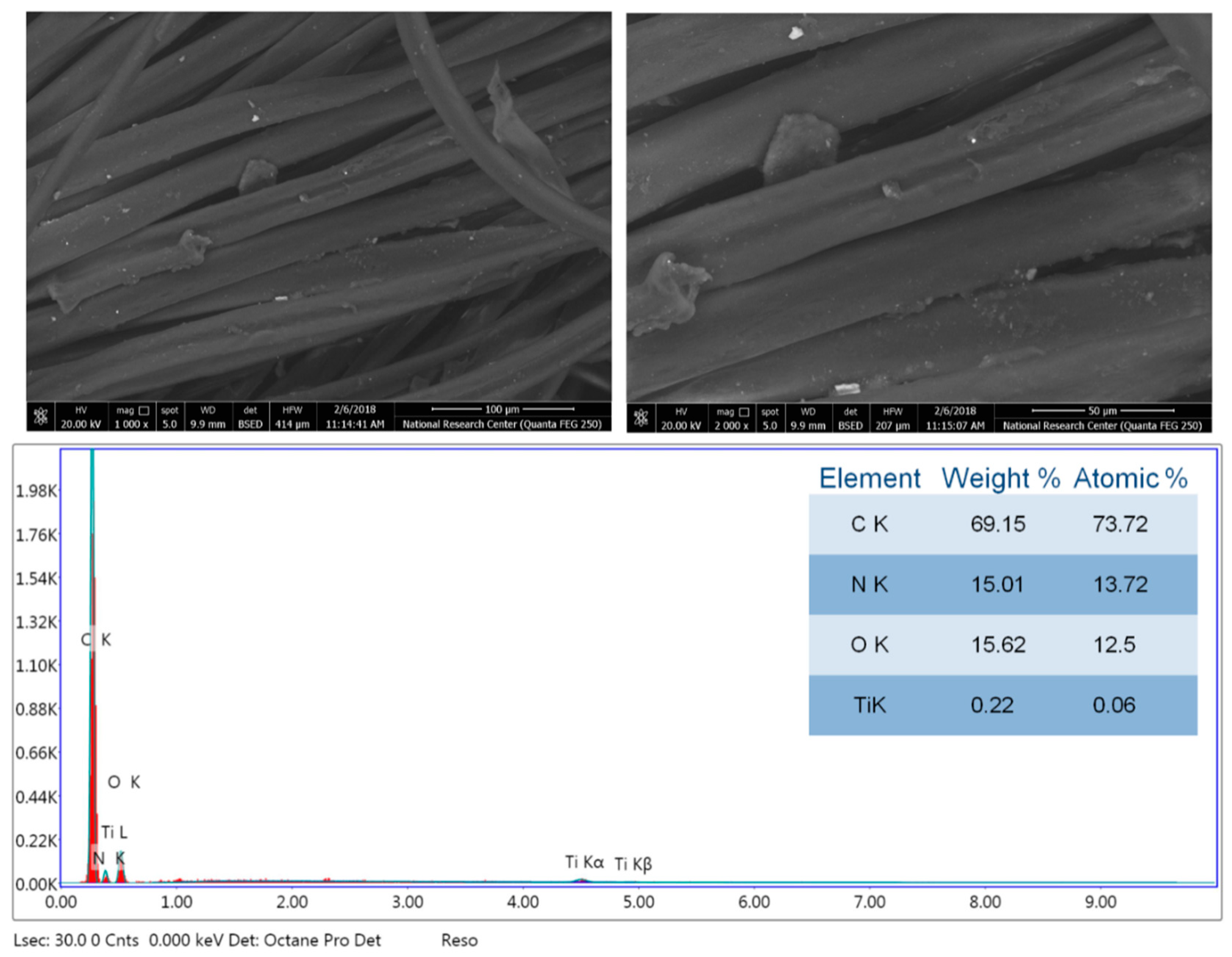
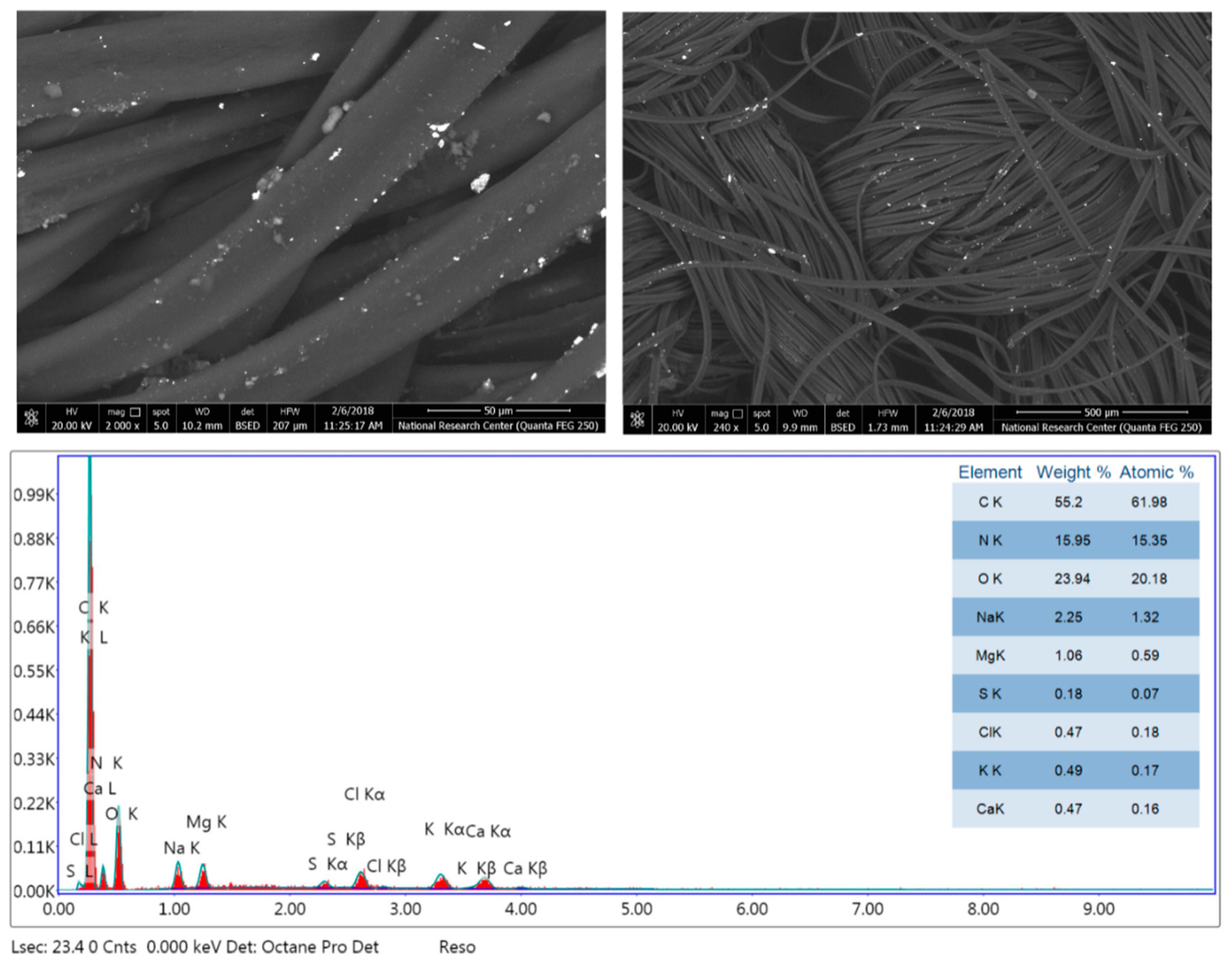
3.2. Morphology and Chemical Composition Properties
3.3. Color Strength and Fastness Properties
3.4. Antibacterial Performance of Treated and Untreated Fabrics
3.5. Ultraviolet Protection Activity
3.6. Self-Cleaning Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milwich, M.; Speck, T.; Speck, O.; Stegmaier, T.; Planck, H. Biomimetics and technical textiles: Solving engineering problems with the help of nature’s wisdom. Am. J. Bot. 2006, 93, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, G.; Straßer, G. Flexible gripping technology for the automated handling of limp technical textiles in composites industry. Prod. Eng. 2011, 5, 301–306. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Fassam, H.A.; Allam, A.A. Co-encapsulation of enzyme and tricyanofuran hydrazone into alginate microcapsules incorporated onto cotton fabric as a biosensor for colorimetric recognition of urea. React. Funct. Polym. 2019, 142, 199–206. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, S.; Huang, G. Anti-static charge character of the plasma treated polyester filter fabric. J. Electrost. 2010, 68, 111–115. [Google Scholar]
- Abdelrahman, M.S.; Tawfik, A.K. Development of One-Step Water-Repellent and Flame-Retardant Finishes for Cotton. ChemistrySelect 2019, 4, 3811–3816. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.Q.; Liu, X.F. Robust superhydrophobic TiO 2@ fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Khattab, T.A.; Mowafi, S.; El-Sayed, H. Development of mechanically durable hydrophobic lanolin/silicone rubber coating on viscose fibers. Cellulose 2019, 26, 9361–9371. [Google Scholar] [CrossRef]
- Zheng, X.; Guo, Z.; Tian, D.; Zhang, X.; Li, W.; Jiang, L. Underwater self-cleaning scaly fabric membrane for oily water separation. ACS Appl. Mater. Interfaces 2015, 7, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Khattab, T.A.; Kassem, N.F.; Adel, A.M.; Kamel, S. Optical Recognition of Ammonia and Amine Vapor Using “Turn-on” Fluorescent Chitosan Nanoparticles Imprinted on Cellulose Strips. J. Fluoresc. 2019, 29, 693–702. [Google Scholar] [CrossRef]
- Lund, A.; van der Velden, N.M.; Persson, N.K.; Hamedi, M.M.; Müller, C. Electrically conducting fibres for e-textiles: An open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R Rep. 2018, 126, 1–29. [Google Scholar] [CrossRef]
- Hu, L.; Cui, Y. Energy and environmental nanotechnology in conductive paper and textiles. Energy Environ. Sci. 2012, 5, 6423–6435. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Barhoum, A.; Khattab, T.A.; Gatjen, L.; Wilken, R. Colored, photocatalytic, antimicrobial and UV-protected viscose fibers decorated with Ag/Ag2CO3 and Ag/Ag3PO4 nanoparticles. Cellulose 2019, 26, 5437–5453. [Google Scholar] [CrossRef]
- Avila, A.G.; Juan, P. Hinestroza. Smart textiles: Tough cotton. Nat. Nanotechnol. 2008, 3, 458. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Rimmele, E.; Wichser, A.; Erni, R.; Height, M.; Nowack, B. Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 2014, 8, 7208–7219. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E.; Behzadnia, A. Novel feature of nano-titanium dioxide on textiles: Antifelting and antibacterial wool. J. Appl. Polym. Sci. 2011, 121, 3407–3413. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Green chemistry approaches to develop antimicrobial textiles based on sustainable biopolymers: A review. Ind. Eng. Chem. Res. 2013, 52, 5245–5260. [Google Scholar] [CrossRef]
- Lee, H.J.; Yeo, S.Y.; Jeong, S.H. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J. Mater. Sci. 2003, 38, 2199–2204. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E. Functionality of nano titanium dioxide on textiles with future aspects: Focus on wool. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 293–303. [Google Scholar] [CrossRef]
- Köhler, A.R.; Som, C. Risk preventative innovation strategies for emerging technologies the cases of nano-textiles and smart textiles. Technovation 2014, 34, 420–430. [Google Scholar] [CrossRef]
- Becheri, A.; Dürr, M.; Nostro, P.L.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Toshniwal, L.; Fan, Q.; Ugbolue, S.C. Dyeable polypropylene fibers via nanotechnology. J. Appl. Polym. Sci. 2007, 106, 706–711. [Google Scholar] [CrossRef]
- He, L.; Gao, C.; Li, S.; Chung, C.T.W.; John, H.X. Non-leaching and durable antibacterial textiles finished with reactive zwitterionic sulfobetaine. J. Ind. Eng. Chem. 2017, 46, 373–378. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, L.; Li, Q.; Li, J.; Zhu, X.; Jiang, Y.; Sha, O.; Yang, X.; Xin, J.H.; Wang, J. Durable antibacterial and nonfouling cotton textiles with enhanced comfort via zwitterionic sulfopropylbetaine coating. Small 2016, 12, 3516–3521. [Google Scholar] [CrossRef]
- Kang, C.K.; Kim, S.S.; Kim, S.; Lee, J.; Lee, J.H.; Roh, C.; Lee, J. Antibacterial cotton fibers treated with silver nanoparticles and quaternary ammonium salts. Carbohydr. Polym. 2016, 151, 1012–1018. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Tang, B.; Yuan, L.; Wang, K.; Liu, X.; Zhu, X.; Li, J.; Ge, Z.; Chen, S. New insights into synergistic antimicrobial and antifouling cotton fabrics via dually finished with quaternary ammonium salt and zwitterionic sulfobetaine. Chem. Eng. J. 2018, 336, 123–132. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; El-Zairy, E.M.R.; Eid, B.M. Eco-friendly modification and antibacterial functionalization of viscose fabric. J. Text. Inst. 2017, 108, 1406–1411. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, L.; Zhang, Z.; Yang, X.; Luo, J.; Gui, Z.; Chen, S. Non-leaching bactericidal cotton fabrics with well-preserved physical properties, no skin irritation and no toxicity. Cellulose 2018, 25, 5415–5426. [Google Scholar] [CrossRef]
- Feng, X.; Zheng, K.; Wang, C.; Chu, F.; Chen, Y. Durable antibacterial cotton fabrics with chitosan based quaternary ammonium salt. Fibers Polym. 2016, 17, 371–379. [Google Scholar] [CrossRef]
- Mwafy, E.A.; Hasanin, M.S.; Mostafa, A.M. Cadmium oxide/TEMPO-oxidized cellulose nanocomposites produced by pulsed laser ablation in liquid environment: Synthesis, characterization, and antimicrobial activity. Opt. Laser Technol. 2019, 120, 105744. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Safari, M.; Mashayekhi, M. Sonocatalyzed decolorization of synthetic textile wastewater using sonochemically synthesized MgO nanostructures. Ultrason. Sonochemistry 2016, 30, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. App. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Karimi, L.; Yazdanshenas, M.E.; Khajavi, R.; Rashidi, A.; Mirjalili, M. Functional finishing of cotton fabrics using graphene oxide nanosheets decorated with titanium dioxide nanoparticles. J. Text. Inst. 2016, 107, 1122–1134. [Google Scholar] [CrossRef]
- Mwafy, E.A.; Mostafa, A.M. Multi walled carbon nanotube decorated cadmium oxide nanoparticles via pulsed laser ablation in liquid media. Opt. Laser Technol. 2019, 111, 249–254. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Khattab, T.A. Environmentally Sound Dyeing of Cellulose-Based Textiles. Text. Cloth. 2019, 79–99. [Google Scholar] [CrossRef]
- Petkova, P.; Francesko, A.; Perelshtein, I.; Gedanken, A.; Tzanov, T. Simultaneous sonochemical-enzymatic coating of medical textiles with antibacterial ZnO nanoparticles. Ultrason. Sonochemistry 2016, 29, 244–250. [Google Scholar] [CrossRef]
- Babar, A.A.; Peerzada, M.H.; Jhatial, A.K. Pad ultrasonic batch dyeing of causticized lyocell fabric with reactive dyes. Ultrason. Sonochemistry 2017, 34, 993–999. [Google Scholar] [CrossRef]
- Gaffer, H.; Khattab, T. Synthesis and characterization of some azo-heterocycles incorporating pyrazolopyridine moiety as disperse dyes. Egypt. J. Chem. 2017, 60, 41–47. [Google Scholar]
- Li, Z.; Dong, Y.; Li, B.; Wang, P.; Chen, Z.; Bian, L. Creation of self-cleaning polyester fabric with TiO2 nanoparticles via a simple exhaustion process: Conditions optimization and stain decomposition pathway. Mater. Des. 2018, 140, 366–375. [Google Scholar] [CrossRef]
- OhadiFar, P.; Shahidi, S.; Dorranian, D. Synthesis of Silver Nanoparticles and Exhaustion on Cotton Fabric Simultaneously Using Laser Ablation Method. J. Nat. Fibers 2018, 1–12. [Google Scholar] [CrossRef]
- Khattab, T.A.; Allam, A.A.; Othman, S.I.; Bin-Jumah, M.; Al-Harbi, H.M.; Fouda, M.M. Synthesis, Solvatochromic Performance, pH Sensing, Dyeing Ability, and Antimicrobial Activity of Novel Hydrazone Dyestuffs. J. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Velmurugan, P.; Shim, J.; Bang, K.S.; Oh, B.T. Gold nanoparticles mediated coloring of fabrics and leather for antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 160, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.S.; Nassar, S.H.; Mashaly, H.; Mahmoud, S.; Maamoun, D.; Khattab, T.A. Polymerization products of lactic acid as synthetic thickening agents for textile printing. J. Mol. Struct. 2020, 1203, 127421. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Sahar, H.N.; Mashaly, H.; Mahmoud, S.; Maamoun, D. Synthesis and Characterization of Biodegradable Synthetic Thickener from Anionic Triglyceride Polylactic Acid. Appl. Ecol. Environ. Sci. 2018, 6, 35–47. [Google Scholar] [CrossRef]
- El-Thalouth, J.; Abd, I.; Mashaly, H.M. Imparting Multi-Functional Performance on Cellulosic Fabrics via Nanotechnology. Int. J. Innov. Appl. Stud. 2016, 18, 140. [Google Scholar]
- Khattab, T.A.; Elnagdi, M.H.; Haggaga, K.M.; Abdelrahmana, A.A.; Abdelmoez Aly, S. Green synthesis, printing performance, and antibacterial activity of disperse dyes incorporating arylazopyrazolopyrimidines. AATCC J. Res. 2017, 4, 1–8. [Google Scholar] [CrossRef]
- Khattab, T.A.; Haggag, K.M.; Elnagdi, M.H.; Abdelrahman, A.A.; Abdelmoez Aly, S. Microwave-assisted synthesis of arylazoaminopyrazoles as disperse dyes for textile printing. Z. Fur Anorg. Und Allg. Chem. 2016, 642, 766–772. [Google Scholar] [CrossRef]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.; Khattab, T.A. Green and sustainable encapsulation of Guava leaf extracts (Psidium guajava, L.) into alginate/starch microcapsules for multifunctional finish over cotton gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Khan, R.; Fulekar, M.H. Biosynthesis of titanium dioxide nanoparticles using Bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye Reactive Red 31. J. Colloid Interface Sci. 2016, 475, 184–191. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Fassam, H.A.; Allam, A.A. Development of Illuminant Glow-in-the-Dark Cotton Fabric Coated by Luminescent Composite with Antimicrobial Activity and Ultraviolet Protection. J. Fluoresc. 2019, 29, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Pisitsak, P.; Hutakamol, J.; Jeenapak, S.; Wanmanee, P.; Nuammaiphum, J.; Thongcharoen, R. Natural dyeing of cotton with xylocarpus granatum bark extract: Dyeing, fastness, and ultraviolet protection properties. Fibers Polym. 2016, 17, 560–568. [Google Scholar] [CrossRef]
- Khattab, T.A.; Abou-Yousef, H.; Kamel, S. Photoluminescent spray-coated paper sheet: Write-in-the-dark. Carbohydr. Polym. 2018, 200, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Pantzas, K.; Patriarche, G.; Troadec, D.; Gautier, S.; Moudakir, T.; Suresh, S.; Largeau, L.; Mauguin, O.; Voss, P.L.; Ougazzaden, A. Nanometer-scale, quantitative composition mappings of InGaN layers from a combination of scanning transmission electron microscopy and energy dispersive X-ray spectroscopy. Nanotechnology 2012, 23, 455707. [Google Scholar] [CrossRef]
- Alghool, S.; El-Halim, H.F.A.; Mostafa, A.M. An eco-friendly synthesis of V 2 O 5 nanoparticles and their catalytic activity for the degradation of 4-nitrophrnol. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1324–1330. [Google Scholar] [CrossRef]
- Eisa, W.H.; Zayed, M.F.; Anis, B.; Abbas, L.M.; Ali, S.S.; Mostafa, A.M. Clean production of powdery silver nanoparticles using Zingiber officinale: The structural and catalytic properties. J. Clean. Prod. 2019, 241, 118398. [Google Scholar] [CrossRef]
| Component | Weight (g) |
|---|---|
| Dye | 30 |
| Urea | 50 |
| Thioethylene glycol | 50 |
| Thickener | Y * |
| Water | X ** |
| Ammonium sulphate | 60 |
| Ludigol | 15 |
| Total | 1000 |
| Component | Weight (g) |
|---|---|
| Dye | 30 |
| Acetic acid 30% | 30 |
| Thioethylene glycol | 3 |
| Thickener | Y * |
| Water | X ** |
| Citric acid | 5 |
| Total | 1000 |
| Metal Oxide Nanoparticles | Concentration | Color Strength (K/S) | |
|---|---|---|---|
| (% owf) | Pre-Treatment | Post-Treatment | |
| Control * | 0 | 6.26 | 6.26 |
| ZnO | 0.5 | 11.19 | 5.45 |
| 1.0 | 10.25 | 7.25 | |
| 1.5 | 6.58 | 9.06 | |
| 2.0 | 5.18 | 10.48 | |
| MgO | 0.5 | 9.45 | 10.4 |
| 1.0 | 11.57 | 7.98 | |
| 1.5 | 12.11 | 7.21 | |
| 2.0 | 13.28 | 6.63 | |
| TiO2 | 0.5 | 15.07 | 8.57 |
| 1.0 | 14.38 | 8.43 | |
| 1.5 | 14.28 | 7.69 | |
| 2.0 | 7.93 | 7.53 | |
| Metal Oxide Nanoparticles | Concentration | K/S | |
|---|---|---|---|
| (% owf) | Pre-Treatment | Post-Treatment | |
| Control * | 0 | 13.99 | 13.99 |
| ZnO | 0.5 | 18.66 | 14.39 |
| 1.0 | 16.54 | 15.78 | |
| 1.5 | 14.61 | 16.82 | |
| 2.0 | 14.32 | 17.2 | |
| MgO | 0.5 | 14.54 | 15.27 |
| 1.0 | 16.51 | 15.36 | |
| 1.5 | 16.78 | 16.75 | |
| 2.0 | 17.43 | 16.96 | |
| TiO2 | 0.5 | 17.64 | 15.3 |
| 1.0 | 16.4 | 13.77 | |
| 1.5 | 11.37 | 13.38 | |
| 2.0 | 10.45 | 12.9 | |
| Conditions | Metal Oxides NPs | Dye | Rubbing | Washing | Perspiration | Light | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wet | Dry | St. | Alt. | Acidic | Alkaline | ||||||
| St. | Alt. | St. | Alt. | ||||||||
| Wool/TGPLA Pre-treatment | Control | Acid dye | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 6–7 |
| ZnO/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 6–7 | ||
| MgO/2.0% | 4 | 3–4 | 4 | 4 | 4 | 4 | 4 | 4 | 7 | ||
| TiO2/0.5% | 3–4 | 3 | 4 | 4 | 3–4 | 3–4 | 4 | 4 | 6–7 | ||
| Wool/TGPLA Post-treatment | Control | 3–4 | 3 | 4 | 4 | 3–4 | 4 | 4 | 4 | 7 | |
| ZnO/2.0% | 4 | 3–4 | 4 | 4 | 4 | 4 | 4 | 4 | 6–7 | ||
| MgO/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 7 | ||
| TiO2/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 6–7 | ||
| Acrylic/CLMA Pre-treatment | Control | Basic dye | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 6–7 |
| ZnO/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4–5 | 4 | 4 | 6–7 | ||
| MgO/2.0% | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 6–7 | ||
| TiO2/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4 | 4 | 4 | 7 | ||
| Acrylic/CLMA Post-treatment | Control | 3–4 | 3 | 4 | 4 | 3–4 | 3–4 | 4 | 4 | 6–7 | |
| ZnO/2.0% | 3–4 | 3 | 4 | 4 | 3–4 | 4 | 4 | 4 | 7 | ||
| MgO/2.0% | 4 | 3–4 | 4 | 4 | 4 | 4 | 4 | 4 | 7 | ||
| TiO2/0.5% | 4 | 3–4 | 4 | 4 | 4 | 4 | 4 | 4 | 6–7 | ||
| Sample | E. Coli (G−) | S. Aureus (G+) | |||
|---|---|---|---|---|---|
| Wool | Acrylic | Wool | Acrylic | ||
| Untreated/Blank | 0.0 | 0.0 | 0.0 | 0.0 | |
| MgO | 0.5% | 27 | 30 | 37 | 30 |
| 1.5% | 27 | 32 | 39 | 26 | |
| 2.0% | 30 | 32 | 39 | 20 | |
| TiO2 | 0.5% | 31 | 36 | 40 | 36 |
| 1.5% | 30 | 37 | 40 | 27 | |
| 2.0% | 29 | 34 | 38 | 27 | |
| ZnO | 0.5% | 30 | 32 | 37 | 29 |
| 1.5% | 26 | 32 | 37 | 28 | |
| 2.0% | 29 | 30 | 36 | 26 | |
| Sample | Pre-Treatment | Post-Treatment | |||
|---|---|---|---|---|---|
| S. aureus (G+) | E. coli (G−) | S. aureus (G+) | E. coli (G−) | ||
| Control | 0 | 0 | 0 | 0 | |
| Wool (TiO2) | 0.50% | 30 | 36 | 31 | 39 |
| 1.00% | 40 | 34 | 26 | 36 | |
| 1.50% | 38 | 36 | 27 | 37 | |
| 2.00% | 30 | 31 | 20 | 33 | |
| Wool (MgO) | 0.50% | 28 | 37 | 27 | 37 |
| 1.00% | 26 | 32 | 24 | 25 | |
| 1.50% | 28 | 35 | 26 | 36 | |
| 2.00% | 31 | 37 | 29 | 37 | |
| Wool (ZnO) | 0.50% | 29 | 40 | 18 | 33 |
| 1.00% | 28 | 32 | 26 | 34 | |
| 1.50% | 25 | 33 | 32 | 38 | |
| 2.00% | 24 | 35 | 29 | 39 | |
| Acrylic (TiO2) | 0.50% | 35 | 32 | 24 | 32 |
| 1.00% | 34 | 34 | 26 | 36 | |
| 1.50% | 30 | 32 | 28 | 36 | |
| 2.00% | 36 | 35 | 28 | 39 | |
| Acrylic (MgO) | 0.50% | 28 | 31 | 27 | 34 |
| 1.00% | 26 | 32 | 24 | 25 | |
| 1.50% | 24 | 32 | 21 | 34 | |
| 2.00% | 25 | 34 | 25 | 36 | |
| Acrylic (ZnO) | 0.50% | 22 | 31 | 28 | 35 |
| 1.00% | 28 | 32 | 26 | 34 | |
| 1.50% | 25 | 32 | 27 | 31 | |
| 2.00% | 21 | 32 | 24 | 30 | |
| Sample | Ultraviolet Protection Factor (UPF) | |||
|---|---|---|---|---|
| Unprinted | Printed | |||
| Pre-Treated | Post-Treated | |||
| Wool (TiO2) | 0.5 | 143.3 | 152.2 | 160.5 |
| 1.0 | 145.4 | 149.8 | 165.2 | |
| 1.5 | 144.3 | 145.0 | 167.8 | |
| 2.0 | 140.9 | 139.1 | 177.6 | |
| Wool (MgO) | 0.5 | 135.2 | 76.6 | 92.7 |
| 1.0 | 141.5 | 102.9 | 117.1 | |
| 1.5 | 147.0 | 105.7 | 122.3 | |
| 2.0 | 148.7 | 108.2 | 125.2 | |
| Wool (ZnO) | 0.5 | 166.5 | 135.8 | 84.6 |
| 1.0 | 153.6 | 111.7 | 109.3 | |
| 1.5 | 123.8 | 90.0 | 124.1 | |
| 2.0 | 99.4 | 72.3 | 157.2 | |
| Acrylic (TiO2) | 0.5 | 136.8 | 111.5 | 129.0 |
| 1.0 | 132.4 | 118.2 | 139.5 | |
| 1.5 | 126.7 | 121.6 | 144.8 | |
| 2.0 | 108.5 | 126.4 | 151.3 | |
| Acrylic (MgO) | 0.5 | 92.5 | 78.0 | 67.6 |
| 1.0 | 91.0 | 69.3 | 73.9 | |
| 1.5 | 78.7 | 61.5 | 82.0 | |
| 2.0 | 76.9 | 57.4 | 94.5 | |
| Acrylic (ZnO) | 0.5 | 77.5 | 59.1 | 51.7 |
| 1.0 | 83.6 | 51.7 | 55.4 | |
| 1.5 | 105.8 | 47.9 | 68.4 | |
| 2.0 | 119.9 | 43.2 | 79.0 | |
| Sample | Dye Removal % | ||
|---|---|---|---|
| Post-Treatment | Pre-Treatment | ||
| Basic Dyestuff (CLMA) | |||
| Acrylic (TiO2) | 0.5 | 90% | 95% |
| 1.0 | 90% | 90% | |
| 1.5 | 95% | 80% | |
| 2.0 | 90% | 90% | |
| Acrylic (MgO) | 0.5 | 90% | 90% |
| 1.0 | 80% | 90% | |
| 1.5 | 90% | 90% | |
| 2.0 | 90% | 80% | |
| Acrylic (ZnO) | 0.5 | 90% | 90% |
| 1.0 | 80% | 90% | |
| 1.5 | 90% | 90% | |
| 2.0 | 90% | 85% | |
| Acid Dyestuff(TGPLA) | |||
| Wool (TiO2) | 0.5 | 90% | 90% |
| 1.0 | 85% | 90% | |
| 1.5 | 90% | 90% | |
| 2.0 | 90% | 90% | |
| Wool (MgO) | 0.5 | 90% | 90% |
| 1.0 | 85% | 90% | |
| 1.5 | 70% | 95% | |
| 2.0 | 80% | 90% | |
| Wool (ZnO) | 0.5 | 90% | 90% |
| 1.0 | 90% | 95% | |
| 1.5 | 90% | 95% | |
| 2.0 | 80% | 90% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, M.S.; Nassar, S.H.; Mashaly, H.; Mahmoud, S.; Maamoun, D.; El-Sakhawy, M.; Khattab, T.A.; Kamel, S. Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings 2020, 10, 58. https://doi.org/10.3390/coatings10010058
Abdelrahman MS, Nassar SH, Mashaly H, Mahmoud S, Maamoun D, El-Sakhawy M, Khattab TA, Kamel S. Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings. 2020; 10(1):58. https://doi.org/10.3390/coatings10010058
Chicago/Turabian StyleAbdelrahman, Meram S., Sahar H. Nassar, Hamada Mashaly, Safia Mahmoud, Dalia Maamoun, Mohamed El-Sakhawy, Tawfik A. Khattab, and Samir Kamel. 2020. "Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints" Coatings 10, no. 1: 58. https://doi.org/10.3390/coatings10010058
APA StyleAbdelrahman, M. S., Nassar, S. H., Mashaly, H., Mahmoud, S., Maamoun, D., El-Sakhawy, M., Khattab, T. A., & Kamel, S. (2020). Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings, 10(1), 58. https://doi.org/10.3390/coatings10010058






