Fabrication of a Thick Crystalline Al2O3 Coating with Insulation and High Thermal Conductivity via Anodic Oxidation and Subsequent Mic Arc Discharge Treatment
Abstract
1. Introduction
2. Experiment
2.1. Materials and Pre-treatment
2.2. Preparation of Aluminum Alloy Oxidation Coatings
2.3. Coating Characterization
3. Results and Discussion
3.1. Thickness of Anodic Coating
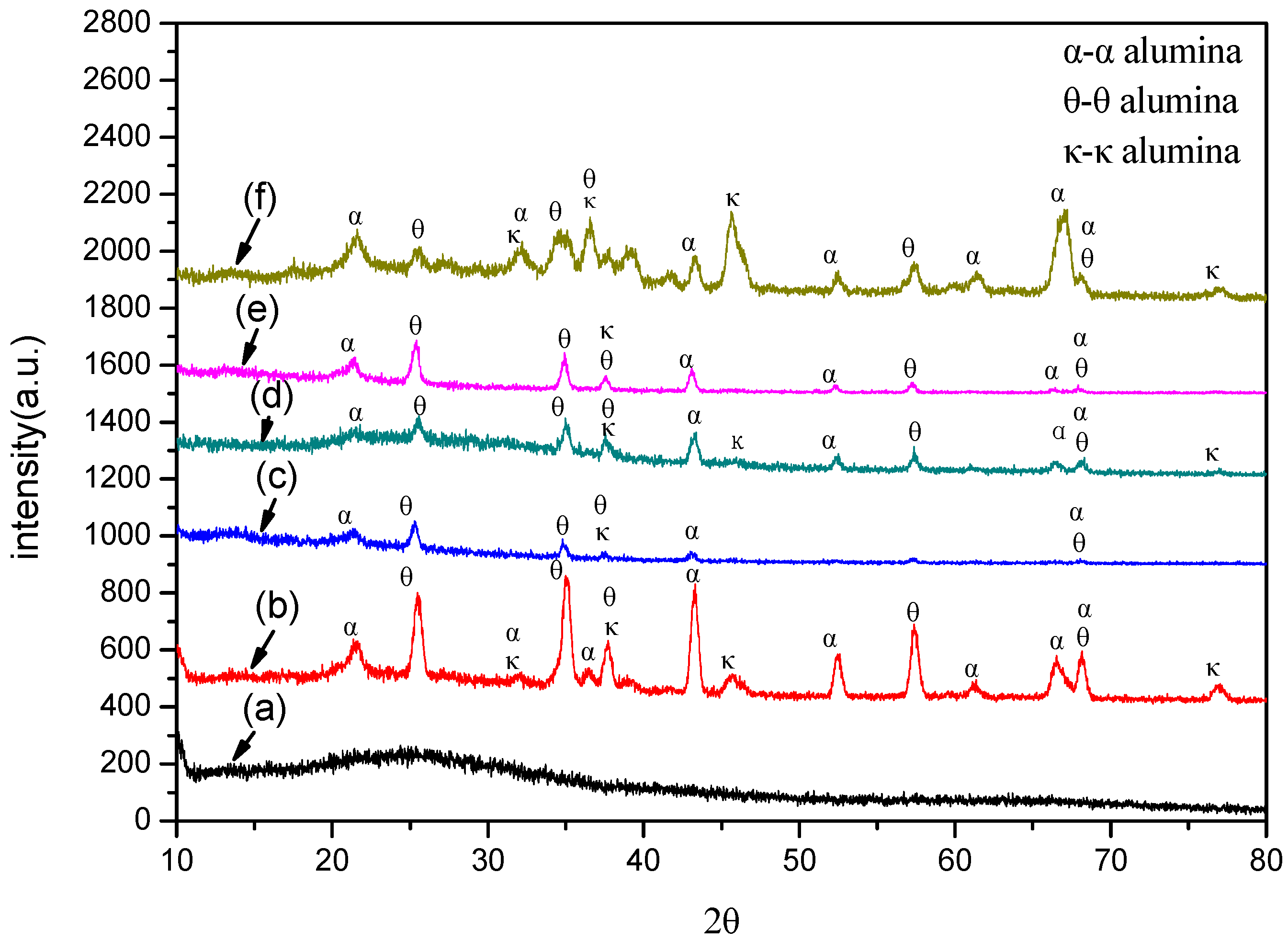
3.2. Phase Structure of the Coatings
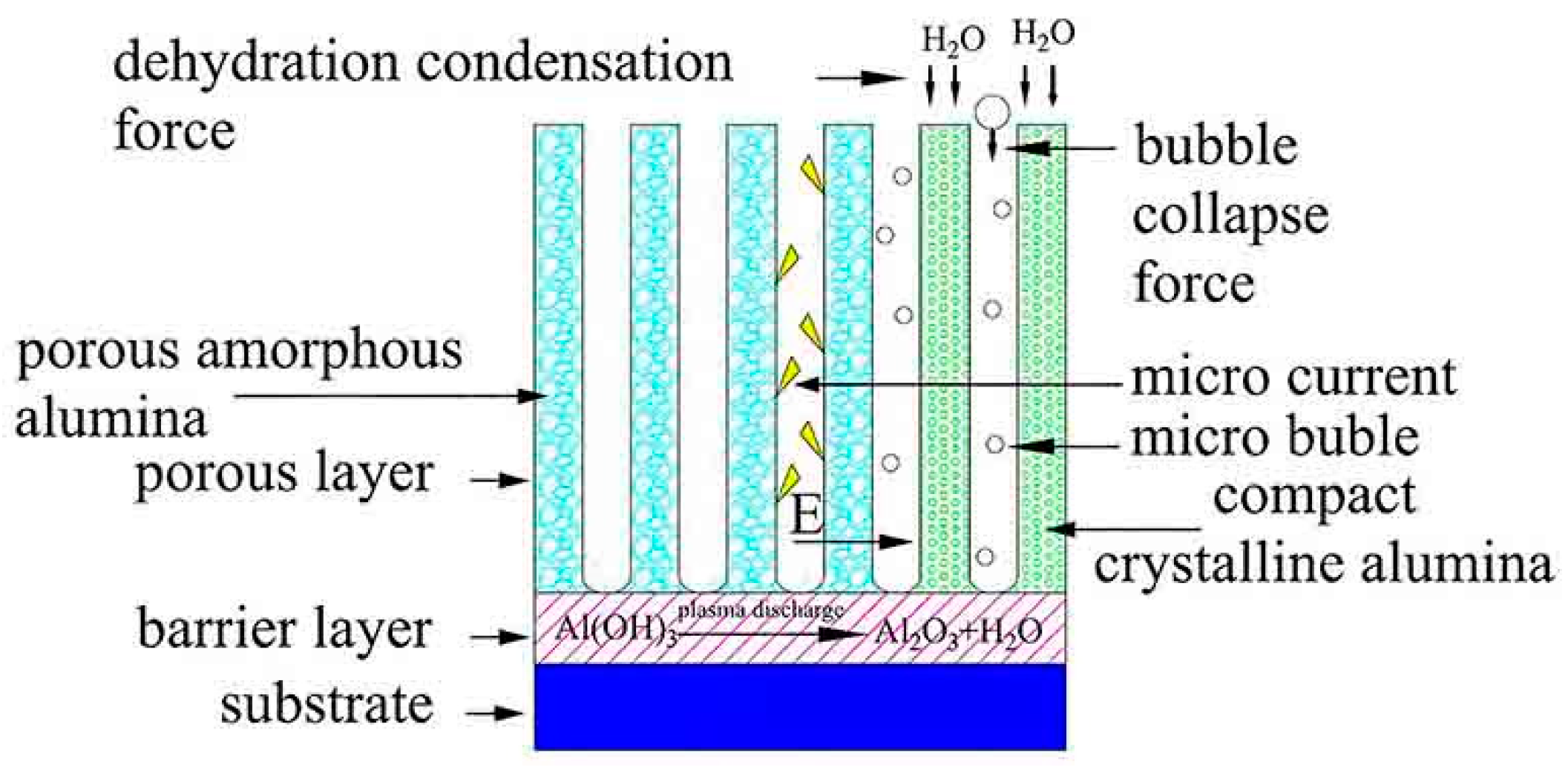
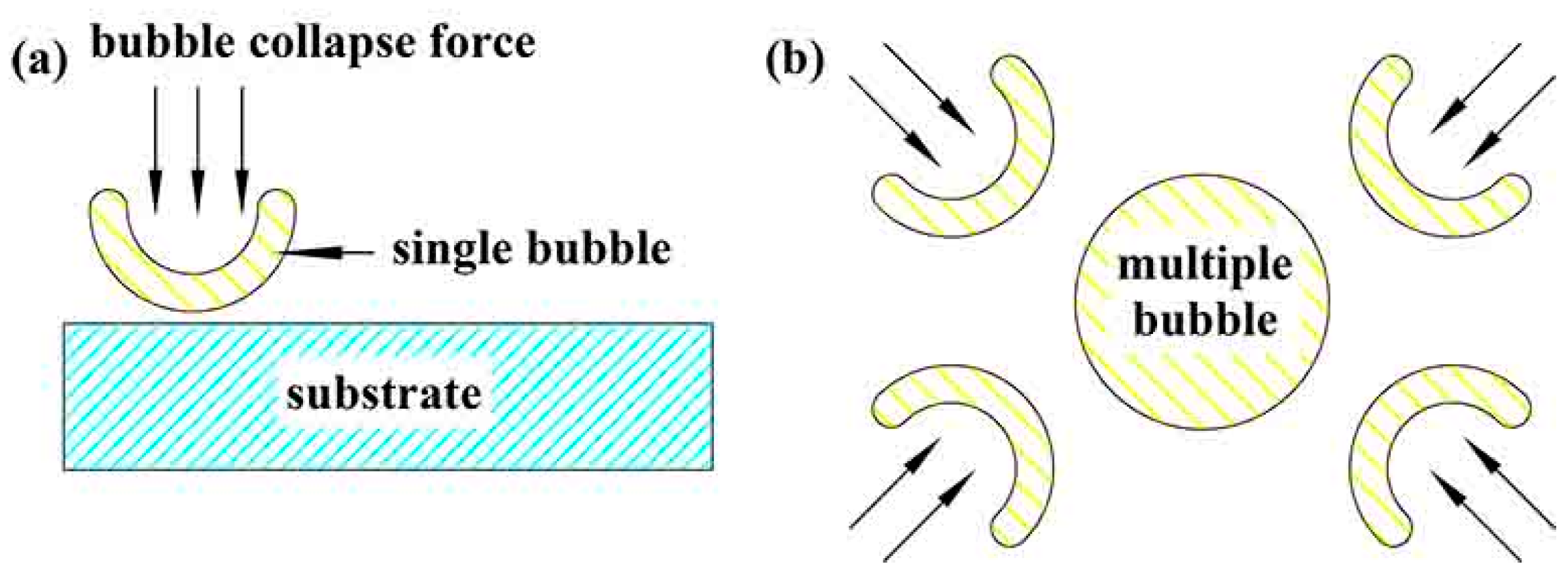
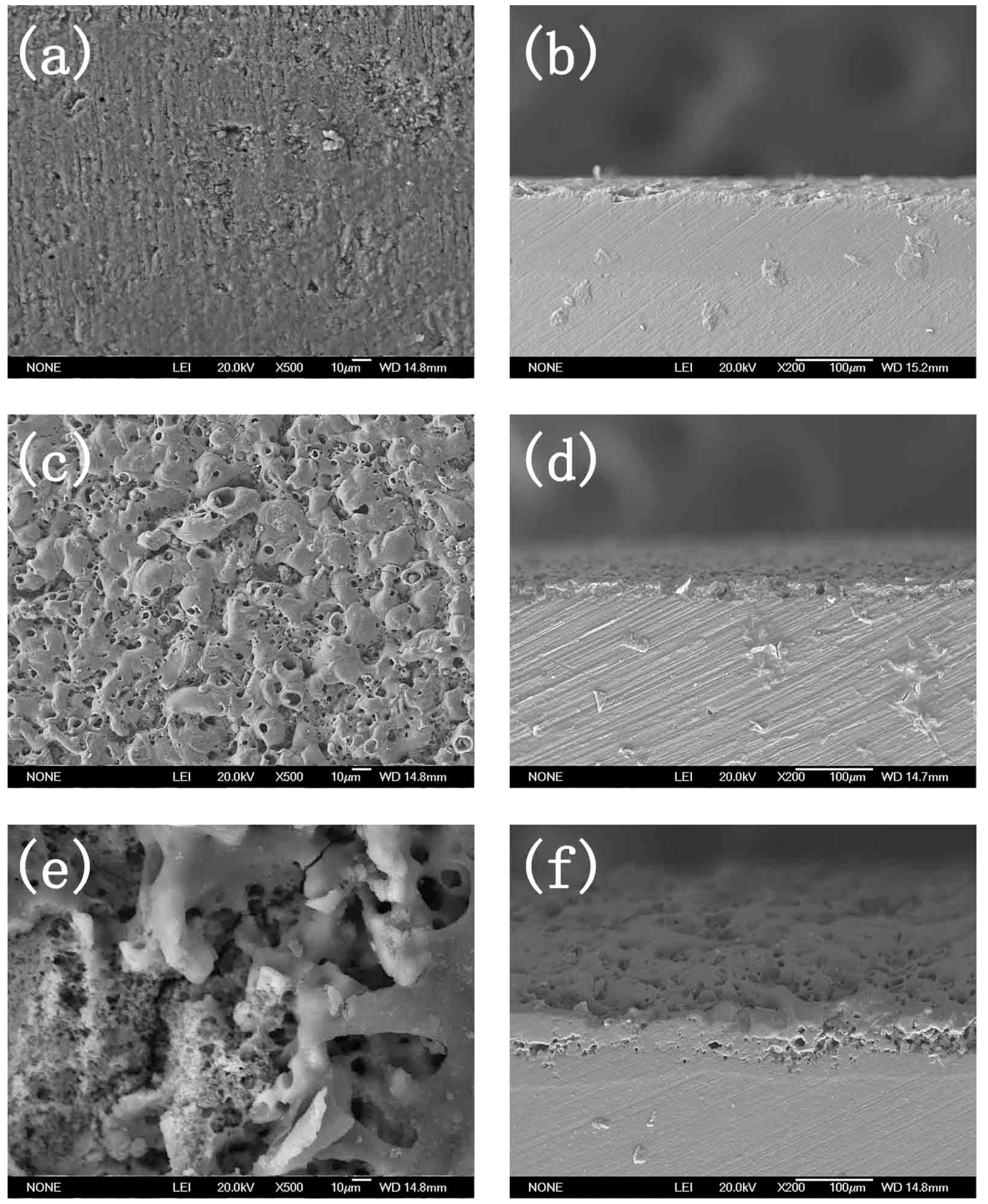
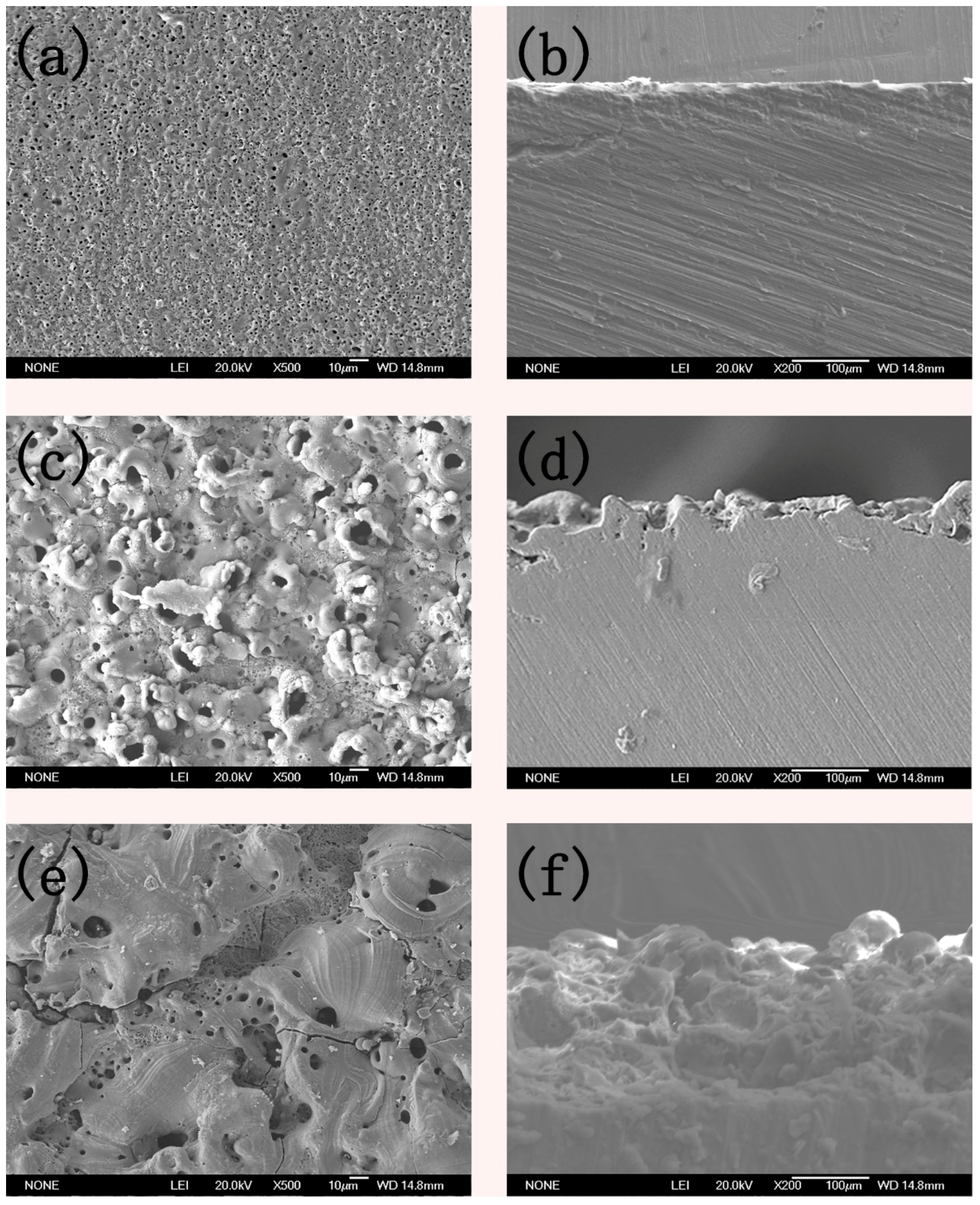
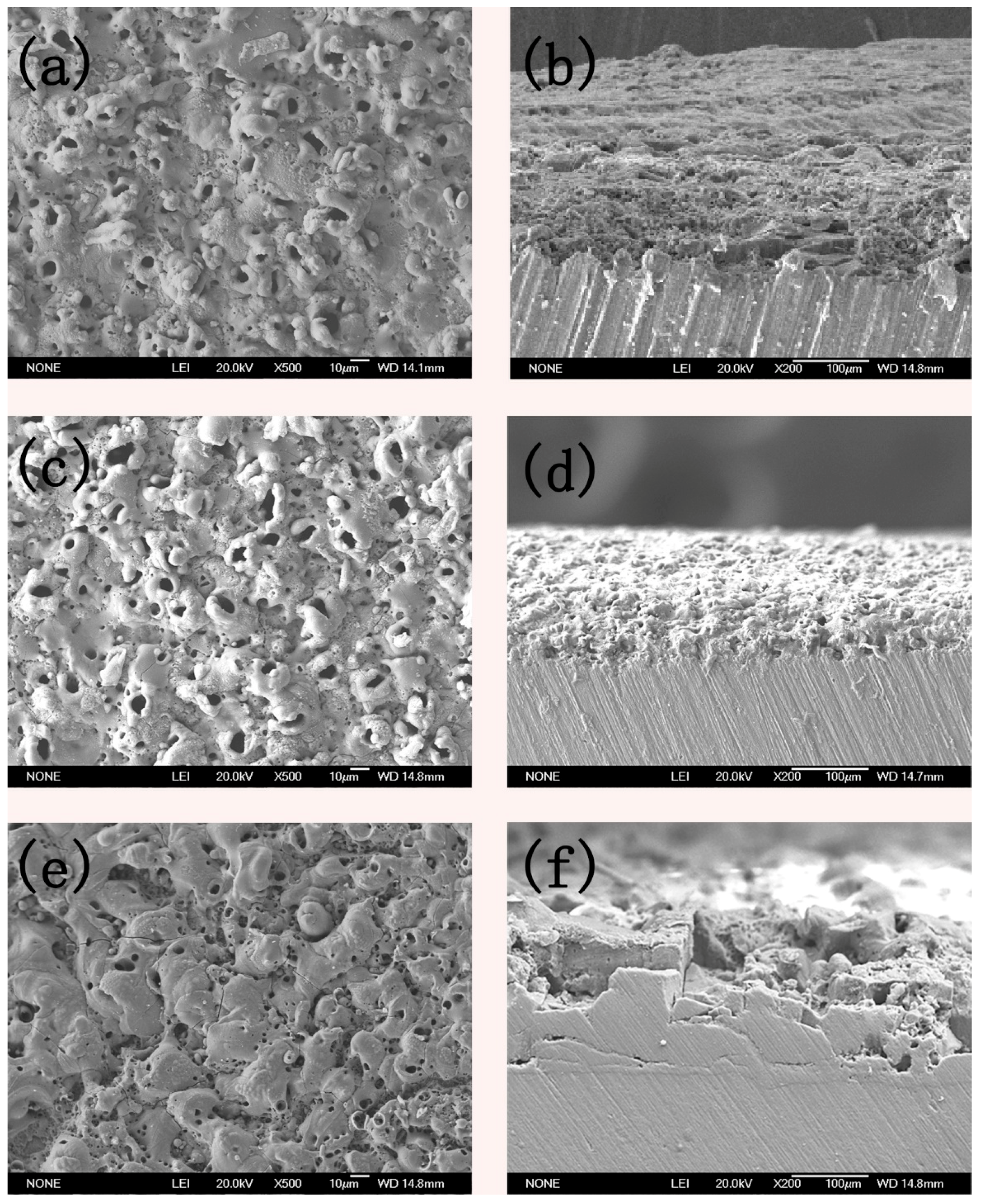
3.3. Micro Morphology of the Coatings
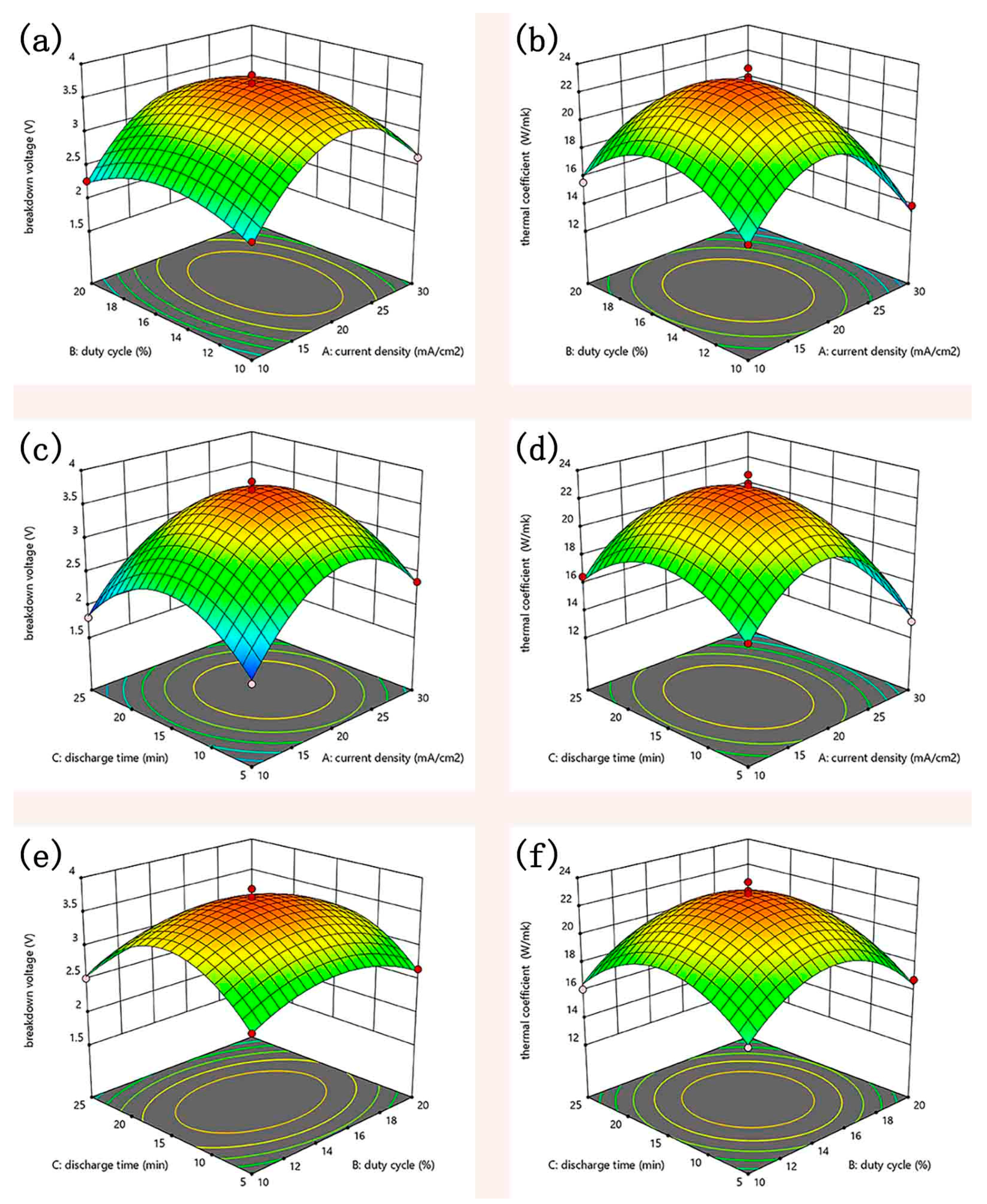
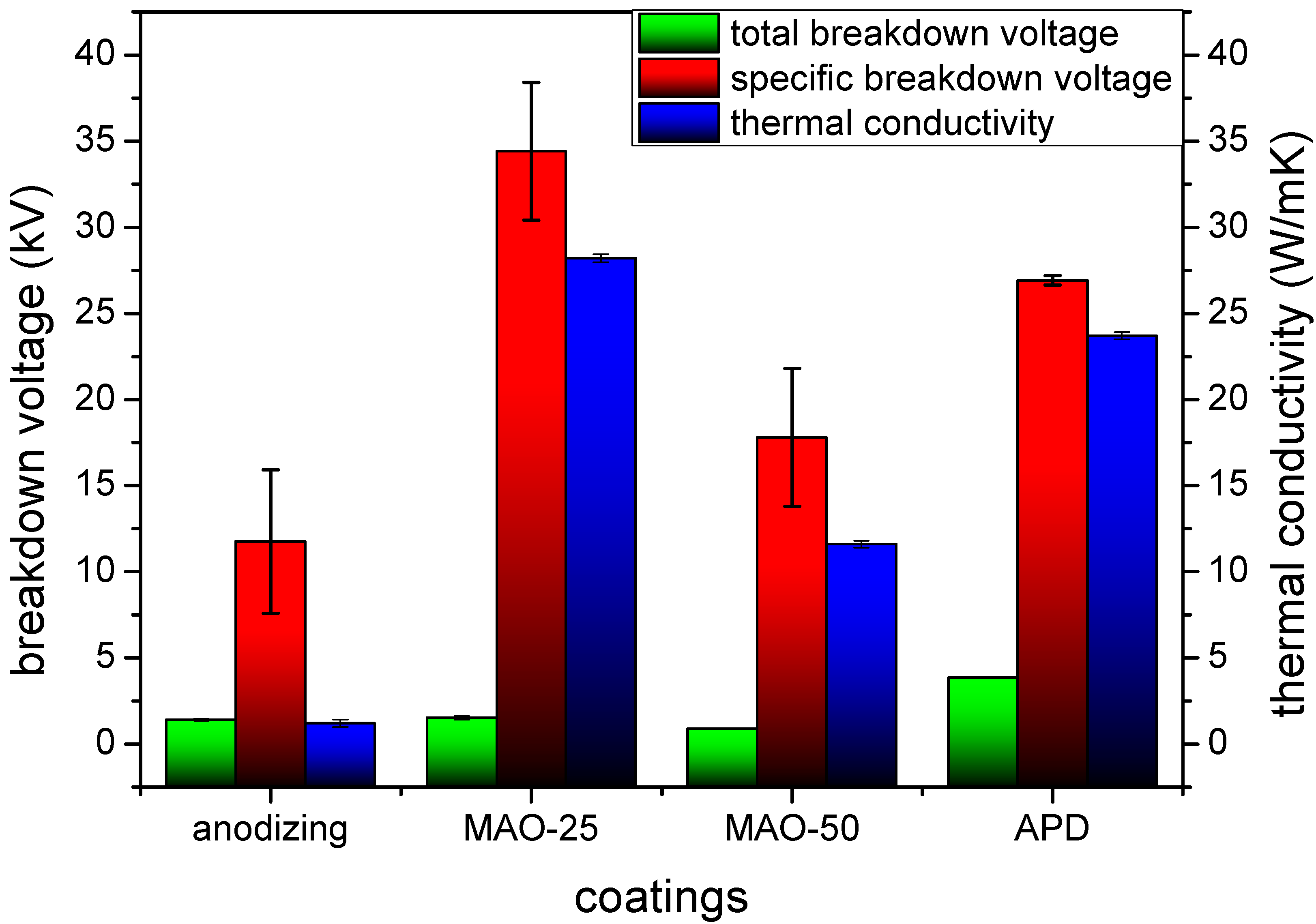
3.4. Coating Performance
4. Conclusions
- A crystalline alumina coating with the thickness of 143 μm was prepared on the pre-anodizing aluminium alloy substrate by the plasma discharge method.
- The crystalline phase composition of the coating had been characterized by XRD. Results proved the amorphous alumina could be transformed into crystalline alumina, the micro-morphology and cross section of the coatings at different plasma discharge conditions had been observed, and results proved the micro pores and micro cracks increased with the plasma discharge intensity.
- The total breakdown voltage and thermal conductivity of APD coating was 3.85 kV and 23.7 W/m·K, much higher than that of anodic and MAO coating. The coating can be applied in the heat dissipation of aluminum alloy, meeting the requirement of the high power and small volume part in the electronic industry, especially in LED development.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsai, W.Y.; Huang, G.R.; Wang, K.K.; Chen, C.F.; Huang, J.C. High thermal dissipation of al heat sink when inserting ceramic powders by ultrasonic mechanical coating and armoring. Materials 2017, 10, 454. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Z.; Guan, Y.C.; Zheng, H.Y. Enhancement of heat dissipation by laser micro structuring for led module. Polymers 2018, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.C.; Wang, Y.M.; Xu, S.M. Superhydrophobic double-layer coating for efficient heat dissipation and corrosion protection. Chem. Eng. J. 2019, 362, 638–649. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, D.; Chung, W. Heat dissipation performance of metal-core printed circuit board prepared by anodic oxidation and electroless deposition. Thermochim. ACTA 2014, 589, 278–283. [Google Scholar] [CrossRef]
- Heo, Y.J.; Kim, H.T.; Kim, K.J.; Nahm, S.; Yoon, Y.J.; Kim, J. Enhanced heat transfer by room temperature deposition of AlN film on aluminum for a light emitting diode package. Appl. Therm. Eng. 2013, 50, 799–804. [Google Scholar] [CrossRef]
- Juntunen, E.; Sitomaniemi, A.; Tapaninen, O.; Persons, R.; Challingsworth, M.; Heikkinen, V. Thermal performance comparison of thick-film insulated aluminum substrates with metal core pcbs for high-power led modules. IEEE. Trans. Compon. Packag. Manuf. Technol. 2012, 2, 1957–1964. [Google Scholar] [CrossRef]
- Zhu, W.L.; Wang, C.Z.; Sun, M.C.; Li, S.M.; Zhai, J.W.; Lai, T.S. Effect of periodic number of [Si/Sb80Te20]x multilayer film on its laser-induced crystallization studied by coherent phonon spectroscopy. Nanoscale Res. Lett. 2012, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Chen, C.M.; Horng, R.H.; Wuu, D.S. An efficient metal-core printed circuit board with a copper-filled through (blind) hole for light-emitting diodes. IEEE. Electron Device Lett. 2012, 34, 105–107. [Google Scholar] [CrossRef]
- Liang, C.; Kumari, N.; Hou, Y. Thermal resistances of crystalline and amorphous few-layer oxide thin films. AIP Adv. 2017, 7, 115205. [Google Scholar]
- Fricke, S.; Friedberger, A.; Schmid, U. The influence of plasma power on the temperature dependant conductivity and on the wet chemical etch rate of sputter-deposited alumina thin films. Surf. Coat. Technol. 2009, 203, 2830–2834. [Google Scholar] [CrossRef]
- Liu, H.G.; Xu, Y.D.; Tang, C.L.; Li, Y.; Chopra, N. SiO2 aerogel-embedded carbon foam composite with Co-Enhanced thermal insulation and mechanical properties. Ceram. Int. 2019, 45, 23393–23398. [Google Scholar] [CrossRef]
- Kume, S.; Yasuoka, M.; Lee, S.K.; Kan, A.; Ogawa, H.; Watari, K. Dielectric and thermal properties of AlN ceramics. J. Eur. Ceram. Soc. 2007, 27, 2967–2971. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.C.; Du, H.Y.; Hou, F. Hierarchical structural silica-fiber-woven/ mullitewhisker material prepared by surface etching and gas-phase reaction. RSC Adv. 2014, 4, 24307–24310. [Google Scholar] [CrossRef]
- Jin, L.; Li, P.Z.; Zhou, H.B.; Zhang, W.; Zhou, G.D.; Wang, C. Improving thermal insulation of TC4 using YSZ-based coating and SiO2 aerogel. Prog. Nat. Sci. 2015, 25, 141–146. [Google Scholar] [CrossRef]
- Wen, X.M.; Xiao, Z.Z.; Jiang, T.; Li, J.; Zhang, W.; Zhang, L.; Shao, H.Q. Constructing novel fiber reinforced plastic (frp) composites through a biomimetic approach: Connecting glass fiber with nanosized boron nitride by polydopamine coating. J. Nanomater. 2013, 2013, 155. [Google Scholar] [CrossRef]
- Sayyar, M.; Weerasiri, R.R.; Soroushian, P.; Lu, J. Experimental and numerical study of shape-stable phase-changenanocomposite toward energy-efficient building constructions. Energy Build. 2014, 75, 249–255. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Jung, U.; Chung, W. Thermal conductivity of anodized aluminum oxide layer:The effect of electrolyte and temperature. Mater. Chem. Phys. 2013, 141, 680–685. [Google Scholar] [CrossRef]
- Mao, F.; Yan, G.Y.; Xuan, Z.J.; Cao, Z.Q.; Wang, T.M. Effect of Eu addition on the microstructures and mechanical properties of A356 aluminum alloys. J. Alloy. Compd. 2015, 650, 896–906. [Google Scholar] [CrossRef]
- Sellarajan, B.; Sharma, M.; Ghost, S.K.; Nagaraja, H.S.; Barshilia, H.C. Effect of electrolyte temperature on the formation of highly ordered nanoporous alumina template. Microporous Mesoporous Mater. 2016, 224, 262–270. [Google Scholar] [CrossRef]
- Ateş, S.; Baran, E.; Yazıcı, B. The nanoporous anodic alumina oxide formed by two-step anodization. Thin Solid Films 2018, 648, 94–102. [Google Scholar] [CrossRef]
- Rogov, A.B.; Slonova, A.I.; Mironov, I.V. The influence of homogeneous electrolyte composition onmicroplasma synthesis and characteristics of Fe-containing coatingson A1050 alloy. Appl. Surf. Sci. 2013, 287, 22–29. [Google Scholar] [CrossRef]
- Deng, J.X.; Yun, D.L.; Tan, Y.Q. Design, fabrication and performance of Al2O3/(W0.7Ti0.3)C + Al2O3/TiC multilayered ceramic nozzles. Int. J. Refractory Met. Hard Mater. 2009, 27, 734–739. [Google Scholar]
- Rogov, A.B.; Shayapov, V.R. Correlations between the optical emission spectra and microstructure of microplasma coatings on aluminum 2024 alloy. Appl. Surf. Sci. 2012, 258, 4871–4876. [Google Scholar] [CrossRef]
- Cheng, F.; Jiang, S.Y.; Liang, J. Cavitation erosion resistance of microarc oxidation coating on aluminium alloy. Appl. Surf. Sci. 2013, 280, 287–296. [Google Scholar] [CrossRef]
- Bononi, M.; Giovanardi, R.; Bozza, A.; Mattioli, P. Pulsed current effect on hard anodizing process of 2024-T3 aluminium alloy. Surf. Coat. Technol. 2016, 289, 110–117. [Google Scholar] [CrossRef]
- Pradhan, N.R.; Duan, H.; Liang, J.; Iannacchione, G.S. Specific heat and thermal conductivity measurements for anisotropic and random macroscopic composites of cobalt nanowires. Nanotechnology 2008, 19, 485712. [Google Scholar] [CrossRef]
- Nampi, P.P.; Ghosh, S.; Warrier, K.G. Calcination and associated structural modifications in boehmite and their influence on high temperature densification of alumina. Ceram. Int. 2011, 37, 3329–3334. [Google Scholar] [CrossRef]
- Bosta, M.M.S.A.; Ma, K.J. Influence of electrolyte temperature on properties and infrared emissivity of MAO ceramic coating on 6061 aluminum alloy. Infrared Phys. Technol. 2014, 67, 63–72. [Google Scholar] [CrossRef]
- Dehghannya, J.; Farshad, P.; Heshmati, M.K. Three-stage hybrid osmotic-intermittent microwave-convective drying of apple at low temperature and short time. Dry. Technol. 2018, 36, 1982–2005. [Google Scholar] [CrossRef]
- Arieli, R.; Marmur, A. Decompression sickness bubbles: Are gas micronuclei formed on a flat hydrophobic surface. Respir. Physiol. Neurobiol. 2011, 177, 19–23. [Google Scholar] [CrossRef]
- Patermarakis, G. Thorough electrochemical kinetic and energy balance models clarifying the mechanisms of normal and abnormal growth of porous anodic alumina films. J. Electroanal. Chem. 2014, 730, 69–85. [Google Scholar] [CrossRef]
- Saffari, H.; Sohrabi, B.; Noori, M.R.; Bahrami, H.R.T. Optimal condition for fabricating superhydrophobic Aluminum surfaces with controlled anodizing processes. Appl. Surf. Sci. 2018, 435, 1322–1328. [Google Scholar] [CrossRef]
- Chung, I.C.; Chung, C.K.; Su, Y.K. Effect of current density and concentration on microstructure and corrosion behavior of 6061 Al alloy in sulfuric acid. Surf. Coat. Technol. 2017, 313, 299–306. [Google Scholar] [CrossRef]


| Materials | Dielectric Strength (kV/mm) | Thermal Conductivity (W/m·K) |
|---|---|---|
| amorphous alumina [9] | 20 | 0.205 |
| α-alumina [10] | 20–40 | 26–50 |
| Silica [11] | 3.8 | 1.4 |
| aluminum nitride [12] | 8 | 70–210 |
| insulation gasket [13] | 3 | 1.0–5.0 |
| insulation grease [14] | 2.75–4.2 | 1.0–5.0 |
| insulation adhesive [15] | 3 | 0.5–2.0 |
| graphite sheets [16] | - | end-wise 3.0–5.0 |
| Air [9] | 1 | 0.025 |
| Kind of Acid | Aperture/nm | Coating Thickness /μm | Hole Wall Thickness /nm | Pore Volume / % | Number of Holes Quantity × 109/cm2 |
|---|---|---|---|---|---|
| sulfuric acid [28] | 12 | 200–400 | 0.80 | 7.5 | 76 |
| phosphate acid [29] | 33 | 50–60 | 1.1 | 4 | 19 |
| chromic acid [30] | 24 | 2–5 | 1.09 | 4 | 22 |
| oxalic acid [31] | 17 | 20 | 0.97 | 2 | 35 |
| Composition | Al | Mg | Si | Cu | Cr | Fe | Mn | Zn | Ti |
|---|---|---|---|---|---|---|---|---|---|
| wt % | balance | 0.8–1.2 | 0.4–0.8 | 0.15–0.4 | 0.04–0.35 | 0.7 | 0.15 | 0.25 | 0.15 |
| Experiment Number | Concentration of H2SO4 (g/L) | Concentration of H3PO4 (g/L) | Experiment Number | Concentration of H2SO4 (g/L) | Concentration of H3PO4 (g/L) |
|---|---|---|---|---|---|
| 1 | 37 | 9.5 | 11 | 73.5 | 28.5 |
| 2 | 19 | 12 | 38 | ||
| 3 | 28.5 | 13 | 92 | 9.5 | |
| 4 | 38 | 14 | 19 | ||
| 5 | 55 | 9.5 | 15 | 28.5 | |
| 6 | 19 | 16 | 38 | ||
| 7 | 28.5 | 17 | 185 | 9.5 | |
| 8 | 38 | 18 | 19 | ||
| 9 | 73.5 | 9.5 | 19 | 28.5 | |
| 10 | 19 | 20 | 38 |
| Experiment Number | Current Density (mA/cm2) | Plasma Discharge Time (min) | Duty Cycle (%) |
|---|---|---|---|
| 1 | 10 | 5 | 10 |
| 2 | 20 | 5 | 10 |
| 3 | 50 | 5 | 10 |
| 4 | 20 | 5 | 10 |
| 5 | 20 | 15 | 10 |
| 6 | 20 | 25 | 10 |
| 7 | 20 | 15 | 15 |
| 8 | 20 | 15 | 20 |
| Coating Performance | Current Density | Duty Cycle | Discharge Time | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mA/cm2 | % | min | |||||||
| 10 | 20 | 50 | 10 | 15 | 20 | 5 | 15 | 25 | |
| Breakdown voltage (kV) | 3.24 | 2.59 | 1.06 | 1.84 | 2.23 | 1.77 | 2.12 | 3.81 | 1.61 |
| Thermal conductivity (W/m·K) | 20.1 | 17.7 | 11.3 | 12.15 | 21.14 | 16.82 | 18.1 | 23.7 | 16.2 |
| Std | Run | Factor 1 A:Current Density (mA/cm2) | Factor 2 B:Duty Cycle (%) | Factor 3 C:Discharge Time (min) | Response 1 Breakdown Voltage (kV) | Response 2 Thermal Coefficient (W/m·K) |
|---|---|---|---|---|---|---|
| 5 | 1 | 10 | 15 | 5 | 1.84 | 16.3 |
| 12 | 2 | 20 | 20 | 25 | 2.41 | 16.5 |
| 1 | 3 | 10 | 10 | 15 | 2.33 | 15.8 |
| 4 | 4 | 30 | 20 | 15 | 2.51 | 12.6 |
| 2 | 5 | 30 | 10 | 15 | 2.63 | 13.9 |
| 14 | 6 | 20 | 15 | 15 | 3.64 | 23.7 |
| 3 | 7 | 10 | 20 | 15 | 2.27 | 15.6 |
| 6 | 8 | 30 | 15 | 5 | 2.36 | 13.2 |
| 11 | 9 | 20 | 10 | 25 | 2.52 | 16.1 |
| 7 | 10 | 10 | 15 | 25 | 1.81 | 16.5 |
| 10 | 11 | 20 | 20 | 5 | 2.66 | 16.8 |
| 15 | 12 | 20 | 15 | 15 | 3.81 | 22.5 |
| 13 | 13 | 20 | 15 | 15 | 3.61 | 23.1 |
| 16 | 14 | 20 | 15 | 15 | 3.72 | 22.9 |
| 8 | 15 | 30 | 15 | 25 | 2.16 | 13.1 |
| 17 | 16 | 20 | 15 | 15 | 3.68 | 21.9 |
| 9 | 17 | 20 | 10 | 5 | 2.63 | 16.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Jiang, B.; Ji, D. Fabrication of a Thick Crystalline Al2O3 Coating with Insulation and High Thermal Conductivity via Anodic Oxidation and Subsequent Mic Arc Discharge Treatment. Coatings 2020, 10, 38. https://doi.org/10.3390/coatings10010038
Song W, Jiang B, Ji D. Fabrication of a Thick Crystalline Al2O3 Coating with Insulation and High Thermal Conductivity via Anodic Oxidation and Subsequent Mic Arc Discharge Treatment. Coatings. 2020; 10(1):38. https://doi.org/10.3390/coatings10010038
Chicago/Turabian StyleSong, Wei, Bailing Jiang, and Dongdong Ji. 2020. "Fabrication of a Thick Crystalline Al2O3 Coating with Insulation and High Thermal Conductivity via Anodic Oxidation and Subsequent Mic Arc Discharge Treatment" Coatings 10, no. 1: 38. https://doi.org/10.3390/coatings10010038
APA StyleSong, W., Jiang, B., & Ji, D. (2020). Fabrication of a Thick Crystalline Al2O3 Coating with Insulation and High Thermal Conductivity via Anodic Oxidation and Subsequent Mic Arc Discharge Treatment. Coatings, 10(1), 38. https://doi.org/10.3390/coatings10010038




