4.1. Thermodynamic Calculation on the Formation Oxides
Due to the short isothermal holding times and the complex thermal cycle used in heat treatments, the selective oxidation behaviors during continuous annealing in an HDG line was a complex process. Therefore, it was difficult to analyze the mechanism of the selective oxidation process for the hot dip galvanized medium manganese lightweight steel. In the present work, aided by thermo-calc software, the effect of dew point and the Mn content on the thermodynamic stability of oxides were investigated based on the work of Suzuki et al. [
9]. In their thermodynamic model of selective oxidation, it was assumed that the local thermodynamic equilibrium was established between the oxide layer and metal ion diffusion layer during annealing process. In addition, the thermodynamic data were provided by the self-built database [
10,
11,
12] and SSUB6, respectively. The oxide stability determined by P(O
2) corresponding to different Mn content could be calculated, as shown in
Figure 7.
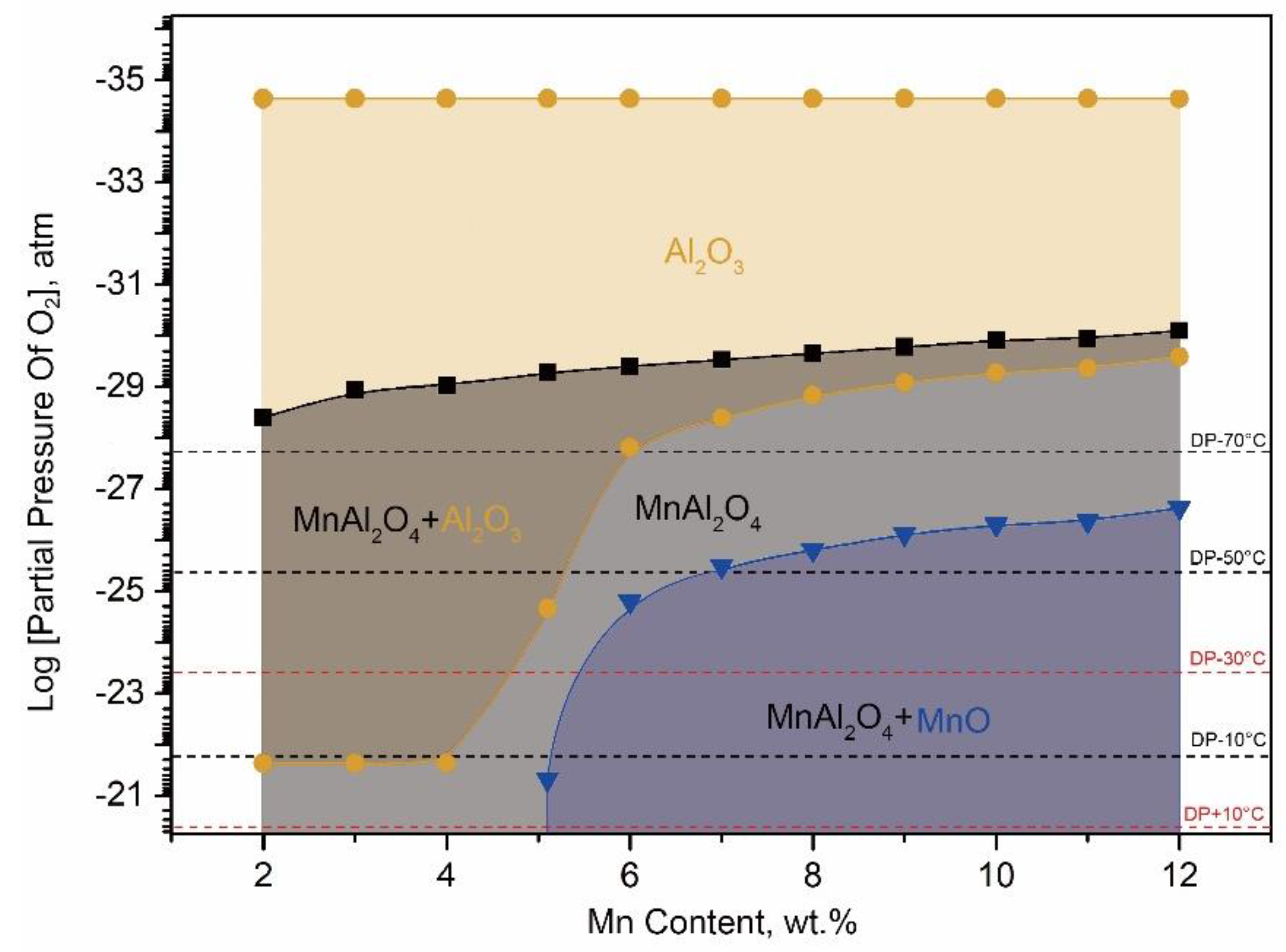
From
Figure 7 and
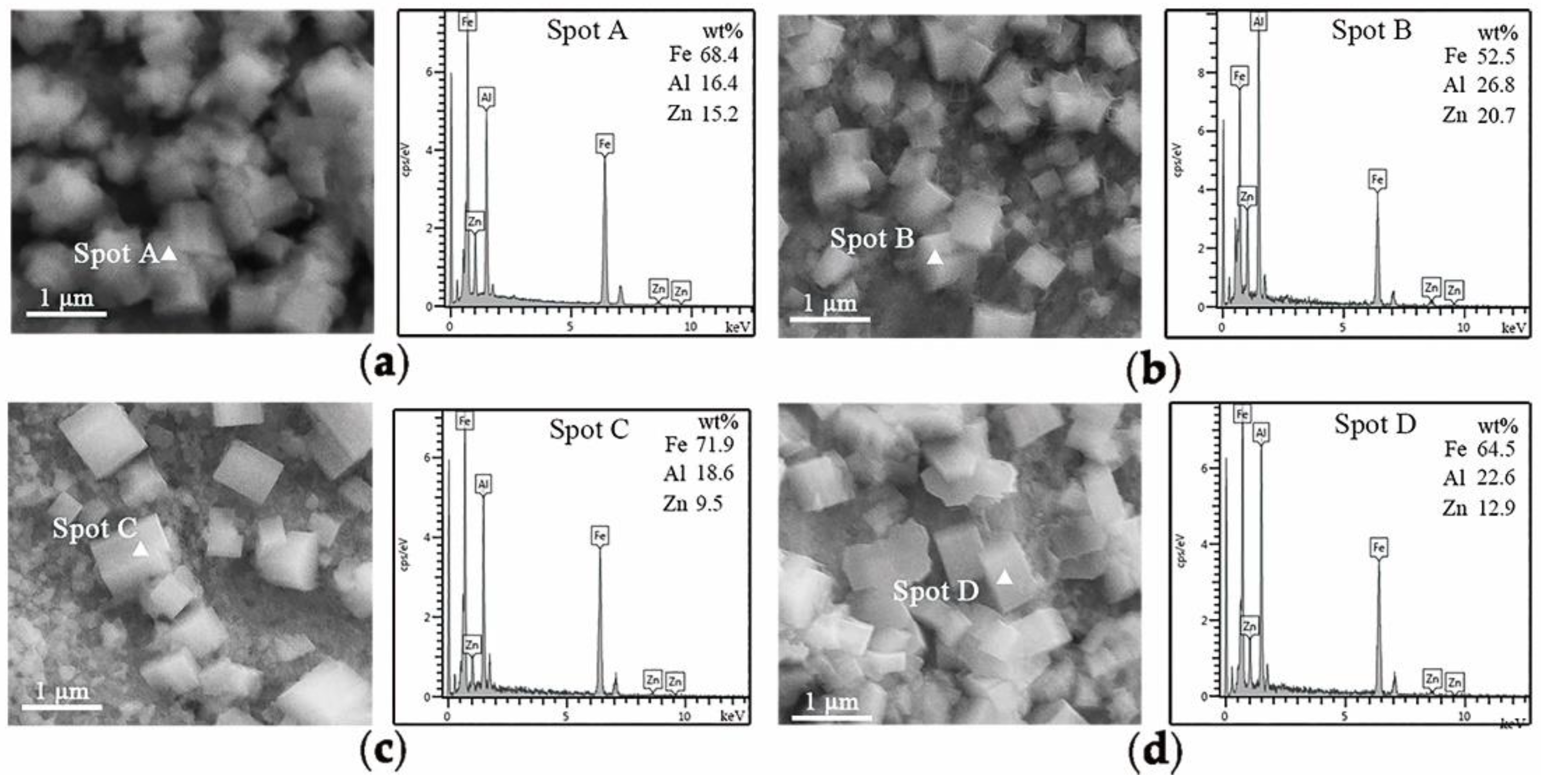
Table 2, it was shown that the stable oxides could be formed on the surface of each kind of experimental steel under the corresponding conditions. It could be seen that MnAl
2O
4 was the most stable surface oxides. This kind of composite oxide composed of alumina oxide and manganese oxide was reported by Bellhouse. [
13], which appeared in the form of xMnO·Al
2O
3. There was a reaction for the composition of the MnAl
2O
4 [
14]:
For 10Mn steels, MnO would be the stable external oxide on the surface. From
Figure 7, it can be seen that the higher oxygen content, the more MnO content. According to the experimental results as mentioned above, from
Figure 3,
Figure 4,
Figure 5 and
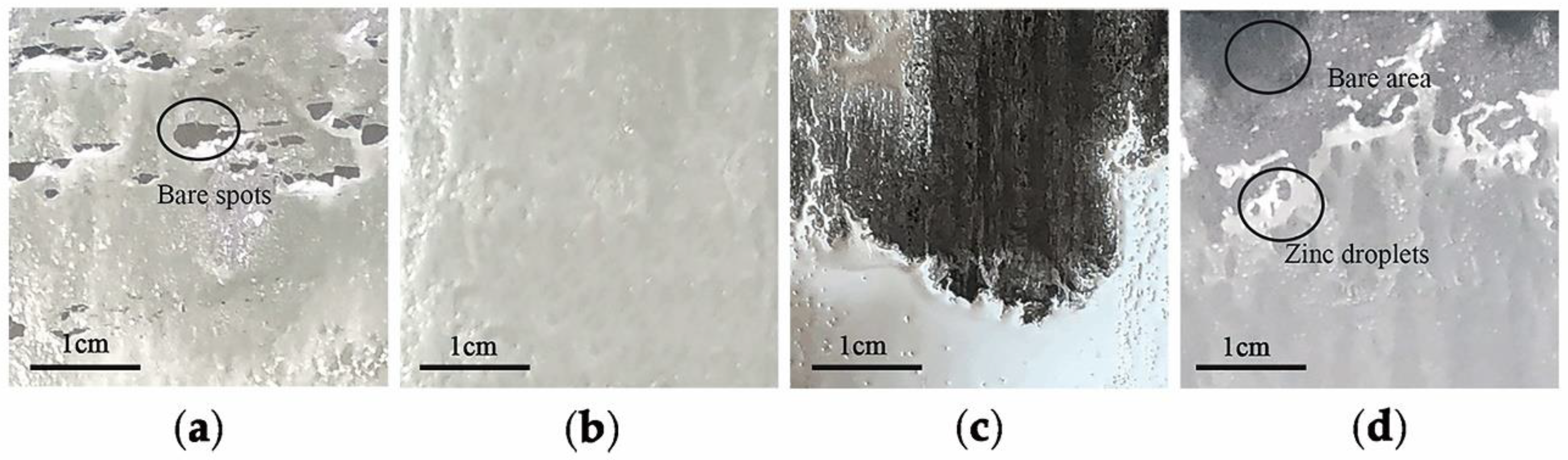
Figure 6, MnO particles remained much less for 3Mn steels than 10Mn steels. Thus, the overall coating quality of 10Mn steels must be worse than 3Mn steels under corresponding conditions because MnO layer led to a severe deterioration of the liquid zinc wettability. It can especially be seen from
Figure 7 that the MnO was able to form a stable external oxide layer on the surface of medium Mn lightweight steel with 5 wt.% Al as long as Mn content exceeded 5.1 wt.%. That is to say, MnO was the stable external oxide for the medium Mn lightweight steel above 5 wt.% Mn content during HDG. It was possible to lead the surface coating defect because the MnO layer was too thick to be reduced in aluminothermic reduction reaction.
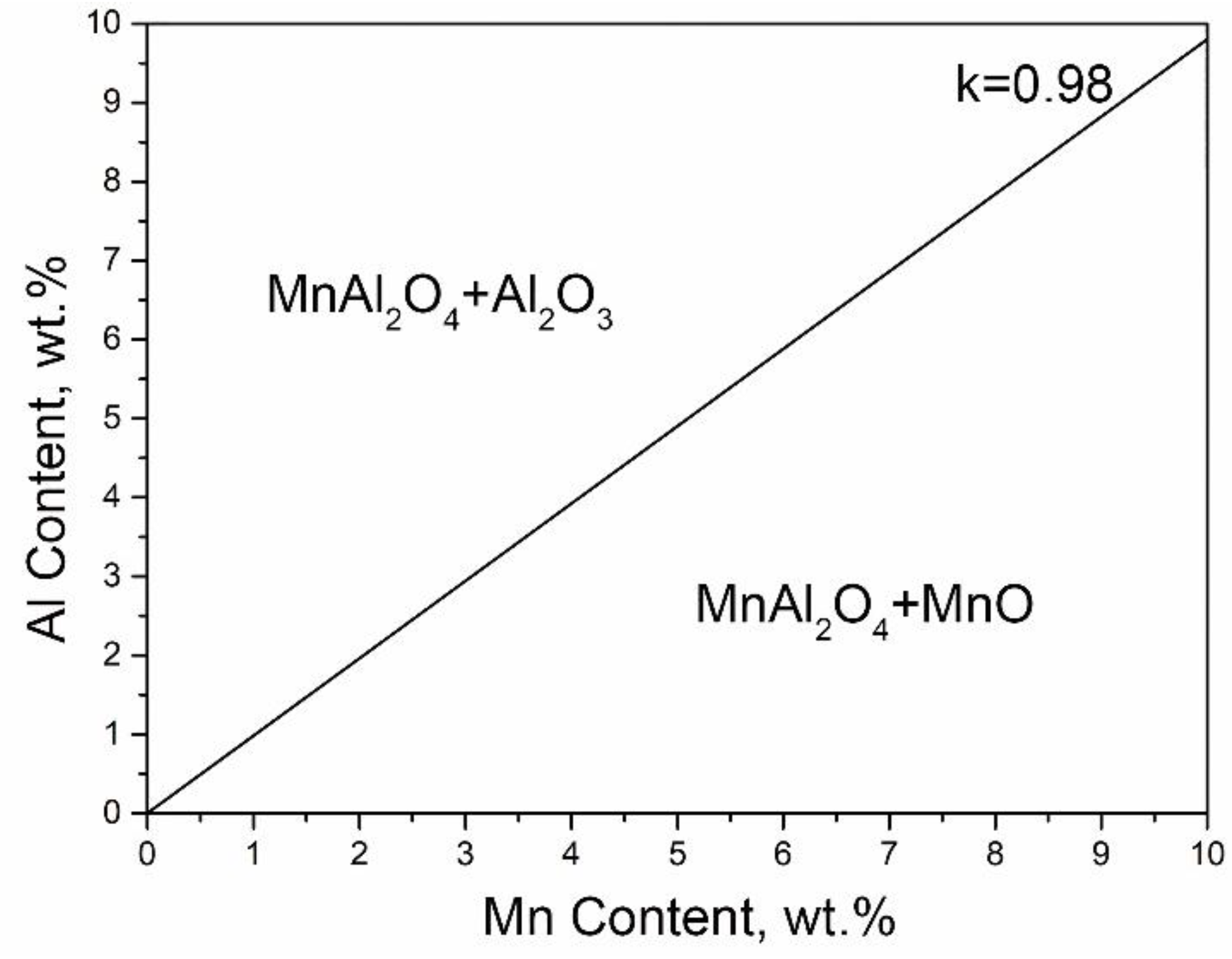
In order to explain the effect of alloy composition on the type of selective oxidation products under the same DP condition, the mass fraction of Al/Mn in MnAl
2O
4 was considered as:
Under the thermodynamic equilibrium condition, the effect of Al/Mn on the type of oxide was calculated. As shown in
Figure 8, if the Al/Mn ratio in the alloy composition was more than 0.98, excess Al would lead to the consumption of Mn to generate MnAl
2O
4, thus the remaining compound may be Al
2O
3. It corresponded to the oxidation behavior of 3Mn steels. On the other hand, once the ratio was less than 0.98, the surplus Mn would oxidize into MnO, which is consistent with 10Mn oxidation behavior.
It was noted that the coexistence of MnAl2O4 and MnO could be observed at the coating/substrate interface of the galvanized 3Mn steel annealed under the +10 °C DP process atmosphere. However, for the 3Mn steel under the −30 °C DP, the coexistence of MnAl2O4 and Al2O3 could be found. One possible reason lied in manganese oxides would form as the “unstable oxides” under the non-thermodynamic equilibrium condition due to the short isothermal holding times. Another possible reason was the preferentially generated Al2O3 formed in deeper layer from the surface because of the higher affinity of Al to oxygen. Moreover, the higher dew point, the less tendency to form Al2O3 at surface.
4.2. Reactive wetting
It was well known that the type, thickness, and morphology of external oxides will influence the reactive wetting behavior. According to the work of Kawano [
2] the wetting angle of the zinc solution on MnO layer would be maintained at 114° and not change with the thermite reduction in the zinc bath, so the presence of MnO always had adverse effects on the wettability. The wetting angle of the zinc solution on Al
2O
3 was always less than 90°, which seemed to be the static wetting. Moreover, the Al
2O
3 tended to form internal oxidation so it had little effect on galvanizability. The MnAl
2O
4, as a common oxide on the surface of galvanized CMnAl steels, also has little effect on coating quality [
15].
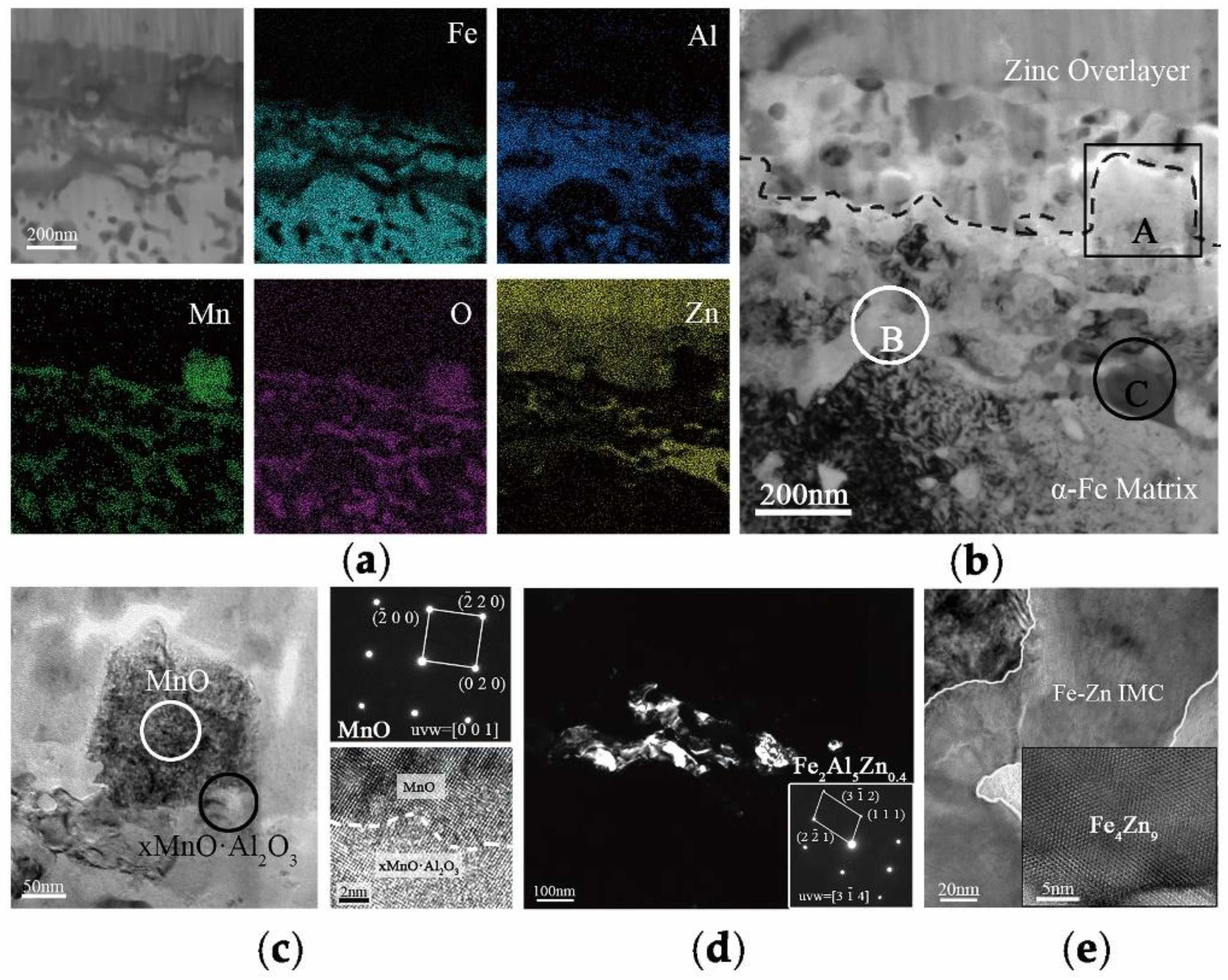
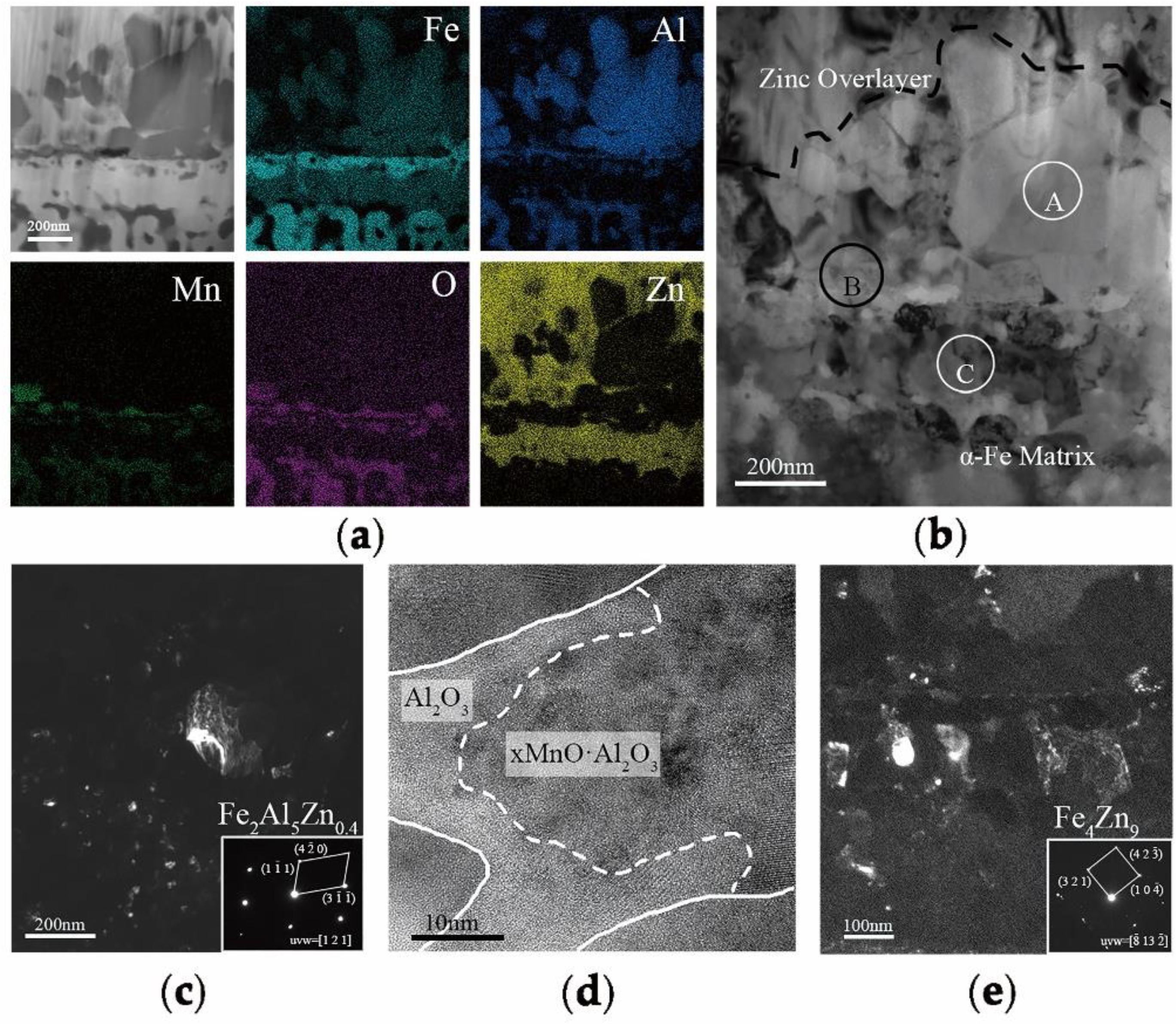
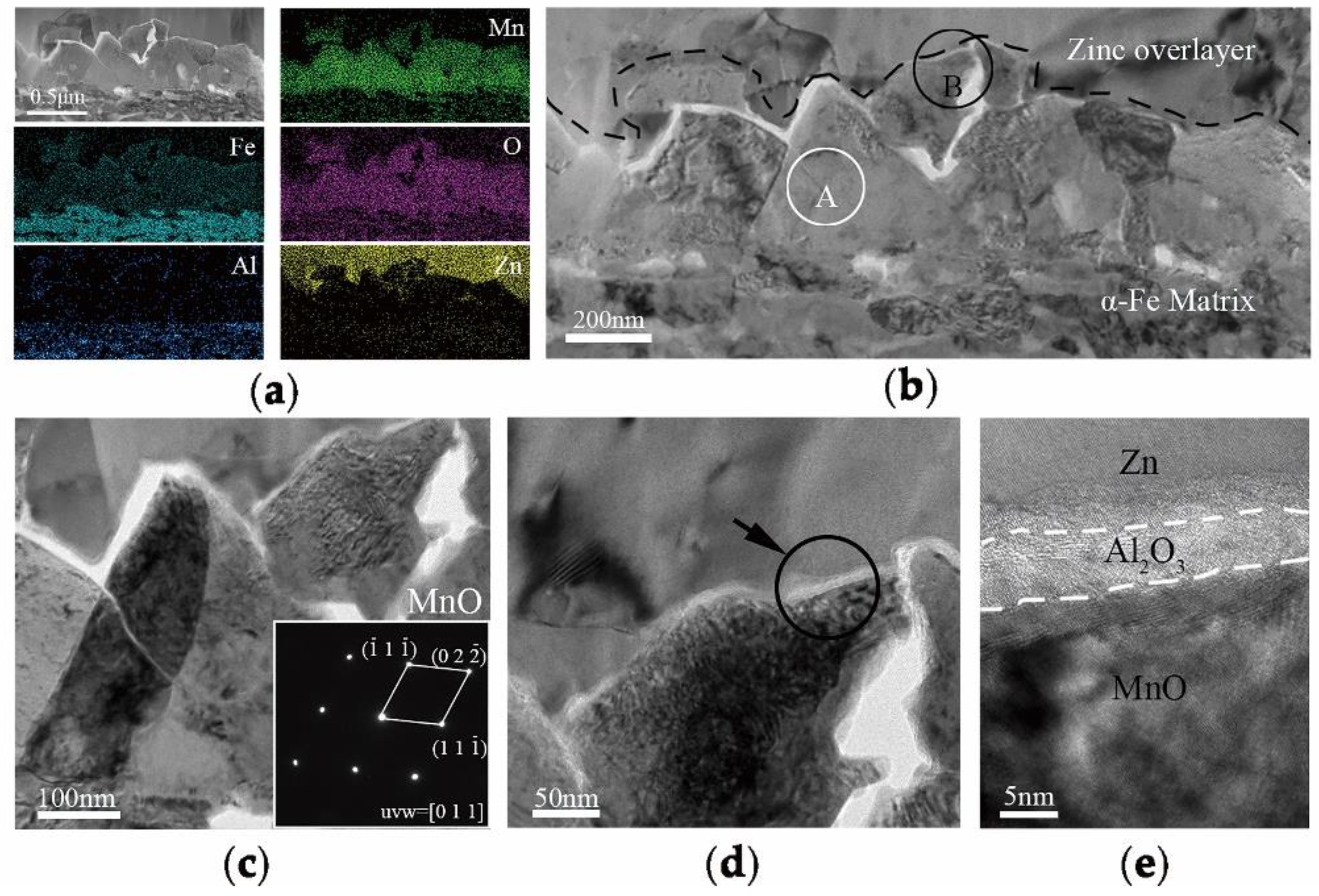
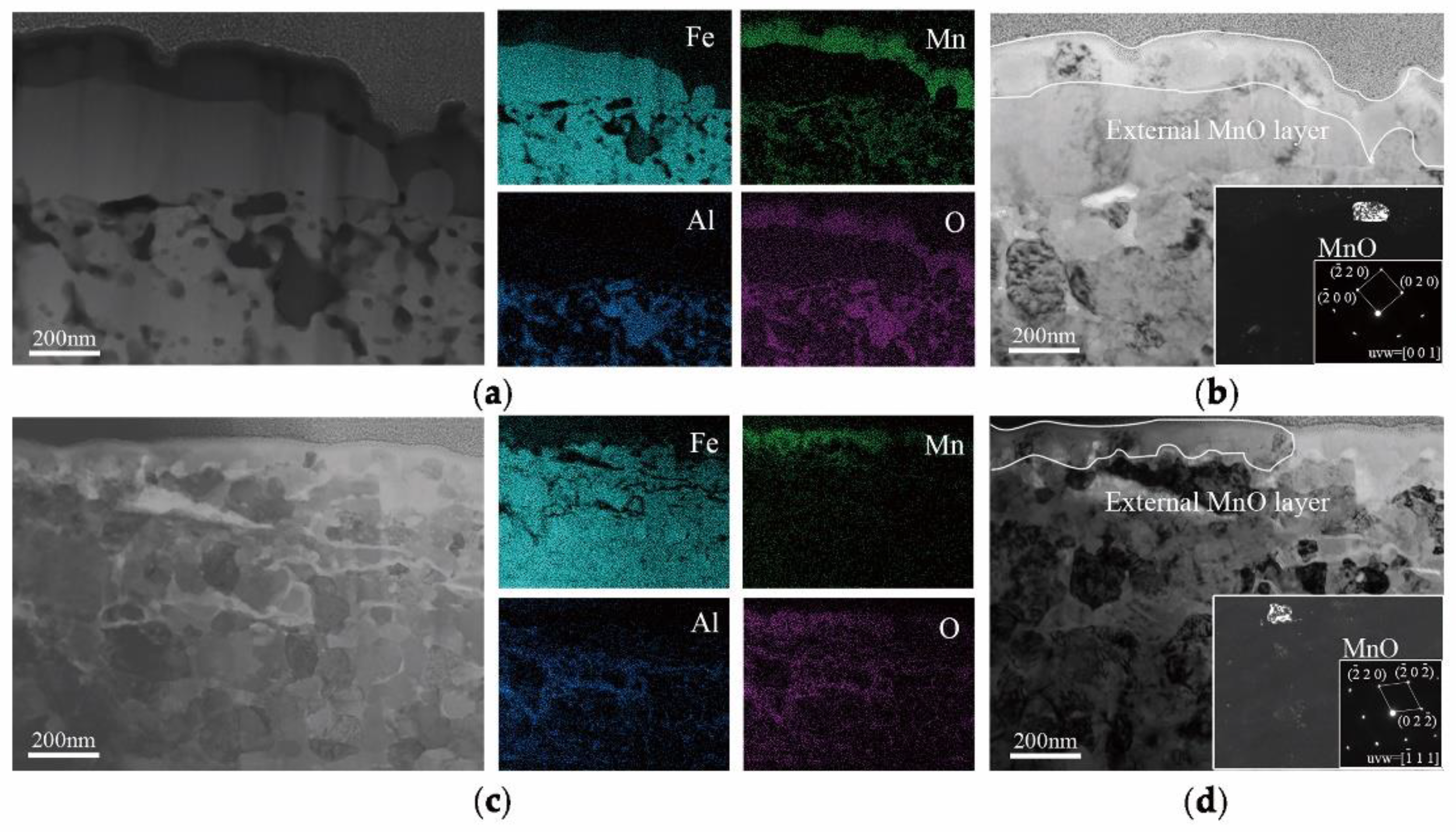
In order to explain the selective oxidation of 3Mn +10 °C and 3Mn −30 °C, the formation of oxide on the surface was also analyzed by TEM, as shown in
Figure 9. It was shown that the thickness and morphology of MnO external oxide layer was different comparing the two samples. For the sample annealed at high dew point +10 °C, the thickness of continuous MnO layer was up to 200 nm, and it was hard to be reduced completely during galvanization process in 4 s. Hence, there was still MnO particles remained on the surface of the sample 3Mn +10 °C, as shown in
Figure 3. On the contrary, for the 3Mn steel annealed at low dew point −30 °C, the maximum thickness MnO layer was only half that of the former and distributed discontinuously. Obviously, it is easily reduced completely during galvanization. It was known that the thickness and morphology of the oxide film was also the key to galvanizing quality, since the different reactive wetting behavior was observed with the same kind of MnO oxides. It could be further concluded that the high DP atmosphere condition results in the formation of thick film-type external oxides, whereas the low DP atmosphere condition could make the MnO layer thinner and form the discontinuous particle-type external MnO.
Khondker et al. [
8] showed that the reduction of MnO by the solute Al in the Zn bath was, in principle, thermodynamically possible. According to Kavitha and McDermid recent work [
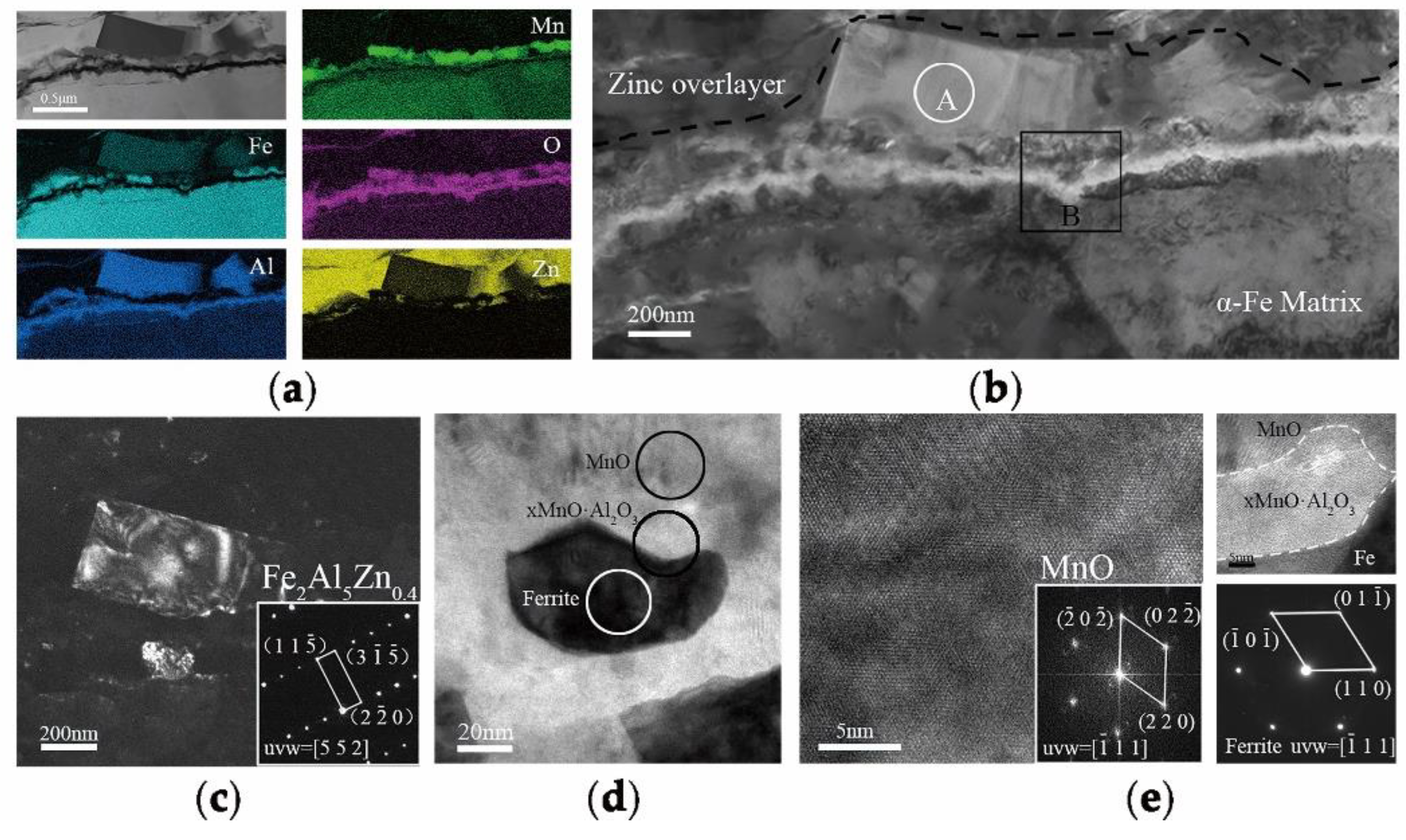
16], the thickness of a MnO layer was significantly reduced for increasing reaction times and the product of the aluminothermic reaction could be detected at the MnO/Zn interface. The abovementioned provided the evidence for the removal of the oxide film by aluminothermic reduction: Compared with the 3Mn oxidation samples shown in
Figure 9, the MnO layer of 3Mn galvanizing samples was reduced a lot, especially at the DP −30 °C. For the two 10Mn steel samples, the reduction product Al
2O
3, which was observed on the outermost of whole interface layer or inside zinc coating also proved the existence of reduction reaction.
Another way to form the inhibition layer depended on the “bridging connection” through grain boundaries below external oxide layer. Lawrence [
17] reported the oxides located on the top of inhibition layer. When the oxide layer was cracked but the iron matrix did not expose enough area to react with the aluminum in the zinc solution, zinc atoms would diffuse across grain boundaries and connected to formed inhibition layer on subsurface. In this study, this kind of formation process of inhibition layer only happened on 3 Mn steels, and it was the main way to form the inhibition layer of 3Mn +10 °C sample. Therefore, it would be likely to generate a lot of brittleness Fe−Zn intermetallic compounds, which may largely deteriorate the mechanical property of medium Mn lightweight steel.
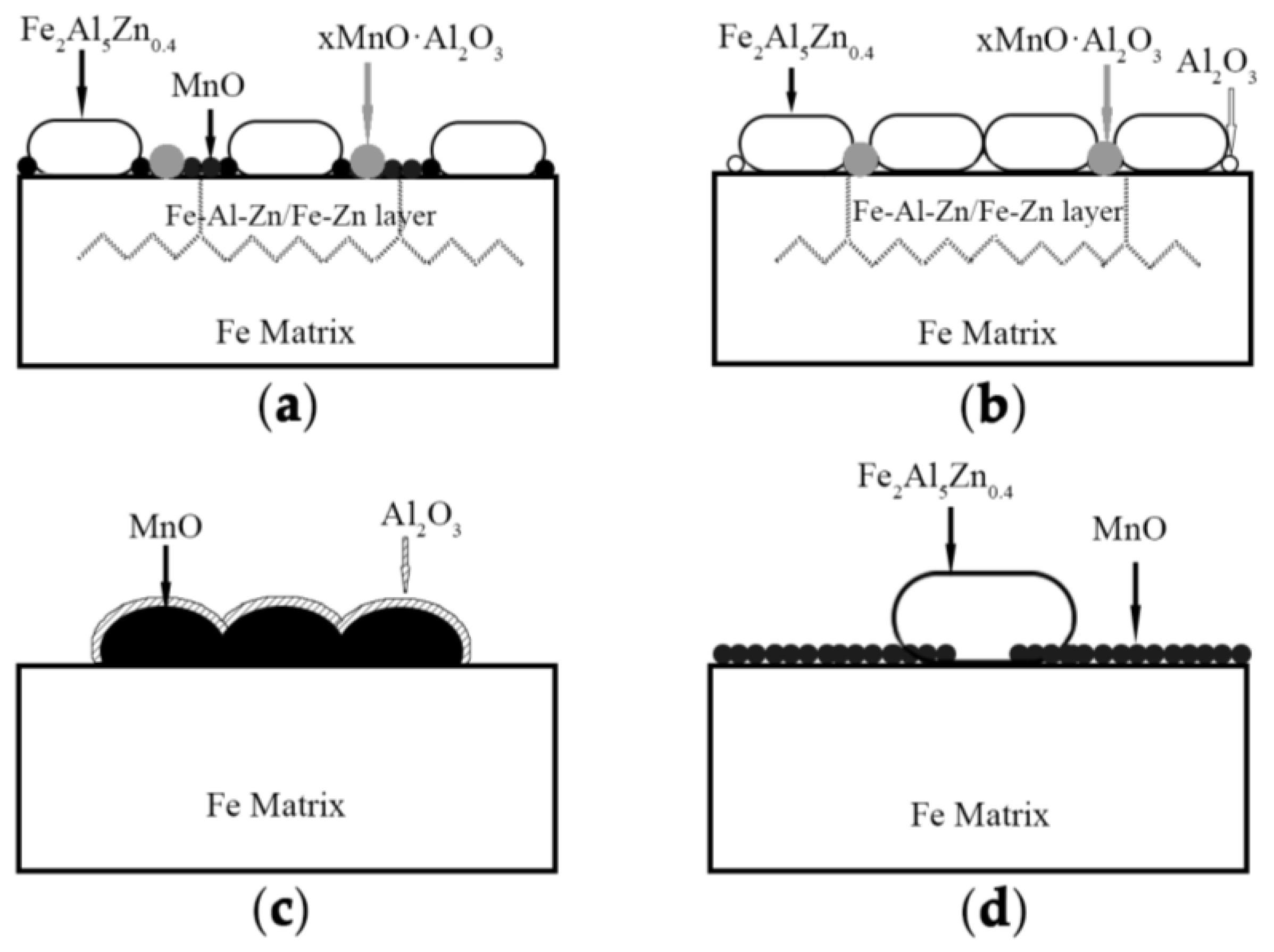
In summary, the reactive wetting mechanism and the comprehensive influence of alloy content and dew points for that steel could be explained by means of the schematic shown in
Figure 10.
At the same dew point, however, there exhibited significantly different ways for reactive wetting between 3Mn steels and 10Mn steels by comparing
Figure 10a with
Figure 10c and
Figure 10b with
Figure 10d, respectively. For the relatively low Mn content samples, see
Figure 10a. Al in zinc bath could partially reduce the MnO, which led to the breaking of the continuity of the external oxide layer. Thus, the small part of Fe was exposed and involved in the reaction to nucleate the Fe
2Al
5Zn
0.4 grains. As for the remaining MnO particles, though it hampered the reactive wetting, it distributed sparsely so that the zinc atoms diffused into the subsurface through grain boundaries. Such formation of the special structure consisted of Fe layer and Fe−Al−Zn layer resulting from “bridging connection”, which improved the good effect of reactive wetting and the galvanizability.
The reactive wetting behavior was quite different for the relatively high Mn content samples, see in
Figure 10c. The surface was covered by manganese oxide layer with about 0.5 μm in thickness, and the Fe−Al−Zn grains were dispersedly distributed in the layer. Compared with low Mn content samples, the one possible reason of poor galvanizability for the higher Mn sample lay in the decreasing exposed areas of reduced Fe substrate brought a consequent destruction of inhibition layer. Another possible reason was the absence of “bridging connection” effect, which weakened the degree of reaction wettability. In addition, comparing
Figure 10b with
Figure 10d, the thicker layer of MnO that formed on the surface of 10 Mn −30 °C resulted in the low nucleation rate and the abnormal grain growth of inhibition layer. For the 3Mn −30 °C, the discontinuous external MnO layer could be completely reduced by aluminothermic reduction and the occurrence of “bridging connection”.
For the same manganese content, the ways of reactive wetting at dew point +10 °C and dew point −30 °C were also different. The reactive wetting was improved at low dew point. Because the thin particle-type MnO layer that formed at low dew point can be reduced completely, together with the diffusion of zinc atoms, the uniform growth of the inhibition layer was promoted. For the high Mn sample at low DP (see
Figure 10d), though MnO was the stable oxide, the remaining surface oxide after aluminum thermal reduction reaction was far less than that at high dew point. It could be seen that the adverse effect of selective oxidation could be alleviated by lowering the dew point. It was noted that the effect of DP on the galvanizability of 10Mn steel was limited, because the external oxide layer (stable MnO) was too thick to be broken by reactive wetting. The influence of manganese content was more than that of dew point once the Mn exceeding 5.1wt.% for medium manganese steel, because the thermodynamically stable MnO would form on the surface of the sample with 10wt.%Mn content and led to the deterioration of galvanizing.