A Novel Polyurethane-Based Polyion Complex Material with Tunable Selectivity against Interferents for Selective Dopamine Determination
Abstract
1. Introduction
| PIC Materials | Analyte | Molecular Weight | Sensitivity (nA/μM×cm2) | Reference | |
|---|---|---|---|---|---|
| Polyanion | Polycation | ||||
| DNA | Poly-L-lysine (PLL) | H2O2 | 34 | 6.6 ± 0.3~7.1 ± 0.3 | [17] |
| L-Ascorbic acid | 176 | 5.1 ± 0.9~11.1 ± 0.9 | |||
| Urate | 168 | 6.3 ± 0.9~15.1 ± 1.1 | |||
| Dopamine | 158 | 4.2 ± 0.4~7.6 ± 1 | |||
| 4-Acetaminophen | 151 | 23.4 ± 1.7~26.3 ± 2 | |||
| DNA-Cu (II) | Poly(allylamine) hydrochloride (PAA) | H2O2 | 34 | 0.03 ~45.8 | [18] |
| Poly(sodium-4-styrenesulfonate) (PSS) | PLL | Urea | 60 | 1.7 | [19] |
| Poly(acrylic acid) | PLL | Glucose | 180 | Not Given | [15] |
| Poly(acrylic acid) | PAA | ||||
| PSS | PLL | Glutamate | 147 | 20 | [13] |
| Lactate (chemiluminescent biosensor) | 90 | Not Given | [10] | ||
| Polyurethane-based anion | Polyurethane-based cation | Dopamine | 158 | 68.9~160.1 | This work |
| L-Ascorbic acid | 176 | 14.1~203.2 | |||
| Uric acid | 168 | 6.9~85.1 | |||
2. Materials and Methods
2.1. Reagents and Materials
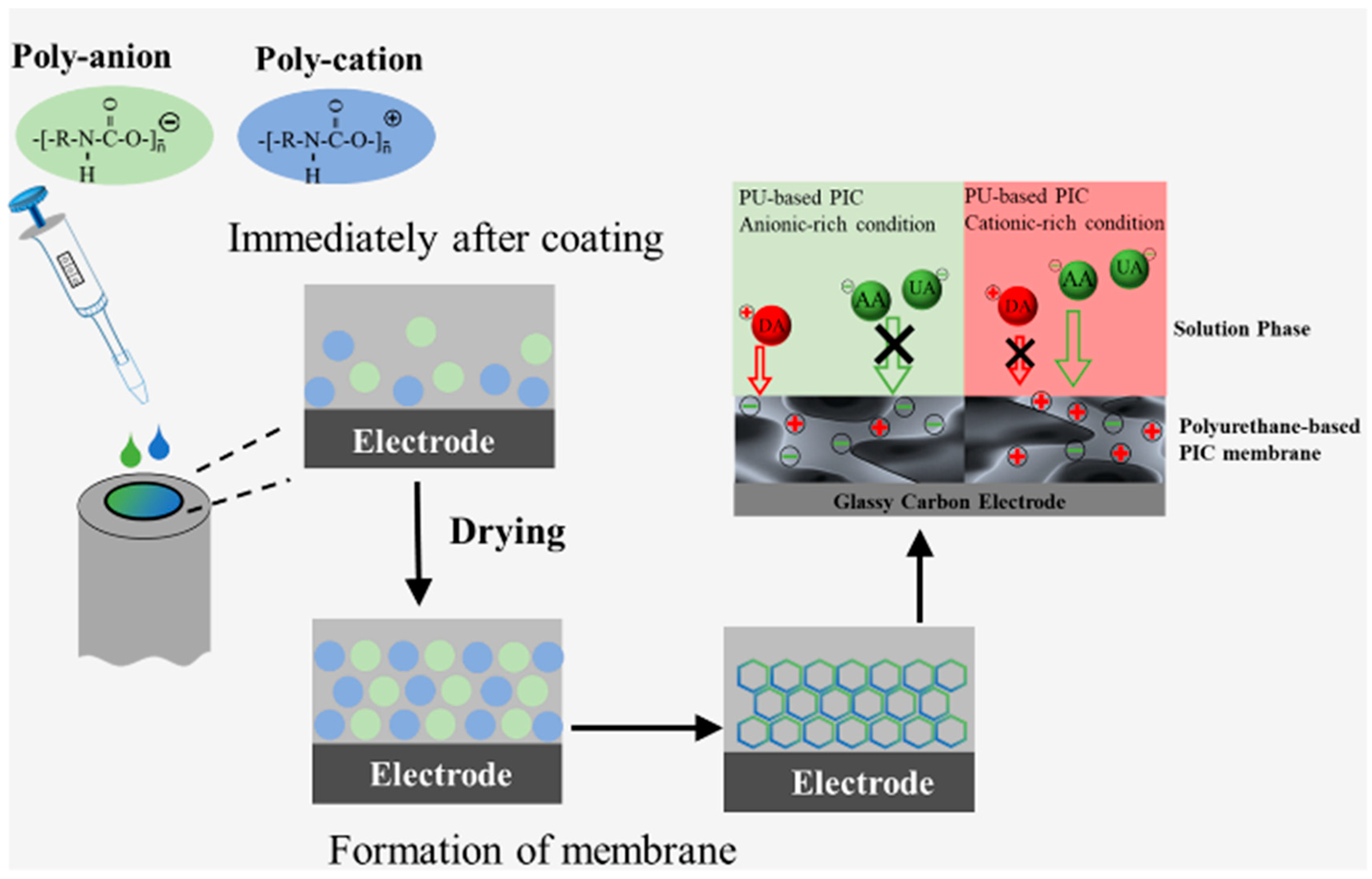
2.2. Preparation of PIC Membrane Modified-Electrodes
2.3. Apparatus
2.4. Electrochemical Analysis
3. Result and Discussion
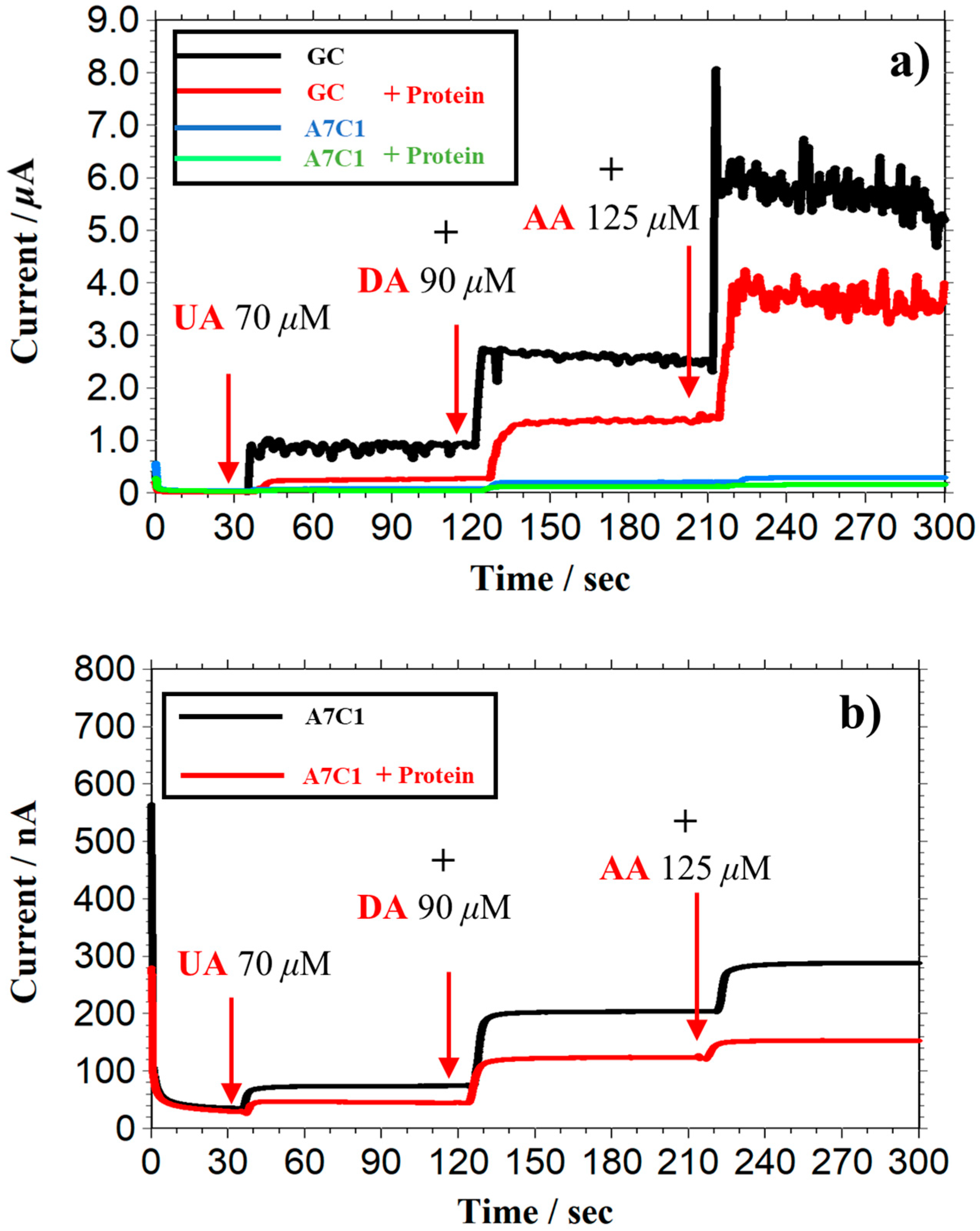
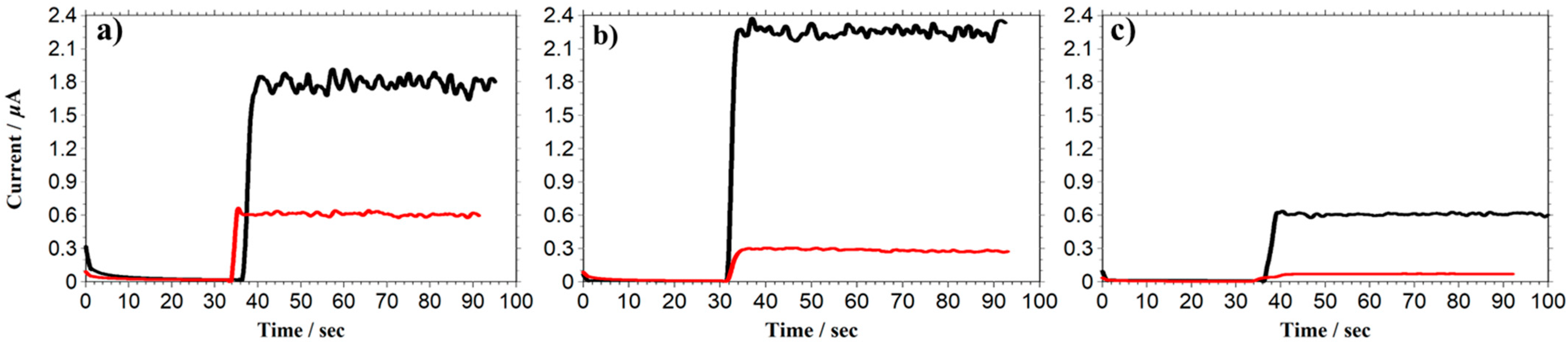
3.1. Selectivity of PU-Based PIC-Membrane-Modified Electrodes
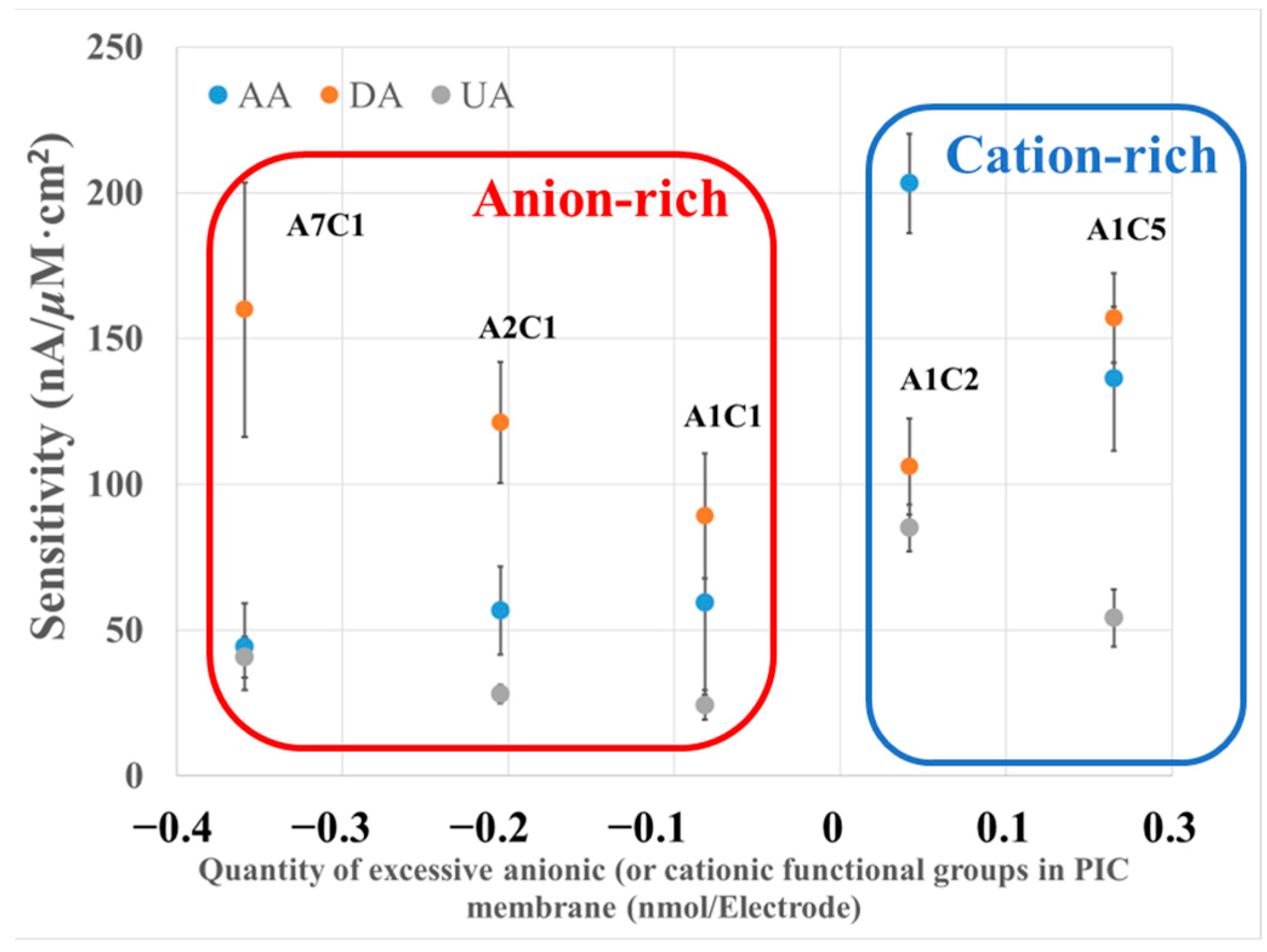
3.2. Study of the Effect of Excessive Functional Groups on PIC Selectivity
3.3. Optimization of PIC Thickness and Density for DA Biosensor Selectivity
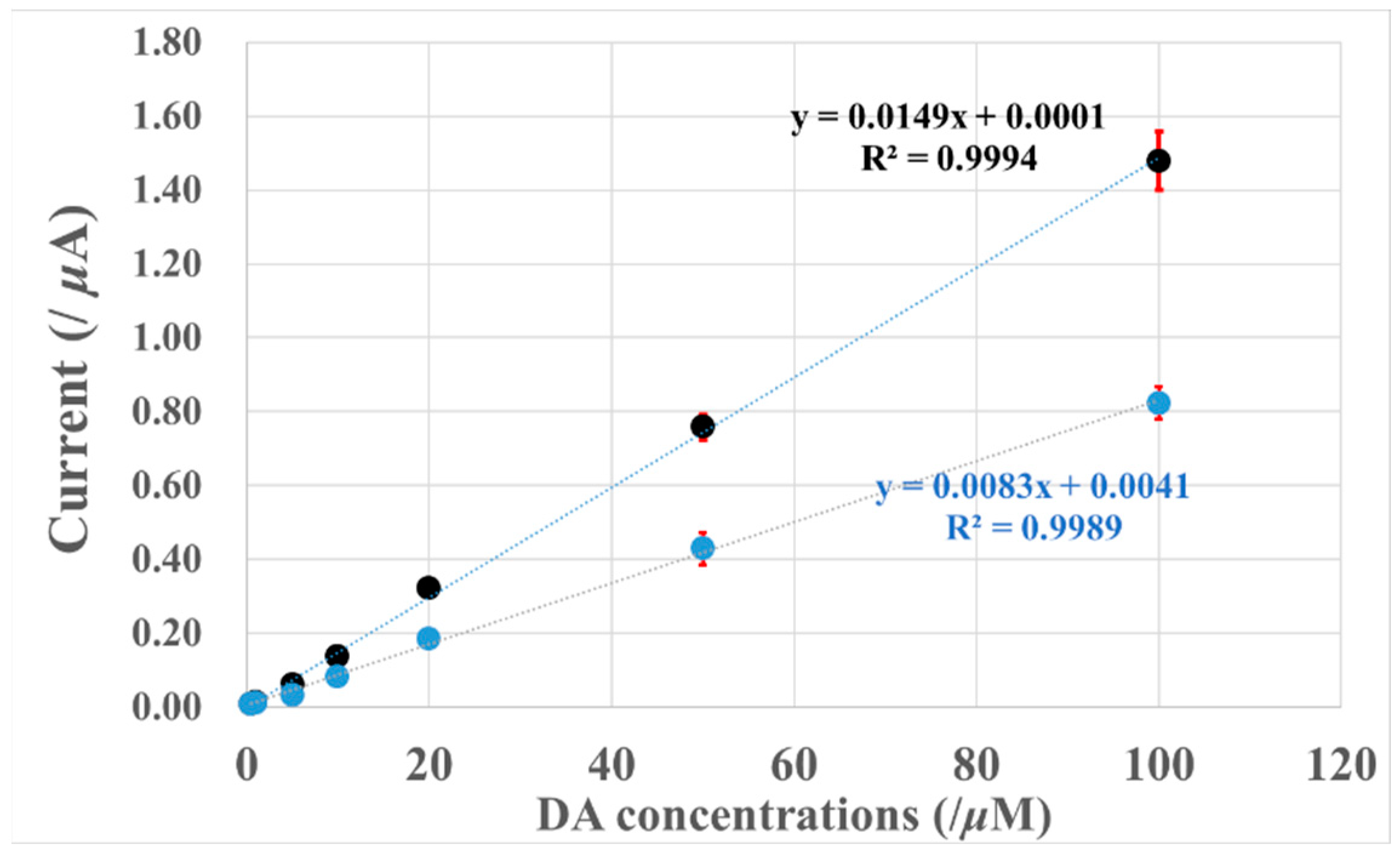
4. Dopamine Calibration Curves
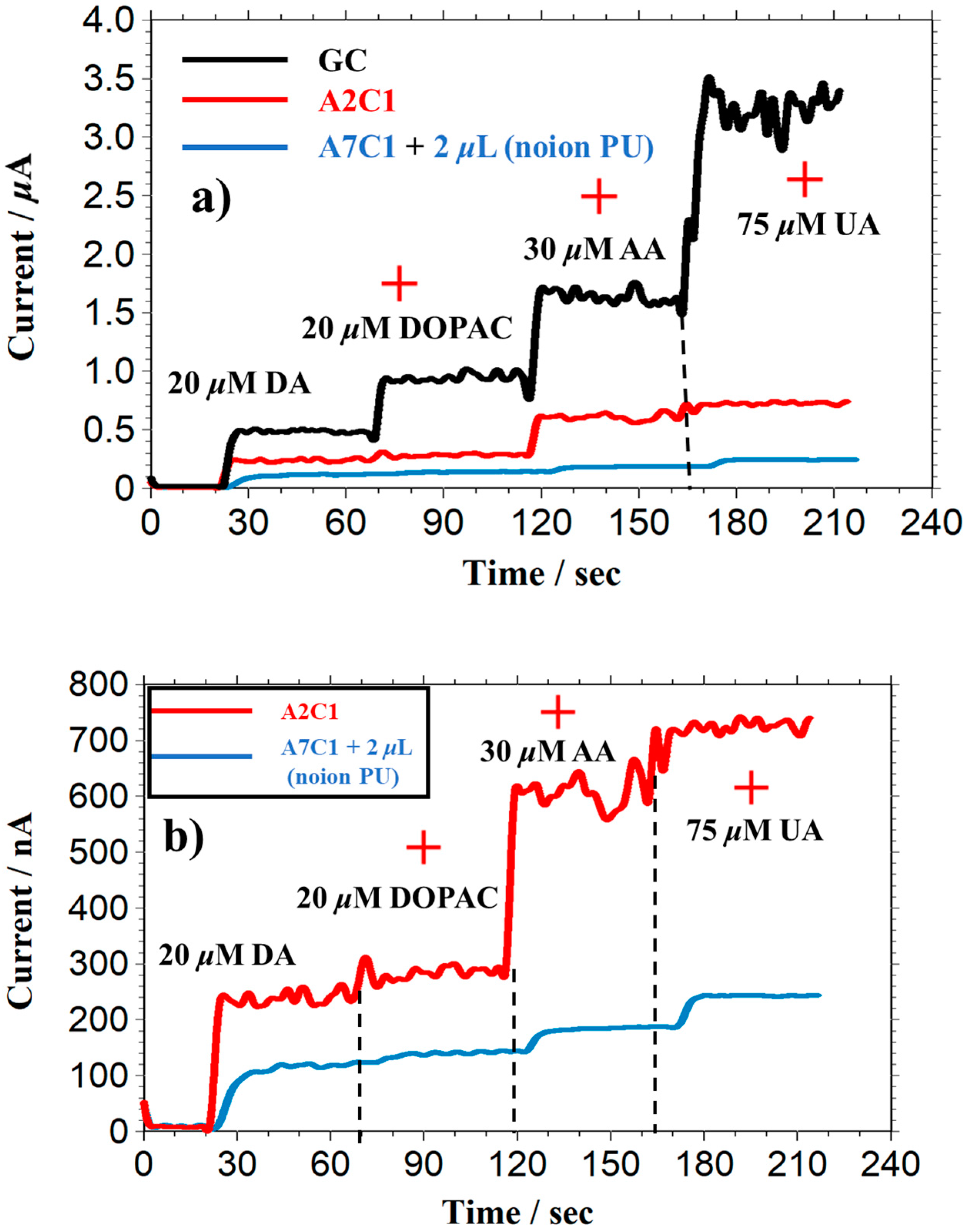
5. Selectivity of Dopamine Biosensors with Interferents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Fuoss, R.M.; Sadek, H. Mutual Interaction of Polyelectrolytes. Science 1949, 110, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leech, D.; Ozsoz, M.; Martinez, S.; Smyth, M.R. One-step fabrication of glucose sensors based on entrapment of glucose oxidase within poly(ester-sulfonic acid) coatings. Anal. Chim. Acta 1991, 245, 139–143. [Google Scholar] [CrossRef]
- Vinogradov, S.; Batrakova, E.; Li, S.; Kabanov, A. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug. Chem. 1999, 10, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Mitsuhashi, T.; Shiraishi, S. Polyion complex nanocomposite electrode incorporating enzyme and carbon nanotube for biofuel cells. Electrochemistry 2008, 76, 55–58. [Google Scholar] [CrossRef]
- Kishimura, A. Development of polyion complex vesicles (PICsomes) from block copolymers for biomedical applications. Polym. J. 2013, 45, 892–897. [Google Scholar] [CrossRef]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 2014, 8, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, R.; Chen, F.; Kent, B.; Stenzel, M.H. Estrone-Decorated Polyion Complex Micelles for Targeted Melittin Delivery to Hormone-Responsive Breast Cancer Cells. Biomacromolecules 2020, 21, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-I.; Cho, S.; Vu, T.T.; Kim, S.; Ryu, S.; Moon, J.; Park, S. Hybrid polyion complex micelles enabling high-performance lithium-metal batteries with universal carbonates. Energy Storage Mater. 2021, 38, 509–519. [Google Scholar] [CrossRef]
- Nagar, H.; Sumana, C.; Rao, V.V.B.; Sridhar, S. Performance evaluation of sodium alginate–Pebax polyion complex membranes for application in direct methanol fuel cells. J. Appl. Polym. Sci. 2017, 134, 44485. [Google Scholar] [CrossRef]
- Ballesta-Claver, J.; Valencia-Mirón, M.C.; Capitán-Vallvey, L.F. One-shot lactate chemiluminescent biosensor. Anal. Chim. Acta 2008, 629, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, F.; Yabuki, S.; Hirata, Y. Amperometric L-lactate-sensing electrode based on a polyion complex layer containing lactate oxidase. Application to serum and milk samples. Anal. Chim. Acta 1995, 314, 233–239. [Google Scholar] [CrossRef]
- Mizutani, F.; Sato, Y.; Hirata, Y.; Sawaguchi, T.; Yabuki, S. Glucose oxidase/polyion complex-bilayer membrane for elimination of electroactive interferents in amperometric glucose sensor. Anal. Chim. Acta 1998, 364, 173–179. [Google Scholar] [CrossRef]
- Mizutani, F.; Sato, Y.; Hirata, Y.; Yabuki, S. High-throughput flow-injection analysis of glucose and glutamate in food and biological samples by using enzyme/polyion complex-bilayer membrane-based electrodes as the detectors. Biosens. Bioelectron. 1998, 13, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, S.; Mizutani, F.; Hirata, Y. Hydrogen peroxide determination based on a glassy carbon electrode covered with polyion complex membrane containing peroxidase and mediator. Sens. Actuators B Chem. 2000, 65, 49–51. [Google Scholar] [CrossRef]
- Katsuno, E.; Yabuuchi, N.; Komaba, S. Double-layered polyion complex for application to biosensing electrodes. Electrochem. Commun. 2014, 47, 88–91. [Google Scholar] [CrossRef]
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.; Zhu, Q.; Ye, X. A needle-type glucose biosensor based on PANI nanofibers and PU/E-PU membrane for long-term invasive continuous monitoring. Biosens. Bioelectron. 2017, 97, 196–202. [Google Scholar] [CrossRef]
- Yabuki, S.; Fujii, S.; Mizutani, F.; Hirata, Y. Permeation regulation of charged species by the component change of polyion complex membranes. Anal. Biochem. 2008, 375, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Hasebe, Y. DNA–Cu(II) poly(amine) complex membrane as novel catalytic layer for highly sensitive amperometric determination of hydrogen peroxide. Biosens. Bioelectron. 2006, 21, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Das, G.; Yoon, H.H. Fabrication of an amperometric urea biosensor using urease and metal catalysts immobilized by a polyion complex. J. Electroanal. Chem. 2015, 747, 143–148. [Google Scholar] [CrossRef]
- SANNCOR Ind Inc. Waterborne Polyurethane Dispersions. European Patent EP0373671(A2), 19891215, 20 June 1990. Available online: https://worldwide.espacenet.com/patent/search/family/023094666/publication/EP0373671A2?q=EP0373671 (accessed on 27 April 2023).
- Kang, S.Y.; Ji, Z.; Tseng, L.F.; Turner, S.A.; Villanueva, D.A.; Johnson, R.; Albano, A.; Langer, R. Design and Synthesis of Waterborne Polyurethanes. Adv. Mater. 2018, 30, e1706237. [Google Scholar] [CrossRef] [PubMed]


| Electrodes | Excessive Functional Group (nM/Electrode) | Normalized Sensitivity (Each Species Selectivity/DA Selectivity) | ||
|---|---|---|---|---|
| AA | DA | UA | ||
| A7C1 | −0.36 | 0.28 | 1.00 | 0.25 |
| A2C1 | −0.21 | 0.47 | 1.00 | 0.23 |
| A1C1 | −0.08 | 0.67 | 1.00 | 0.27 |
| A1C2 | 0.04 | 1.92 | 1.00 | 0.80 |
| A1C5 | 0.14 | 0.87 | 1.00 | 0.34 |
| GC | 1.27 | 1.00 | 0.35 | |
| Electrodes | Anionic Functional Group Density (nM/mm3·Electrode) | Thickness of Membrane (μm) | Normalized Sensitivity (Each Species Selectivity/DA Selectivity) | ||
|---|---|---|---|---|---|
| AA | DA | UA | |||
| A2C1-10-fold | 1.11 | 27 | 0.47 | 1.00 | 0.23 |
| A2C1-20-fold | 0.77 | 19 | 0.58 | 1.00 | 0.27 |
| A2C1-30-fold | 0.89 | 11 | 0.47 | 1.00 | 0.21 |
| Electrodes | Polymer Density (g/cm3) | Thickness of Membrane (μm) | Normalized Sensitivity (Each Species Selectivity/DA Selectivity) | ||
|---|---|---|---|---|---|
| AA | DA | UA | |||
| A7C1 | 0.57 | 18 | 0.28 | 1 | 0.25 |
| A7C1 + 0.5 μL | 0.60 | 24 | 0.48 | 1 | 0.13 |
| A7C1 + 1 μL | 0.77 | 28 | 0.33 | 1 | 0.09 |
| A7C1 + 2 μL | 0.92 | 35 | 0.21 | 1 | 0.10 |
| Electrodes | Electrolyte | Normalized Sensitivity (Each Species Selectivity/DA Selectivity) | ||
|---|---|---|---|---|
| AA | DA | UA | ||
| GC | Protein-free | 1.08 | 1.00 | 0.43 |
| Protein-containing | 1.12 | 1.00 | 0.29 | |
| A7C1 + 2 μL no-ion PU | Protein-free | 0.27 | 1.00 | 0.14 |
| Protein-containing | 0.19 | 1.00 | 0.12 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Guo, H.; Hirai, Y.; Takeda, K.; Asai, C.; Takamura, N.; Niwa, O. A Novel Polyurethane-Based Polyion Complex Material with Tunable Selectivity against Interferents for Selective Dopamine Determination. Biosensors 2023, 13, 638. https://doi.org/10.3390/bios13060638
Zhang Z, Guo H, Hirai Y, Takeda K, Asai C, Takamura N, Niwa O. A Novel Polyurethane-Based Polyion Complex Material with Tunable Selectivity against Interferents for Selective Dopamine Determination. Biosensors. 2023; 13(6):638. https://doi.org/10.3390/bios13060638
Chicago/Turabian StyleZhang, Zixin, Hongchen Guo, Yuugo Hirai, Katsunori Takeda, Chiho Asai, Naohiro Takamura, and Osamu Niwa. 2023. "A Novel Polyurethane-Based Polyion Complex Material with Tunable Selectivity against Interferents for Selective Dopamine Determination" Biosensors 13, no. 6: 638. https://doi.org/10.3390/bios13060638
APA StyleZhang, Z., Guo, H., Hirai, Y., Takeda, K., Asai, C., Takamura, N., & Niwa, O. (2023). A Novel Polyurethane-Based Polyion Complex Material with Tunable Selectivity against Interferents for Selective Dopamine Determination. Biosensors, 13(6), 638. https://doi.org/10.3390/bios13060638





