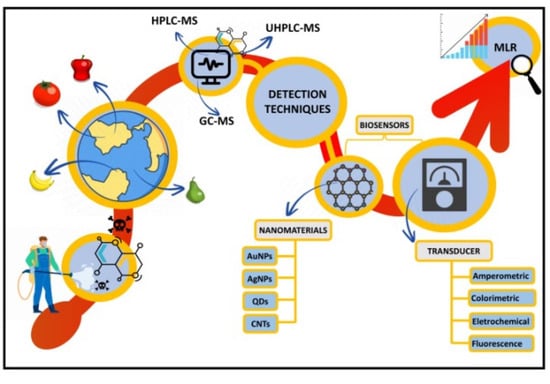
Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods
Abstract
:1. Introduction
2. Systematic Research Methods
2.1. Focus Questions
2.2. Search Strategy and Selection Criteria
- SC1 = biosensor *
- SC2 = pesticide * OR agrochemical *
2.3. Data Extraction
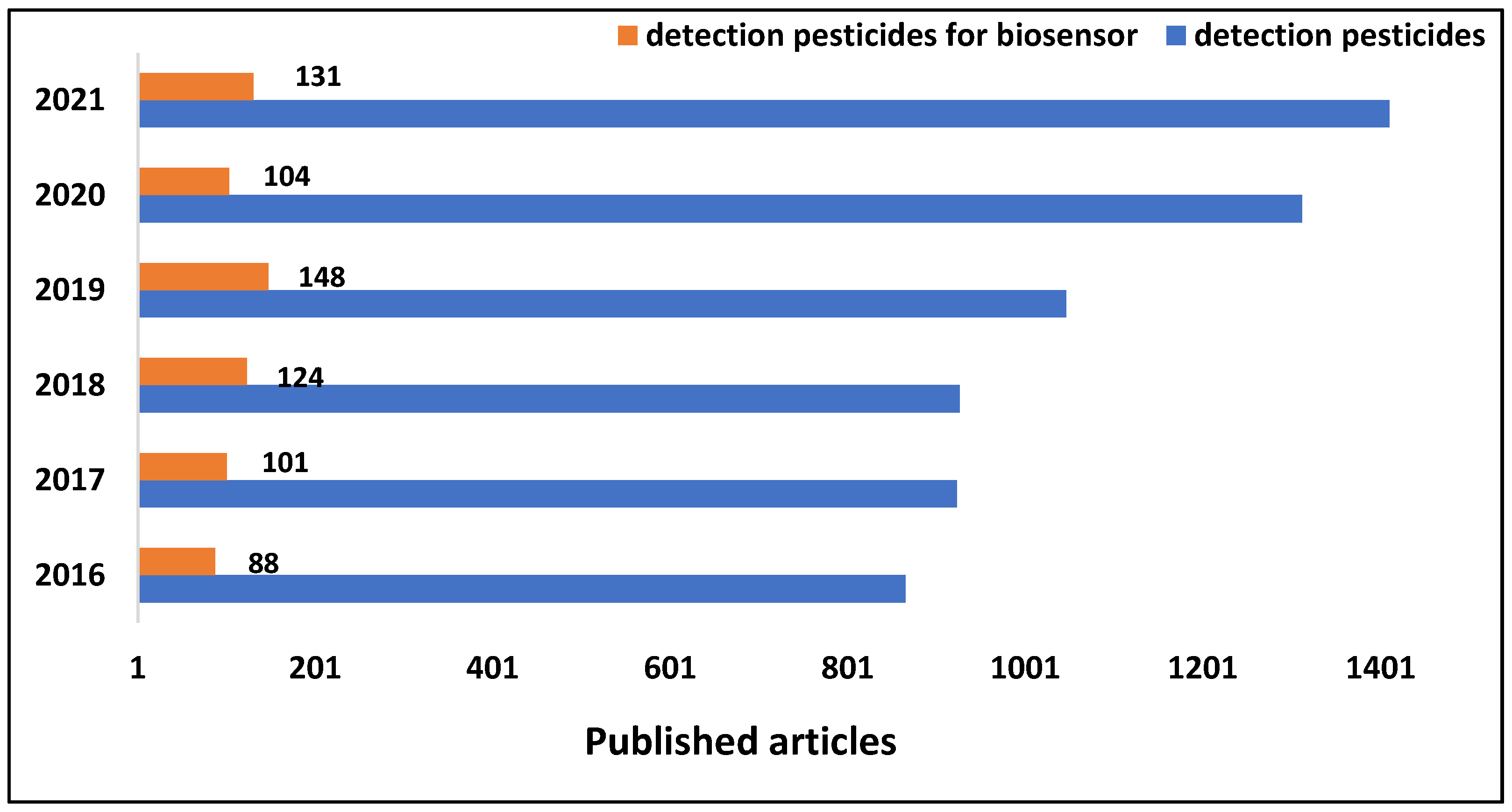
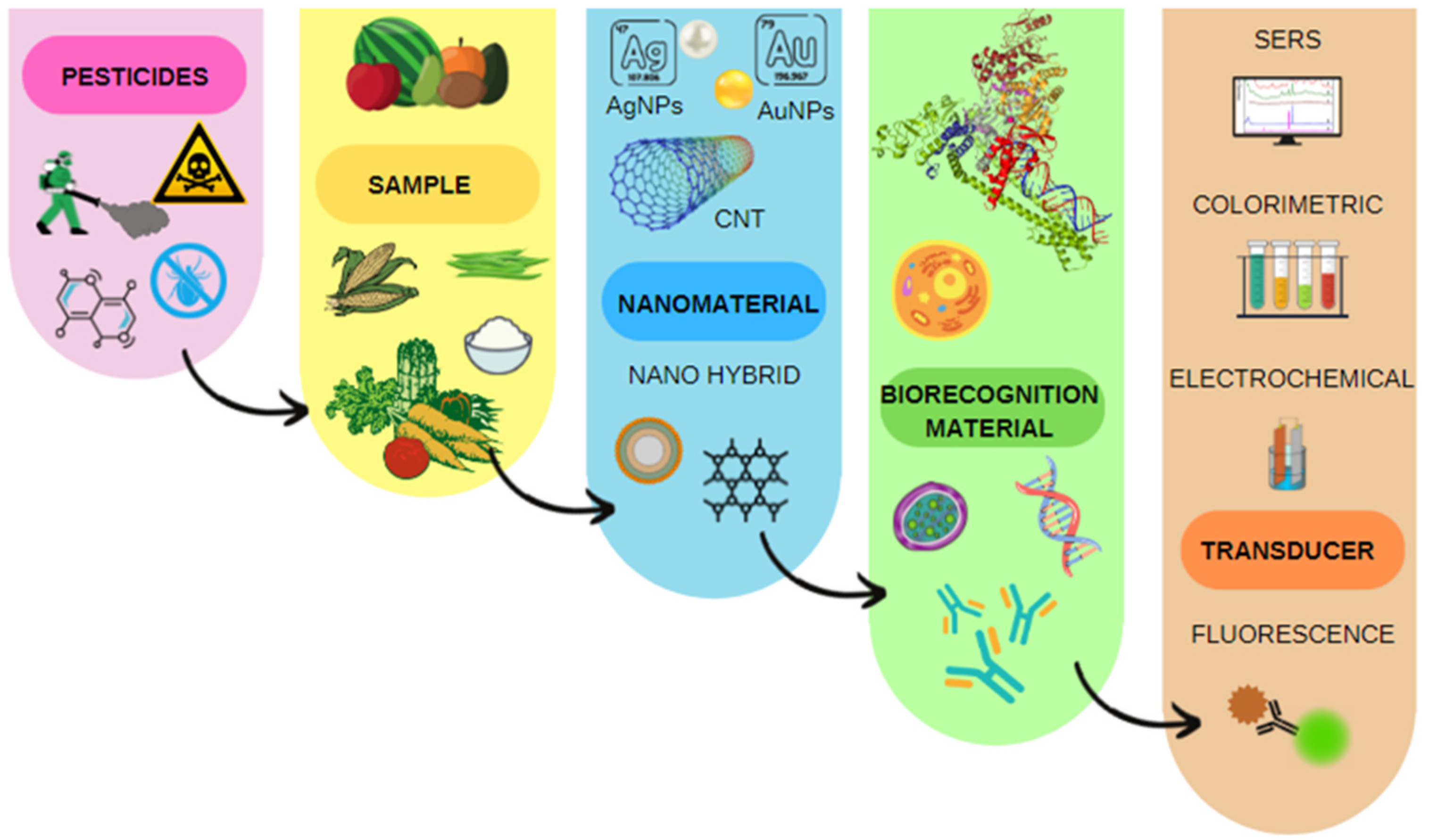
3. Dataset Visual Approaches
3.1. Influence of the Type of Nanomaterial on the Sensitivity of the Biosensor to Pesticides
3.1.1. Gold Nanomaterials (AuNMs)
3.1.2. Silver Nanomaterials (AgNMs)
3.1.3. Carbon Nanotubes (CNTs)
3.1.4. Graphene and Graphene Oxide (rGO)
3.1.5. Quantum Dots (QDs)
3.1.6. Titanium Nanomaterials (TiNMs)
3.1.7. Hybrid Nanostructures
3.2. Effect of the Transducer Type on the Limit of Detection
3.2.1. Colorimetric Transducer
3.2.2. Electrochemical Transducer
3.2.3. Fluorescence Transducer
3.2.4. Microcantilever-Array Sensor
3.2.5. Piezoelectric Transducer
3.2.6. SERS Transducer
3.3. Biosensors with Contaminant Analysis Devices
3.4. Highlights and Futures Perspectives
3.5. Efficiency of Biosensors in Legislation: MRL, ARfD and LD50
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AgNMs | Silver nanomaterials |
| AgNPs | Silver nanoparticles |
| AgNWs | Silver nanowires |
| AuNPS | Gold nanoparticles |
| AuNMs | Gold nanomaterials |
| BChE | Butyrylcholinesterase |
| CNTs | Carbon nanotubes |
| GCE | Glassy carbon electrode |
| LFB | Lateral flow biosensor |
| LFI | Lateral flow immunoassay |
| LOD | Limit of detection |
| M-Cell | Mineralized cell |
| MHCS | Mesoporous hollow carbon spheres |
| MNPs | Magnetic nanoparticles |
| MRL | Maximum residue limit |
| MWCNTs | Multi-walled carbon nanotubes |
| NMs | Nanomaterials |
| NSs | Nanosheets |
| SERS | Surface-Enhanced Raman Scattering |
| SPR | Surface plasmon resonance |
| SWCNTs | Single-walled carbon nanotubes |
| VNSWCNTs | Vertical nitrogen-doped single-walled carbon nanotubes |
References
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Sikora, J.; Niemiec, M.; Szeląg-Sikora, A.; Gródek-Szostak, Z.; Kuboń, M.; Komorowska, M. The impact of a controlled-release fertilizer on greenhouse gas emissions and the efficiency of the production of Chinese cabbage. Energies 2020, 13, 2063. [Google Scholar] [CrossRef]
- El-Nahhal, I.; El-Nahhal, Y. Pesticide residues in drinking water, their potential risk to human health and removal options. J. Environ. Manag. 2021, 299, 113611. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Karanth, S.; Liu, J. Pharmacology and toxicology of cholinesterase inhibitors: Uses and misuses of a common mechanism of action. Environ. Toxicol. Pharmacol. 2005, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.A.; Alshumrani, E.S.; Bin Saeed, M.S.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.; Wanderley, K.A.; Paiva-Santos, C.D.O.; De Sá, G.F.; Alexandre, M.D.R.; Júnior, S.A.; Navickiene, S. Coordination polymer adsorbent for matrix solid-phase dispersion extraction of pesticides during analysis of dehydrated Hyptis pectinata medicinal plant by GC/MS. Talanta 2010, 83, 631–636. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What are the main sensor methods for quantifying pesticides in agricultural activities? A review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef]
- Bilal, S.; Hassan, M.M.; ur Rehman, M.F.; Nasir, M.; Sami, A.J.; Hayat, A. An insect acetylcholinesterase biosensor utilizing WO3/g-C3N4 nanocomposite modified pencil graphite electrode for phosmet detection in stored grains. Food Chem. 2021, 346, 128894. [Google Scholar] [CrossRef]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Batool, R.; Rhouati, A.; Nawaz, M.H.; Hayat, A.; Marty, J.L. A review of the construction of nano-hybrids for electrochemical biosensing of glucose. Biosensors 2019, 9, 46. [Google Scholar] [CrossRef]
- Tepeli, Y.; Ülkü, A. Electrochemical biosensors for influenza virus a detection: The potential of adaptation of these devices to POC systems. Sens. Actuators B Chem. 2018, 254, 377–384. [Google Scholar] [CrossRef]
- Vermisoglou, E.; Panáček, D.; Jayaramulu, K.; Pykal, M.; Frébort, I.; Kolář, M.; Hajdúch, M.; Zbořil, R.; Otyepka, M. Human virus detection with graphene-based materials. Biosens. Bioelectron. 2020, 166, 112436. [Google Scholar] [CrossRef]
- Kumar, N.; Bhatia, S.; Pateriya, A.K.; Sood, R.; Nagarajan, S.; Murugkar, H.V.; Kumar, S.; Singh, P.; Singh, V.P. Label-free peptide nucleic acid biosensor for visual detection of multiple strains of influenza A virus suitable for field applications. Anal. Chim. Acta 2020, 1093, 123–130. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Tessaro, L.; Aquino, A.; de Carvalho, A.P.A.; Conte-Junior, C.A. A systematic review on gold nanoparticles based-optical biosensors for Influenza virus detection. Sens. Actuators Rep. 2021, 3, 100060. [Google Scholar] [CrossRef]
- Uniyal, S.; Sharma, R.K. Technological advancement in electrochemical biosensor based detection of Organophosphate pesticide chlorpyrifos in the environment: A review of status and prospects. Biosens. Bioelectron. 2018, 116, 37–50. [Google Scholar] [CrossRef]
- Kalyani, N.; Goel, S.; Jaiswal, S. On-site sensing of pesticides using point-of-care biosensors: A review. Environ. Chem. Lett. 2021, 19, 345–354. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kaya, S.I.; Ozkan, S.A. Recent advances of enzyme biosensors for pesticide detection in foods. J. Food Meas. Charact. 2021, 15, 4582–4595. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Cheng, N.; Luo, Y.; Lin, Y.; Xu, W.; Du, D. Recent advances in nanomaterials-based electrochemical (bio)sensors for pesticides detection. TrAC—Trends Anal. Chem. 2020, 132, 116041. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097-6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, B.; Wang, X.; Shen, J.; Zhao, B. Detection of organophosphorus pesticides by nanogold/Mercaptomethamidophos multi-residue electrochemical biosensor. Food Chem. 2021, 354, 129511. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Song, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Li, Y.; Li, C.; Yang, L.; Ma, K.; Zhang, Z.; Huang, H. Electrochemical aptasensor based on Mo2C/Mo2N and gold nanoparticles for determination of chlorpyrifos. Microchim. Acta 2021, 188, 170. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Kong, M.; Jiang, G.; Sun, Y.; Mo, W.; Lin, T.; Ye, F.; Zhao, S. A competitive immunoassay for electrochemical impedimetric determination of chlorpyrifos using a nanogold-modified glassy carbon electrode based on enzymatic biocatalytic precipitation. Microchim. Acta 2020, 187, 204. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tang, Y.; Yang, Y.; Wu, Y. Design an aptamer-based sensitive lateral flow biosensor for rapid determination of isocarbophos pesticide in foods. Food Control 2021, 129, 108208. [Google Scholar] [CrossRef]
- Kaur, N.; Bhatnagar, A.; Bhalla, A.; Prabhakar, N. Determination of an organophosphate pesticide using antibody immobilised hybrid nanocomposites. Int. J. Environ. Anal. Chem. 2019, 101, 1485–1498. [Google Scholar] [CrossRef]
- Xu, G.; Huo, D.; Hou, J.; Zhang, C.; Zhao, Y.; Hou, C.; Bao, J.; Yao, X.; Yang, M. An electrochemical aptasensor of malathion based on ferrocene/DNA-hybridized MOF, DNA coupling-gold nanoparticles and competitive DNA strand reaction. Microchem. J. 2021, 162, 105829. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y. A lanthanide-based ratiometric fluorescent biosensor for the enzyme-free detection of organophosphorus pesticides. Anal. Methods 2021, 13, 2005–2010. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, Y.; Fan, K.; Ji, F.; Wu, J.; Ying, Y. A nano-silver enzyme electrode for organophosphorus pesticide detection. Anal. Bioanal. Chem. 2016, 408, 5819–5827. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Mittal, S.; Sharma, R.K.; Wangoo, N. A supersensitive silver nanoprobe based aptasensor for low cost detection of malathion residues in water and food samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Turan, J.; Kesik, M.; Soylemez, S.; Goker, S.; Coskun, S.; Emrah, H.; Toppare, L. Sensors and Actuators B: Chemical An effective surface design based on a conjugated polymer and silver nanowires for the detection of paraoxon in tap water and milk. Sens. Actuators B Chem. 2016, 228, 278–286. [Google Scholar] [CrossRef]
- Lu, Y.; Tan, Y.; Xiao, Y.; Li, Z.; Sheng, E.; Dai, Z. A silver @ gold nanoparticle tetrahedron biosensor for multiple pesticides detection based on surface-enhanced Raman scattering. Talanta 2021, 234, 122585. [Google Scholar] [CrossRef]
- Rahmani, T.; Hajian, A.; Afkhami, A.; Bagheri, H. A novel and high performance enzyme-less sensing layer for electrochemical detection of methyl parathion based on BSA templated Au-Ag bimetallic nanoclusters. New J. Chem. 2018, 42, 7213–7222. [Google Scholar] [CrossRef]
- Cui, H.-F.; Zhang, T.-T.; Lv, Q.-Y.; Song, X.; Zhai, X.-J.; Wang, G.-G. An acetylcholinesterase biosensor based on doping Au nanorod@SiO2 nanoparticles into TiO2-chitosan hydrogel for detection of organophosphate pesticides. Biosens. Bioelectron. 2019, 141, 111452. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, H.; Wang, X.; Yao, W.; Zhang, W.; Jiang, L. An aptamer based aggregation assay for the neonicotinoid insecticide acetamiprid using fluorescent upconversion nanoparticles and DNA functionalized gold nanoparticles. Microchim. Acta 2019, 186, 308. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, S.; Jiang, B.; Zheng, J. Organophosphorus pesticides detection using acetylcholinesterase biosensor based on gold nanoparticles constructed by electroless plating on vertical nitrogen-doped single-walled carbon nanotubes Organophosphorus pesticides detection using acetylcholinest. Int. J. Environ. Anal. Chem. 2019, 99, 913–927. [Google Scholar] [CrossRef]
- Sharma, D.; Wangoo, N.; Sharma, R.K. Sensing platform for pico-molar level detection of ethyl parathion using Au-Ag nanoclusters based enzymatic strategy. Talanta 2021, 221, 121267. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Feng, Z.; Shen, G.; Xiu, Y.; Zhou, Y.; Niu, X.; Wang, H. Acetylcholinesterase Biosensor Based On Mesoporous Hollow Carbon Spheres/Core-Shell Magnetic Nanoparticles-Modified Electrode for the Detection of Organophosphorus Pesticides. Sensors 2018, 18, 4429. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, L.; He, Y.; Wang, L.; Huang, Z.; Jiang, Y.; Gao, J. Hierarchical nanocomposites with an N-doped carbon shell and bimetal core: Novel enzyme nanocarriers for electrochemical pesticide detection. Biosens. Bioelectron. 2018, 121, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Song, Y.; Fu, Q.; Du, D.; Luo, Y.; Wang, Y.; Xu, W.; Lin, Y. Aptasensor based on fluorophore-quencher nano-pair and smartphone spectrum reader for on-site quantification of multi-pesticides. Biosens. Bioelectron. 2018, 117, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jin, M.; Yan, M.; Cui, X.; Wang, Y.; Zheng, W.; Qin, G.; Zhang, Y.; Li, M.; Liao, Y.; et al. Colorimetric bio-barcode immunoassay for parathion based on amplification by using platinum nanoparticles acting as a nanozyme. Microchim. Acta 2019, 186, 339. [Google Scholar] [CrossRef]
- Chen, G.; Jin, M.; Ma, J.; Yan, M.; Cui, X.; Wang, Y.; Zhang, X.; Li, H.; Zheng, W.; Zhang, Y.; et al. Competitive Bio-Barcode Immunoassay for Highly Sensitive Detection of Parathion Based on Bimetallic Nanozyme Catalysis. J. Agric. Food Chem. 2020, 68, 660–668. [Google Scholar] [CrossRef]
- Zhao, R.; Jia, D.; Wen, Y.; Yu, X. Cantilever-based aptasensor for trace level detection of nerve agent simulant in aqueous matrices. Sens. Actuators B Chem. 2017, 238, 1231–1239. [Google Scholar] [CrossRef]
- Xu, G.; Hou, J.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H.; Yang, Y.; Li, L.; Huo, D.; Hou, C. Dual-signal aptamer sensor based on polydopamine-gold nanoparticles and exonuclease I for ultrasensitive malathion detection. Sens. Actuators B Chem. 2019, 287, 428–436. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, Y.; Wu, K.; Feng, Q.; Wang, C.; He, P. Bioelectrochemistry biosensor: Towards ef fi cient fabrication and application in paraoxon detection. Bioelectrochemistry 2020, 131, 107392. [Google Scholar] [CrossRef]
- Chen, D.; Fu, J.; Liu, Z.; Guo, Y.; Sun, X.; Wang, X.; Wang, Z. A Simple acetylcholinesterase biosensor based on ionic liquid/multiwalled carbon nanotubes-modified screen-printed electrode for rapid detecting chlorpyrifos. Int. J. Electrochem. Sci. 2017, 12, 9465–9477. [Google Scholar] [CrossRef]
- Kaur, N.; Thakur, H.; Prabhakar, N. Conducting polymer and multi-walled carbon nanotubes nanocomposites based amperometric biosensor for detection of organophosphate. J. Electroanal. Chem. 2016, 775, 121–128. [Google Scholar] [CrossRef]
- Zare, A.R.; Ensafi, A.A.; Rezaei, B. An impedimetric biosensor based on poly(l-lysine)-decorated multiwall carbon nanotubes for the determination of diazinon in water and fruits. J. Iran. Chem. Soc. 2019, 16, 2777–2785. [Google Scholar] [CrossRef]
- Thakkar, J.B.; Gupta, S.; Prabha, C.R. Acetylcholine esterase enzyme doped multiwalled carbon nanotubes for the detection of organophosphorus pesticide using cyclic voltammetry. Int. J. Biol. Macromol. 2019, 137, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Fakhri, H.; Hajian, A.; Afkhami, A.; Bagheri, H. High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modi fi ed metal-organic framework sensing platforms. Bioelectrochemistry 2019, 130, 107348. [Google Scholar] [CrossRef]
- Kaur, N.; Thakur, H.; Kumar, R.; Prabhakar, N. An electrochemical sensor modified with poly(3,4-ethylenedioxythiophene)-wrapped multi-walled carbon nanotubes for enzyme inhibition-based determination of organophosphates. Microchim. Acta 2016, 183, 2307–2315. [Google Scholar] [CrossRef]
- Lin, B.; Yu, Y.; Li, R.; Cao, Y.; Guo, M. Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sens. Actuators B Chem. 2016, 229, 100–109. [Google Scholar] [CrossRef]
- Han, L.; Chen, D.; Li, F. Rational Integration of Biomineralization, Microbial Surface Display, and Carbon Nanocomposites: Ultrasensitive and Selective Biosensor for Traces of Pesticides. Adv. Mater. Interfaces 2018, 5, 1801332. [Google Scholar] [CrossRef]
- Jiang, B.; Lu, M.; Xu, M. Amperometric sensing of organophosphorus pesticides based on covalently attached multilayer assemblies of diazo-resin, Prussian blue single-walled carbon nanotubes, and acetylcholinesterase. Rev. Roum. Chim. 2019, 64, 763–774. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Chen, D.; Wang, Z.; Liu, G. A sensitive acetylcholinesterase biosensor based on screen printed electrode modified with Fe3O4 nanoparticle and graphene for chlorpyrifos determination. Int. J. Electrochem. Sci. 2016, 11, 10906–10918. [Google Scholar] [CrossRef]
- Hu, H.; Wang, B.; Li, Y.; Wang, P.; Yang, L. Acetylcholinesterase Sensor with Patterned Structure for Detecting Organophosphorus Pesticides Based on Titanium Dioxide Sol-gel Carrier. Electroanalysis 2020, 32, 1834–1842. [Google Scholar] [CrossRef]
- Qiu, L.; Lv, P.; Zhao, C.; Feng, X.; Fang, G.; Liu, J. Sensors and Actuators B: Chemical Electrochemical detection of organophosphorus pesticides based on amino acids conjugated nanoenzyme modi fi ed electrodes. Sens. Actuators B Chem. 2019, 286, 386–393. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Wu, S.; Zhang, Q. Analytica Chimica Acta Aptamer-based microcantilever-array biosensor for profenofos detection. Anal. Chim. Acta 2018, 1020, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Palanivelu, J.; Chidambaram, R. Acetylcholinesterase with mesoporous silica: Covalent immobilization, physiochemical characterization, and its application in food for pesticide detection. J. Cell. Biochem. 2019, 120, 10777–10786. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.Y.; Soliman, E.A.; Ibrahim, H.Z.; Marty, J.-L.; Istamboulie, G.; Noguer, T. Biosensor based on electrospun blended chitosan-poly (vinyl alcohol) nanofibrous enzymatically sensitized membranes for pirimiphosmethyl detection in olive oil. Talanta 2016, 155, 258–264. [Google Scholar] [CrossRef]
- Korram, J.; Dewangan, L.; Karbhal, I.; Nagwanshi, R.; Vaishanav, S.K.; Ghosh, K.K.; Satnami, M.L. CdTe QD-based inhibition and reactivation assay of acetylcholinesterase for the detection of organophosphorus pesticides. RSC Adv. 2020, 10, 24190–24202. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Thakur, H.; Bharti, A.; Kaur, N. Chitosan-iron oxide nanocomposite based electrochemical aptasensor for determination of malathion. Anal. Chim. Acta 2016, 939, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Jiang, B.; Zheng, J. A novel acetylcholinesterase biosensor based on gold nanoparticles obtained by electroless plating on three-dimensional graphene for detecting organophosphorus pesticides in water and vegetable samples. Anal. Methods 2019, 11, 2428–2434. [Google Scholar] [CrossRef]
- Silva, M.K.L.; Vanzela, H.C.; Defavari, L.M.; Cesarino, I. Sensors and Actuators B: Chemical Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sens. Actuators B Chem. 2018, 277, 555–561. [Google Scholar] [CrossRef]
- Yi, J.; Liu, Z.; Liu, J.; Liu, H.; Xia, F.; Tian, D.; Zhou, C. A label-free electrochemical aptasensor based on 3D porous CS/rGO/GCE for acetamiprid residue detection. Biosens. Bioelectron. 2020, 148, 111827. [Google Scholar] [CrossRef]
- Li, S.; Qu, L.M.; Wang, J.F.; Ran, X.Q.; Niu, X. Acetylcholinesterase based rGO-TEPA-Copper nanowires biosensor for detecting malathion. Int. J. Electrochem. Sci. 2020, 15, 505–514. [Google Scholar] [CrossRef]
- Cui, H.; Wu, W.; Li, M.; Song, X.; Lv, Y.; Zhang, T. Biosensors and Bioelectronics graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, L.; He, Y.; Wang, L.; Huang, Z.; Jiang, Y.; Gao, J. Mesoporous Bimetallic PtPd Nanoflowers as a Platform to Enhance Electrocatalytic Activity of Acetylcholinesterase for Organophosphate Pesticide Detection. Electroanalysis 2018, 30, 1793–1802. [Google Scholar] [CrossRef]
- Apilux, A.; Siangproh, W.; Insin, N.; Chailapakul, O.; Prachayasittikul, V. Paper-based thioglycolic acid (TGA)-capped CdTe QD device for rapid screening of organophosphorus and carbamate insecticides. Anal. Methods 2017, 9, 519–527. [Google Scholar] [CrossRef]
- Cheng, Y.; Lai, O.; Tan, C.-P.C.; Panpipat, W.; Cheong, L.L.-Z.; Shen, C. Proline-Modified UIO-66 as Nanocarriers to Enhance Candida rugosa Lipase Catalytic Activity and Stability for Electrochemical Detection of Nitrofen. ACS Appl. Mater. Interfaces 2021, 13, 4146–4155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Shi, Q.; Zhu, C.; Li, S.; Lin, Y.; Du, D. Pt-Ni(OH)(2) nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides. Biosens. Bioelectron. 2019, 142, 111498. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; He, Y.; Zhao, J.; Chen, S.; Yuan, R. Ratiometric electrochemiluminescence biosensor based on Ir nanorods and CdS quantum dots for the detection of organophosphorus pesticides. Sens. Actuators B Chem. 2021, 341, 130008. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Ahmad, W.; Rong, Y.; Li, H.; Hu, Y.; Chen, Q. A highly sensitive detection of carbendazim pesticide in food based on the upconversion-MnO2 luminescent resonance energy transfer biosensor. Food Chem. 2021, 349, 129157. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Huo, D.; Hou, C.; Zhao, Y.; Bao, J.; Yang, M.; Fa, H. A regenerative and selective electrochemical aptasensor based on copper oxide nanoflowers-single walled carbon nanotubes nanocomposite for chlorpyrifos detection. Talanta 2018, 178, 1046–1052. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-Templated Gold Nanoparticles on Optical Fibers for Enhanced-Sensitivity Localized Surface Plasmon Resonance Biosensors. ACS Sens. 2019, 4, 613–622. [Google Scholar] [CrossRef]
- Takemura, K.; Adegoke, O.; Takahashi, N.; Kato, T.; Li, T.C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Versatility of a localized surface plasmon resonance-based gold nanoparticle-alloyed quantum dot nanobiosensor for immunofluorescence detection of viruses. Biosens. Bioelectron. 2017, 89, 998–1005. [Google Scholar] [CrossRef]
- Dissanayake, N.M.; Arachchilage, J.S.; Samuels, T.A.; Obare, S.O. Highly sensitive plasmonic metal nanoparticle-based sensors for the detection of organophosphorus pesticides. Talanta 2019, 200, 218–227. [Google Scholar] [CrossRef]
- Rui, Y.; Wu, X.; Ma, B.; Xu, Y. Immobilization of acetylcholinesterase on functionalized SBA-15 mesoporous molecular sieve for detection of organophosphorus and carbamate pesticide. Chin. Chem. Lett. 2018, 29, 1387–1390. [Google Scholar] [CrossRef]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.J.; Vilian, A.T.E.; Kim, J.; Lee, S.Y.; Han, Y.K.; Yoo, S.M.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 10130. [Google Scholar] [CrossRef]
- Zarei, A.R.; Barghak, F. Application of the Localized Surface Plasmon Resonance of Gold Nanoparticles for the Determination of 1, 1-Dimethylhydrazine in Water: Toward Green Analytical Chemistry. J. Anal. Chem. 2017, 72, 430–436. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A review on carbon nanotubes in biosensor devices and their applications in medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Dhull, V. Fabrication of AChE/SnO2-cMWCNTs/Cu Nanocomposite-Based Sensor Electrode for Detection of Methyl Parathion in Water. Int. J. Anal. Chem. 2018, 2018, 2874059. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12–18. [Google Scholar] [CrossRef]
- Chakravarty, M.; Vora, A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021, 11, 748–787. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Hassan, S.; Jafri, M. Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.F.; Chaniotakis, N. Semiconductor Quantum Dots in Chemical Sensors and Biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Kulandaiswamy, A.J.; Sharma, N.; Nesakumar, N.; Kailasam, K.; Rayappan, J.B.B. S,N-GQDs Enzyme Mimicked Electrochemical Sensor to Detect the Hazardous Level of Monocrotophos in Water. Electroanalysis 2020, 32, 971–977. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Bao, J.; Hou, C.; Dong, Q.; Ma, X.; Chen, J.; Huo, D.; Yang, M.; Galil, K.H.A.E.; Chen, W.; Lei, Y. ELP-OPH/BSA/TiO2 nanofibers/c-MWCNTs based biosensor for sensitive and selective determination of p-nitrophenyl substituted organophosphate pesticides in aqueous system. Biosens. Bioelectron. 2016, 85, 935–942. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Yang, L. Ultra-highly sensitive and stable acetylcholinesterase biosensor based on TiO2-NRs and rGO. Microchem. J. 2021, 168, 106435. [Google Scholar] [CrossRef]
- Cheng, W.; Zheng, Z.; Yang, J.; Chen, M.; Yao, Q.; Chen, Y.; Gao, W. The visible light-driven and self-powered photoelectrochemical biosensor for organophosphate pesticides detection based on nitrogen doped carbon quantum dots for the signal amplification. Electrochim. Acta 2019, 296, 627–636. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, M. Il Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. Trends Anal. Chem. 2020, 132, 116038. [Google Scholar] [CrossRef] [PubMed]
- Kangas, M.J.; Burks, R.M.; Atwater, J.; Lukowicz, R.M.; Williams, P.; Holmes, A.E. Colorimetric Sensor Arrays for the Detection and Identification of Chemical Weapons and Explosives. Crit. Rev. Anal. Chem. 2017, 47, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, F.; Wu, J.; Yang, Q.; Li, Q. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Srivastava, K.R.; Awasthi, S.; Mishra, P.K.; Srivastava, P.K. Biosensors/Molecular Tools for Detection of Waterborne Pathogens; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978012-818783-8.00013-X. [Google Scholar]
- Tessaro, L.; Aquino, A.; de Almeida Rodrigues, P.; Joshi, N.; Ferrari, R.G.; Conte-Junior, C.A. Nucleic Acid-Based Nanobiosensor (NAB) Used for Salmonella Detection in Foods: A Systematic Review. Nanomaterials 2022, 12, 821. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing-A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Aquino, A.; Souza, M.R.R.; Maciel, S.T.A.; Da Rosa Alexandre, M.; Navickiene, S. Multiclass MSPD method for pesticide determination in dehydrated Hyptis pectinata (Sambacaitá) medicinal plant by GC-MS. J. Braz. Chem. Soc. 2011, 22, 1525–1530. [Google Scholar] [CrossRef]
- De Andrade, J.C.; Galvan, D.; Effting, L.; Tessaro, L.; Aquino, A.; Conte-Junior, C.A. Multiclass Pesticide Residues in Fruits and Vegetables from Brazil: A Systematic Review of Sample Preparation Until Post-Harvest. Crit. Rev. Anal. Chem. 2021. online ahead of print. [Google Scholar] [CrossRef]
- WHO/FAO. Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session; Report of the Thirty Eight Session of the Codex Committee on Food Hygiene; WHO/FAO: Rome, Italy, 2007; 104p. [Google Scholar]
- FAO/WHO. Pesticides Residues in Food 2016. FAO Plant Production and Protection Paper 229; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; ISBN 9789251095522. ISSN 0259-2517. [Google Scholar]






| Inclusion Criteria | Exclusion Criteria |
|---|---|
| English language | Non-English language articles |
| Original research articles | Thesis, review articles, and short communications |
| Use of biosensors for pesticide detection | Use of biosensors to detect non-pesticide |
| Biosensor application in food matrices | Biosensors not applied in food matrices |
| Articles published from 2016 to 2021 | Articles published outside of this timeline |
| Nanomaterial | Biorecognition Material | LOD | Pesticide or Pesticide Class | Food Matrix | Ref. |
|---|---|---|---|---|---|
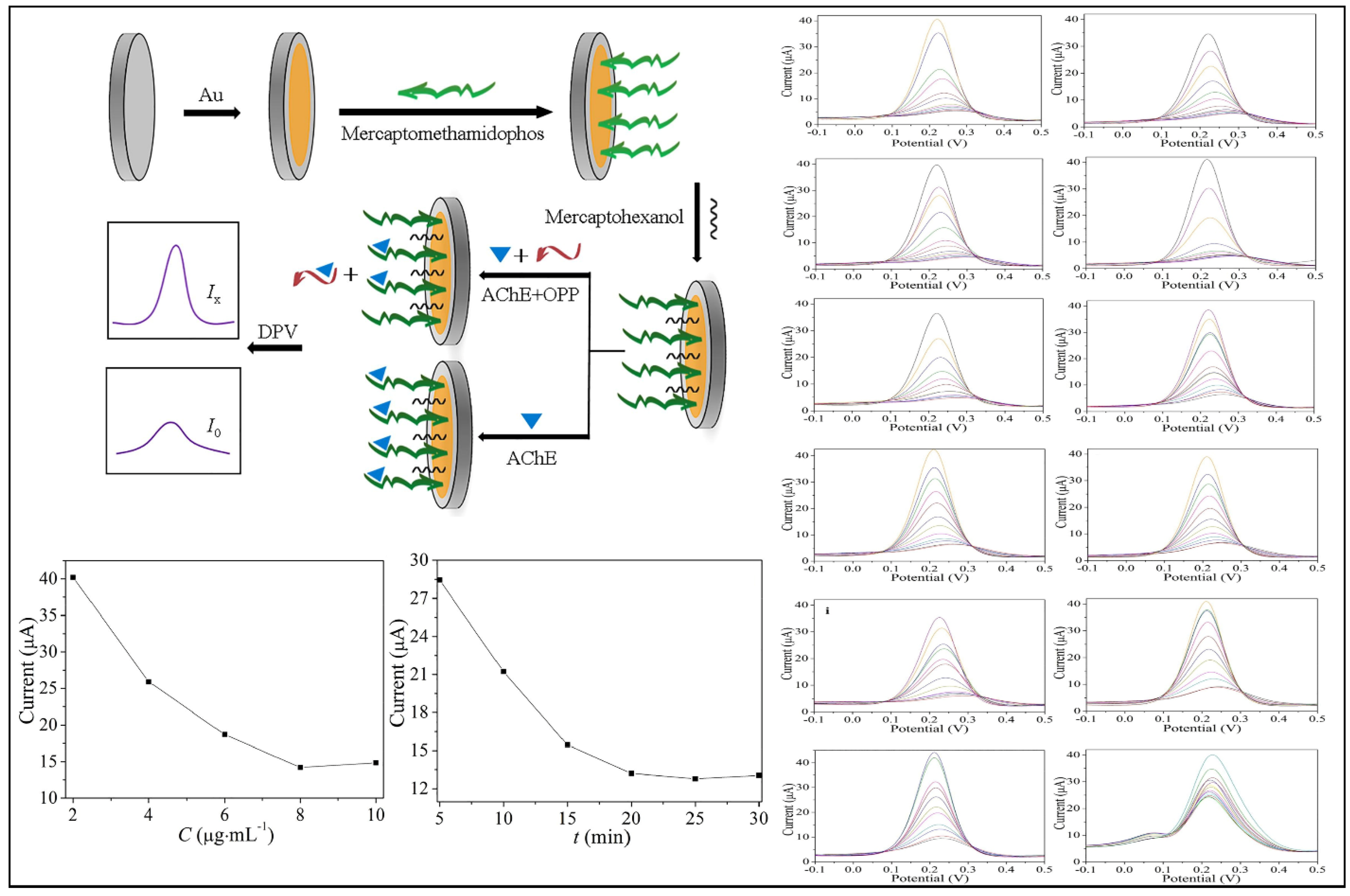
| AuNPs | AChE | Organophosphorus: 19–77 ng L−1 Methomyl: 81 ng L−1 | 11 Organophosphorus pesticides and Methomyl | Apple and Cabbage | [24] |
| AuNPs | AChE | 1.0 nM | Carbamate | Fruit | [25] |
| AuNPs | Aptamer | 36 ng L−1 | Chlorpyrifos | Apple and Pak choi | [26] |
| AuNPs | Antibody | 70 × 10−3 ng L−1 | Chlorpyrifos | Chinese cabbage and Lettuce | [27] |
| AuNPs | Aptamer | 2.48 × 103 ng L−1 | Isocarbophos | Cabbage, Peach and Tea | [28] |
| PEDOT-MWCNTs | Antibody | 1 × 10−6 nM | Malathion | Lettuce | [29] |
| AuNPs CP-MOF-Fc | Aptamer | 17.18 ng L−1 | Malathion | Cucumber and Long bean | [30] |
| AgNPs GQDs | AChE | 17 × 103 ng L−1 | Paraoxon | Apple and Carrot | [31] |
| AgNPs | G-DNA | 34 ng L−1 | Organophosphorus | Apple | [32] |
| AgNPs | AChE | 4 × 103 ng L−1 | Paraoxon | Chives and Cabbage | [33] |
| AgNPs | Aptamer | 5 × 10−4 nM | Malathion | Apple | [34] |
| AgNWs | BChE | 212 nM | Paraoxon | Milk | [35] |
| Ag@AuNPs | Aptamers | Profenofos: 2.1 ng L−1; Acetamiprid: 4.6 ng L−1 Carbendazim: 6.1 ng L−1 | Profenophos, Carbendazim and Acetamiprid | Rice and Apple | [36] |
| Au–Ag NC | BSA | 8.2 nmol L−1 | Methyl parathion | Apple, Cabbage, Spinach and Lettuce | [37] |
| AuNRs and MS SiO2 | AChE | Fenthion: 1.3 nM Dichlovos: 5.3 nM | Fenthion and Dichlorvos | Cabbage juice | [38] |
| AuNPs and UCNPs | ABA | 0.36 nM | Acetamiprid | Celery leaves and Chinese green tea | [39] |
| AuNPs and VNSWCNTs | AChE | Methyl parathion: 3.04 × 10−3 ng L−1 Malathion: 1.96 × 10−3 ng L−1 Chlorpyrifos: 2.06 × 10−3 ng L−1 | Methyl parathion, Malathion and Chlorpyrifos | Cabbage juice | [40] |
| Au-Ag NC | AChE | 2.40 × 10−3 nM | Ethyl parathion | Orange and Apple juice | [41] |
| MHCS and Fe3O4@MHCS | AChE | MHCS: 14.8 ng L−1 Fe3O4@MHCS: 18.2 ng L−1 | Malathion | Pear | [42] |
| PtPd@NCS | AChE | 8.6 × 10−6–7.1 × 10−5 nM | Malathion, Chlorpyrifos and Methyl parathion | Potato and Corn grans | [43] |
| QDs-AuNSs | Antibodies | Chlorpyrifos: 730 ng L−1 Diazinon: 6.7 × 103 ng L−1 Malathion: 740 ng L−1 | Chlorpyrifos, Malathion and Diazinon | Maize, Long bean, Cauliflower, Eggplant, Oyster mushroom, Shiitake mushroom, Apple, Orange, Tomato, Blueberry, Spinach, Lettuce and Cabbage | [44] |
| PtNPs AuNPs and MNPs | mAbs ssDNA C-ssDNA | 2 ng L−1 | Parathion | Pear, Cabbage and Rice | [45] |
| Au@PtNPs MNPs | ssDNAs and mAbs | 2.13 ng kg−1 | Parathion | Rice, Pear, Apple and Cabbage | [46] |
| SiO2 and Cr/Au modified layer | Aptamer | 50 nM | Dimethyl-methylphosphonate | Apple juice | [47] |
| PDA-AuNPs | Aptamer | 5 × 10−1 ng L−1 | Malathion | Cauliflower and Cabbage | [48] |
| AuNPs | AChE | 1.4 × 103 ng L−1 | Paraoxon | Vegetable (not specified) | [49] |
| MWCNTs | AChE | 50 ng L−1. | Chlorpyrifos | Cabbage, Rape and Lettuce | [50] |
| MWCNTs | AChE | 1 × 10−6 ng L−1 | Malathion | Lettuce | [51] |
| MWCNTs | ds-DNA | 0.3 nmol L−1 | Diazinon | Lettuce and Tomato juice | [52] |
| MWCNT | AChE | 0.1 nM | Paraoxon | Potato | [53] |
| MWCNTs | AChE | 4 × 10−3 nM | Organophosphate | Spinach and Cabbage | [54] |
| f-MWCNTs | AChE | 1 ng L−1 | Chlorpyrifos-methyl | Lettuce | [55] |
| ZnS:Mn-QDs and MWCNTs | Aptamer | 0.7 nM | Acetamiprid | Cabbage leaves | [56] |
| CNT | M-Cell | 3 × 10−6 nmol L−1 | Paraoxon | Spinach juice | [57] |
| PB-SWCNTs | AChE | Malathion: 3.11 × 10−4 ng L−1 Metyl parathion: 1.88 × 10−4 ng L−1 | Malathiona and Methyl parathion | Chinese cabbage | [58] |
| Fe3O4 and graphene | AChE | 20 ng L−1 | Chlorpyrifos | Cabbage and Spinach | [59] |
| TiO2 NP | AChE | 0.23 nM | Dichlorvos | Cabbage juice | [60] |
| TiO2NP | Nanoenzymes | Methyl paraoxon: 240 nM Methyl parathion: 260 nM Ethyl paraoxon: 220 nM | Organophosphorus | Lettuce | [61] |
| Film titanium with AuNP | Aptamer | 1.3 × 103 ng L−1 | Profenofos | Chinese chives | [62] |
| SBA-15 | AChE | Monocrotophos: 2510 ng L−1 Dimethoate: 1500 ng L−1 | Monocrotophs and Dimethoate | Soft drinks | [63] |
| WO3/g-C3N4 | Tc-AChE | 3.6 nM | Phosmet | Wheat flour | [8] |
| CS-PVA | AChE | 0.2 nM | Pirimiphosmethyl | Olive oil | [64] |
| CdTe-QD | AChE and CHOx | Paraoxon: 1.62 × 10−6 nM Dichlorvos: 7.53 × 10−5 nM Malathion: 0.23 nM Triazophos: 1.06 × 10−2 nM | Paraoxon, Dichlorvos, Malathion and Triazophos | Apple and Tomato juice | [65] |
| CHIT-IO | Biotinylated DNA | 1 ng L−1 | Malathion | Lettuce leaves | [66] |
| rGO/AuNPs | AChE | Malathion: 2.78 × 10−2 ng L−1 Methyl parathion: 2.17 × 10−2 ng L−1 | Malathion and Methyl parathion | Chinese cabbage | [67] |
| rGO | AChE | 1.9 nmol L−1 | Carbamate | Tomatoes | [68] |
| rGO | Aptamer | 7.12 × 10−5 nM | Acetamiprid | Tea | [69] |
| rGO-TEPA-CuNW | AChE | 3.9 × 102 ng L−1 | Malathion | Cabbage and Carrot | [70] |
| CS@TiO2-CS/rGO | AChE | 29 nM | Dichlorvos | Cabbage juice | [71] |
| ZIF-8 | AChE | 1.70 × 103 ng/L | Paraoxon | Apple and Eggplant | [31] |
| MPtPdN | AChE | 1.7 × 10−3 nM | Organophosphate | Cabbage and Cucumber | [72] |
| CdTe-QD | AChE | Pirimicarb: 5 × 104 ng L−1 Dichlorvos: 1 × 104 ng L−1 Carbaryl: 1 × 104 ng L−1 | Organophosphorus and Carbamate | Lettuce, Choy and Rice | [73] |
| Nanocarriers (Proline- UIO-66) | Candida Rugosa Lipase | 26 nM | Nitrofen | Apricot | [74] |
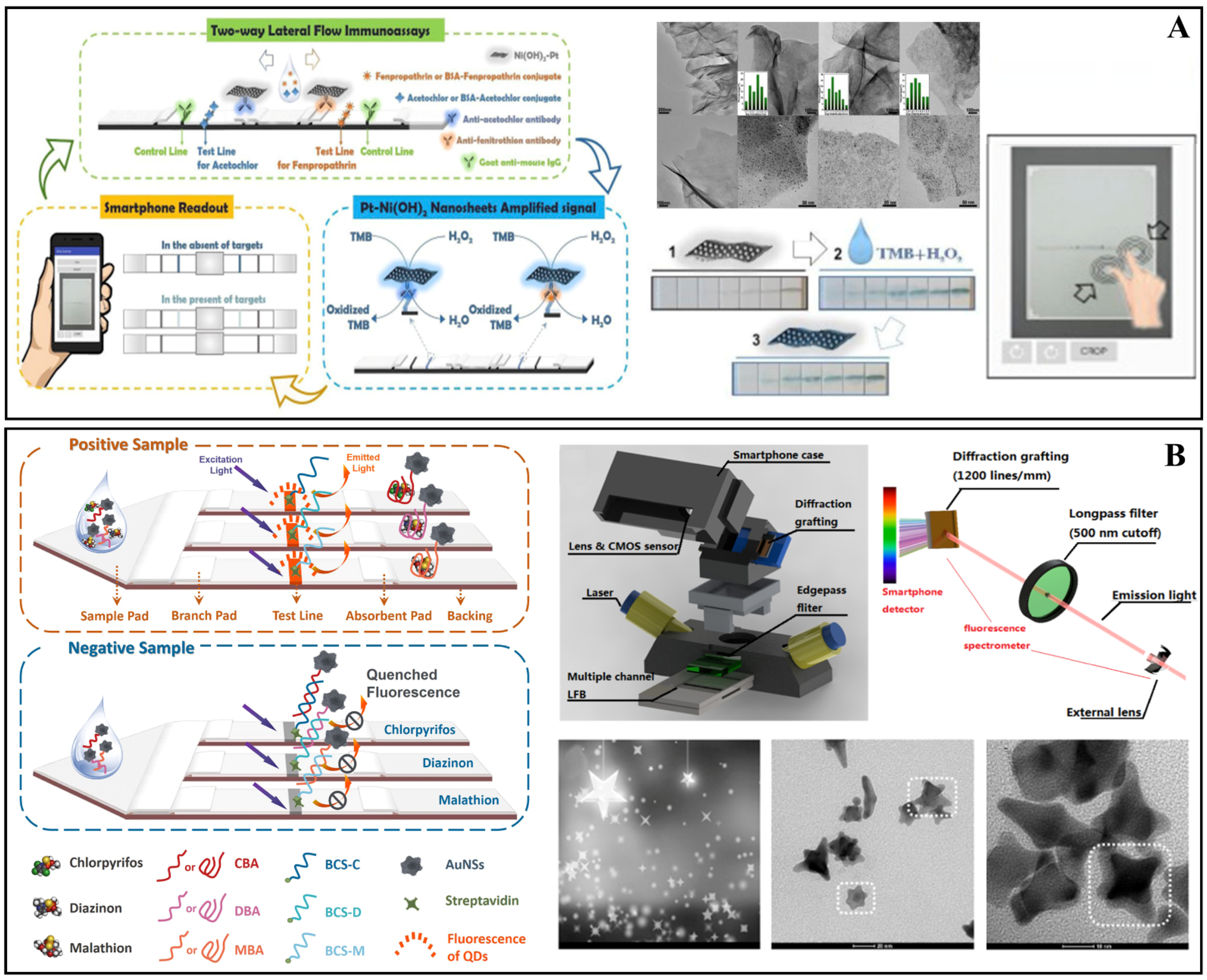
| Pt–Ni(OH)2 and nanosheets | Antibodies and Nitrocellulose membrane | Acetochlor: 6.3 × 102 ng L−1 Fenpropathrin: 2.4 × 102 ng L−1 | Acetochlor and Fenpropathrin | Corn, Sorghum, Soybean, Apple, Orange, Peach, Cabbage, Broccoli, Tomato and Drinking water | [75] |
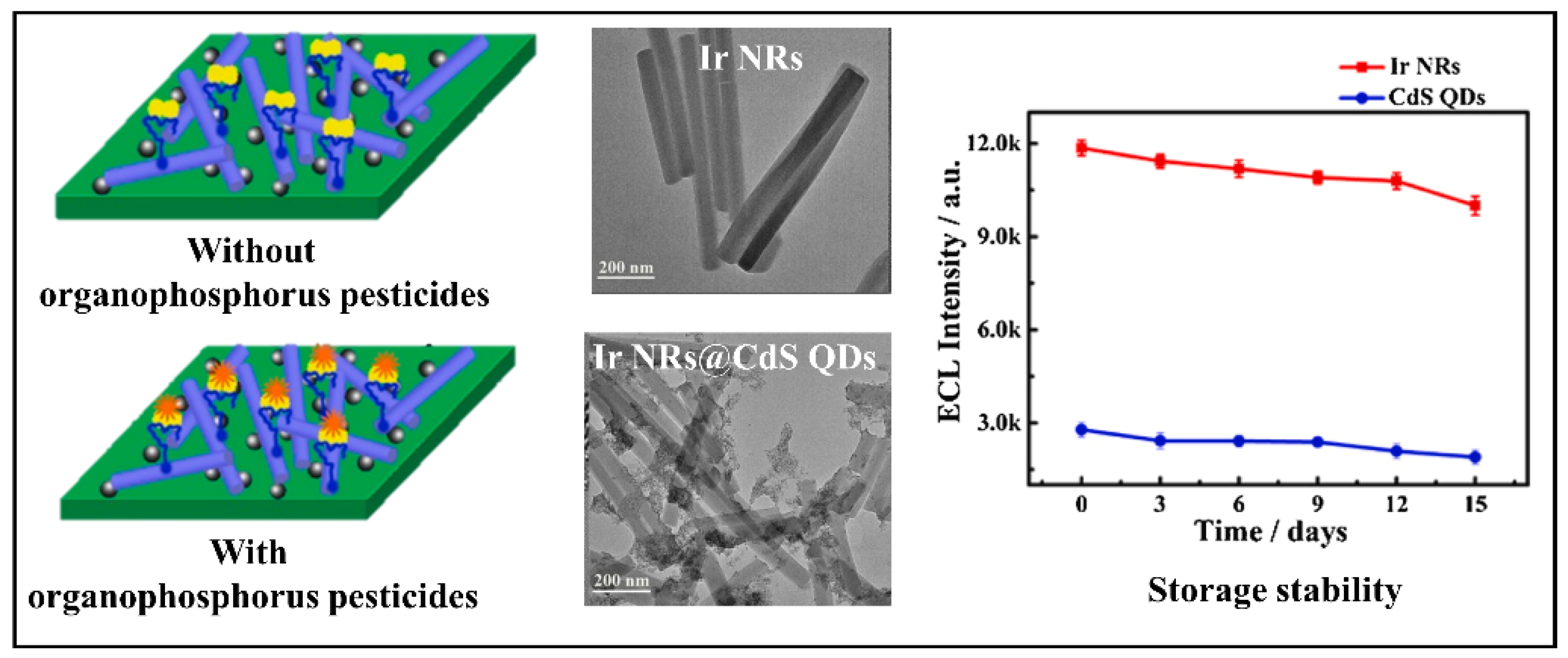
| Ir NRs@CdS QDs | AChE-ChOx biocomposite | 1.67 × 10−3 nM | Organophosphorus | Pakchoi, Cabbage and Lettuce | [76] |
| UCNPs | Aptamer | 50 ng⋅L−1 | Carbendazim | Apple, Cucumber and Matcha powder | [77] |
| CuO NFs and c-SWCNTs | Oligonucleotides | 70 ng L−1 | Chlorpyrifos | Apple and Cabbage | [78] |
| Biosensor-Based | Biorecognition Material | Pesticide or Pesticide Class | Transducer Type | LOD | RSD(%) | Ref. |
|---|---|---|---|---|---|---|
| DNA | Aptamer | Malathion | Colorimetric | 5 × 10−4 nM | 2.98 | [34] |
| DNA | C-ssDNA | Parathion | Colorimetric | 2 ng L−1 | Pear: 5.19 Cabagge: 9.81 Rice: 15.75 | [45] |
| Enzyme | Nanozyme | Parathion | Colorimetric | 2.13 ng kg−1 | Rice: 5.59 Pear: 6.09 Apple: 10.18 Cabbage: 10.87 | [46] |
| DNA | Aptamer | Isocarbophos | Colorimetric | 2.48 × 10 3 ng L−1 | 2.37–7.13 | [28] |
| Antibodies | Antibodies | Acetochlor and Fenpropathrin | Colorimetric | Acetochlor: 6.3 × 102 ng L−1 Fenpropathrin: 2.4 × 102 ng L−1 | 3.30 | [75] |
| Enzyme | BChE | Paraoxon | Electrochemical (Amperometric) | 212 nM | - | [35] |
| Enzyme | AChE | Paraoxon | Electrochemical (CV) | 4 × 103 ng L−1 | Chinese chives: 2.39 Cabbage: 5.86 | [33] |
| Antibodies | BSA | Methyl parathion | Electrochemical (CV) | 8.2 nmol L−1 | 4.7 | [37] |
| DNA | Oligonucleotides | Chlorpyrifos | Electrochemical (CV) | 70 ng L−1 | Apple: 2.52 Celery cabbage: 2.25 | [78] |
| Enzyme | AChE | Chlorpyrifos | Electrochemical | 20 ng L−1 | Cabbage: 3.86 Spinach: 2.46 | [59] |
| Enzyme | AChE | Chlorpyrifos | Electrochemical (CV) | 50 ng L−1 | Cabbage: 4.35 Rape: 2.57 Lettuce: 3.17 | [50] |
| Enzyme | AChE | Dichlorvos | Electrochemical (CV and DPV) | 0.23 nM | 7.3 | [60] |
| Enzyme | AChE | Malathion | Electrochemical (DPV) | 1 × 10−6 nM | - | [51] |
| Enzyme | AChE | Malathion and Methyl parathion | Electrochemical (CV) | Malathion: 3.11 × 10−4 ng L−1 Methyl parathion: 1.88 × 10−4 ng L−1 | 4.59 | [58] |
| Enzyme | AChE | Monocrotophos and Dimethoate | Electrochemical (CV) | Monocrotophos 2.51 × 103 ng L−1 Dimethoate 1.50 × 103 ng L−1 | Monocrotophos: 1.05 Dimethoate: 0.95 | [63] |
| Enzyme | AChE | Fenthion | Electrochemical (CV and EIS) | 1.3 nM | 11.5 | [38] |
| Enzyme | AChE | Chlorpyrifos-methyl | Electrochemical (DPV) | 1 ng L−1 | - | [55] |
| DNA | ds-DNA | Diazinon | Electrochemical (EIS) | 0.3 nmol L−1 | - | [52] |
| Enzyme | Tc-AChE | Phosmet | Electrochemical (CV and EIS) | 3.6 nM | 2.5 | [8] |
| Enzyme | AChE | Malathion | Electrochemical (CV and EIS) | 3.9 × 102 ng L−1 | 2.3 | [70] |
| Enzyme | AChE | Pirimiphos methyl | Electrochemical (Amperometric) | 0.2 nM | - | [64] |
| Enzyme | AChE and CHOx | Malathion | Electrochemical (DPV) | 1 ng L−1 | - | [66] |
| Enzyme | AChE | 11 Organophosphorus pesticides and Methomyl | Electrochemical (DPV and EIS) | Organophosphorus: 19–77 ng L−1 Methomyl: 81 ng L−1 | Trichlorfon: 1.80–8.63 Dichlorvos: 3.21–9.20 | [24] |
| Enzyme | Nanoenzyme | Organophosphorus | Electrochemical (DPV) | Methyl paraoxon: 240 nM Methyl parathion: 260 nM Ethyl paraoxon: 220 nM | Methyl paraoxon: 3.41 Methyl parathion: 2.41 Ethyl paraoxon: 2.56 | [61] |
| DNA | Aptamer | Chlopyrifos | Electrochemical (CV) | 36 ng L−1 | 2.57–7.08 | [26] |
| Enzyme | AChE | Organophosphorus | Electrochemical (CV) | Malathion: 2.78 × 10−2 ng L−1 Methyl parathion: 2.17 × 10−2 ng L−1 | 4.07 | [67] |
| Enzyme | AChE | Paraoxon | Electrochemical (CV) | 0.1 nM and 500 nM | - | [53] |
| Enzyme | AChE | Paraoxon | Electrochemical (DPV) | 1.4 × 103 ng L−1 | 4.68 | [49] |
| Enzyme | AChE | Malathion | Electrochemical (CV and EIS) | 3.9 × 102 ng L−1 | 2.30 | [70] |
| Enzyme | AChE | Organophosphorus | Electrochemical (CV and EIS) | 14.8 ng L−1–18.2 ng L−1 | 5.6–7.1 | [42] |
| Enzyme | AChE | Carbaryl | Electrochemical (CV) | 1.0 nM | 5.32 | [25] |
| Enzyme | AChE | Malathion, Chlorpyrifos and Methyl parathion | Electrochemical (DPV) | 8.6 × 10−6–7.1 × 10−5 nM | 3.31–5.24 | [43] |
| Enzyme | AChE | Methyl parathion, Malathion and Chlorpyrifos | Electrochemical (CV, DPV and EIS) | Methyl parathion: 3.04 × 10−3 ng L−1 Malathion: 1.96 × 10−3 ng L−1 Chlorpyrifos: 2.06 × 10−3 ng L−1 | 3.74 | [40] |
| Enzyme | AChE | Carbaryl | Electrochemical (CV and EIS) | 1.9 nmol L−1 | - | [68] |
| Enzyme | Candida Rugosa Lipase | Nitrofen | Electrochemical (DPV) | 26 nM | 1.75–4.12 | [74] |
| Enzyme | AChE-ChOx | Organophosphorus | Electrochemical (CV) | 1.67 × 10−3 nM | 5 | [76] |
| Cell | M-Cell | Paraoxon | Electrochemical (DPV) | 3 × 10−6 nmol L−1 | 3.5 | [57] |
| Antibodies | Antibody | Chlorpyrifos | Electrochemical (EIS) | 70 × 10−3 ng L−1 | 2.6 | [27] |
| Enzyme | AChE | Dichlorvos | Electrochemical (CV and EIS) | 29 nM | 1.44 | [71] |
| DNA | Aptamer | Acetamiprid | Electrochemical (CV and EIS) | 7.12 × 10−5 nM | 5.9 | [69] |
| Enzyme | AChE | Paraoxon | Electrochemical (CV and EIS) | 4 × 10−3 nM | Spinach: 10.2–96 Cabbage: 3.4–4.1 | [54] |
| Enzyme | AChE | Organophosphorus | Electrochemical (DPV) | 1.73 × 10−3 nM | 3.84 and 5.91 | [72] |
| DNA | Aptamer | Malathion | Eletrochemical (DPV) | 5 × 10−1 ng L −1 | 1.04–6.14 | [48] |
| Enzyme | AChE | Paraoxon | Electrochemical (DPV) | 1.7 × 10 3 ng L−1 | Apple: 3.2–3.7 Eggplant 5.0–4.6 | [31] |
| DNA | Aptamer | Malathion | Electrochemical (DPV) | 17.18 ng L−1 | Cucumber: 1.17–1.33 Beans: 0.52–5.90 | [30] |
| Antibodies | Antibodies | Malathion | Electrochemical (DPV) | 1 × 10−6 nM | 1.15–3.21 | [29] |
| DNA | Aptamer | Cabendazim | Fluorescence | 50 ng L−1 | Apple: 2.02–4.39 Cucumber: 2.90–4.30 Matcha powder: 1.87–3.51 | [77] |
| DNA | G-DNA | Organophosphorus | Fluorescence | 34 ng L−1 | Apple: < 2.8 | [61] |
| DNA | Aptamer | Chlorpyrifos, Diazinon and Malathion | Fluorescence | Chlorpyrifos: 730 ng L−1 Diazinon: 6.7 × 103 ng L−1 Malathion: 740 ng L−1 | - | [44] |
| Enzyme | AChE and CHOx | Paraoxon, Dichlorvos, Malathion and Triazophos | Fluorescence | 1.62 × 10 −6–0.23 nM | 2.23–7.19 | [65] |
| DNA | ABA | Acetamiprid | Fluorescence | 0.36 nM | <4.54 | [39] |
| Enzyme | AChE | Pirimicarb, Dichlorvos and Carbaryl | Fluorescence | Pirimicarb: 5 × 104 ng L−1 Dichlorvos: 1 × 104 ng L−1 Carbaryl: 1 × 10 4 ng L−1 | - | [73] |
| Enzyme | AChE | Ethylparathion | Fluorescence | 2.40 × 10−3 nM | - | [41] |
| DNA | Aptamer | Acetamiprid | Fluorescence | 0.7 nM | Cabbage leaves: 1.0–2.1 | [56] |
| DNA | Aptamer | Profenofos | Microcantilever | 1.3 × 103 ng L−1 | - | [62] |
| DNA | Aptamer | Dimethyl methylphosphonate | Piezoelectric | 50 nM | - | [47] |
| DNA | Aptamer | Profenofos, Acetamiprid and Carbendazim | SERS | Profenofos: 2.1 ng L−1 Acetamiprid: 4.6 ng L−1 Carbendazin: 6.1 ng L−1 | - | [36] |
| Pesticides | ARfD (mg/kg bw) | ADI (mg/kg) | LD50 (mg/kg) |
|---|---|---|---|
| Acetamiprid | 0.025 | 0.070 | 146 |
| Fenpropathrin | 0.061 | 0.030 | 870 |
| Acetochlor | 1.5 | 1.0 | 1929 |
| Carbendazim | 0.020 | 0.020 | 15,000 |
| Carbaryl | 0.10 | 0.20 | 303 |
| Chlorpyrifos | 0.011 | 0.12 | 500 |
| Diazinon | 0.00020 | 0.025 | 1160 |
| Dichlorvos | 0.10 | 0.0040 | 80 |
| Dimethyl-methylphosphonate | - | - | 5000 |
| Ethylparathion | 0.0050 | 0.00061 | 2.0 |
| Fenthion | 0.0010 | 0.0072 | 190 |
| Isocarbophos | - | - | 50 |
| Malathion | 0.30 | 0.030 | 1778 |
| Methyl parathion | 0.030 | 0.0030 | 3.0 |
| Monocrotophos | 0.0020 | 0.00060 | 112 |
| Dimethoate | - | 0.013 | 240 |
| Nitrofen | - | - | 5000 |
| Paraoxon | - | - | 1800 |
| Phosmet | 0.045 | 0.010 | 113 |
| Pirimicarb | 0.11 | 0.035 | 142 |
| Pirimiphos methyl | 0.15 | 0.004 | 1250 |
| Profenofos | 1.0 | 0.030 | 450 |
| Triazophos | 0.0012 | 0.00020 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirres, A.C.d.M.; Silva, B.E.P.d.M.d.; Tessaro, L.; Galvan, D.; Andrade, J.C.d.; Aquino, A.; Joshi, N.; Conte-Junior, C.A. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors 2022, 12, 572. https://doi.org/10.3390/bios12080572
Mirres ACdM, Silva BEPdMd, Tessaro L, Galvan D, Andrade JCd, Aquino A, Joshi N, Conte-Junior CA. Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors. 2022; 12(8):572. https://doi.org/10.3390/bios12080572
Chicago/Turabian StyleMirres, Ana Carolina de Morais, Brenno Enrique Pereira de Matos da Silva, Leticia Tessaro, Diego Galvan, Jelmir Craveiro de Andrade, Adriano Aquino, Nirav Joshi, and Carlos Adam Conte-Junior. 2022. "Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods" Biosensors 12, no. 8: 572. https://doi.org/10.3390/bios12080572
APA StyleMirres, A. C. d. M., Silva, B. E. P. d. M. d., Tessaro, L., Galvan, D., Andrade, J. C. d., Aquino, A., Joshi, N., & Conte-Junior, C. A. (2022). Recent Advances in Nanomaterial-Based Biosensors for Pesticide Detection in Foods. Biosensors, 12(8), 572. https://doi.org/10.3390/bios12080572