A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
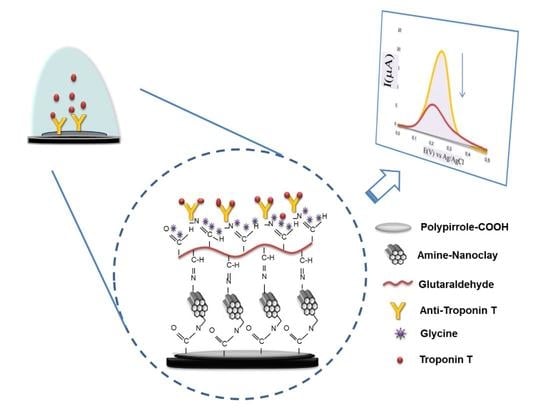
2.3. Sensor Preparation
2.4. Electrochemical Immunoassay
3. Results and Discussion
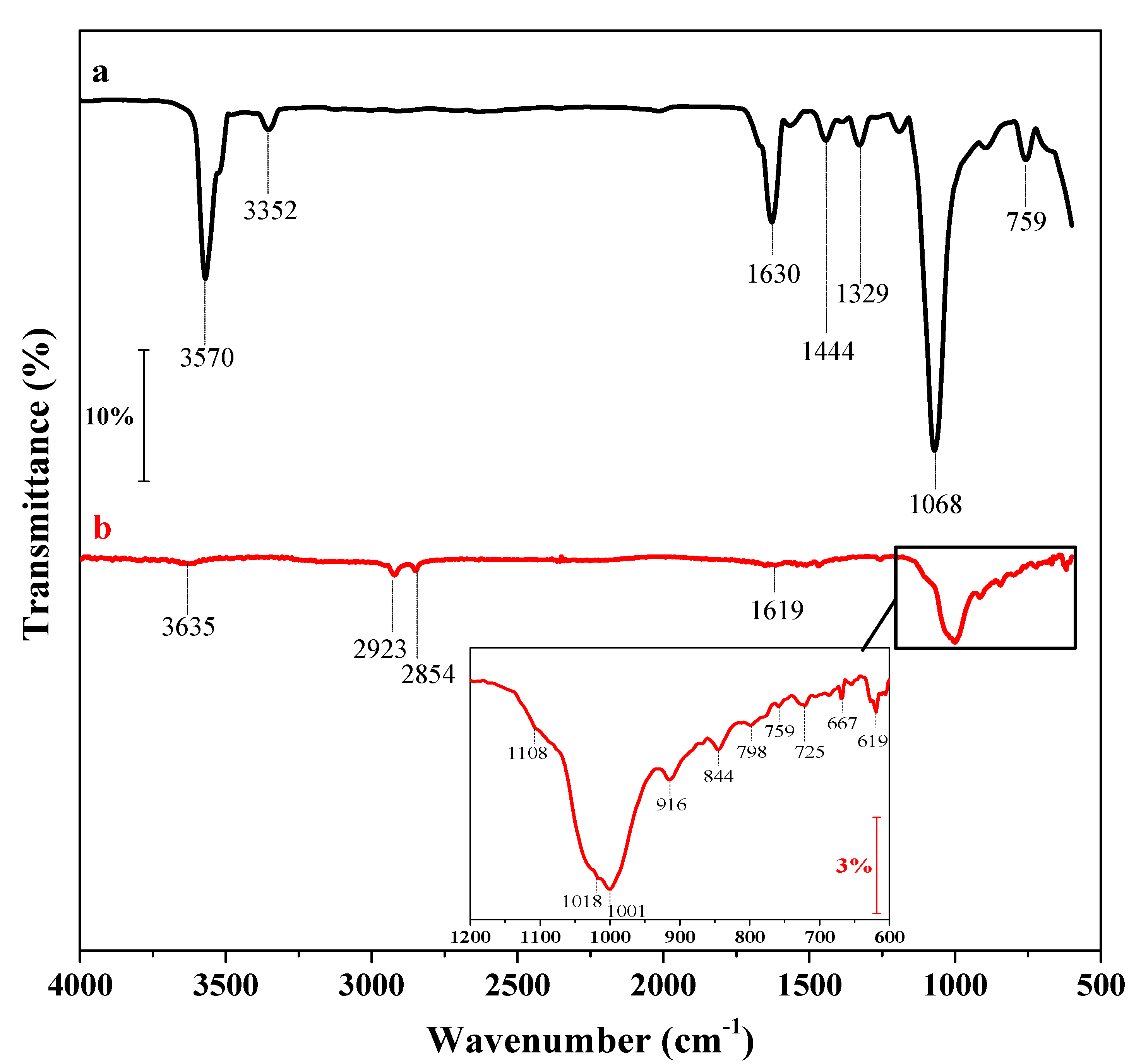
3.1. Characterization of the Nanohybrid Film
3.2. Immunosensor Preparation
3.3. Optimization of the Experimental Conditions
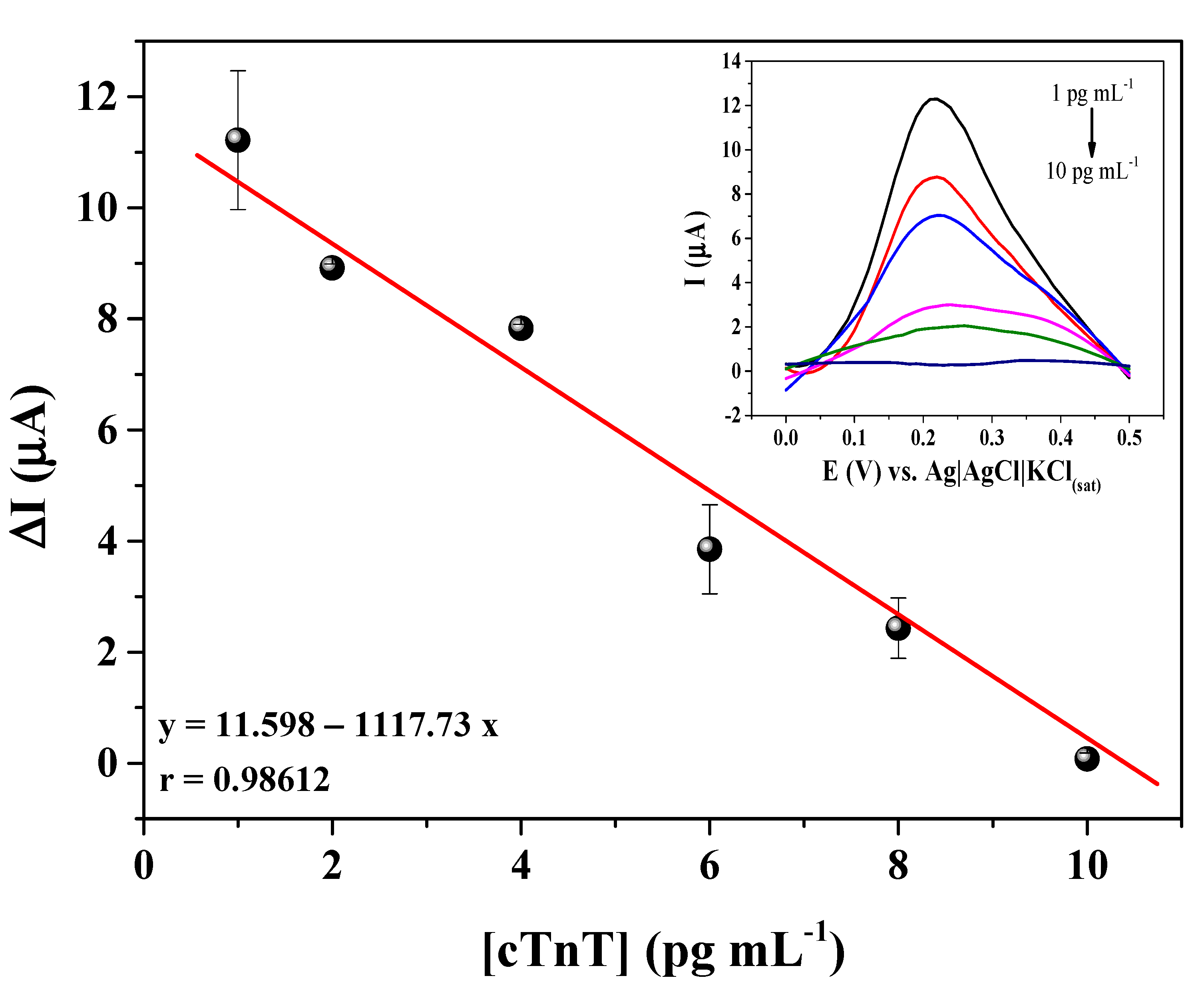
3.4. Analytical Response
3.5. Immunosensor Response to Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Ahmed, M.U. Introduction to Immunosensors; The Royal Society of Chemistry: London, UK, 2019; pp. 1–20. ISBN 9781788016162. [Google Scholar]
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef] [PubMed]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-carbons in biosensor applications: An overview of carbon nanotubes (CNTs) and fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef] [Green Version]
- Malekzad, H.; Sahandi Zangabad, P.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Zumpano, R.; Polli, F.; D’Agostino, C.; Antiochia, R.; Favero, G.; Mazzei, F. Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem 2021, 2, 10–28. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for biosensing, with a brief overview on cancer detection, IoT, and the role of machine learning in smart biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; Pinyou, P.; Zampardi, G. Research Frontiers in Energy-Related Materials and Applications for 2020–2030. Adv. Sustain. Syst. 2020, 4, 1900145. [Google Scholar] [CrossRef]
- Pavón, E.; Martín-Rodríguez, R.; Perdigón, A.C.; Alba, M.D. New trends in nanoclay-modified sensors. Inorganics 2021, 9, 43. [Google Scholar] [CrossRef]
- Mousty, C. Sensors and biosensors based on clay-modified electrodes—New trends. Appl. Clay Sci. 2004, 27, 159–177. [Google Scholar] [CrossRef]
- Hosseini, H.; Shojaee-Aliabadi, S.; Hosseini, S.M.; Mirmoghtadaie, L. Nanoantimicrobials in Food Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128119433. [Google Scholar]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zari, N.; Raji, M.; El Mghari, H.; Bouhfid, R.; Qaiss, A.E.K. Nanoclay and Polymer-Based Nanocomposites: Materials for Energy Efficiency; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081019115. [Google Scholar]
- Zare, Y.; Rhee, K.Y. Effects of critical interfacial shear strength between polymer and nanoclay on the Pukanszky’s “B” interphase factor and tensile strength of polymer nanocomposites. Mech. Mater. 2020, 149, 103562. [Google Scholar] [CrossRef]
- Hussain, S.A.; Chakraborty, S.; Bhattacharjee, D.; Schoonheydt, R.A. Incorporation of nano-clay saponite layers in the organo-clay hybrid films using anionic amphiphile stearic acid by Langmuir-Blodgett technique. Thin Solid Films 2013, 536, 261–268. [Google Scholar] [CrossRef]
- Wajs, E.; Fernández, N.; Fragoso, A. Supramolecular biosensors based on electropolymerised pyrrole-cyclodextrin modified surfaces for antibody detection. Analyst 2016, 141, 3274–3279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanavicius, A.; Oztekin, Y.; Ramanaviciene, A. Electrochemical formation of polypyrrole-based layer for immunosensor design. Sens. Actuators B Chem. 2014, 197, 237–243. [Google Scholar] [CrossRef]
- Foschini, M.; Silva, H.S.; Silva, R.A.; Marletta, A.; Gonçalves, D. Theoretical and experimental studies on the electronic, optical, and structural properties of poly-pyrrole-2-carboxylic acid films. Chem. Phys. 2013, 425, 91–95. [Google Scholar] [CrossRef]
- Chung, M.J.; Brown, D.L. Diagnosis of Acute Myocardial Infarction, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780323529938. [Google Scholar]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Katrukha, I.A.; Katrukha, A.G. Myocardial Injury and the Release of Troponins I and T in the Blood of Patients. Clin. Chem. 2021, 67, 124–130. [Google Scholar] [CrossRef]
- Kitte, S.A.; Tafese, T.; Xu, C.; Saqib, M.; Li, H.; Jin, Y. Plasmon-enhanced quantum dots electrochemiluminescence aptasensor for selective and sensitive detection of cardiac troponin I. Talanta 2021, 221, 121674. [Google Scholar] [CrossRef]
- Müller-Bardorff, M.; Hallermayer, K.; Schröder, A.; Ebert, C.; Borgya, A.; Gerhardt, W.; Remppis, A.; Zehelein, J.; Katus, H.A. Improved troponin T ELISA specific for cardiac troponin T isoform: Assay development and analytical and clinical validation. Clin. Chem. 1997, 43, 458–466. [Google Scholar] [CrossRef]
- Sinha, R.K. Wavelength modulation based surface plasmon resonance sensor for detection of cardiac marker proteins troponin I and troponin T. Sens. Actuators A Phys. 2021, 332, 113104. [Google Scholar] [CrossRef]
- Taranova, N.A.; Slobodenuyk, V.D.; Zherdev, A.V.; Dzantiev, B.B. Network of gold conjugates for enhanced sensitive immunochromatographic assays of troponins. RSC Adv. 2021, 11, 16445–16452. [Google Scholar] [CrossRef] [PubMed]
- Santana, G.M.; Silva, A.K.S.; Foguel, M.V.; Dutra, R.F. An ultrasensitive electrochemical immunosensor for hepatitis C antibodies based on one-stepeletrosynthetized polypyrrole–graphene nanocomposite. J. Mater. Sci. 2022, 57, 5586–5595. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Gao, R.; Yang, H. Application of layered nanoclay in electrochemical energy: Current status and future. EnergyChem 2021, 3, 100062. [Google Scholar] [CrossRef]
- Silva, B.V.M.; Cordeiro, M.T.; Rodrigues, M.A.B.; Marques, E.T.A.; Dutra, R.F. A label and probe-free zika virus immunosensor prussian blue@carbon nanotube-based for amperometric detection of the ns2b protein. Biosensors 2021, 11, 157. [Google Scholar] [CrossRef]
- Makaraviciute, A.; Ramanaviciene, A. Site-directed antibody immobilization techniques for immunosensors. Biosens. Bioelectron. 2013, 50, 460–471. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G.; Bond, A.M. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques. Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landim, V.P.A.; Foguel, M.V.; Prado, C.M.; Sotomayor, M.P.T.; Vieira, I.C.; Silva, B.V.M.; Dutra, R.F. A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors 2022, 12, 545. https://doi.org/10.3390/bios12070545
Landim VPA, Foguel MV, Prado CM, Sotomayor MPT, Vieira IC, Silva BVM, Dutra RF. A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors. 2022; 12(7):545. https://doi.org/10.3390/bios12070545
Chicago/Turabian StyleLandim, Vicente P. A., Marcos V. Foguel, Cecília M. Prado, Maria P. T. Sotomayor, Iolanda C. Vieira, Bárbara V. M. Silva, and Rosa F. Dutra. 2022. "A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor" Biosensors 12, no. 7: 545. https://doi.org/10.3390/bios12070545
APA StyleLandim, V. P. A., Foguel, M. V., Prado, C. M., Sotomayor, M. P. T., Vieira, I. C., Silva, B. V. M., & Dutra, R. F. (2022). A Polypyrrole/Nanoclay Hybrid Film for Ultra-Sensitive Cardiac Troponin T Electrochemical Immunosensor. Biosensors, 12(7), 545. https://doi.org/10.3390/bios12070545