NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
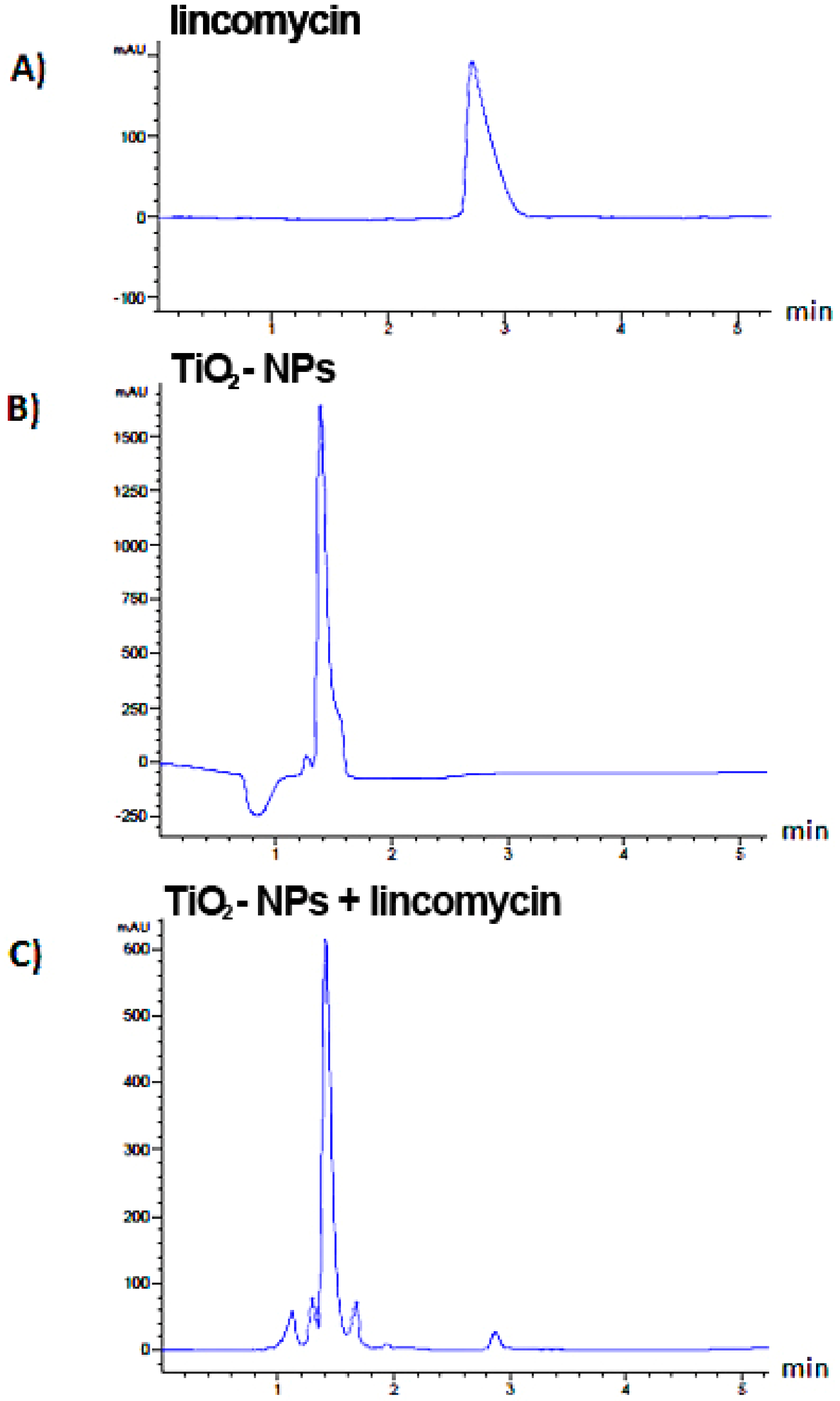
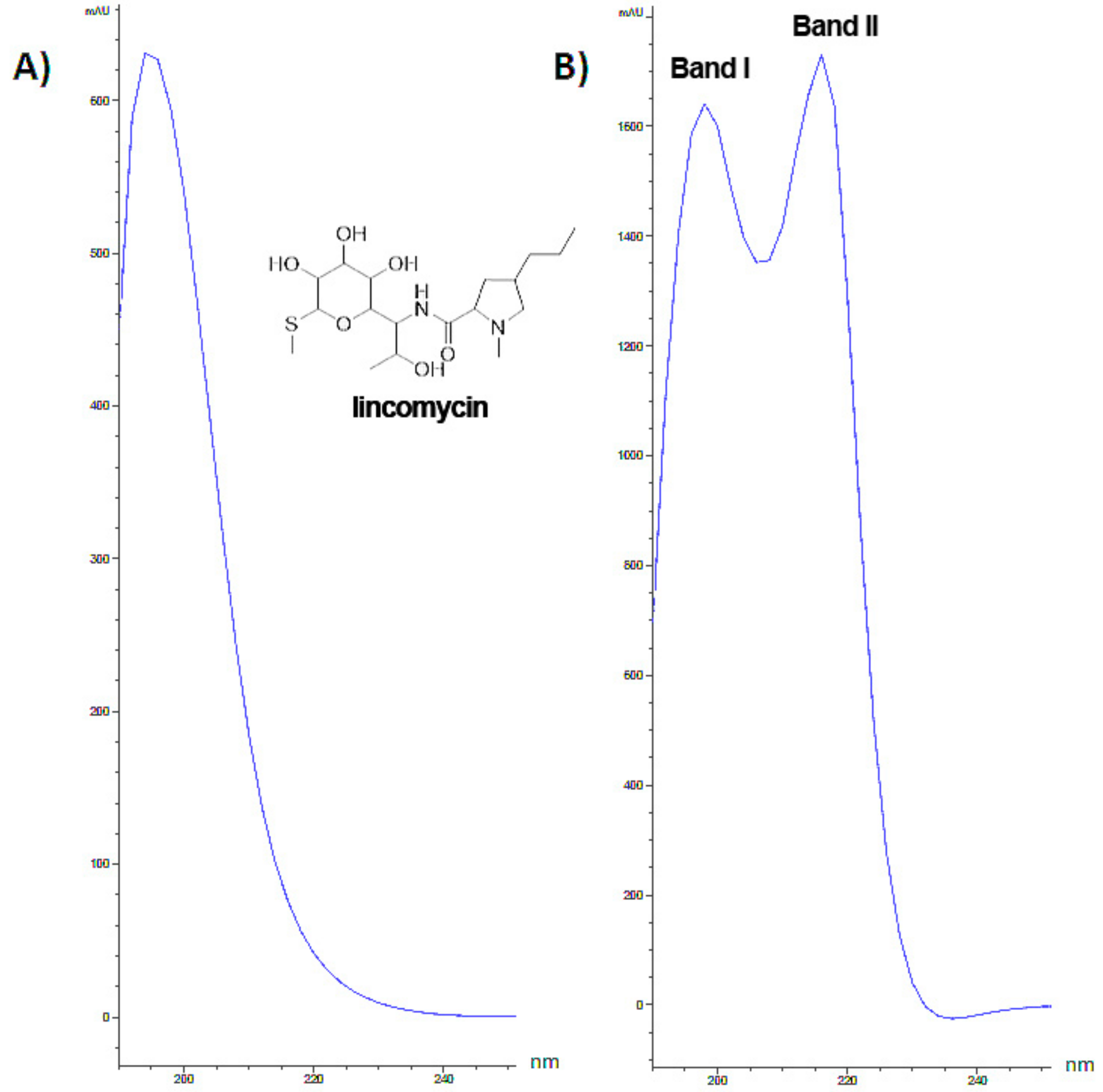
2.2. Chromatographic Analysis
2.3. Cell Culture and Exposure Procedure
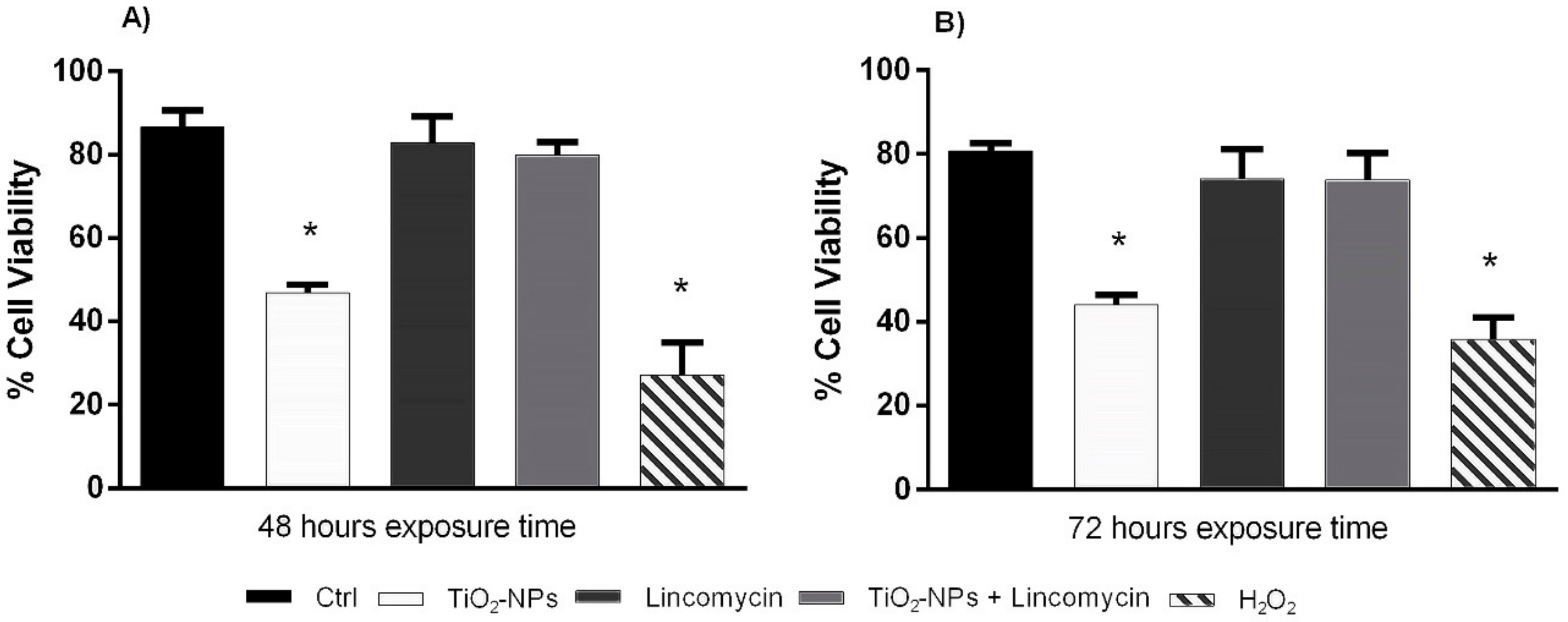
2.4. Viability Assay
2.5. Comet Assay
2.6. Diffusion Assay
2.7. RAPD-PCR Technique
2.8. Statistical Analysis
3. Results
3.1. Characterization and Analytical Determinations
3.2. TiO2-NPs Reduces Amniotic Cells Viability
3.3. TiO2-NPs and Lincomycin Co-Exposure Induces an Increase of Amniocyte DNA Fragmentation
3.4. TiO2-NPs in Combination with Lincomycin Cause Amniotic Cells Apoptosis
3.5. TiO2-NPs and Lincomycin Determines a Change of DNA Polymorphic Profiles
3.6. TiO2-NPs and Lincomycin Co-Exposure Decreases Amniotic Cells Genomic Stability
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, S.C.; Boyko, V.; Meyers, G.; Voetz, M.; Wohlleben, W. Toward advancing nano-object count metrology: A best practice framework. Environ. Health Perspect. 2013, 121, 1282. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhu, Y.; Zhang, M.; Yu, H.; Chen, W.; Qin, J. A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicol. In vitro 2019, 54, 105–113. [Google Scholar] [CrossRef]
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the fiel forward! Nanomedicine 2016, 11, 941–957. [Google Scholar] [CrossRef]
- Taylor, U.; Barchanski, A.; Garrels, W.; Klein, S.; Kues, W.; Barcikowski, S.; Rath, D. Toxicity of gold nanoparticles on somatic and reproductive cells. Adv. Exp. Med. Biol. 2012, 733, 125–133. [Google Scholar]
- Jorge de Souza, T.A.; Rosa Souza, L.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Envron. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Singh, S. Zinc oxide nanoparticles impacts: Cytotoxicity, genotoxicity, developmental toxicity, and neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef]
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology 2018, 12, 357–374. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In vitro genotoxic effects of titanium dioxide nanoparticles (n-TiO2) in human sperm cells. Mol. Reprod. Dev. 2019. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Santonastaso, M.; Mottola, F.; Costagliola, D.; Suero, T.; Pacifico, S.; Stingo, V. Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol. Environ. Saf. 2015, 113, 223–230. [Google Scholar] [CrossRef]
- Ren, W.; Zeng, L.; Shen, Z.; Xiang, L.; Gong, A.N.; Zhang, J.; Mao, C.; Li, A.; Paunesku, T.; Woloschak, G.E.; et al. Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv. 2013, 3, 20855–20861. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019. [Google Scholar] [CrossRef]
- Du, Y.; Ren, W.; Li, Y.; Zhang, Q.; Zeng, L.; Chi, C.; Wu, A.; Tian, J. The enhanced chemotherapeutic effects of doxorubicin loaded PEG coated TiO2 nanocarriers in an orthotopic breast tumor bearing mouse model. J. Mater. Chem. B 2015, 3, 1518–1528. [Google Scholar] [CrossRef]
- Haugen, H.J.; Will, J.C.O.; Koehler, A.G.; Hopfner, U.; Aigner, J.; Wintermantel, E. Ceramic TiO2-foams: Characterisation of a potential scaffold. J. Eur. Ceram. Soc. 2004, 24, 661–666. [Google Scholar] [CrossRef]
- Cui, C.; Liu, H.; Li, Y.; Sun, J.; Wanga, R.U.; Liu, S.A.; Greer, L. Fabrication and biocompatibility of nano-TiO2/titanium alloys biomaterials. Mater. Lett. 2005, 59, 3144–3148. [Google Scholar] [CrossRef]
- Peters, R.J.; Bemmel, V.G.; Herrera-Rivera, Z.; Helsper, H.P.; Marvin, H.J.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of titanium dioxide nanoparticles in food products: Analytical methods to define nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef]
- Lorenz, C.; Tiede, K.; Tear, S.; Boxall, A.; Von Goetz, N.; Hungerbühler, K. Imaging and characterization of engineered nanoparticles in sunscreens by electron microscopy, under wet and dry conditions. Int. J. Occup. Environ. Health 2010, 16, 406–428. [Google Scholar] [CrossRef]
- Contado, C.; Pagnoni, A. TiO2 nano-and micro-particles in commercial foundation creams: Field flow-fractionation techniques together with ICP-AES and SQW voltammetry for their characterization. Anal. Methods 2010, 2, 1112–1124. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Mahmoud, W.M.; Rastogi, T.; Kümmerer, K. Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Rezaei, B.; Mosaddeghi, H. Applications of titanium dioxide nanoparticles. In Proceedings of the Nano-Technology in Environments Conference, Isfahan University of Technology, Isfahan, Iran, 19–20 May 2009. [Google Scholar]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol. 2013, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of nano-titanium dioxide (TiO2-NP) through various routes of exposure: A review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Elgrabli, D.; Beaudouin, R.; Jbilou, N.; Floriani, M.; Pery, A.; Rogerieux, F.; Lacroix, G. Biodistribution and clearance of TiO2 nanoparticles in rats after intravenous injection. PLoS ONE 2015, 10, e012449. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C.; Devoy, J.; Cosnefroy, A.; Chalansonnet, M.; Herlin-Boime, N.; Brun, E.; Lund, A.; Mabondzo, A. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part Fibre Toxicol. 2015, 12, 27. [Google Scholar] [CrossRef]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef]
- Mao, Z.; Li, Y.; Dong, T.; Zhang, L.; Zhang, Y.; Li, S.; Hu, H.; Sun, C.; Xia, Y. Exposure to titanium dioxide nanoparticles during pregnancy changed maternal gut microbiota and increased blood glucose of rat. Nanoscale Res. Lett. 2019, 14, 26. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, J.; Chen, C.; Hong, F. Retardation of axonal and dendritic outgrowth is associated with the MAPK signaling pathway in offspring mice following maternal exposure to nanosized titanium dioxide. Agric. Food Chem. 2019, 67, 2709–2715. [Google Scholar] [CrossRef]
- Notter, T.; Aengenheister, L.; Weber-Stadlbauer, U.; Naegeli, H.; Wick, P.; Meyer, U.; Buerki-Thurnherr, T. Prenatal exposure to TiO2 nanoparticles in mice causes behavioral deficits with relevance to autism spectrum disorder and beyond. Transl. Psychiatr. 2018, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, X.; Zhou, Y.; Yu, D.; Deng, Y.; Ouyang, J.; Yang, B.; Luo, D.; Zhang, D.; Kuang, H. Gestational exposure to titanium dioxide nanoparticles impairs the placentation through dysregulation of vascularization, proliferation and apoptosis in mice. Int. J. Nanomed. 2018, 13, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol. 2011, 6, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Zhou, Y.; Zhao, X.; Sheng, L.; Wang, L. Maternal exposure to nanosized titanium dioxide suppresses embryonic development in mice. Int. J. Nanomed. 2017, 12, 6197–6204. [Google Scholar] [CrossRef] [PubMed]
- Ascar, M.S.; Bulut, Z.B.; Ateş, A.; Nami, B.; Koçak, N.; Yıldız, B. Titanium dioxide nanoparticles induce cytotoxicity and reduce mitotic index in human amniotic fluid-derived cells. Hum. Exp. Toxicol. 2015, 34, 74–82. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Nigro, M.; Bernardeschi, M.; Costagliola, D.; Della Torre, C.; Frenzilli, G.; Guidi, P.; Lucchesi, P.; Mottola, F.; Santonastaso, M.; Scarcelli, V.; et al. n-TiO2 and CdCl2 co-exposure to titanium dioxide nanoparticles and cadmium: Genomic, DNA and chromosomal damage evaluation in the marine fish European sea bass (Dicentrarchus labrax). Aquat. Toxicol. 2015, 168, 72–77. [Google Scholar] [CrossRef]
- Della Torre, C.; Buonocore, F.; Frenzilli, G.; Corsolini, S.; Brunelli, A.; Guidi, P.; Kocan, A.; Mariottini, M.; Mottola, F.; Nigro, M.; et al. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environ. Pollut. 2015, 196, 185–193. [Google Scholar] [CrossRef]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della Torre, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, T.J.; Tsai, B.Y.; Chen, L.K.; Lai, Y.H.; Li, M.J.; Tsai, C.Y.; Tsai, P.J.; Shieh, D.B. Vancomycin-loaded nanoparticles enhance sporicidal and antibacterial efficacy for clostridium difficile Infection. Front Microbiol. 2019, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Spížek, J.; Řezanka, T. Lincosamides: Chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharm. 2017, 133, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Ersbøll, A.S.; Karlsen, M.A.; Svare, J.; Sneider, K.; Hee, L.; Weile, L.K.; Ziobrowska-Bech, A.; Østergaard, C.; Jensen, J.S.; et al. Treatment of bacterial vaginosis in pregnancy in order to reduce the risk of spontaneous preterm delivery - a clinical recommendation. Acta Obs. Gynecol. Scand. 2016, 95, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.F.; Nhan-Chang, C.L.; Sobel, J.D.; Workowski, K.; Conde-Agudelo, A.; Romero, R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: A systematic review and metaanalysis. Am. J. Obs. Gynecol. 2011, 205, 177–190. [Google Scholar] [CrossRef]
- Saquib, Q.; Al-Khedhairy, A.A.; Siddiqui, M.A.; Abou-Tarboush, F.M.; Azam, A.; Musarrat, J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol. In vitro 2012, 26, 351–361. [Google Scholar] [CrossRef]
- Rocco, L.; Peluso, C.; Stingo, V. Micronucleus test and comet assay for the evaluation of zebrafish genomic damage induced by erythromycin and lincomycin. Environ. Toxicol. 2012, 27, 598–604. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 21, A.3B.1-A.3B.2. [Google Scholar] [CrossRef]
- Frenzilli, G.; Nigro, M.; Lyons, B.P. The comet assay for the evaluation of genotoxic impact in aquatic environments. Mutat. Res. Rev. 2009, 681, 80–89. [Google Scholar] [CrossRef]
- Gyori, B.M.; Venkatachalam, G.; Thiagarajan, P.S.; Hsu, D.; Clement, M.V. OpenComet: An automated tool for comet assay image analysis. Redox Biol. 2014, 2, 457–465. [Google Scholar]
- Meintières, S.; Nesslany, F.; Pallardy, M.; Marzin, D. Detection of ghost cells in the standard alkaline comet assay is not a good measure of apoptosis. Environ. Mol. Mutagen. 2003, 41, 260–269. [Google Scholar] [CrossRef]
- Singh, N.P. A simple method for accurate estimation of apoptotic cells. Exp. Cell Res. 2000, 256, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Cantafora, E.; Sean Giorgi, F.; Frenzilli, G.; Scarcelli, V.; Busceti, C.L.; Nigro, M.; Bernardeschi, M.; Fornai, F. Region-specific DNA alterations in focally induced seizures. J. Neural. Transm. 2014, 121, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Valentino, I.V.; Scapigliati, G.; Stingo, V. RAPD-PCR analysis for molecular characterization and genotoxic studies of a new marine fish cell line derived from Dicentrarchus labrax. Cytotechnology 2014, 66, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Costa, C.; Sharma, V.; Kilic, G.; Pásaro, E.; Teixeira, J.P.; Dhawan, A.; Laffon, B. Comparative study on effects of two different types of titanium dioxide nanoparticles on human neuronal cells. Food Chem. Toxicol. 2013, 57, 352–361. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, L.; Zhang, T.; Gao, G.; Cui, Y.; Cheng, Z.; Cheng, J.; Hong, M.; Tang, M.; Hong, F. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J. Hazard Mater. 2011, 191, 32–40. [Google Scholar] [CrossRef]
- Petković, J.; Žegura, B.; Stevanović, M. DNA damage and alteration in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology 2011, 5, 341–353. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of titanium dioxide nanoparticles exposure on human health—A review. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; Mandach, U.V. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, K.I.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef]
- Hussain, S.; Boland, S.; Baeza-Squiban, A.; Hamel, R.; Thomassen, L.C.; Martens, J.A.; Billon-Galland, M.A.; Fleury-Feith, J.; Moisan, F.; Pairon, J.C.; et al. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: Role of particle surface area and internalized amount. Toxicology 2009, 260, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Thomassen, L.C.J.; Ferecatu, I.; Borot, M.C.; Andreau, K.; Martens, J.A.; Fleury, J.; Baeza-Squiban, A.; Marano, F.; Boland, S. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part. Fiber Toxicol. 2010, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; Wiedmann, T.S.; Panyama, J. Enhancing therapeutic efficacy through designed aggregation of nanoparticles. Biomaterials 2014, 35, 7860–7869. [Google Scholar] [CrossRef] [PubMed]
- Boland, S.; Hussain, S.; Baeza-Squiban, A. Carbon black and titanium dioxide nanoparticles Induce distinct molecular mechanisms of toxicity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Dalton, S.; Janes, P.A.; Jones, N.G.; Nicholson, J.A.; Hallam, K.R.; Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: A surface spectroscopic approach. Environ. Pollut. 2002, 120, 415–422. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann. Ist. Super. Sanità 2003, 39, 405–410. [Google Scholar]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Shukla, R.K.; Sharma, V.; Pandey, A.K.; Singh, S.; Sultana, S.; Dhawan, A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In vitro 2011, 25, 231–241. [Google Scholar] [CrossRef]
- Kazimirova, A.; Baranokova, M.; Staruchova, M.; Drlickova, M.; Volkovova, K.; Dusinska, M. Titanium dioxide nanoparticles tested for genotoxicity with the comet and micronucleus assays in vitro, ex vivo and in vivo. Mutat. Res. 2019, 843, 57–65. [Google Scholar] [CrossRef]
- Stoccoro, A.; Di Bucchianico, S.; Coppedè, F.; Ponti, J.; Uboldi, C.; Blosi, M.; Delpivo, C.; Ortelli, S.; Costa, A.L.; Migliore, L. Multiple endpoints to evaluate pristine and remediated titanium dioxide nanoparticles genotoxicity in lung epithelial A549 cells. Toxicol. Lett. 2017, 276, 48–61. [Google Scholar] [CrossRef]
- Patel, S.; Patel, P.; Bakshi Sonal, R. Titanium dioxide nanoparticles: An in vitro study of DNA binding, chromosome aberration assay, and comet assay. Cytotechnology 2017, 69, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.F.; Davies, S.J.; Dodd, N.J.F.; Jha, A.N. Hydroxyl radicals (•OH) are associated with titanium dioxide (TiO 2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat. Res. 2008, 640, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Challier, J.C.; Panigel, M.; Meyer, E. Uptake of colloidal 198Au by fetal liver in rat, after direct intrafetal administration. Int. J. Nucl. Med. Biol. 1973, 1, 103–106. [Google Scholar] [PubMed]
- Bosman, S.J.; Nieto, S.P.; Patton, W.C.; Jacobson, J.D.; Corselli, J.U.; Chan, P.J. Development of mammalian embryos exposed to mixed-size nanoparticles. Clin. Exp. Obs. Gynecol. 2005, 32, 222–224. [Google Scholar]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in foodpackaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef]
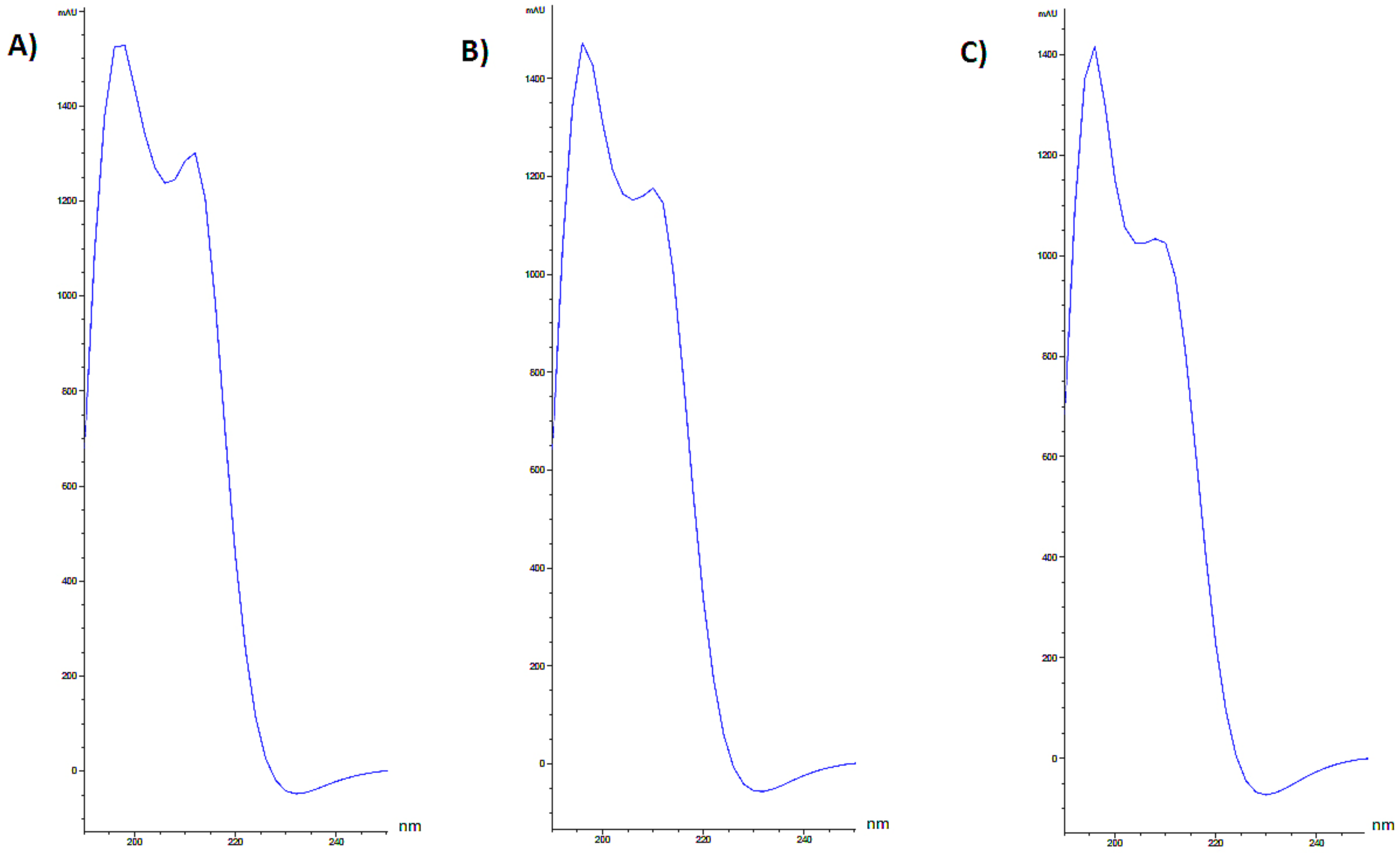
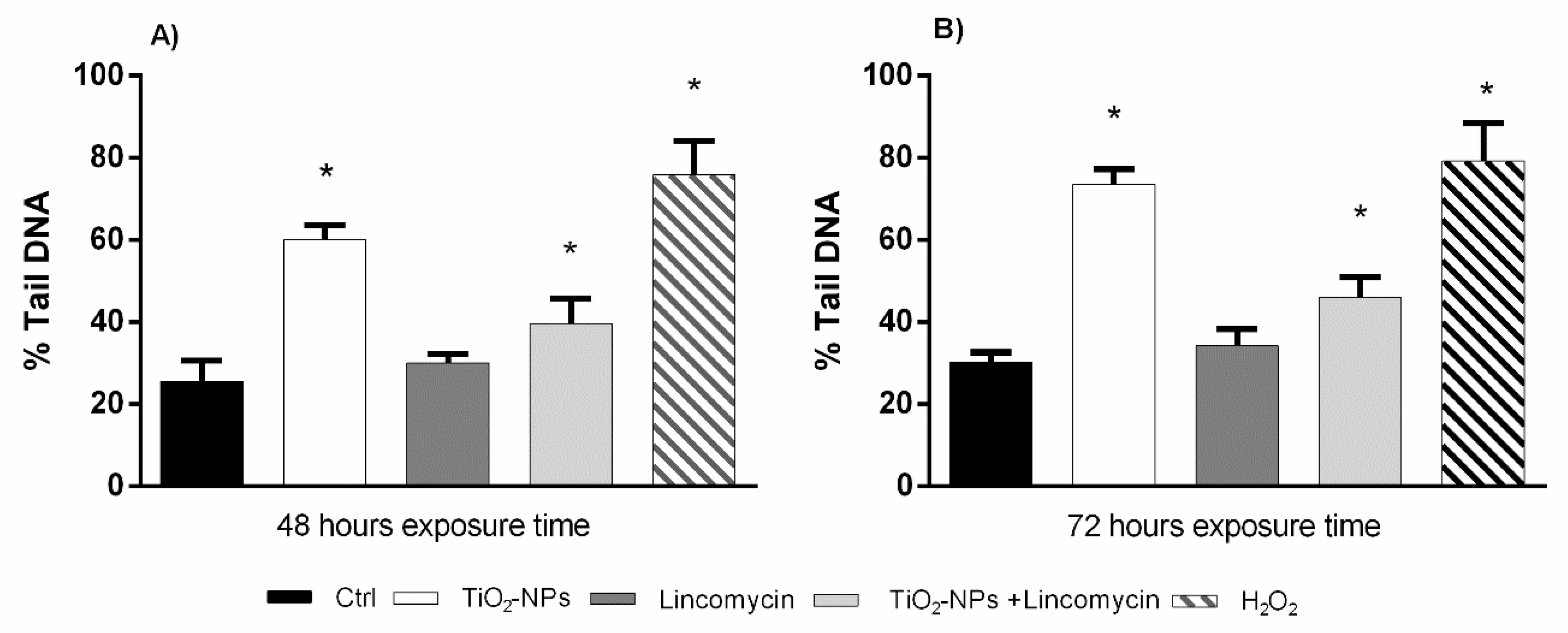
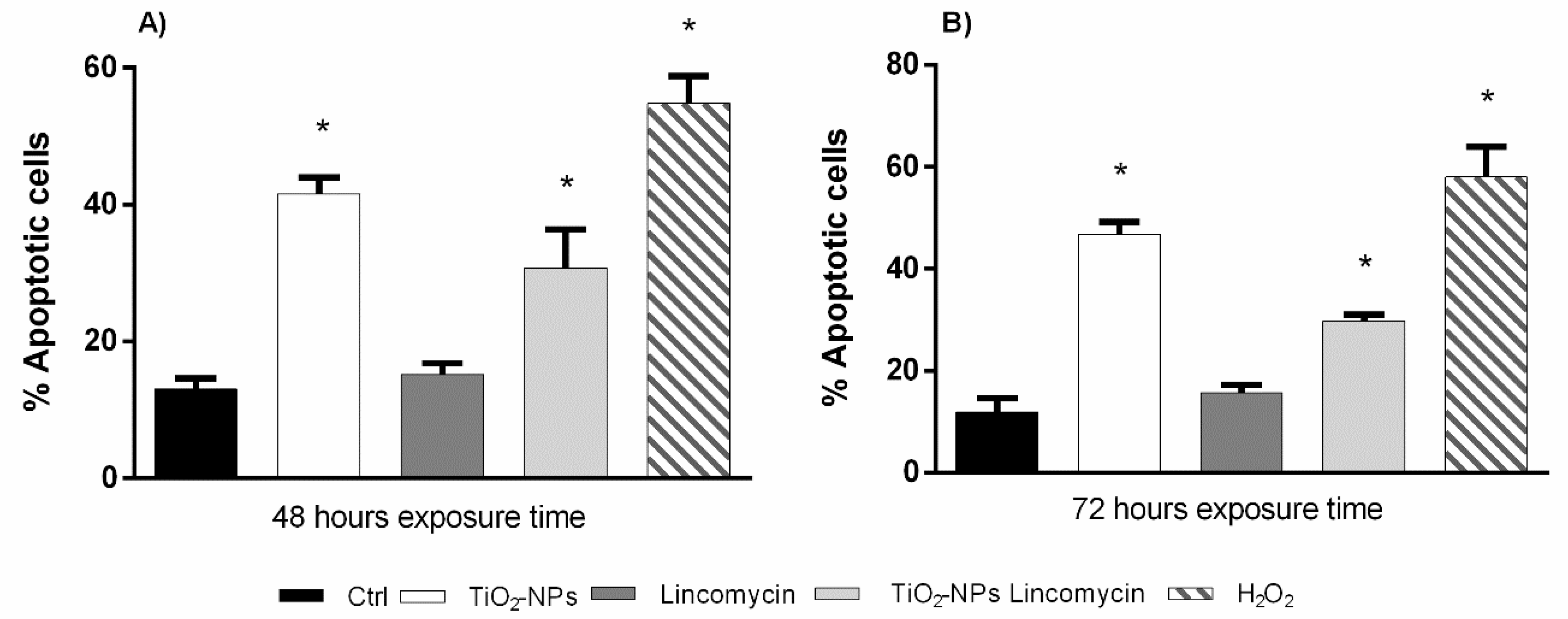
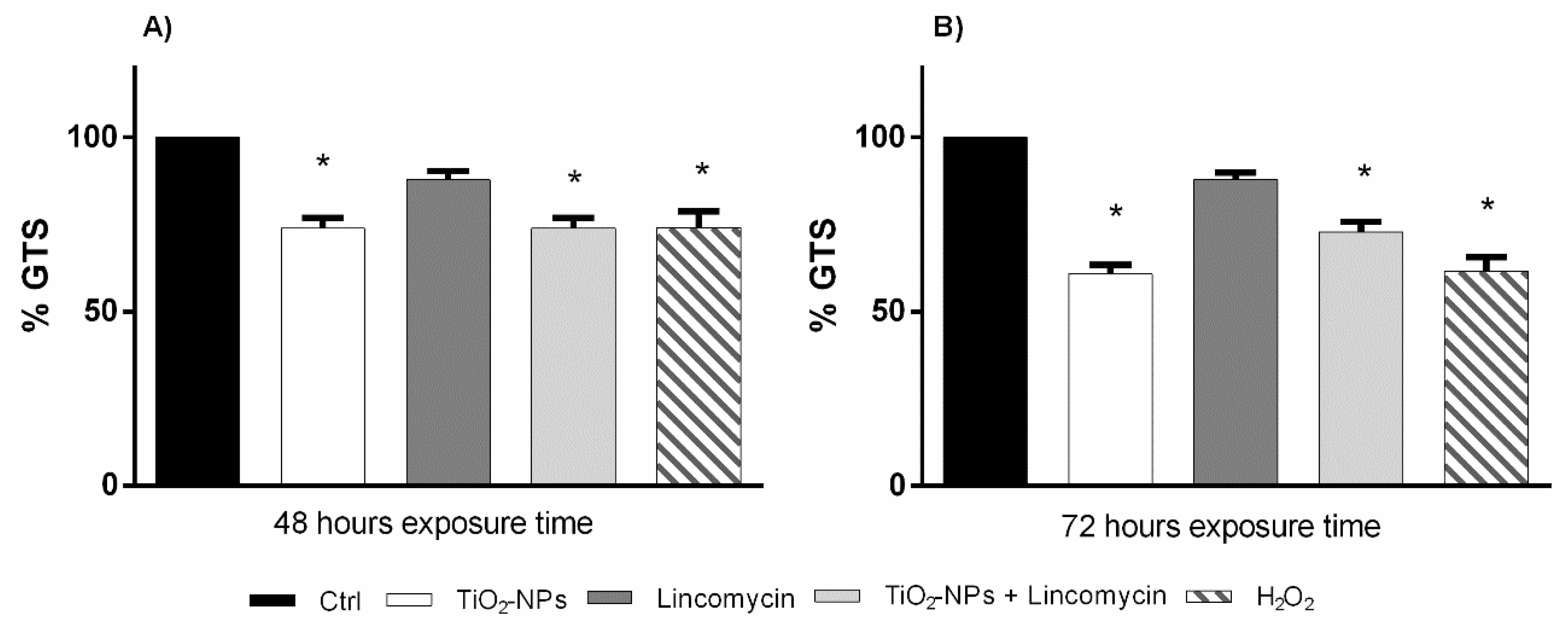
| Substances Concentration | Hours of Exposure | Gained Bands * | Lost Bands * |
|---|---|---|---|
| TiO2-NPs 10 µg/L | 48 72 | 850, 530 650 | - 320, 400 |
| Lincomycin 100 mg/L | 48 72 | - 650 | 400 - |
| TiO2-NPs 10 µg/L + Lincomycin 100 mg/L | 48 72 | - 650 | 400, 520 300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottola, F.; Iovine, C.; Santonastaso, M.; Romeo, M.L.; Pacifico, S.; Cobellis, L.; Rocco, L. NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials 2019, 9, 1511. https://doi.org/10.3390/nano9111511
Mottola F, Iovine C, Santonastaso M, Romeo ML, Pacifico S, Cobellis L, Rocco L. NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials. 2019; 9(11):1511. https://doi.org/10.3390/nano9111511
Chicago/Turabian StyleMottola, Filomena, Concetta Iovine, Marianna Santonastaso, Maria Luisa Romeo, Severina Pacifico, Luigi Cobellis, and Lucia Rocco. 2019. "NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells" Nanomaterials 9, no. 11: 1511. https://doi.org/10.3390/nano9111511
APA StyleMottola, F., Iovine, C., Santonastaso, M., Romeo, M. L., Pacifico, S., Cobellis, L., & Rocco, L. (2019). NPs-TiO2 and Lincomycin Coexposure Induces DNA Damage in Cultured Human Amniotic Cells. Nanomaterials, 9(11), 1511. https://doi.org/10.3390/nano9111511








