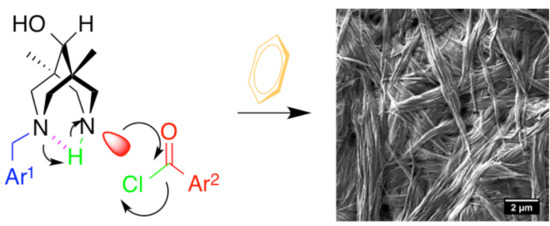
Supramolecular Organogels Based on N-Benzyl, N′-Acylbispidinols
Abstract
:1. Introduction
2. Materials and Methods
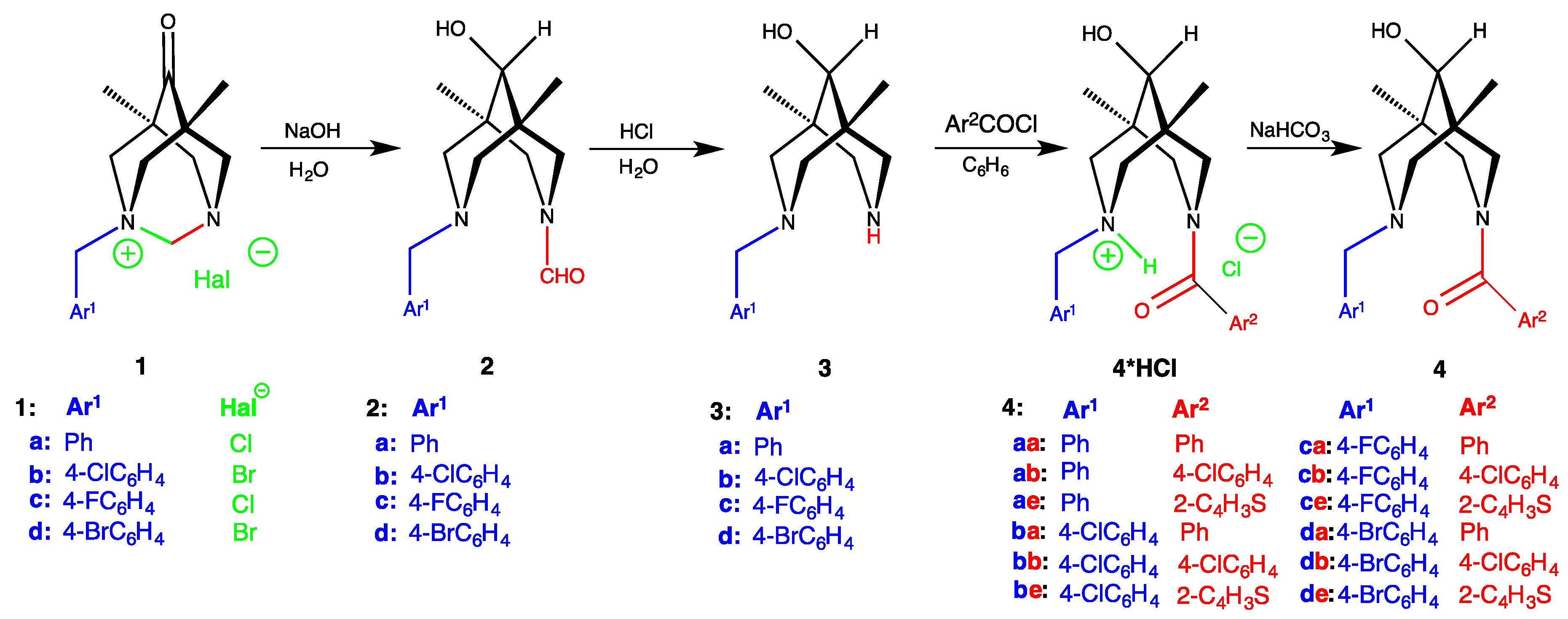
Synthesis. General Methods
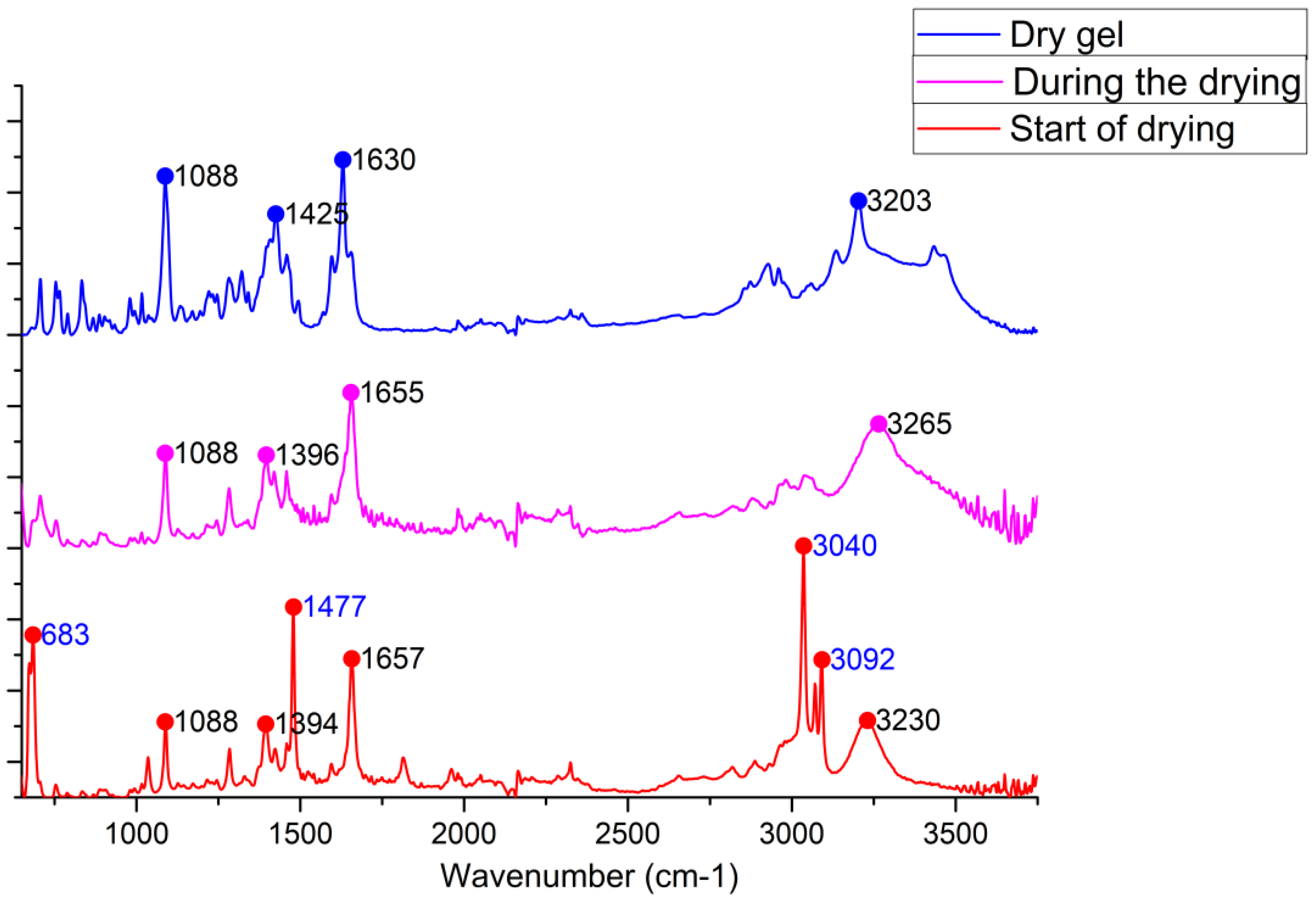
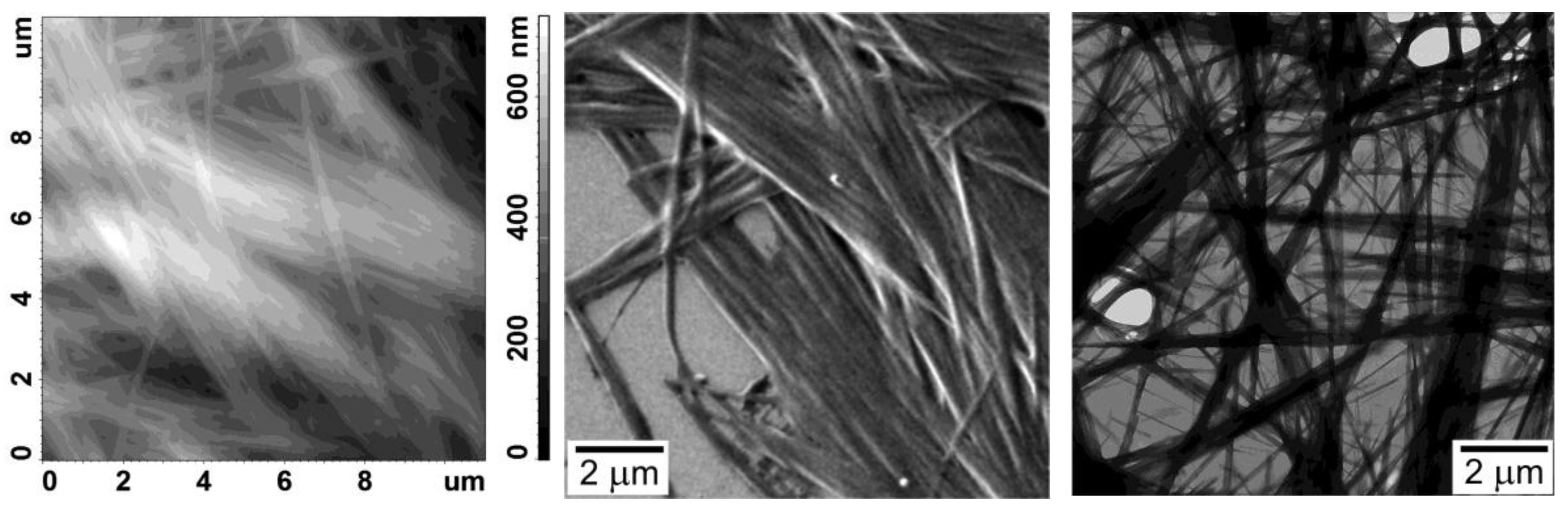
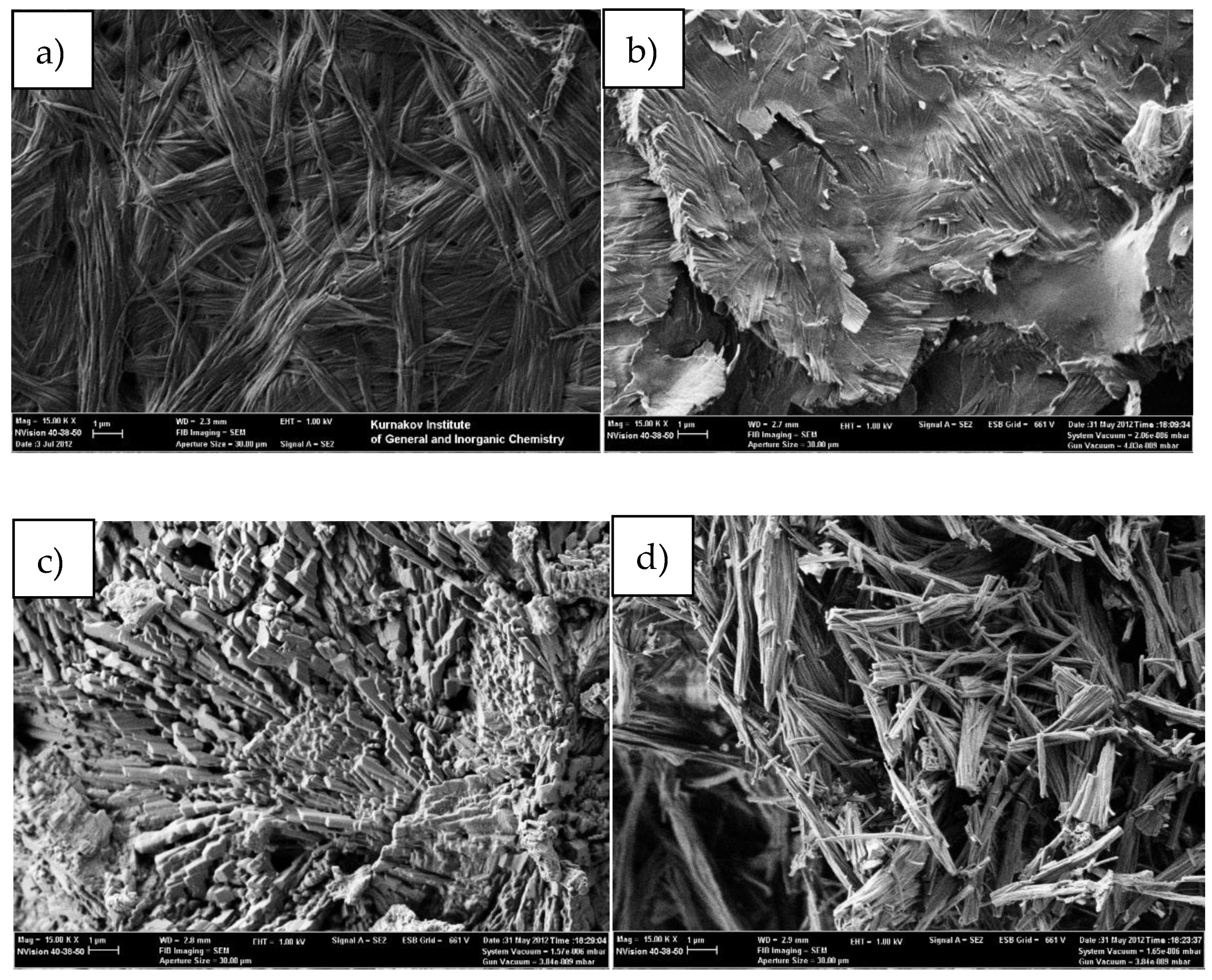
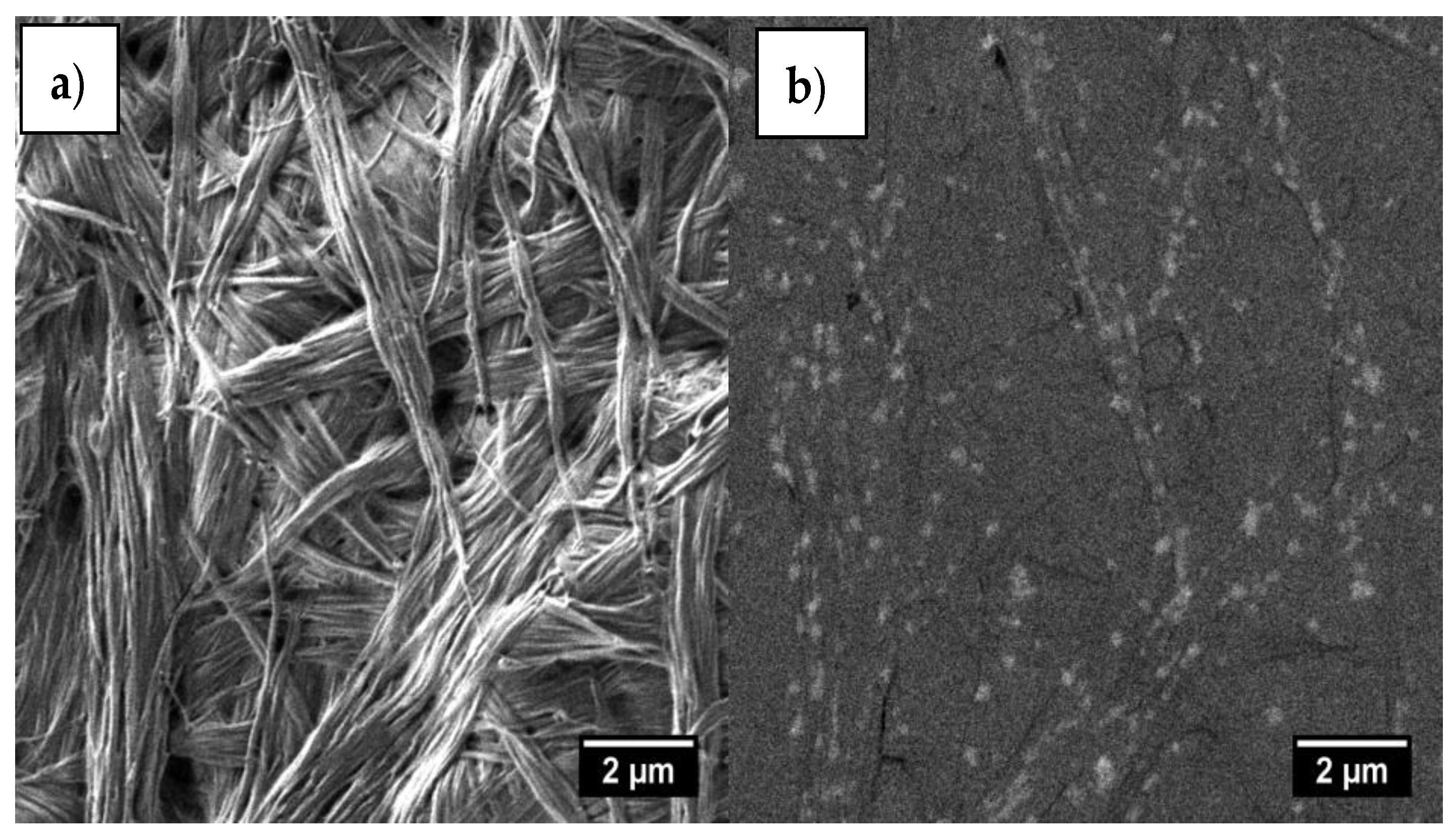
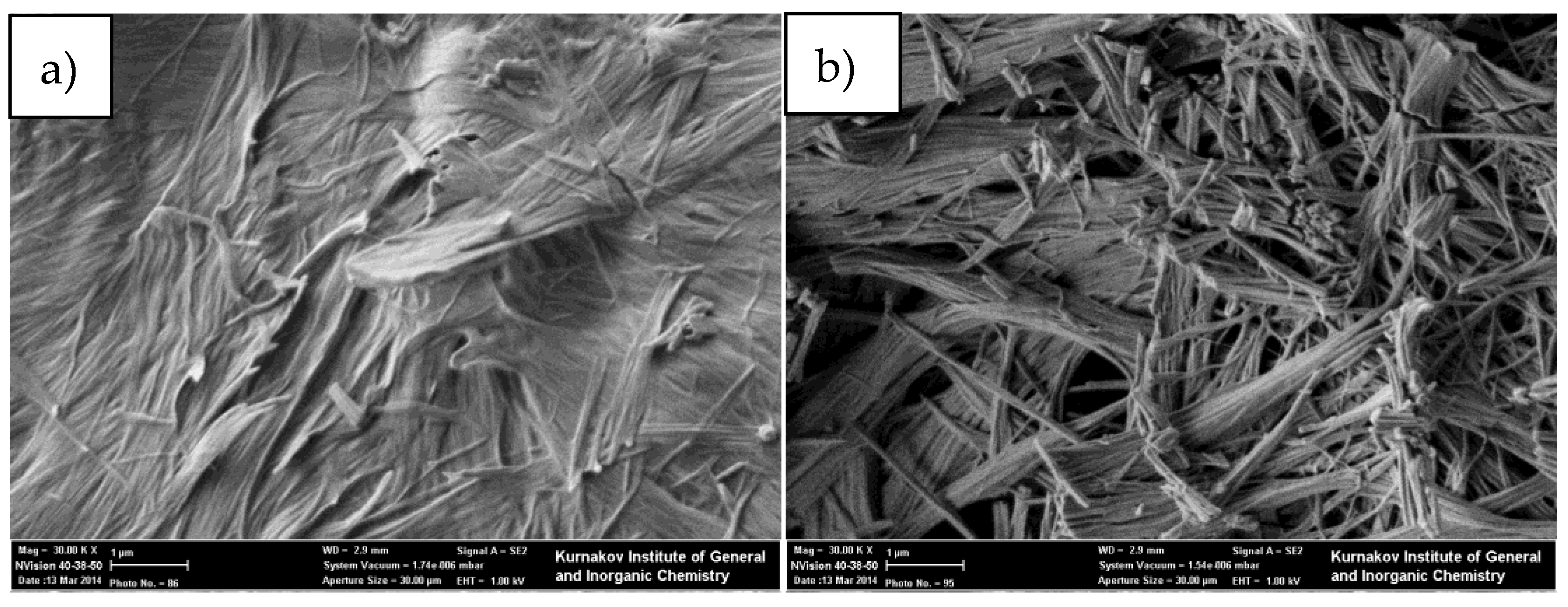
3. Results
4. Discussion
- the gels are formed only during the acylation of secondary amines of N-benzylbispidine-9-ols, but not upon purging the HCl gas through the solution/suspension of isolated amino-amides 4 in benzene;
- during the removal of benzene from the gel, the latter undergoes some irreversible structural rearrangements which lead to the further insolubility of dried material in benzene;
- the gels are formed neither in the presence of the external base (e.g., triethylamine), nor from the chloroanhydrides of picolinic acids (beta or gamma) or pyrazole-containing acid chlorides;
- the removal of the chloride anion by the action of aqueous silver nitrate destroys the supramolecular gel;
- the gels are not formed upon alkylation (e.g., by benzylchloride);
- the gels are formed only from aromatic (benzoic, para-chlorobenzoic acid) or heteroaromatic (2-thiophencarboxylic acid) acid chlorides, but not with the use of aliphatic reagents (acetylchloride, chloroanhydride, or cyclopropanecarboxylic acid);
- the gels are formed also in nitrobenzene, ethoxybenzene, and mesitylene at elevated temperatures (nearly 100 °C), but their stability depends on the substituents at acylating agents (SI pp. 6–9).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hendel, R.C.; Kimmelstiel, C. Cardiology Procedures; Springer-Verlag: London, UK, 2017; ISBN 978-1-4471-7288-8. [Google Scholar]
- Vatsadze, S.Z.; Eremina, O.E.; Veselova, I.A.; Kalmykov, S.N.; Nenajdenko, V.G. 18F-Labelled catecholamine type radiopharmaceuticals in the diagnosis of neurodegenerative diseases and neuroendocrine tumours: Approaches to synthesis and development prospects. Russ. Chem. Rev. 2018, 87. [Google Scholar] [CrossRef]
- Jødal, L.; Le Loirec, C.; Champion, C. Positron range in PET imaging: Non-conventional isotopes. Phys. Med. Biol. 2014, 59, 7419–7434. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Williamson, M.J.; Lewis, J.S. Unconventional Nuclides for Radiopharmaceuticals. Mol. Imaging 2010, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Schubiger, A.P.; Lehmann, L.; Friebe, M. PET Chemistry (Ernst Schering Research Foundation Workshop 62); Springer-Verlag: Berlin-Heidelberg, Germany, 2007; ISBN 3540326235. [Google Scholar]
- Paterson, B.M.; Donnelly, P.S. Macrocyclic Bifunctional Chelators and Conjugation Strategies for Copper-64 Radiopharmaceuticals. In Advances in Inorganic Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 68, pp. 223–251. ISBN 9780128035269. [Google Scholar]
- Lee, S.G.; Gangangari, K.; Kalidindi, T.M.; Punzalan, B.; Larson, S.M.; Pillarsetty, N.V.K. Copper-64 labeled liposomes for imaging bone marrow. Nucl. Med. Biol. 2016, 43, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comba, P.; Kerscher, M.; Rück, K.; Starke, M. Bispidines for radiopharmaceuticals. Dalton Trans. 2018, 47, 9202–9220. [Google Scholar] [CrossRef] [PubMed]
- Comba, P.; Jermilova, U.; Orvig, C.; Patrick, B.O.; Ramogida, C.F.; Rück, K.; Schneider, C.; Starke, M. Octadentate Picolinic Acid-Based Bispidine Ligand for Radiometal Ions. Chem. A Eur. J. 2017, 23, 15945–15956. [Google Scholar] [CrossRef] [PubMed]
- Medved’Ko, A.V.; Egorova, B.V.; Komarova, A.A.; Rakhimov, R.D.; Krut’Ko, D.P.; Kalmykov, S.N.; Vatsadze, S.Z. Copper-Bispidine Complexes: Synthesis and Complex Stability Study. ACS Omega 2016, 1, 854–867. [Google Scholar] [CrossRef]
- Stephan, H.; Walther, M.; Fahnemann, S.; Ceroni, P.; Molloy, J.K.; Bergamini, G.; Heisig, F.; Muller, C.E.; Kraus, W.; Comba, P. Bispidines for Dual Imaging. Chem. A Eur. J. 2014, 20, 17011–17018. [Google Scholar] [CrossRef] [PubMed]
- Tomassoli, I.; Gündisch, D. Bispidine as a Privileged Scaffold. Curr. Top. Med. Chem. 2016, 16, 1314–1342. [Google Scholar] [CrossRef]
- Comba, P.; Morgen, M.; Wadepohl, H. Tuning of the properties of transition-metal bispidine complexes by variation of the basicity of the aromatic donor groups. Inorg. Chem. 2013, 52, 6481–6501. [Google Scholar] [CrossRef] [PubMed]
- Toom, L.; Kütt, A.; Kaljurand, I.; Leito, I.; Ottosson, H.; Grennberg, H.; Gogoll, A. Substituent Effects on the Basicity of 3,7-Diazabicyclo[3.3.1]nonanes. J. Org. Chem. 2006, 71, 7155–7164. [Google Scholar] [CrossRef]
- Breuning, M.; Steiner, M. Chiral Bispidines. Synthesis 2008, 2008, 2841–2867. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Z.; Wang, Z.; Wang, F.; Chen, X.; Liu, X.; Feng, X.; Su, Z.; Hu, C. Asymmetric Aldol Reaction Using New Bispidine Catalysts. Synfacts 2008, 2008, 0647. [Google Scholar] [CrossRef]
- Haridas, V.; Sadanandan, S.; Sharma, Y.K.; Chinthalapalli, S.; Shandilya, A. Bispidine as a secondary structure nucleator in peptides. Tetrahedron Lett. 2012, 53, 623–626. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Gao, J.Y.; Shi, N.Y.; Zhao, J.Q. Synthesis of Chiral Tridentate Ligands Embodying the Bispidine Framework and their Application in the Enantioselective Addition of Diethylzinc to Aldehydes. Adv. Mater. Res. 2011, 396–398, 1236–1243. [Google Scholar] [CrossRef]
- Stucchi, M.; Lesma, G. Split- Ugi Reaction with Chiral Compounds: Synthesis of Piperazine- and Bispidine-Based Peptidomimetics. Helv. Chim. Acta 2016, 99, 315–320. [Google Scholar] [CrossRef]
- Rossetti, A.; Landoni, S.; Meneghetti, F.; Castellano, C.; Mori, M.; Colombo Dugoni, G.; Sacchetti, A. Application of chiral bi- and tetra-dentate bispidine-derived ligands in the copper(II)-catalyzed asymmetric Henry reaction. New J. Chem. 2018, 42, 12072–12081. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A. Topics in Stereochemistry. Top. Stereochem. 1991, 20, 171. [Google Scholar]
- Vatsadze, S.Z.; Krut’ko, D.P.; Zyk, N.V.; Zefirov, N.S.; Churakov, A.V.; Howard, J.A. First 1H NMR observation of chair-boat conformers in bispidinone system. Molecular structure of 3,7-diisopropyl-1,5-diphenyl-3,7-diazabicyclo-[3.3.1]nonane-9-one. Mendeleev Commun. 1999, 9, XXVI–XXVII. [Google Scholar] [CrossRef]
- Palyulin, V.A.; Emets, S.V.; Chertkov, V.A.; Kasper, C.; Schneider, H.J. Conformational switching of 3,7-diacyl-3,7-diazabicyclo[3.3.1]nonanes by metal binding and by solvent changes. Eur. J. Org. Chem. 1999, 2, 3479–3482. [Google Scholar] [CrossRef]
- Kudryavtsev, K.V.; Shulga, D.A.; Chupakhin, V.I.; Sinauridze, E.I.; Ataullakhanov, F.I.; Vatsadze, S.Z. Synthesis of novel bridged dinitrogen heterocycles and their evaluation as potential fragments for the design of biologically active compounds. Tetrahedron 2014, 70, 7854–7864. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Shulga, D.A.; Loginova, Y.D.; Vatsadze, I.A.; Wang, L.; Yu, H.; Kudryavtsev, K.V. Computer modeling of ferrocene-substituted 3,7-diazabicyclo[3.3.1]nonanes as serine protease inhibitors. Mendeleev Commun. 2016, 26, 212–213. [Google Scholar] [CrossRef]
- Comba, P.; Kerscher, M.; Schiek, W. Bispidine Coordination Chemistry. Prog. Inorg. Chem. 2007, 55, 613–704. [Google Scholar] [CrossRef]
- Comba, P.; Jakob, M.; Rück, K.; Wadepohl, H. Tuning of the properties of a picolinic acid-based bispidine ligand for stable copper(II) complexation. Inorg. Chim. Acta 2017. [Google Scholar] [CrossRef]
- Comba, P.; Hunoldt, S.; Morgen, M.; Pietzsch, J.; Stephan, H.; Wadepohl, H. Optimization of Pentadentate Bispidines as Bifunctional Chelators for 64 Cu Positron Emission Tomography (PET). Inorg. Chem. 2013, 52, 8131–8143. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, M.; Comba, P.; Martin, B.; Müller, V.; Rajaraman, G.; Rohwer, H.; Wunderlich, S. DFT models for copper(II) bispidine complexes: Structures, stabilities, isomerism, spin distribution, and spectroscopy. J. Comput. Chem. 2006, 27, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Smith, D.K.; Steed, J.W. Supramolecular materials. Chem. Soc. Rev. 2017, 46, 2404–2420. [Google Scholar] [CrossRef] [Green Version]
- Vieira, V.M.P.; Hay, L.L.; Smith, D.K. Multi-component hybrid hydrogels – understanding the extent of orthogonal assembly and its impact on controlled release. Chem. Sci. 2017. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: From regenerative medicine to electronic devices. Angew. Chem. (Int. Ed. Engl.) 2008, 47, 8002–8018. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.G. The past, present, and future of molecular gels. What is the status of the field, and where is it going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Weiss, R.G. Organogels and Low Molecular Mass Organic Gelators. Adv. Mater. 2000, 12, 1237–1247. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Molecular Gels—Nanostructured Soft Materials. In Organic Nanostructures; Atwood, J.L., Steed, J.W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 978-3-527-31836-0. [Google Scholar]
- Cornwell, D.J.; Smith, D.K. Expanding the scope of gels – combining polymers with low-molecular-weight gelators to yield modified self-assembling smart materials with high-tech applications. Mater. Horiz. 2015, 2. [Google Scholar] [CrossRef]
- Hirst, A.R.; Coates, I.A.; Boucheteau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-molecular-weight gelators: Elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P.; Khemchyan, L.L.; Ivanova, Y.V.; Bukhtiyarov, V.I.; Sorokin, A.M.; Prosvirin, I.P.; Vatsadze, S.Z.; Medved’ko, A.V.; Nuriev, V.N.; Dilman, A.D.; et al. Development of new methods in modern selective organic synthesis: Preparation of functionalized molecules with atomic precision. Russ. Chem. Rev. 2014, 83, 885–985. [Google Scholar] [CrossRef]
- Desvergne, J.-P. Organic gelators and hydrogelators. Beilstein J. Org. Chem. 2010, 6, 846–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.K. Molecular gels-underpinning nanoscale materials with organic chemistry. Tetrahedron 2007, 63, 7283–7284. [Google Scholar] [CrossRef]
- Liu, X.Y. Low Molecular Mass Gelator; Springer-Verlag Berlin Heidelberg: Berlin, NY, USA; New York, NY, USA, 2005; Volume 256, ISBN 978-3-540-25321-1. [Google Scholar]
- Weiss, R.G.; Terech, P. Molecular Gels: Materials with Self-Assembled Fibrillar Networks, 1st ed.; Springer: Dordrecht, The Netherlands, 2006; ISBN 1402033524. [Google Scholar]
- Jones, C.D.; Steed, J.W. Gels with sense: Supramolecular materials that respond to heat, light and sound. Chem. Soc. Rev. 2016, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yi, T.; Zhou, Z.; Zhou, Y.; Wu, J.; Xu, M.; Li, F.; Huang, C. Switchable fluorescent organogels and mesomorphic superstructure based on naphthalene derivatives. Langmuir ACS J. Surf. Colloids 2007, 23, 8224–8230. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Sankolli, R.; Lee, H.-Y.; Raghavan, S.R.; Dastidar, P. Gel sculpture: Moldable, load-bearing and self-healing non-polymeric supramolecular gel derived from a simple organic salt. Chemistry 2012, 18, 8057–8063. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, D.J.; Okesola, B.O.; Smith, D.K. Multidomain hybrid hydrogels: Spatially resolved photopatterned synthetic nanomaterials combining polymer and low-molecular-weight gelators. Angew. Chem. Int. Ed. 2014, 53, 12461–12465. [Google Scholar] [CrossRef]
- Morris, K.L.; Chen, L.; Raeburn, J.; Sellick, O.R.; Cotanda, P.; Paul, A.; Griffiths, P.C.; King, S.M.; O’Reilly, R.K.; Serpell, L.C.; et al. Chemically programmed self-sorting of gelator networks. Nat. Commun. 2013, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, S.H.; Lee, S.S.; Kwon, K.-Y.; Sakurai, K.; Jaworski, J.; Jung, J.H. Ultraviolet Patterned Calixarene-Derived Supramolecular Gels and Films with Spatially Resolved Mechanical and Fluorescent Properties. Acs Nano 2017, 11, 4155–4164. [Google Scholar] [CrossRef] [PubMed]
- Ajayaghosh, A.; Praveen, V.K.; Vijayakumar, C. Organogels as scaffolds for excitation energy transfer and light harvesting. Chem. Soc. Rev. 2008, 37, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting—self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45. [Google Scholar] [CrossRef]
- Vieira, V.; Lima, A.; de Jong, M.; Smith, D.K. Commercially-relevant orthogonal multi-component supramolecular hydrogels for programmed cell growth. Chem. A Eur. J. 2018. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Tyurin, V.S.; Zatsman, A.I.; Manaenkova, M.A.; Semashko, V.S.; Krut’ko, D.P.; Zyk, N.V.; Churakov, A.V.; Kuz’mina, L.G. New stereoselective intramolecular redox reaction in the system of 3,7-diazabicyclo[3.3.1]nonan-9-one. Russ. J. Org. Chem. 2006, 42, 1225–1231. [Google Scholar] [CrossRef]
- Semashko, V.S.; Vatsadze, S.Z.; Zyk, N.V.; Godovikov, I.A. An NMR study of quaternary ammonium salts of 5,7-dimethyl-1,3- diazaadamantan-6-one. Russ. Chem. Bull. 2008, 57, 2207–2209. [Google Scholar] [CrossRef]
- Balakhonov, S.V.; Vatsadze, S.Z.; Churagulov, B.R. Effect of supercritical drying parameters on the phase composition and morphology of aerogels based on vanadium oxide. Russ. J. Inorg. Chem. 2015, 60, 9–15. [Google Scholar] [CrossRef]
- Shlyakhtin, A.V.; Vatsadze, S.Z.; Krut’ko, D.P.; Lemenovskii, D.A.; Zabalov, M.V. Carboxylation of aromatic compounds in a supercritical carbon dioxide medium. Russ. J. Phys. Chem. B 2012, 6, 818–826. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yan, X.; Han, C.; Huang, F. Characterization of supramolecular gels. Chem. Soc. Rev. 2013, 42. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. How should multicomponent supramolecular gels be characterised? Chem. Soc. Rev. 2018, 47, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawn, A.; Kumari, H. Low Molecular Weight Supramolecular Gels Under Shear: Rheology as the Tool for Elucidating Structure–Function Correlation. Chem. A Eur. J. 2018, 24, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Goddard, R.; Pörschke, K.R. Degradation of dichloromethane by bispidine. J. Phys. Org. Chem. 2012, 25, 814–827. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Palyulin, V.A. Conformational Analysis of Bicyclo[3.3.1]nonanes and Their Hetero Analogs. In Topics in Stereochemistry; Iliel, E.L., Wilen, S.H., Eds.; Wiley Online Library: Hoboken, NJ, USA, 1991; Volume 20, pp. 171–230. ISBN 0-471-60026-1. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. Sect. B: Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtsev, K.V.; Vatsadze, S.Z.; Semashko, V.S.; Churakov, A.V. syn -3-(4-Chlorobenzyl)-1,5-dimethyl-3,7-diazabicyclo[3.3.1]nonan-9-ol. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o2373. [Google Scholar] [CrossRef]
- Maekawa, H.; Nishiyama, Y. Selective introduction of a trifluoroacetyl group onto 4-vinylpyridines through magnesium-promoted reduction. Tetrahedron 2015, 71, 6694–6700. [Google Scholar] [CrossRef]
- Ebead, A.; Fournier, R.; Lee-Ruff, E. Synthesis of cyclobutane nucleosides. Nucleosidesnucleotides Nucl. Acids 2011, 30, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Kurkutova, E.N.; Goncharov, A.V.; Zefirov, N.S.; Palyulin, V.A. Molecular Structure of 1-Methyl-5,7- diphenyl-1,3-diazaadamantan-6-one Iodide. J. Struct. Chem. 1976, 17, 591–593. [Google Scholar] [CrossRef]
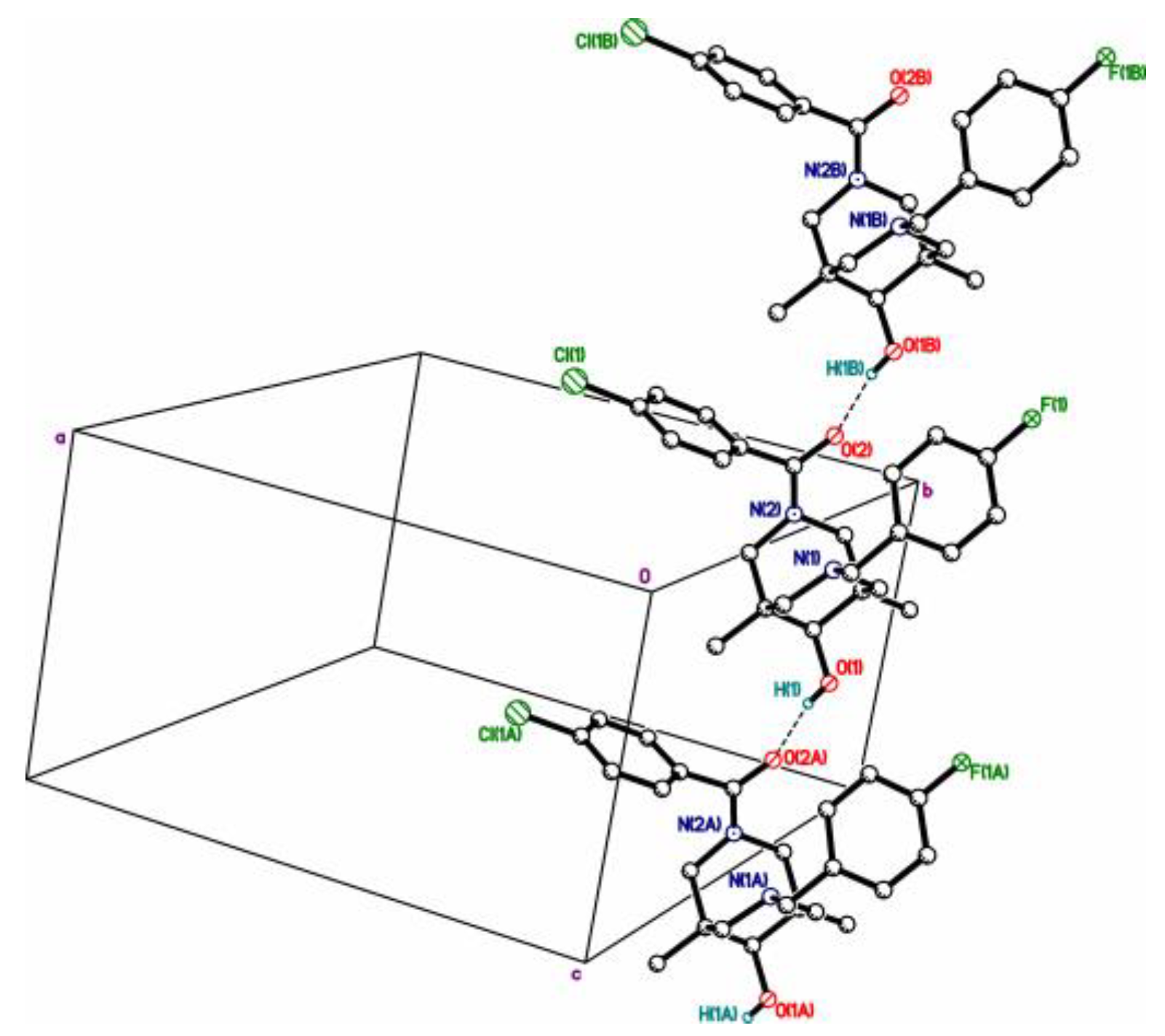
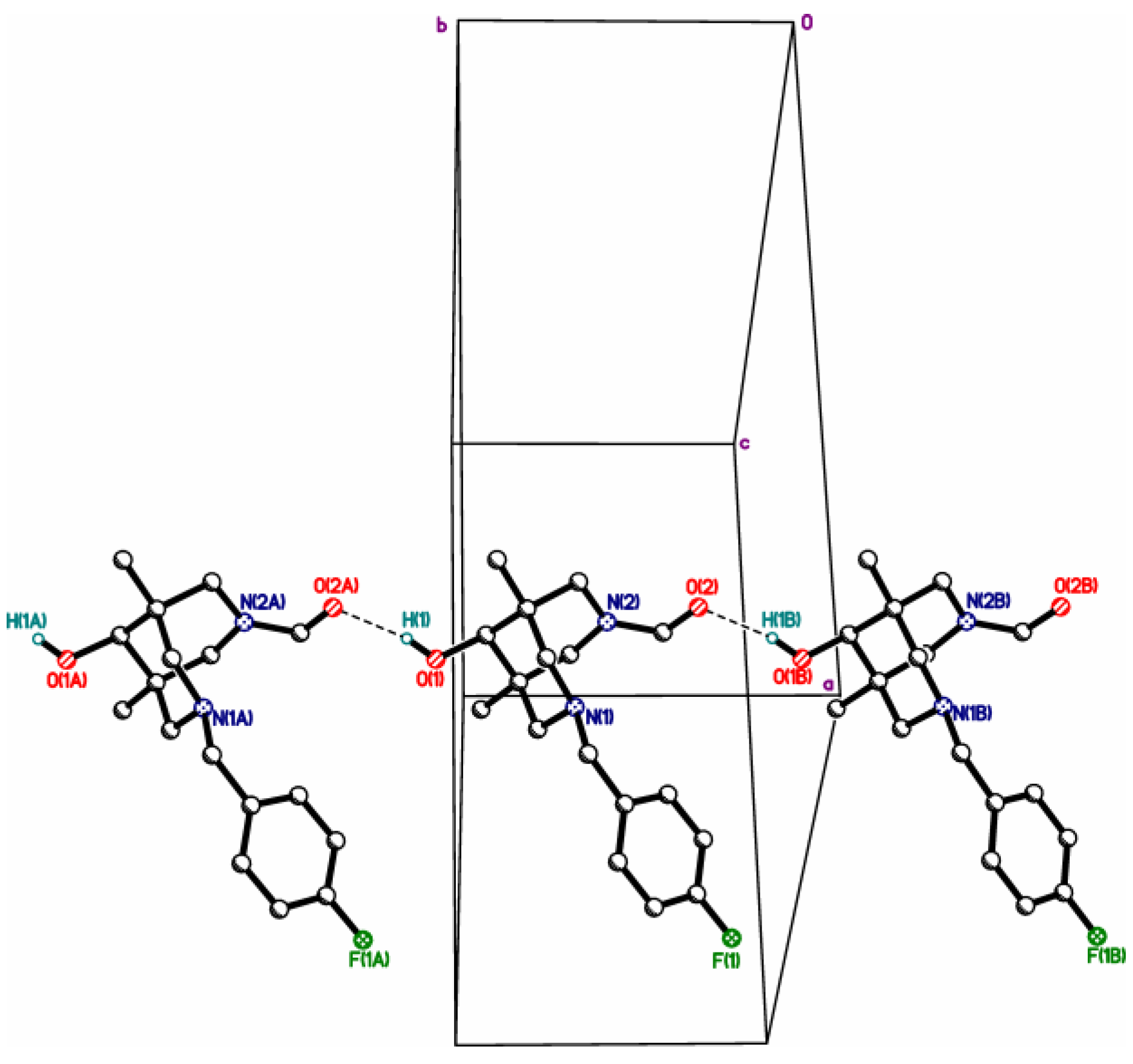
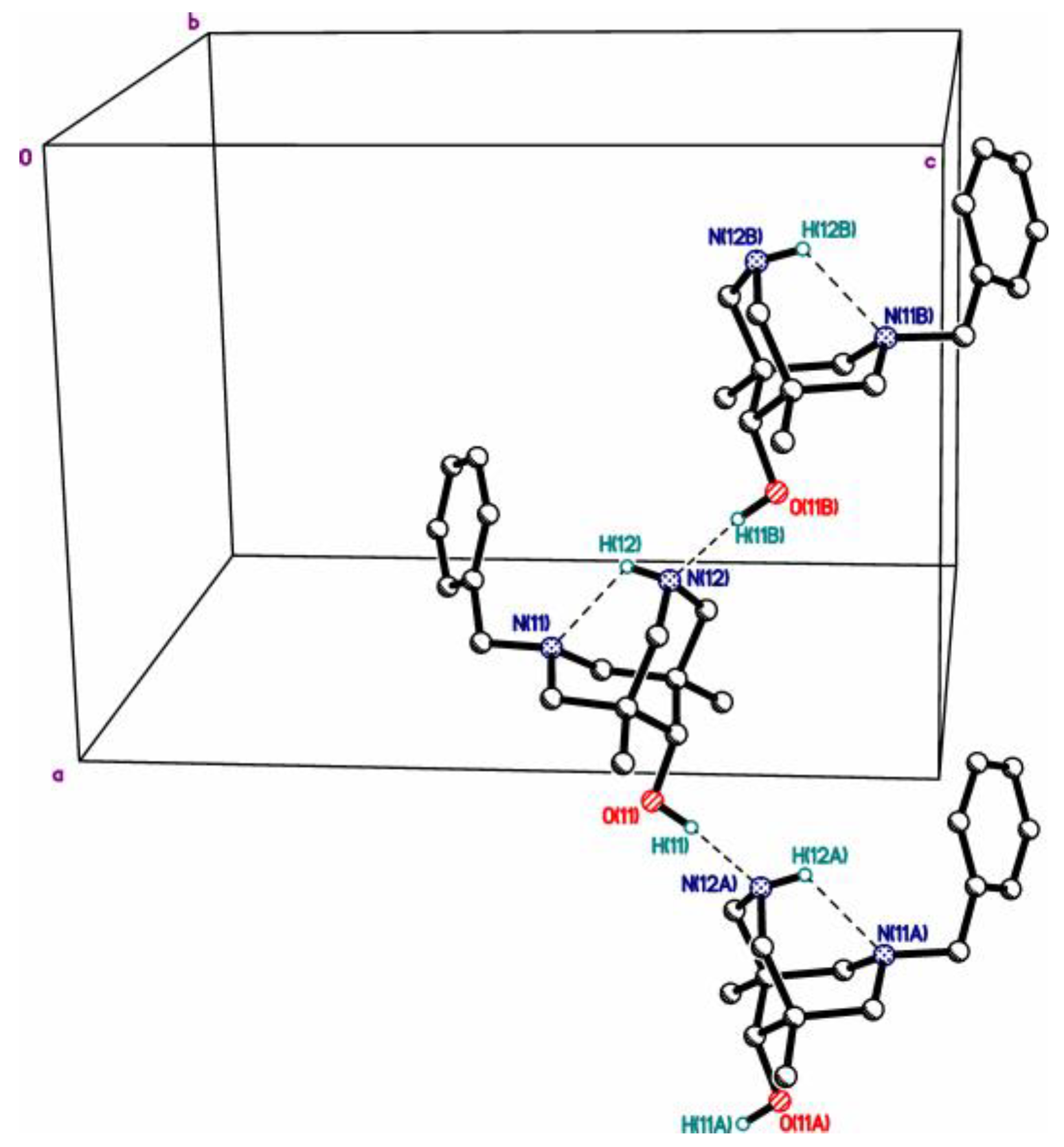
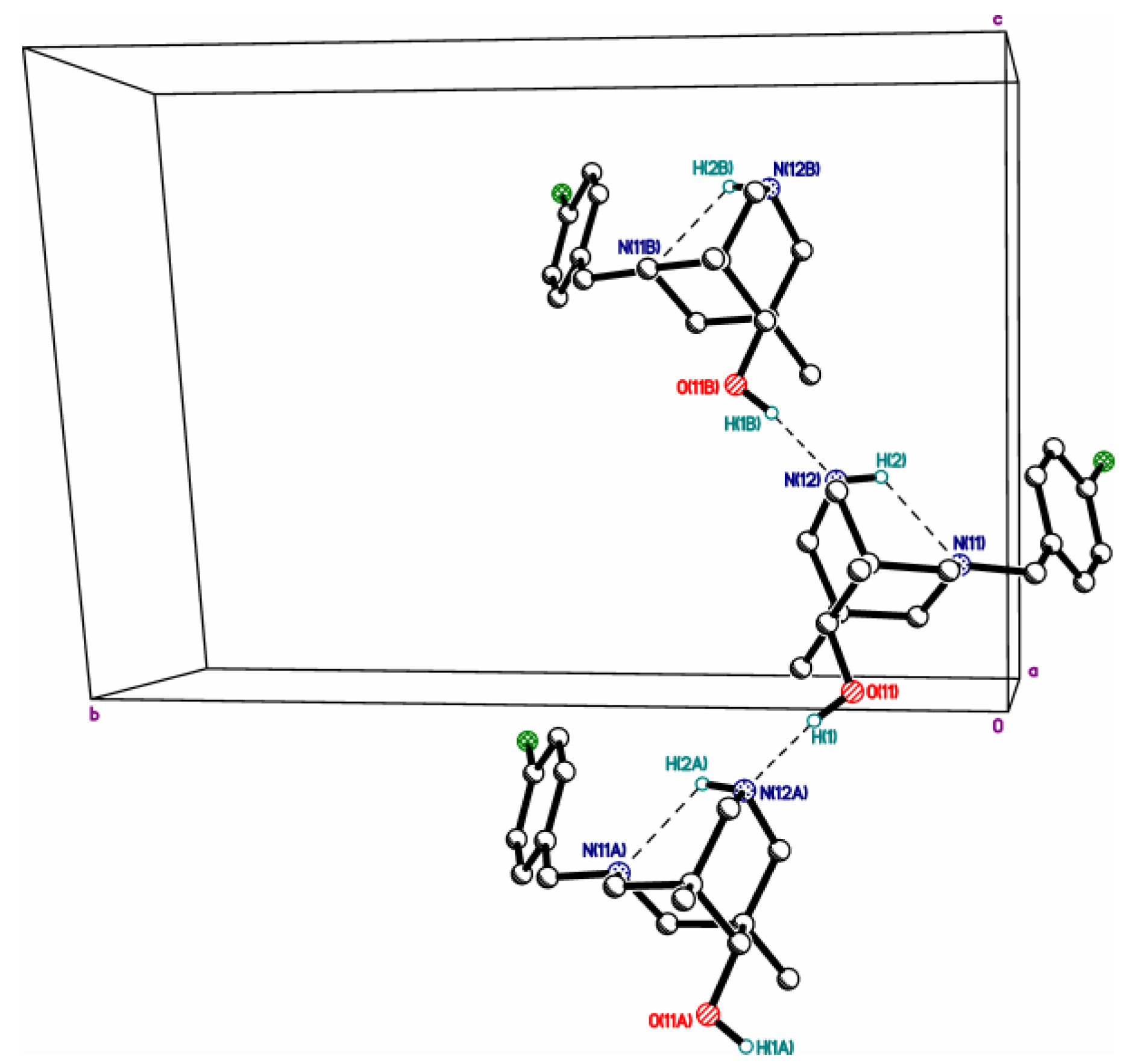
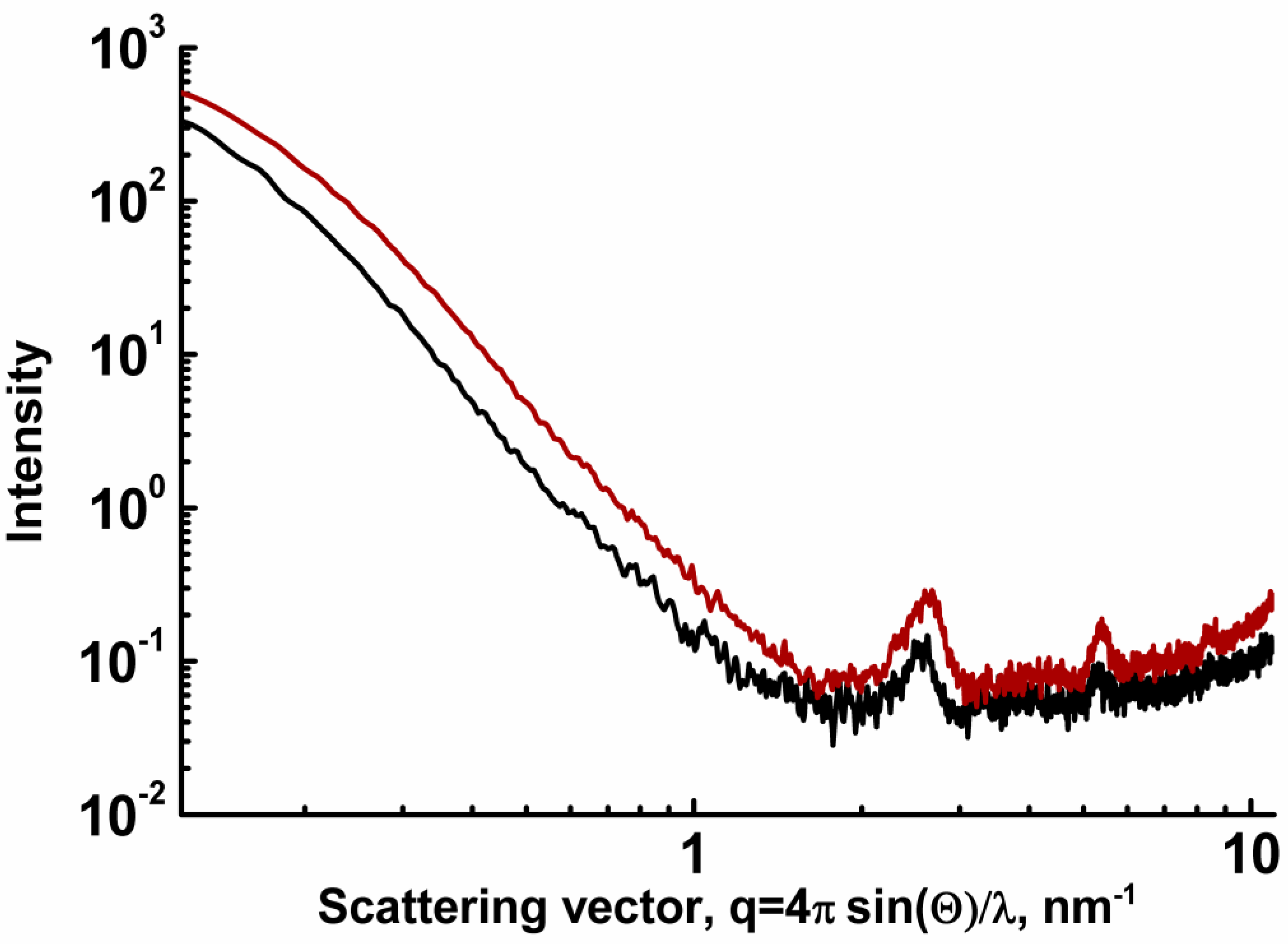
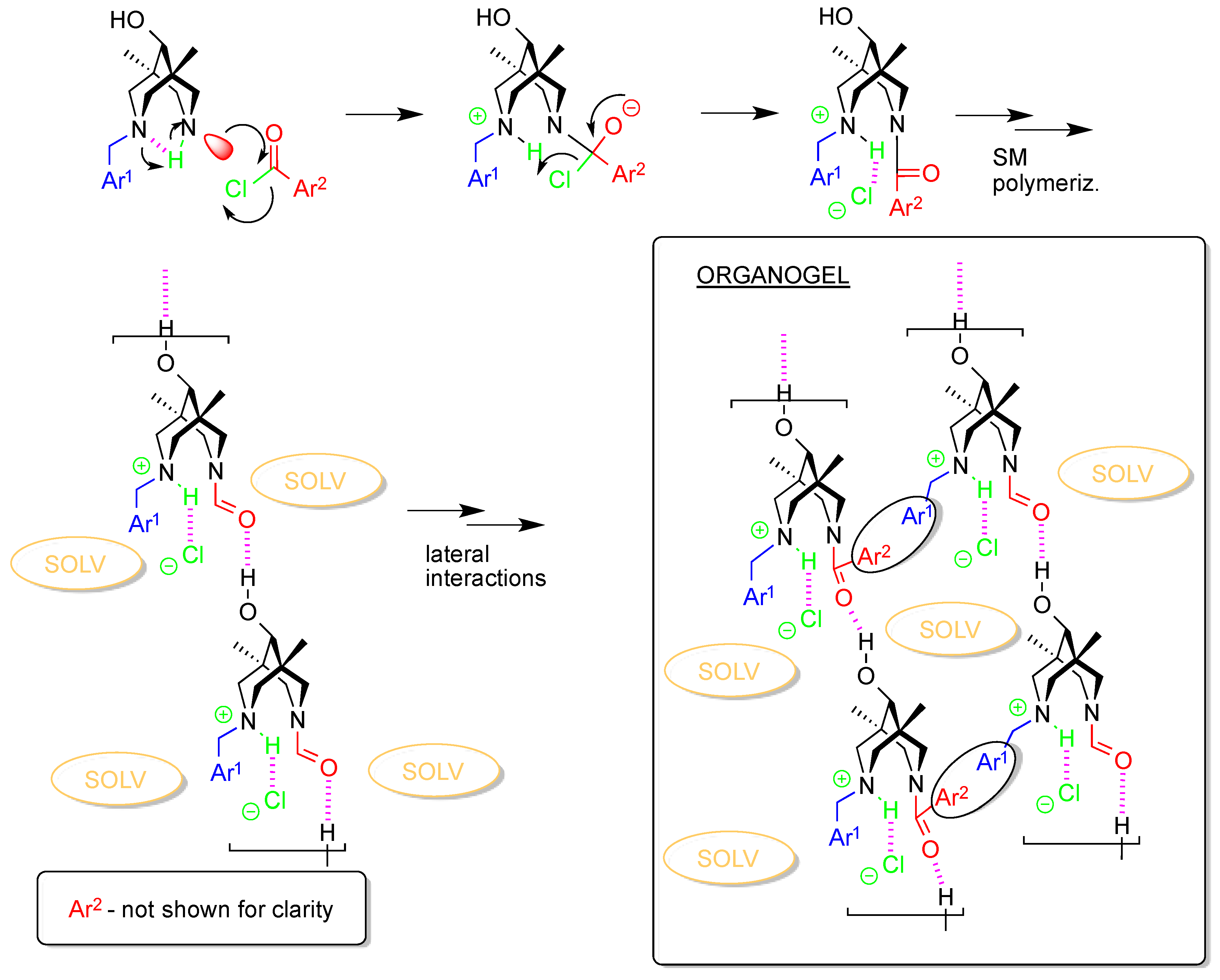
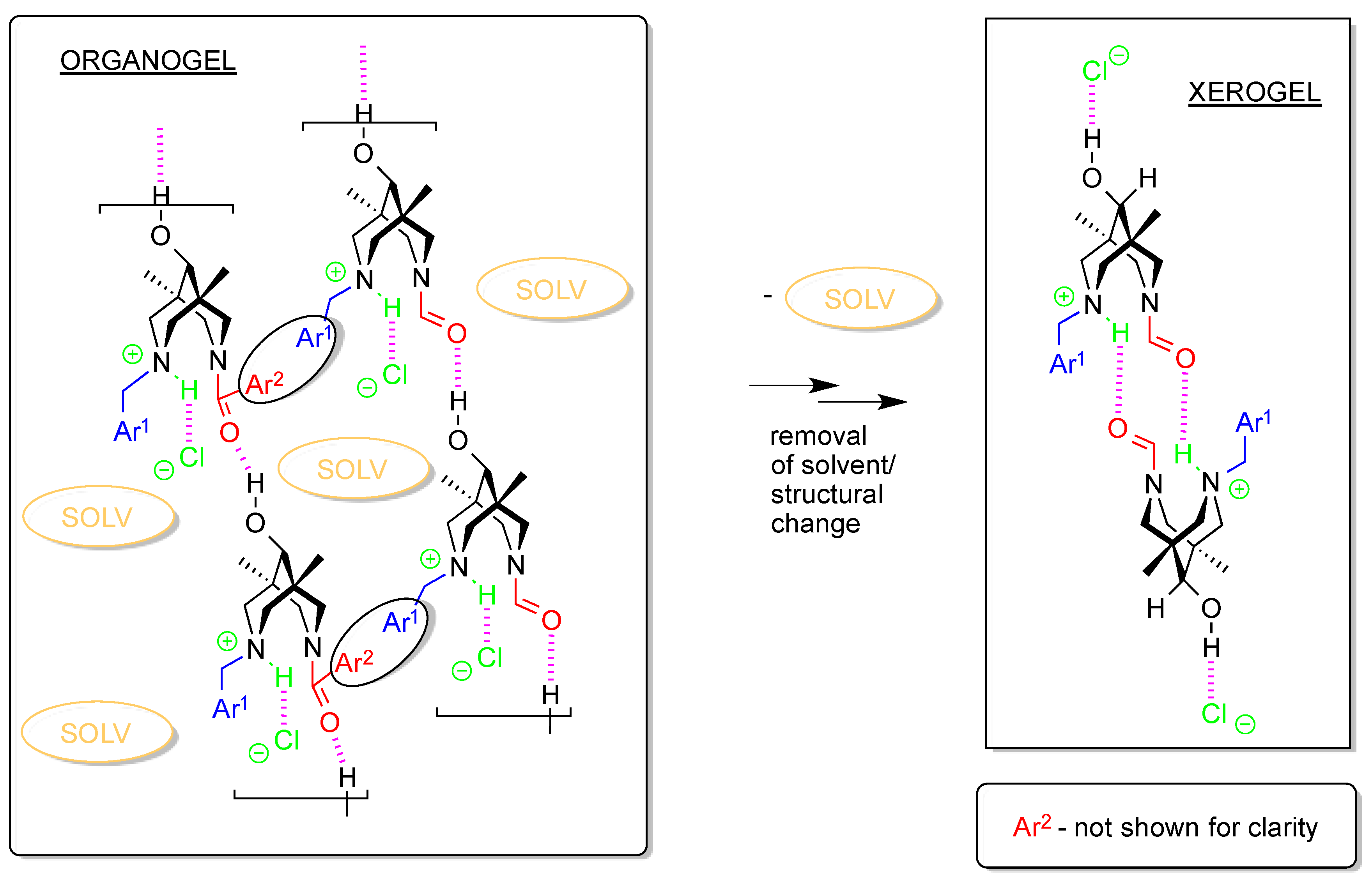
| 4cb | 2c | 3a | 3c | 4da*HCl | |
|---|---|---|---|---|---|
| R1, R2 R3 | p-FC6H4CH2-, —p-ClC6H4C(=O)- | p-FC6H4CH2-, —-CH(=O) | C6H4CH2-, —H | p-FC6H4CH2-, —H | p-BrC6H4CH2-, HC6H4C(=O)- |
| C-OH | 1.423(3) | 1.472(6)* | 1.4195(12) | 1.4178(12) | 1.416(12) |
| av N-Cendo ** | 1.469(3) | 1.458(3) | 1.4695(15) | 1.4691(14) | 1.458(14) |
| NBz-C ** | 1.465(3) | 1.471(3) | 1.4653(14) | 1.4658(13) | 1.505(13) |
| N-CO ** | 1.339(3) | 1.331(3) | — | — | 1.375(13) |
| ΣC-NBz-C *** | 330.3 | 330.0 | 331.4 | 331.9 | 332.1 |
| ΣC-Namid-C *** | 359.4 | 359.5 | — | — | 353.7 |
| Σ ang NH *** | — | — | 325.0 | 325.8 | — |
| X, O…X **** | =O, 2.784(3) | =O, 2.665(5) | R2HN, 2.7250(12) | R2HN, 2.7157(12) | Cl-, 3.049(10) |
| N…Nintra ***** | 2.825(3) | 2.830(4) | 2.8372(13) | 2.8309(13) | 2.790(14) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medved’ko, A.V.; Dalinger, A.I.; Nuriev, V.N.; Semashko, V.S.; Filatov, A.V.; Ezhov, A.A.; Churakov, A.V.; Howard, J.A.K.; Shiryaev, A.A.; Baranchikov, A.E.; et al. Supramolecular Organogels Based on N-Benzyl, N′-Acylbispidinols. Nanomaterials 2019, 9, 89. https://doi.org/10.3390/nano9010089
Medved’ko AV, Dalinger AI, Nuriev VN, Semashko VS, Filatov AV, Ezhov AA, Churakov AV, Howard JAK, Shiryaev AA, Baranchikov AE, et al. Supramolecular Organogels Based on N-Benzyl, N′-Acylbispidinols. Nanomaterials. 2019; 9(1):89. https://doi.org/10.3390/nano9010089
Chicago/Turabian StyleMedved’ko, Alexey V., Alexander I. Dalinger, Vyacheslav N. Nuriev, Vera S. Semashko, Andrei V. Filatov, Alexander A. Ezhov, Andrei V. Churakov, Judith A. K. Howard, Andrey A. Shiryaev, Alexander E. Baranchikov, and et al. 2019. "Supramolecular Organogels Based on N-Benzyl, N′-Acylbispidinols" Nanomaterials 9, no. 1: 89. https://doi.org/10.3390/nano9010089
APA StyleMedved’ko, A. V., Dalinger, A. I., Nuriev, V. N., Semashko, V. S., Filatov, A. V., Ezhov, A. A., Churakov, A. V., Howard, J. A. K., Shiryaev, A. A., Baranchikov, A. E., Ivanov, V. K., & Vatsadze, S. Z. (2019). Supramolecular Organogels Based on N-Benzyl, N′-Acylbispidinols. Nanomaterials, 9(1), 89. https://doi.org/10.3390/nano9010089