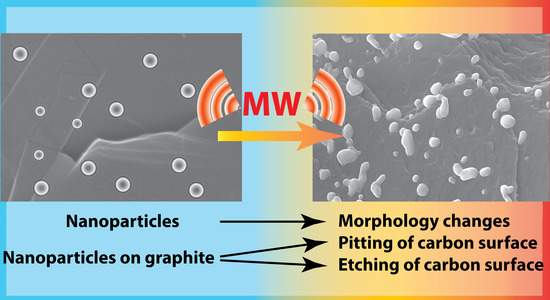
Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Samples Preparation
2.2. General Procedure
2.3. Scanning Electron Microscope (SEM) and Energy Dispersive X-Ray Spectroscopy (EDX) Studies
2.4. Surface Area Measurements and X-Ray Phase Analysis (XRD) Study
3. Results and Discussion
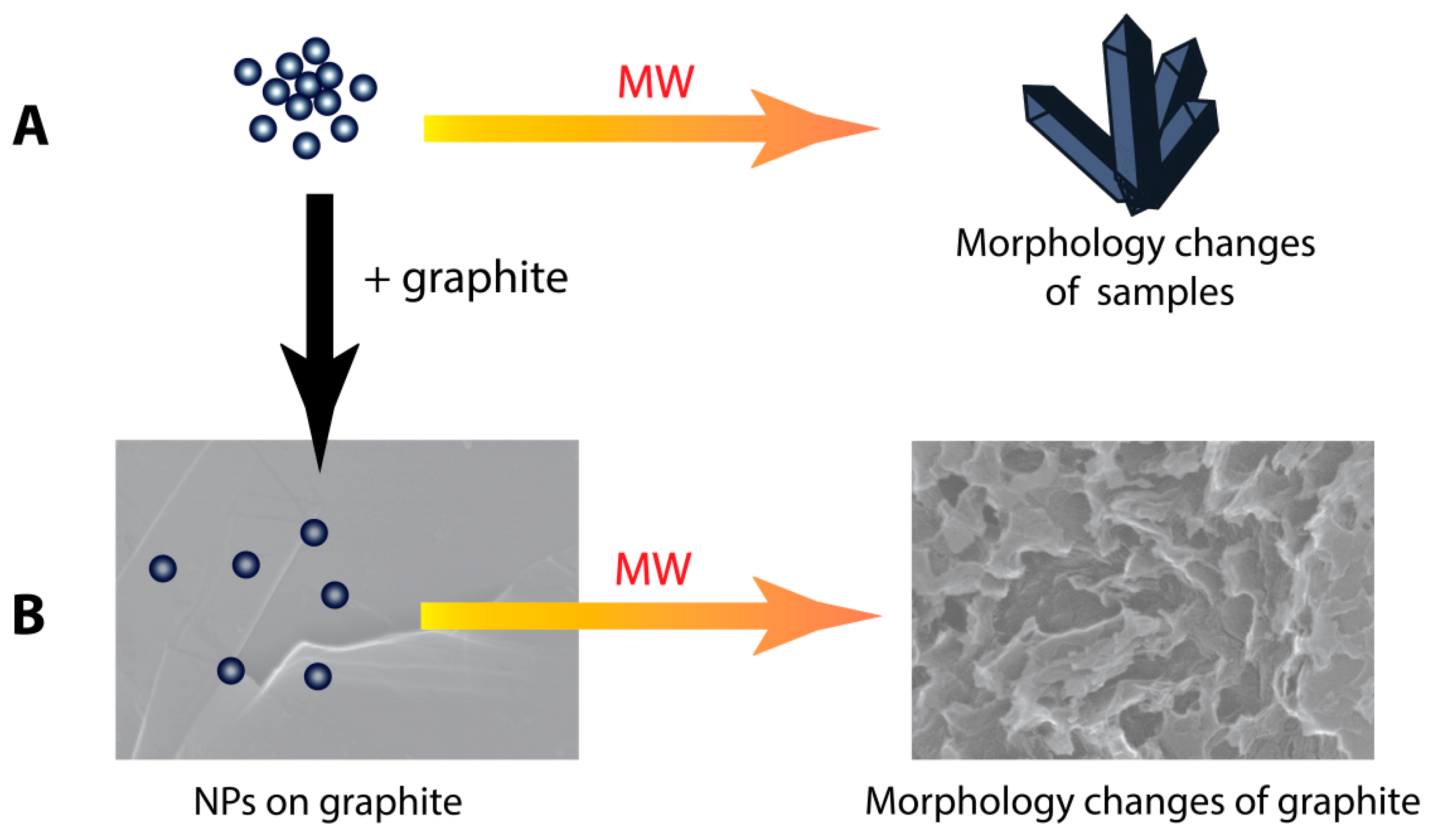
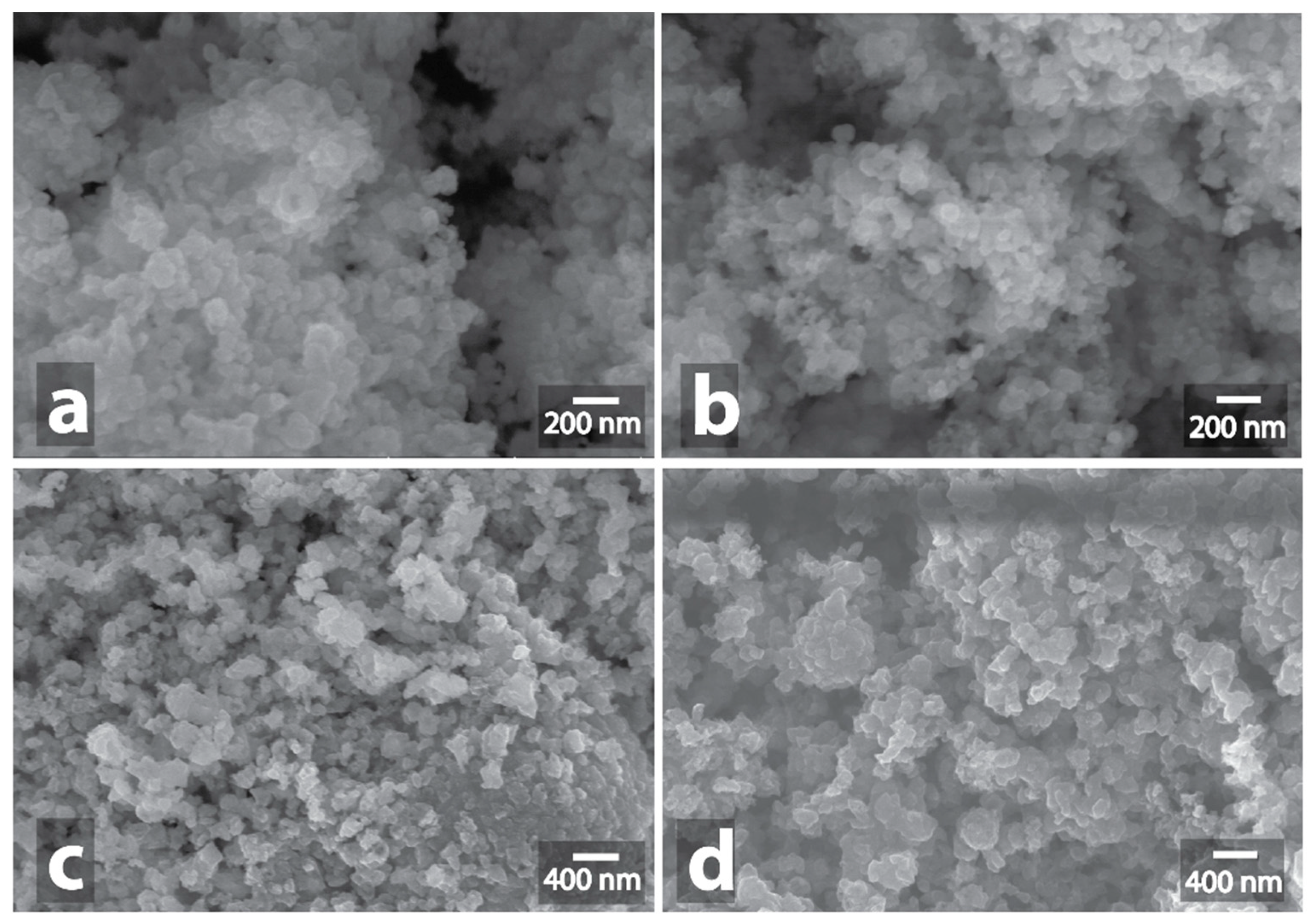
3.1. Investigation of Morphological Changes in Metal-Containing Substances under Microwave Treatment Conditions
3.2. Changes in the Morphology of Graphite in the Presence of Metal-Containing Substances under the Microwave Treatment Conditions
4. Conclusions
- -
- type 1: very weak MW-absorption with a maximum heating up to 200 °C (Al2O3, SiO2, WO3, ZnO, ZrO2, TiO2, CoO, Fe2O3, SnO2, CuO, Y2O3, MgO, Cr2O3, ZrO2-SiO2, Al-B, SiC, AlN, AlON, TiN, TiCN, Cu-W, W-Cu, W-Ni-Fe, Ni, Co);
- -
- type 2: weak MW-absorption or reflection of microwaves, a single spark discharge (Ag, Pt, Cu, Cu/C);
- -
- type 3: moderate MW-absorption, red-colored heat and/or red sparks (Re, W-C, V-C, Cr-C,);
- -
- type 4: intensive MW-absorption, spark discharges, glow of plasma, flame appearance with red-colored heat (Fe/C, Mo/C, Mo-Fe-C, WC, TiC, MoS2, W-V-C).
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave Chemical and Materials Processing; Springer Nature Singapore Pte Ltd.: Singapore, 2018; ISBN 978-981-10-6466-1. [Google Scholar]
- de la Hoz, A.; Loupy, A. (Eds.) Microwaves in Organic Synthesis, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527651313. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 9783527623907. [Google Scholar]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Serpone, N. Preparation of Heterogeneous Catalysts by a Microwave Selective Heating Method. In Microwaves in Catalysis: Methodology and Applications; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Chapter 5; pp. 77–108. ISBN 9783527688111. [Google Scholar]
- Vivas-Castro, J.; Rueda-Morales, G.; Ortega-Cervantez, G.; Moreno-Ruiz, L.; Ortega-Aviles, M.; Ortiz-Lopez, J. Synthesis of Carbon Nanostructures by Microwave Irradiation. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; InTech: London, UK, 2011; Chapter 3; pp. 47–60. ISBN 978-953-307-497-9. [Google Scholar]
- Guiotoku, M.; Rambo, C.; Maia, C.; Hotza, D. Synthesis of carbon-based materials by microwave-assisted hydrothermal process. In Microwave Heating; Chandra, U., Ed.; InTech: London, UK, 2011; Chapter 13; pp. 291–308. ISBN 978-953-307-573-0. [Google Scholar]
- Horikoshi, S.; Serpone, N. (Eds.) Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527648122. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwave-assisted synthesis of nanoparticles. In Microwave Chemistry; Cravotto, G., Carnaroglio, D., Eds.; Walter de Gruyter GmbH: Berlin, Germany, 2017; Chapter 14; pp. 248–269. ISBN 978-3-11-047993-5. [Google Scholar]
- Tsodikov, M.D.; Ellert, O.G.; Arapova, O.V.; Nikolaev, S.A.; Chistyakov, A.V.; Maksimov, Y.V. Benefit of Fe-containing catalytic systems for dry reforming of lignin to syngas under microwave radiation. Chem. Eng. Trans. 2018, 65, 367–372. [Google Scholar] [CrossRef]
- Kustov, L.M. Microwave-Stimulated Oil and Gas Processing. In Microwaves in Catalysis: Methodology and Applications; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Chapter 14; pp. 281–300. ISBN 9783527688111. [Google Scholar]
- Varma, R.S. Solvent-free organic syntheses. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ondruschka, B.; Bonrath, W.; Stuerga, D. Development and Design of Reactors in Microwave-Assisted Chemistry. In Microwaves in Organic Synthesis, 3rd ed.; de la Hoz, A., Loupy, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Chapter 2; pp. 57–103. ISBN 9783527651313. [Google Scholar]
- Horikoshi, S.; Serpone, N. Managing Microwave-Induced Hot Spots in Heterogeneous Catalytic Systems. In Microwaves in Catalysis: Methodology and Applications; Horikoshi, S., Serpone, N., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Chapter 4; pp. 61–77. ISBN 9783527688111. [Google Scholar]
- Shore, G.; Yoo, W.-J.; Li, C.-J.; Organ, M.G. Propargyl amine synthesis catalysed by gold and copper thin films by using microwave-assisted continuous-flow organic synthesis (MACOS). Chem. Eur. J. 2010, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Mathe, M. Hydrogen evolution reaction on single crystal WO3/C nanoparticles supported on carbon in acid and alkaline solution. Int. J. Hydrogen Energy 2011, 36, 1960–1964. [Google Scholar] [CrossRef]
- Qi, S.; Yang, B. Methane aromatization using Mo-based catalysts prepared by microwave heating. Catal. Today 2004, 98, 639–645. [Google Scholar] [CrossRef]
- Kustov, L.M.; Sinev, I.M. Microwave activation of catalysts and catalytic processes. Russ. J. Phys. Chem. A 2010, 84, 1676–1694. [Google Scholar] [CrossRef]
- Ng, S.; Fairbridge, C.; Mutyala, S.; Liu, Y.; Bélanger, J.M.R.; Paré, J.R.J. Microwave-assisted conversion of ethane to ethylene. Appl. Petrochem. Res. 2013, 3, 55–61. [Google Scholar] [CrossRef]
- Sinev, I.; Kardash, T.; Kramareva, N.; Sinev, M.; Tkachenko, O.; Kucherov, A.; Kustov, L.M. Interaction of vanadium containing catalysts with microwaves and their activation in oxidative dehydrogenation of ethane. Catal. Today 2009, 141, 300–305. [Google Scholar] [CrossRef]
- Tsodikov, M.V.; Konstantinov, G.I.; Chistyakov, A.V.; Arapova, O.V.; Perederii, M.A. Utilization of petroleum residues under microwave irradiation. Chem. Eng. J. 2016, 292, 315–320. [Google Scholar] [CrossRef]
- Liu, B.; Slocombe, D.R.; Wang, J.; Aldawsari, A.; Gonzalez-Cortes, S.; Arden, J.; Kuznetsov, V.L.; AlMegren, H.; AlKinany, M.; Xiao, T.; et al. Microwaves effectively examine the extent and type of coking over acid zeolite catalysts. Nat. Commun. 2017, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Münch, J.; Herrmann, R.; Schwieger, W. Effects of microwave radiation on one-step oxidation of benzene to phenol with nitrous oxide over Fe-ZSM-5 catalyst. Chem. Eng. J. 2006, 120, 99–105. [Google Scholar] [CrossRef]
- Jou, C.-J.G.; Lo, C.C. Using a microwave-induced method to regenerate platinum catalyst. Sustain. Environ. Res. 2017, 27, 279–282. [Google Scholar] [CrossRef]
- Deutschmann, O.; Knözinger, H.; Kochloefl, K.; Turek, T. Heterogeneous Catalysis and Solid Catalysts. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2009. [Google Scholar]
- Gupta, M.; Wong Wai Leong, E. Microwaves and Metals; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2011; ISBN 9780470822746. [Google Scholar]
- Roy, R.; Agrawal, D.; Cheng, J.; Gedevanishvili, S. Full sintering of powdered-metal bodies in a microwave field. Nature 1999, 399, 668–670. [Google Scholar] [CrossRef]
- Saitou, K. Microwave sintering of iron, cobalt, nickel, copper and stainless steel powders. Scr. Mater. 2006, 54, 875–879. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave Processing of Materials and Applications in Manufacturing Industries: A Review. Mater. Manuf. Process. 2014, 30, 1–29. [Google Scholar] [CrossRef]
- Rodŕíguez-Reinoso, F.; Seṕulveda-Escribano, A. Carbon as Catalyst Support. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Chapter 4; pp. 131–155. ISBN 9780470403709. [Google Scholar]
- Kim, J.; McNamara, N.D.; Hicks, J.C. Catalytic activity and stability of carbon supported V oxides and carbides synthesized via pyrolysis of MIL-47 (V). Appl. Catal. A 2016, 517, 141–150. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Hu, H.; Wang, Y.; Chen, H.; Lou, X.W.D. Ultrathin MoS2 Nanosheets Supported on N-doped Carbon Nanoboxes with Enhanced Lithium Storage and Electrocatalytic Properties. Angew. Chem. Int. Ed. Engl. 2015, 127, 7503–7506. [Google Scholar] [CrossRef]
- Gotterbarm, K.; Späth, F.; Bauer, U.; Bronnbauer, C.; Steinrück, H.-P.; Papp, C. Reactivity of Graphene-Supported Pt Nanocluster Arrays. ACS Catal. 2015, 5, 2397–2403. [Google Scholar] [CrossRef]
- Cui, S.-C.; Sun, X.-Z.; Liu, J.-G. Photo-reduction of CO2 Using a Rhenium Complex Covalently Supported on a Graphene/TiO2 Composite. ChemSusChem 2016, 9, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xu, K.; Nguyen, L.; Qiao, M.; Tao, F.F. Preparation and Catalysis of Carbon-Supported Iron Catalysts for Fischer-Tropsch Synthesis. ChemCatChem 2012, 4, 1498–1511. [Google Scholar] [CrossRef]
- Wang, Q.-N.; Shi, L.; Lu, A.-H. Highly Selective Copper Catalyst Supported on Mesoporous Carbon for the Dehydrogenation of Ethanol to Acetaldehyde. ChemCatChem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Patel, S.B.; Vasava, D.V. Carbon Nitride-Supported Silver Nanoparticles: Microwave- Assisted Synthesis of Propargylamine and Oxidative C-C Coupling Reaction. ChemistrySelect 2018, 3, 471–480. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Cherepanova, V.A.; Ananikov, V.P. Dynamic Behavior of Metal Nanoparticles in Pd/C and Pt/C Catalytic Systems under Microwave and Conventional Heating. ACS Appl. Mater. Interfaces 2017, 9, 36723–36732. [Google Scholar] [CrossRef] [PubMed]
- Pentsak, E.O.; Gordeev, E.G.; Ananikov, V.P. Noninnocent Nature of Carbon Support in Metal/Carbon Catalysts: Etching/Pitting vs Nanotube Growth under Microwave Irradiation. ACS Catal. 2014, 4, 3806–3814. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Basak, T.; Srinivasan, R. Microwave heating characteristics of graphite based powder mixtures. Int. Commun. Heat Mass Transf. 2013, 48, 22–27. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Wu, Y. Absorption properties of carbon black/silicon carbide microwave absorbers. Compos. Part B 2011, 42, 326–329. [Google Scholar] [CrossRef]
- Severin, N.; Kirstein, S.; Sokolov, I.M.; Rabe, J.P. Rapid trench channeling of graphenes with catalytic silver nanoparticles. Nano Lett. 2009, 9, 457–461. [Google Scholar] [CrossRef]
- Lukas, M.; Meded, V.; Vijayaraghavan, A.; Song, L.; Ajayan, P.M.; Fink, K.; Wenzel, W.; Krupke, R. Catalytic subsurface etching of nanoscale channels in graphite. Nat. Commun. 2013, 4, 1379. [Google Scholar] [CrossRef]
- Sinaiskii, M.A.; Samokhin, A.V.; Alekseev, N.V.; Tsvetkov, Y.V. Extended characteristics of dispersed composition for nanopowders of plasmachemical synthesis. Nanotechnol. Russ. 2016, 11, 805–814. [Google Scholar] [CrossRef]
- Samokhin, A.; Alekseev, N.; Sinayskiy, M.; Astashov, A.; Kirpichev, D.; Fadeev, A.; Tsvetkov, Y.; Kolesnikov, A. Nanopowders Production and Micron-Sized Powders Spheroidization in DC Plasma Reactors. In Powder Technology; Cavalheiro, A.A., Ed.; IntechOpen: London, UK, 2018; Chapter 1. [Google Scholar]
- Samokhin, A.V.; Alexeev, N.V.; Vodopyanov, A.V.; Mansfeld, D.A.; Tsvetkov, Y.V. Metal Oxide Nanopowder Production by Evaporation-Condensation Using a Focused Microwave Radiation at a Frequency of 24 GHz. J. Nanotechnol. Eng. Med. 2015, 6, 011008. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Kashin, A.S.; Polynski, M.V.; Kvashnina, K.O.; Glatzel, P.; Ananikov, V.P. Spatial imaging of carbon reactivity centers in Pd/C catalytic systems. Chem. Sci. 2015, 6, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, A.E.; Gordeev, E.G.; Pentsak, E.O.; Ananikov, V.P. Shielding the chemical reactivity using graphene layers for controlling the surface properties of carbon materials. Phys. Chem. Chem. Phys. 2016, 18, 4608–4616. [Google Scholar] [CrossRef] [PubMed]
- Pentsak, E.O.; Ananikov, V.P. Modulation of chemical interactions across graphene layers and metastable domains in carbon materials. Mendeleev Commun. 2014, 24, 327–328. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. Int. Ed. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Tiwari, S.K.; Mishra, P. Microwave sintering of W–Ni–Fe alloy. Scr. Mater. 2007, 56, 5–8. [Google Scholar] [CrossRef]
- Mondal, A.; Agrawal, D.; Upadhyaya, A. Microwave Sintering of Refractory Metals/alloys: W, Mo, Re, W-Cu, W-Ni-Cu and W-Ni-Fe Alloys. J. Microw. Power 2010, 44, 28–44. [Google Scholar] [CrossRef]
- Mellodge, P.; Folz, D.; Clark, D.; West, J. Heating Rates of Silicon Carbide in a Microwave Field. In Mechanical Properties and Performance of Engineering Ceramics II: Ceramic Engineering and Science Proceedings; Tandon, R., Wereszczak, A., Lara-Curzio, E., Eds.; Wiley: Hoboken, NJ, USA, 2008; Volume 27, Chapter 48. [Google Scholar] [CrossRef]
- Sugawara, H.; Kashimura, K.; Hayashi, M.; Ishihara, S.; Mitani, T.; Shinohara, N. Behavior of microwave-heated silicon carbide particles at frequencies of 2.0–13.5 GHz. Appl. Phys. Lett. 2014, 105, 034103. [Google Scholar] [CrossRef]
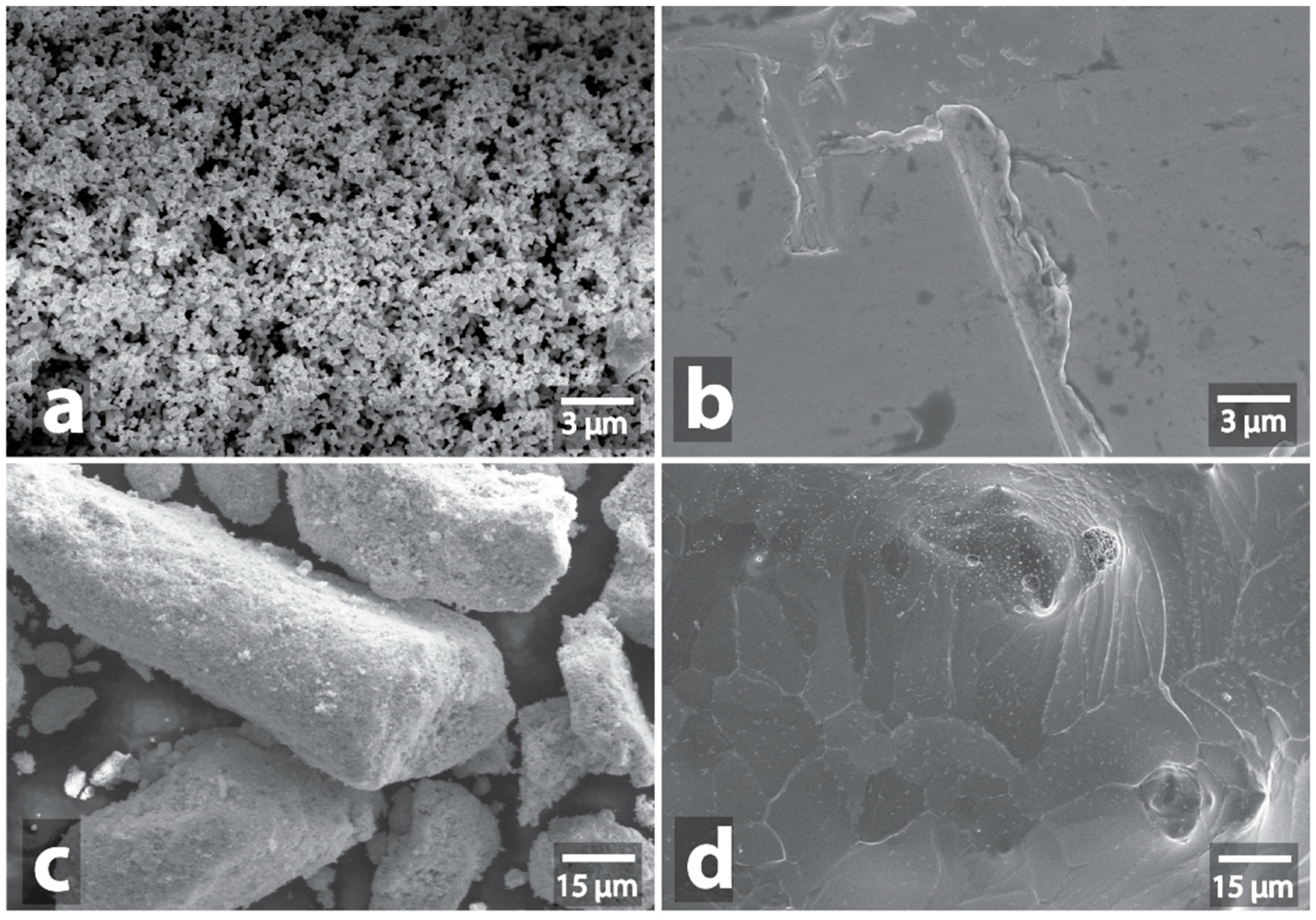
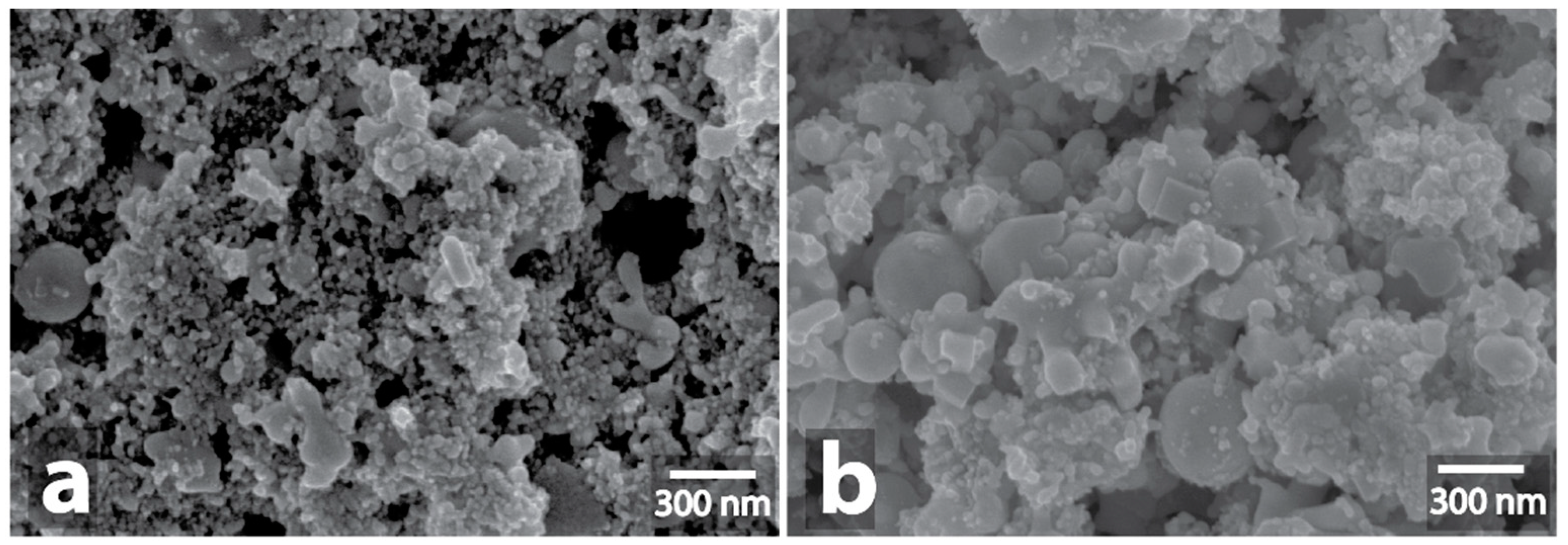
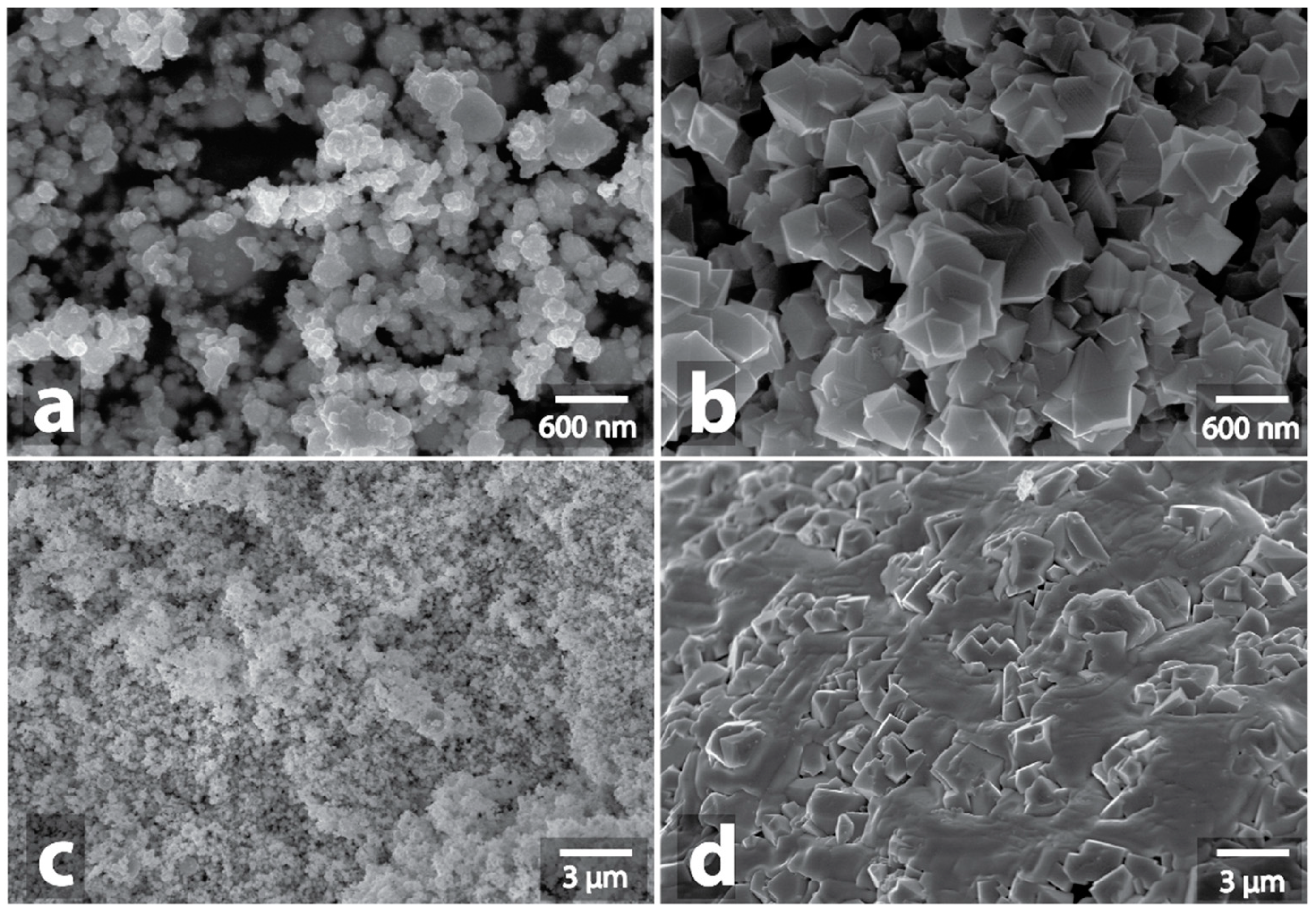
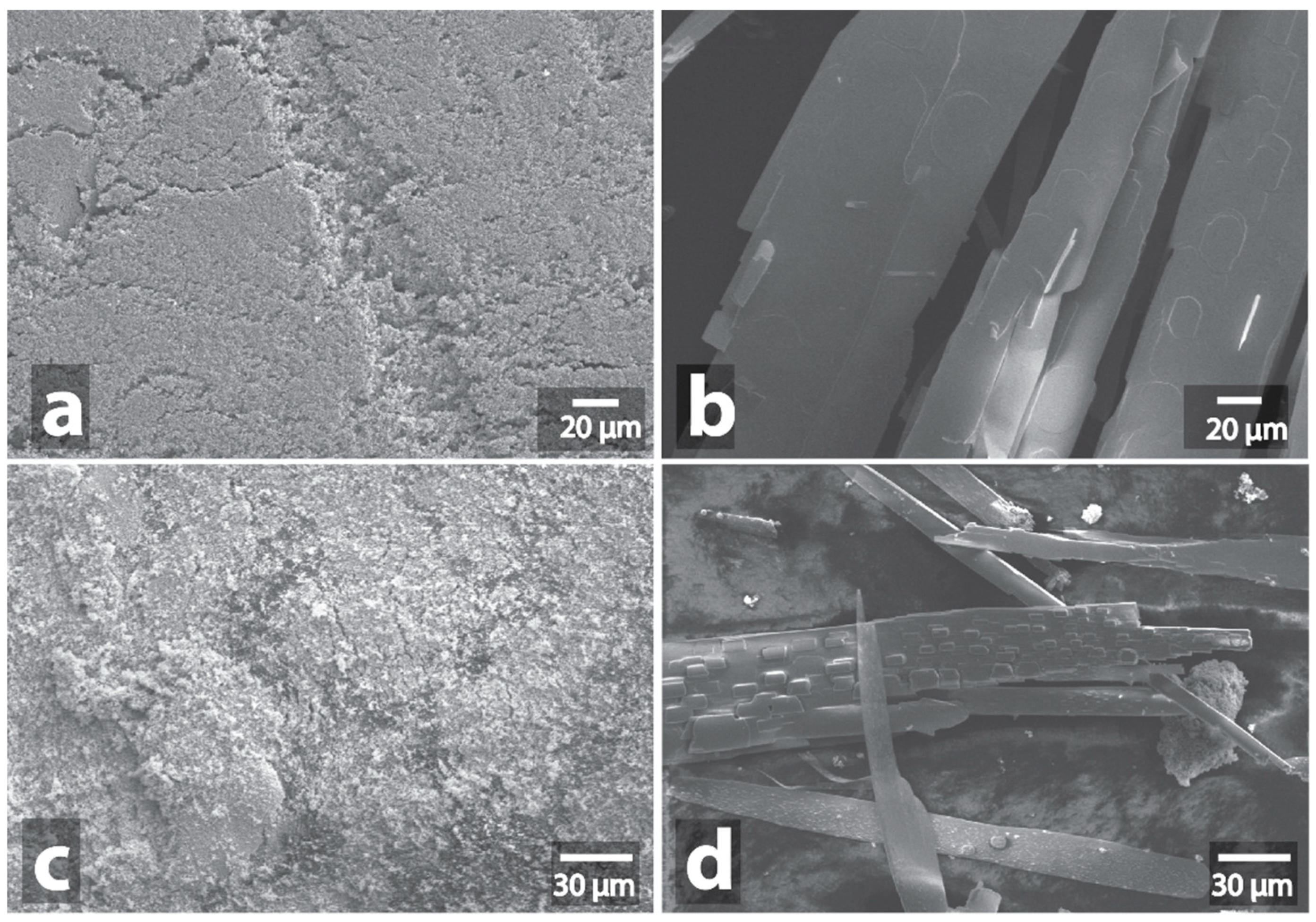
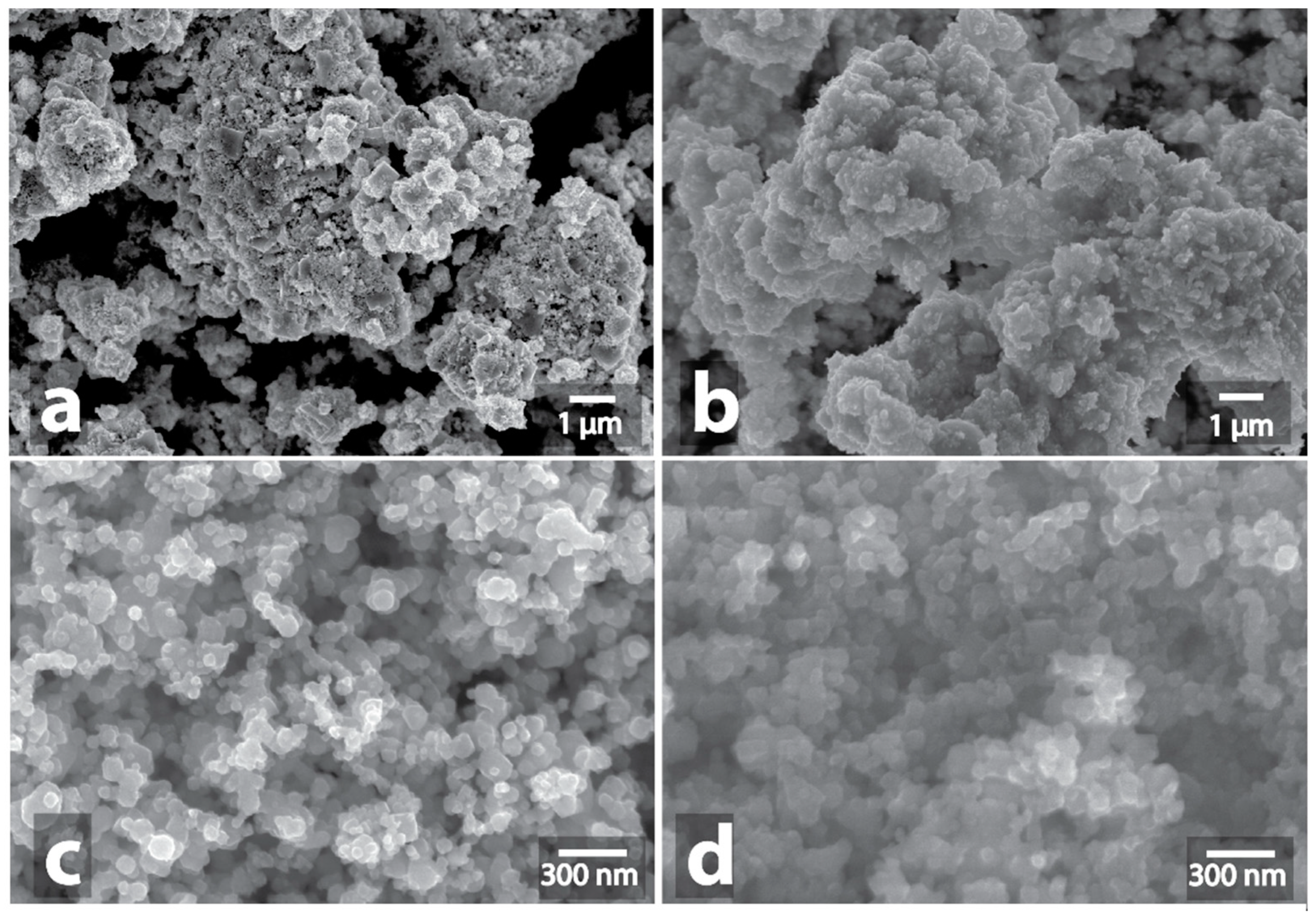
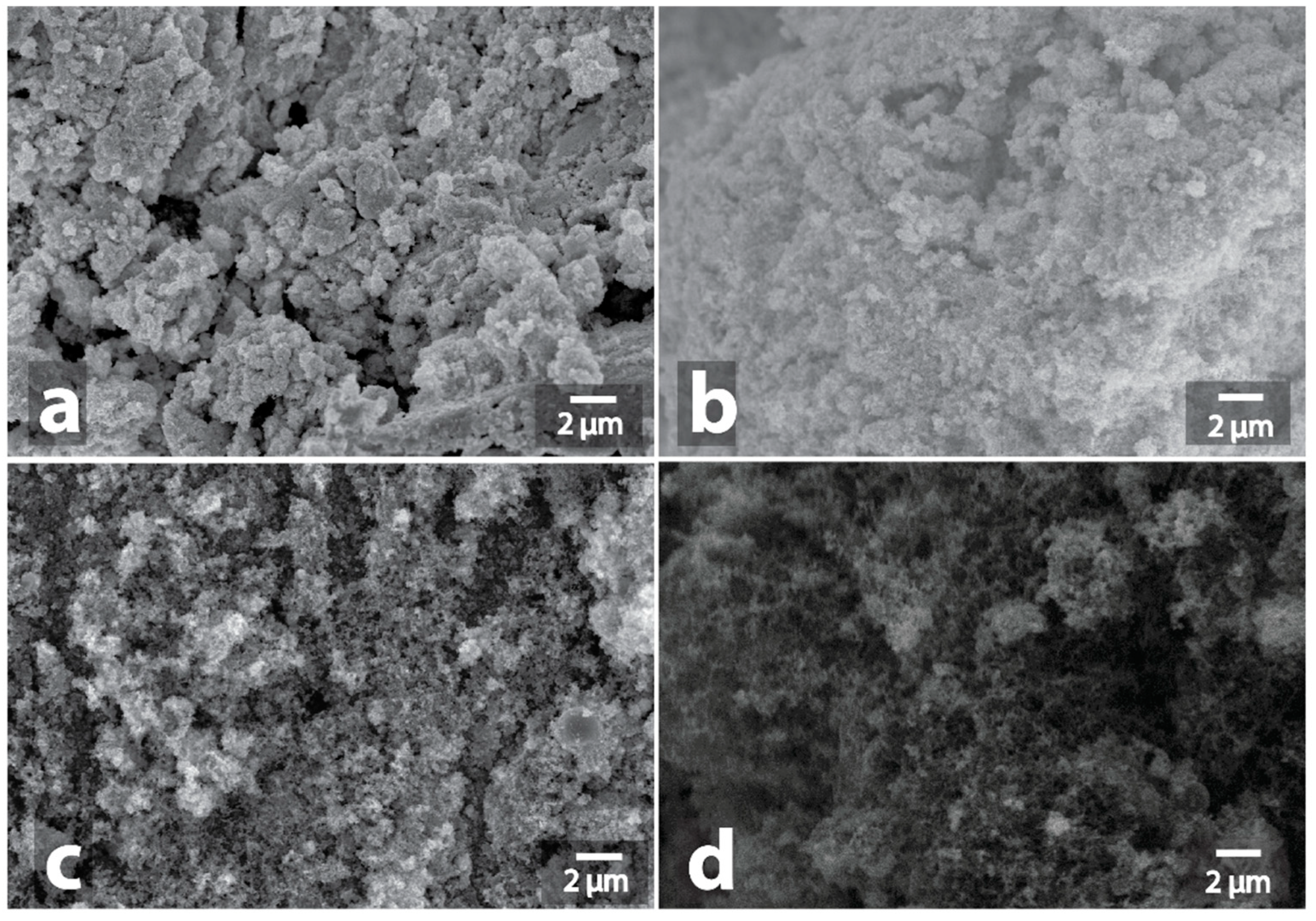
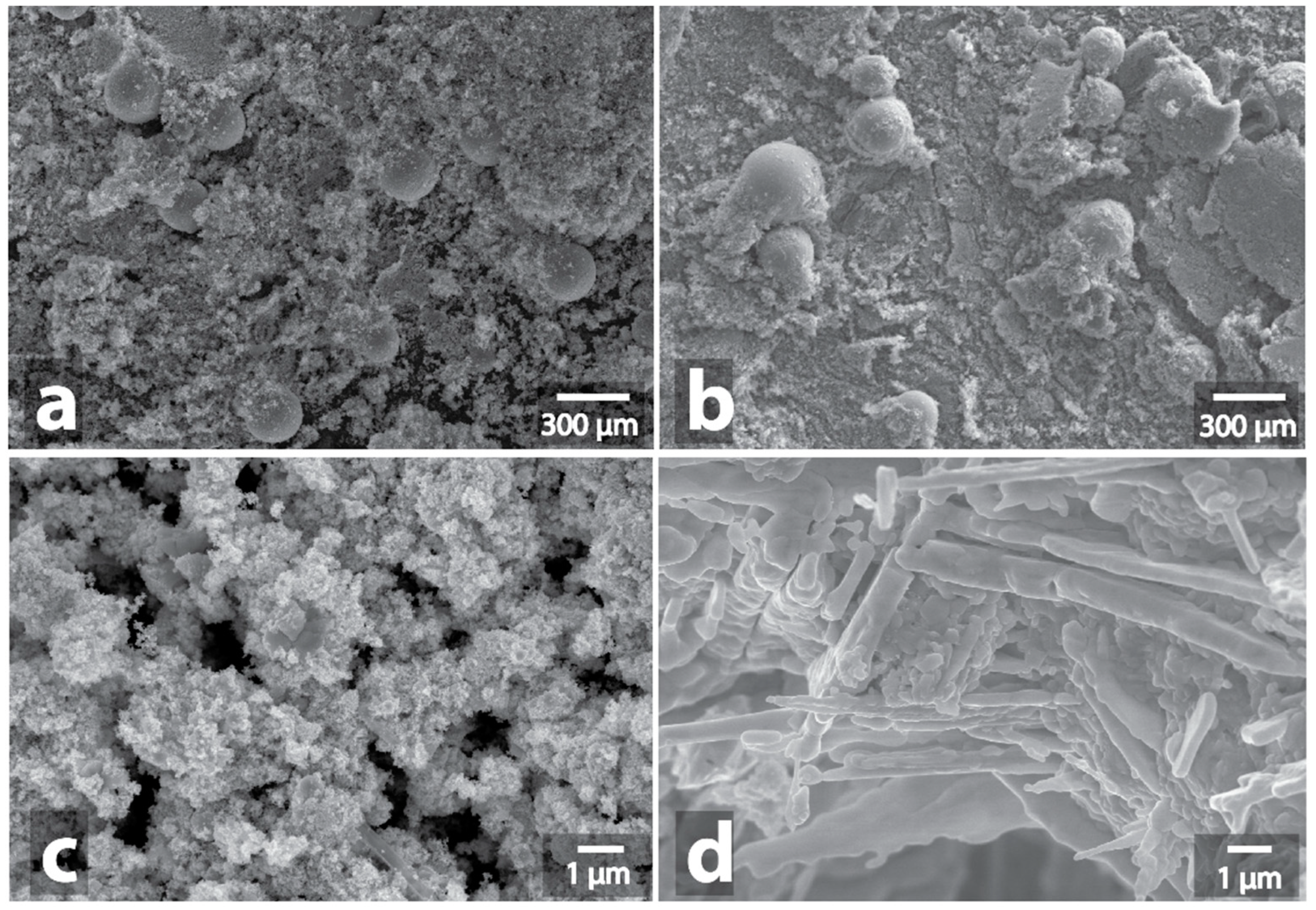

| Sample | Heating a | Morphology Changes of Initial Samples after MW b | Morphology Changes of Graphite after MW Treatment with the Samples c |
|---|---|---|---|
| Pt | type 2 | + | + |
| Re | type 3 | +/- | + |
| Ag | type 2 | + | + |
| Co | type 1 | - | + |
| Fe/C | type 4 | + | - |
| Ni | type 1 | - | + |
| Cu | type 2 | - | + |
| Cu/C | type 2 | - | + |
| W-Ni-Fe (90-7-3%) | type 1 | - | + |
| Mo/C | type 4 | + | - |
| Mo-Fe-C | type 4 | + | + |
| Cu-W (9:1) | type 1 | - | + |
| W-Cu (1:1) | type 1 | - | + |
| WC | type 4 | +/- | + |
| TiN | type 1 | - | + |
| TiC | type 4 | - | + |
| MoS2 | type 4 | + | - |
| W-C | type 3 | - | +/- |
| V-C | type 3 | - | +/- |
| Cr-C | type 3 | - | + |
| W-V-C | type 4 | + | + |
| WO3 | type 1 | - | + |
| ZnO | type 1 | - | +/- |
| TiO2 | type 1 | - | + |
| Fe2O3 | type 1 | - | +/- |
| CuO | type 1 | - | + |
| Y2O3 | type 1 | - | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentsak, E.O.; Cherepanova, V.A.; Sinayskiy, M.A.; Samokhin, A.V.; Ananikov, V.P. Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology. Nanomaterials 2019, 9, 19. https://doi.org/10.3390/nano9010019
Pentsak EO, Cherepanova VA, Sinayskiy MA, Samokhin AV, Ananikov VP. Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology. Nanomaterials. 2019; 9(1):19. https://doi.org/10.3390/nano9010019
Chicago/Turabian StylePentsak, Evgeniy O., Vera A. Cherepanova, Mikhail A. Sinayskiy, Andrey V. Samokhin, and Valentine P. Ananikov. 2019. "Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology" Nanomaterials 9, no. 1: 19. https://doi.org/10.3390/nano9010019
APA StylePentsak, E. O., Cherepanova, V. A., Sinayskiy, M. A., Samokhin, A. V., & Ananikov, V. P. (2019). Systematic Study of the Behavior of Different Metal and Metal-Containing Particles under the Microwave Irradiation and Transformation of Nanoscale and Microscale Morphology. Nanomaterials, 9(1), 19. https://doi.org/10.3390/nano9010019