Fracture Mechanics and Oxygen Gas Barrier Properties of Al2O3/ZnO Nanolaminates on PET Deposited by Atomic Layer Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. ALD of Al2O3/ZnO Nanolaminate Films
2.2. Microstructural Characterization
2.3. Mechanical Tensile Tests
2.4. Gas Permeability Measurement
3. Results
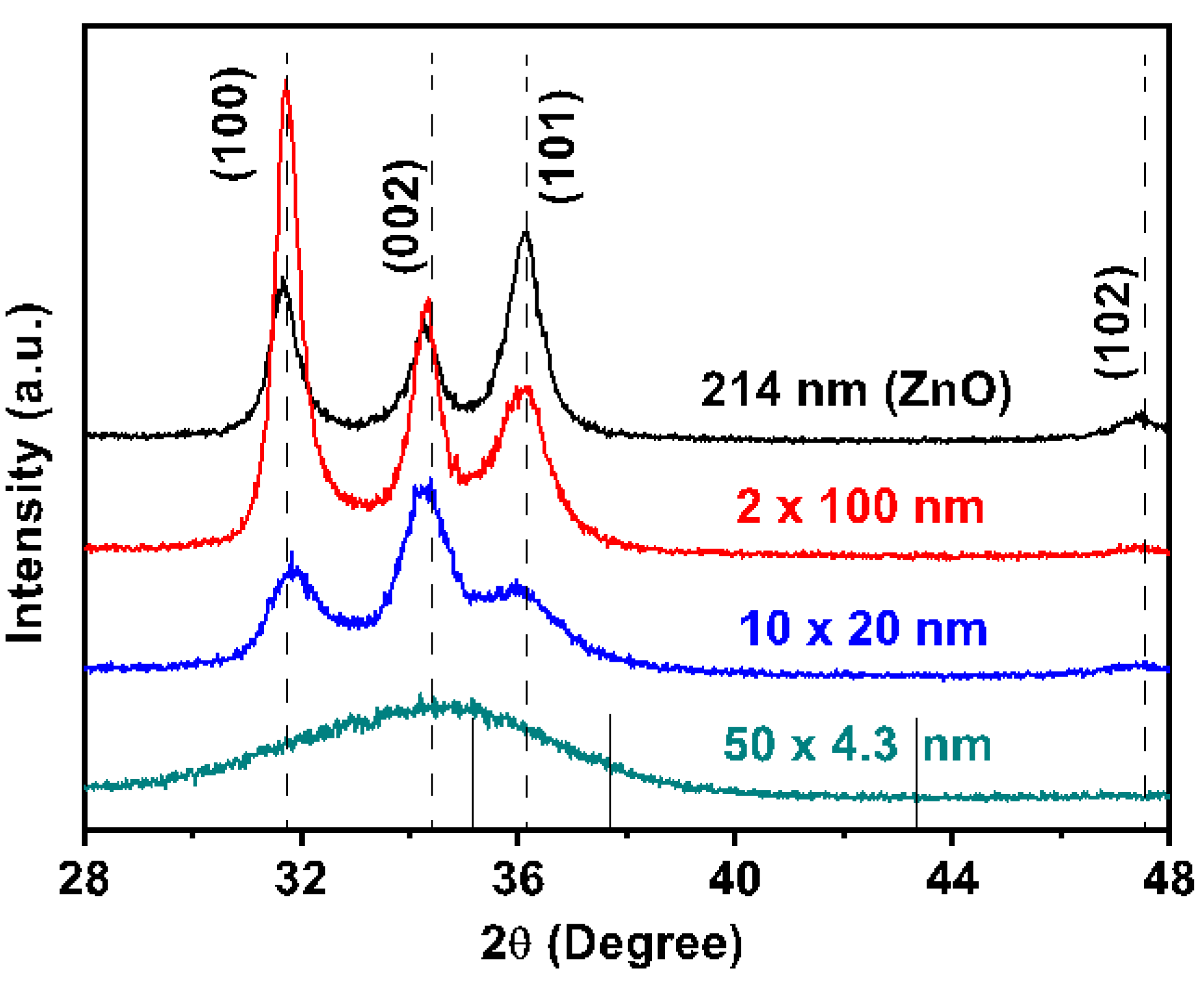
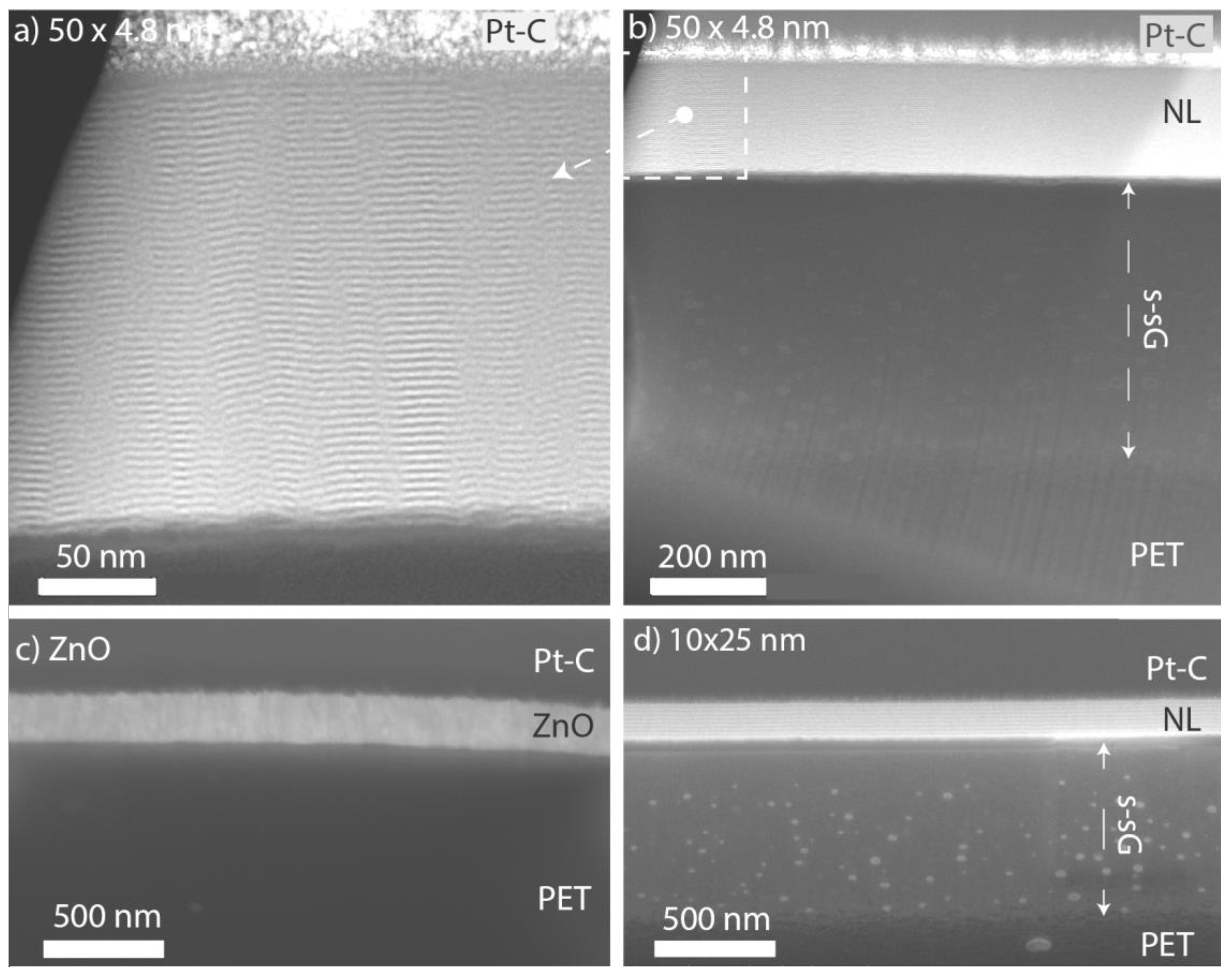
3.1. ALD Film Thickness and Microstructure
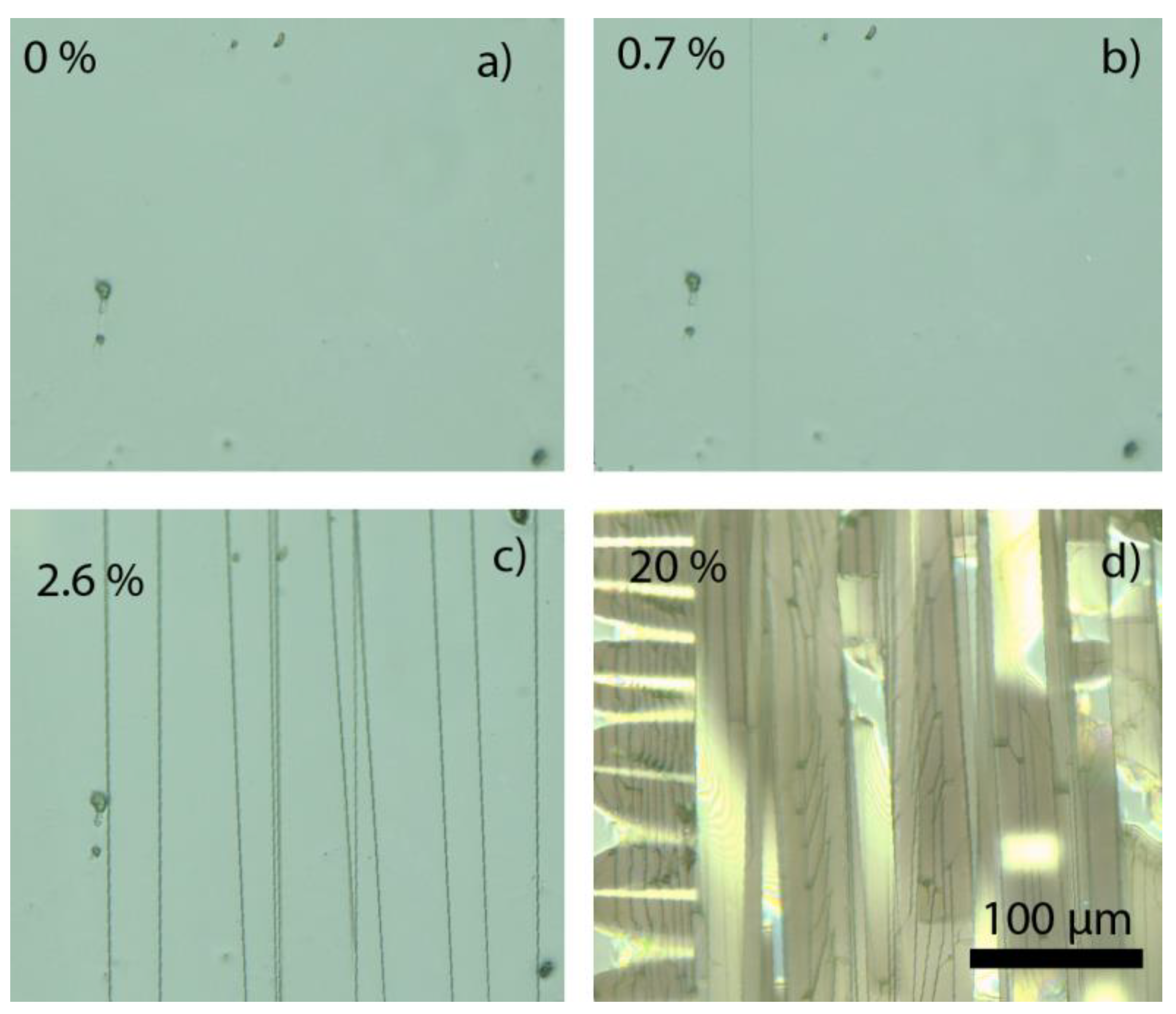
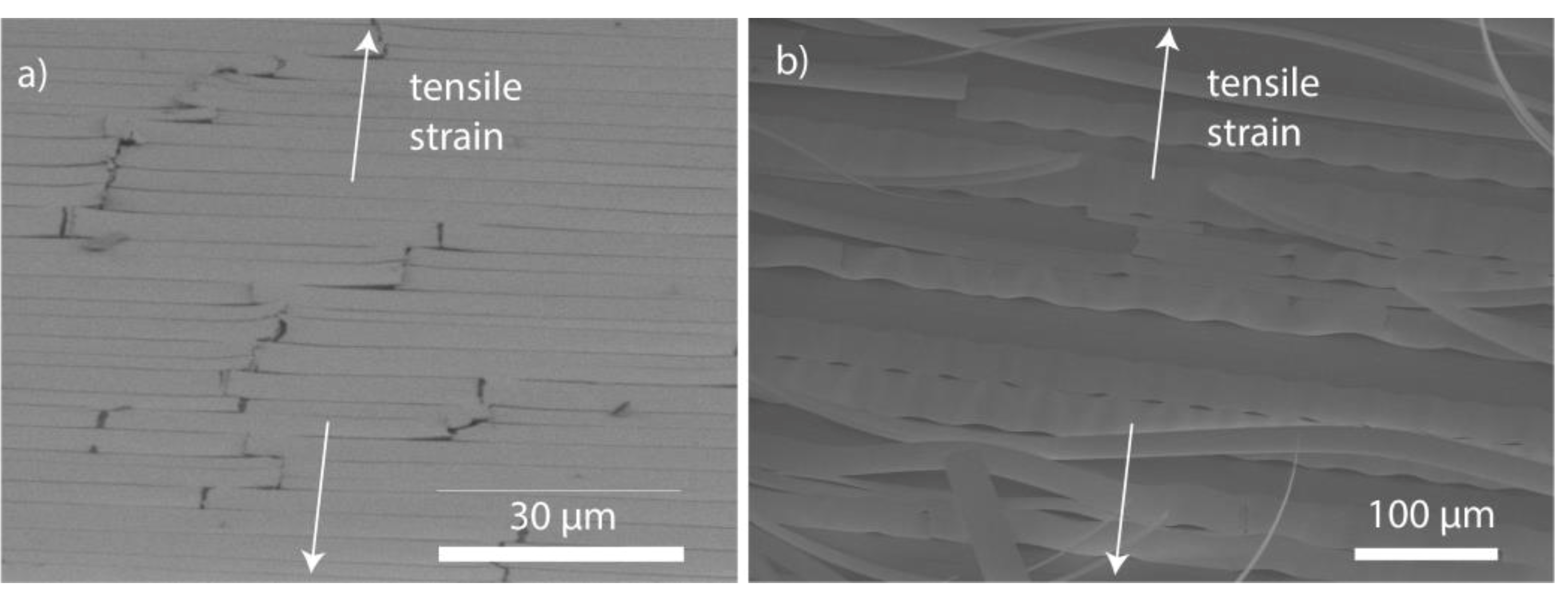
3.2. Mechanical Properties
3.3. Gas Permeability Testing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Zaera, F. The surface chemistry of thin film atomic layer deposition (ALD) processes for electronic device manufacturing. J. Mater. Chem. 2008, 18, 3521–3526. [Google Scholar] [CrossRef]
- van Delft, J.A.; Garcia-Alonso, D.; Kessels, W.M.M. Atomic layer deposition for photovoltaics: Applications and prospects for solar cell manufacturing. Semicond. Sci. Technol. 2012, 27, 074002. [Google Scholar] [CrossRef]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Membranes: Basics, Challenges, and Opportunities. Chem. Mater. 2018. [Google Scholar] [CrossRef]
- Jarvis, K.L.; Evans, P.J. Growth of thin barrier films on flexible polymer substrates by atomic layer deposition. Thin Solid Films 2017, 624, 111–135. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-temperature Al2O3 atomic layer deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H.; Groner, M.D.; George, S.M. Ca test of Al2O3 gas diffusion barriers grown by atomic layer deposition on polymers. Appl. Phys. Lett. 2006, 89, 031915. [Google Scholar] [CrossRef]
- Chou, C.T.; Yu, P.W.; Tseng, M.H.; Hsu, C.C.; Shyue, J.J.; Wang, C.C.; Tsai, F.Y. Transparent Conductive Gas-Permeation Barriers on Plastics by Atomic Layer Deposition. Adv. Mater. 2013, 25, 1750–1754. [Google Scholar] [CrossRef]
- Behrendt, A.; Friedenberger, C.; Gahlmann, T.; Trost, S.; Becker, T.; Zilberberg, K.; Polywka, A.; Gorrn, P.; Riedl, T. Highly Robust Transparent and Conductive Gas Diffusion Barriers Based on Tin Oxide. Adv. Mater. 2015, 27, 5961–5967. [Google Scholar] [CrossRef]
- Meyer, J.; Schmidt, H.; Kowalsky, W.; Riedl, T.; Kahn, A. The origin of low water vapor transmission rates through Al2O3/ZrO2 nanolaminate gas-diffusion barriers grown by atomic layer deposition. Appl. Phys. Lett. 2010, 96, 243308. [Google Scholar] [CrossRef]
- Kim, L.H.; Kim, K.; Park, S.; Jeong, Y.J.; Kim, H.; Chung, D.S.; Kim, S.H.; Park, C.E. Al2O3/TiO2 nanolaminate thin film encapsulation for organic thin film transistors via plasma-enhanced atomic layer deposition. ACS Appl. Mater. Interfaces 2014, 6, 6731–6738. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tynell, T.; Karppinen, M. Atomic layer deposition of ZnO: A review. Semicond. Sci. Technol. 2014, 29, 043001. [Google Scholar] [CrossRef]
- Ylivaara, O.M.E.; Liu, X.W.; Kilpi, L.; Lyytinen, J.; Schneider, D.; Laitinen, M.; Julin, J.; Ali, S.; Sintonen, S.; Berdova, M.; et al. Aluminum oxide from trimethylaluminum and water by atomic layer deposition: The temperature dependence of residual stress, elastic modulus, hardness and adhesion. Thin Solid Films 2014, 552, 124–135. [Google Scholar] [CrossRef]
- Tripp, M.K.; Stampfer, C.; Miller, D.C.; Helbling, T.; Hermann, C.F.; Hierold, C.; Gall, K.; George, S.M.; Bright, V.M. The mechanical properties of atomic layer deposited alumina for use in micro- and nano-electromechanical systems. Sens. Actuators A-Phys. 2006, 130, 419–429. [Google Scholar] [CrossRef]
- Tapily, K.; Jakes, J.E.; Stone, D.S.; Shrestha, P.; Gu, D.; Baumgart, H.; Elmustafa, A.A. Nanoindentation investigation of HfO2 and Al2O3 films grown by atomic layer deposition. J. Electrochem. Soc. 2008, 155, H545–H551. [Google Scholar] [CrossRef]
- Miller, D.C.; Foster, R.R.; Zhang, Y.; Jen, S.-H.; Bertrand, J.A.; Lu, Z.; Seghete, D.; O’Patchen, J.L.; Yang, R.; Lee, Y.-C.; et al. The mechanical robustness of atomic-layer- and molecular-layer-deposited coatings on polymer substrates. J. Appl. Phys. 2009, 105, 093527. [Google Scholar] [CrossRef]
- Herrmann, C.F.; DelRio, F.W.; Miller, D.C.; George, S.M.; Bright, V.M.; Ebel, J.L.; Strawser, R.E.; Cortez, R.; Leedy, K.D. Alternative dielectric films for rf MEMS capacitive switches deposited using atomic layer deposited Al2O3/ZnO alloys. Sens. Actuators A Phys. 2007, 135, 262–272. [Google Scholar] [CrossRef]
- Ding, J.N.; Wang, X.F.; Yuan, N.Y.; Li, C.L.; Zhu, Y.Y.; Kan, B. The influence of substrate on the adhesion behaviors of atomic layer deposited aluminum oxide films. Surf. Coat. Technol. 2011, 205, 2846–2851. [Google Scholar] [CrossRef]
- Bull, S.J. Mechanical response of atomic layer deposition alumina coatings on stiff and compliant substrates. J. Vac. Sci. Technol. A 2012, 30, 01A160. [Google Scholar] [CrossRef]
- Yuan, N.Y.; Wang, S.Y.; Tan, C.B.; Wang, X.Q.; Chen, G.G.; Ding, J.N. The influence of deposition temperature on growth mode, optical and mechanical properties of ZnO films prepared by the ALD method. J. Cryst. Growth 2013, 366, 43–46. [Google Scholar] [CrossRef]
- Jian, S.-R.; Lee, Y.-H. Nanoindentation-induced interfacial fracture of ZnO thin films deposited on Si(111) substrates by atomic layer deposition. J. Alloys Compd. 2014, 587, 313–317. [Google Scholar] [CrossRef]
- Sah, R.E.; Driad, R.; Bernhardt, F.; Kirste, L.; Leancu, C.-C.; Czap, H.; Benkhelifa, F.; Mikulla, M.; Ambacher, O. Mechanical and electrical properties of plasma and thermal atomic layer deposited Al2O3 films on GaAs and Si. J. Vac. Sci. Technol. A 2013, 31, 041502. [Google Scholar] [CrossRef]
- Lyytinen, J.; Berdova, M.; Franssila, S.; Koskinen, J. Adhesion testing of atomic layer deposited TiO2 on glass substrate by the use of embedded SiO2 microspheres. J. Vac. Sci. Technol. A 2014, 32, 01A102. [Google Scholar] [CrossRef]
- Weber, M.; Coy, E.; Iatsunskyi, I.; Yate, L.; Miele, P.; Bechelany, M. Mechanical properties of boron nitride thin films prepared by atomic layer deposition. Crystengcomm 2017, 19, 6089–6094. [Google Scholar] [CrossRef]
- Lyytinen, J.; Berdova, M.; Hirvonen, P.; Liu, X.W.; Franssila, S.; Zhou, Q.; Koskinen, J. Interfacial mechanical testing of atomic layer deposited TiO2 and Al2O3 on a silicon substrate by the use of embedded SiO2 microspheres. RSC Adv. 2014, 4, 37320–37328. [Google Scholar] [CrossRef]
- Jõgiaas, T.; Zabels, R.; Tamm, A.; Merisalu, M.; Hussainova, I.; Heikkilä, M.; Mändar, H.; Kukli, K.; Ritala, M.; Leskelä, M. Mechanical properties of aluminum, zirconium, hafnium and tantalum oxides and their nanolaminates grown by atomic layer deposition. Surf. Coat. Technol. 2015, 282, 36–42. [Google Scholar] [CrossRef]
- Raghavan, R.; Bechelany, M.; Parlinska, M.; Frey, D.; Mook, W.M.; Beyer, A.; Michler, J.; Utke, I. Nanocrystalline-to-amorphous transition in nanolaminates grown by low temperature atomic layer deposition and related mechanical properties. Appl. Phys. Lett. 2012, 100, 191912. [Google Scholar] [CrossRef]
- Herrmann, C.F.; DelRio, F.W.; George, S.M.; Bright, V.M. Properties of atomic layer deposited Al2O3/ZnO dielectric films grown at low temperature for RF MEMS. Micromach. Microfabr. Process Technol. X 2005, 5715, 159–166. [Google Scholar]
- Iatsunskyi, I.; Coy, E.; Viter, R.; Nowaczyk, G.; Jancelewicz, M.; Baleviciute, I.; Zaleski, K.; Jurga, S. Study on Structural, Mechanical, and Optical Properties of Al2O3-TiO2 Nanolaminates Prepared by Atomic Layer Deposition. J. Phys. Chem. C 2015, 119, 20591–20599. [Google Scholar] [CrossRef]
- Marin, E.; Guzman, L.; Lanzutti, A.; Ensinger, W.; Fedrizzi, L. Multilayer Al2O3/TiO2 atomic layer deposition coatings for the corrosion protection of stainless steel. Thin Solid Films 2012, 522, 283–288. [Google Scholar] [CrossRef]
- Härkönen, E.; Daz, B.; Wiatowska, J.; Maurice, V.; Seyeux, A.; Vehkamki, M.; Sajavaara, T.; Fenker, M.; Marcus, P.; Ritala, M. Corrosion protection of steel with oxide nanolaminates grown by atomic layer deposition. J. Electrochem. Soc. 2011, 158, C369–C378. [Google Scholar] [CrossRef]
- Mohseni, H.; Scharf, T.W. Atomic layer deposition of ZnO/Al2O3/ZrO2 nanolaminates for improved thermal and wear resistance in carbon-carbon composites. J. Vac. Sci. Technol. A Vac. Surf. Films 2012, 30, 01A149. [Google Scholar] [CrossRef]
- Jen, S.-H.; Bertrand, J.A.; George, S.M. Critical tensile and compressive strains for cracking of Al2O3 films grown by atomic layer deposition. J. Appl. Phys. 2011, 109, 084305. [Google Scholar] [CrossRef]
- Latella, B.A.; Triani, G.; Evans, P.J. Toughness and adhesion of atomic layer deposited alumina films on polycarbonate substrates. Scr. Mater. 2007, 56, 493–496. [Google Scholar] [CrossRef]
- Triani, G.; Campbell, J.A.; Evans, P.J.; Davis, J.; Latella, B.A.; Burford, R.P. Low temperature atomic layer deposition of titania thin films. Thin Solid Films 2010, 518, 3182–3189. [Google Scholar] [CrossRef]
- Jen, S.H.; Lee, B.H.; George, S.M.; McLean, R.S.; Carcia, P.F. Critical tensile strain and water vapor transmission rate for nanolaminate films grown using Al2O3 atomic layer deposition and alucone molecular layer deposition. Appl. Phys. Lett. 2012, 101, 234103. [Google Scholar] [CrossRef]
- Ruoho, M.; Tarasiuk, N.; Rohbeck, N.; Kapusta, C.; Michler, J.; Utke, I. Stability of mechanical properties of molecular layer–deposited alucone. Mater. Today Chem. 2018, 10, 187–194. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Elam, J.W.; George, S.M. Growth of ZnO/Al2O3 alloy films using atomic layer deposition techniques. Chem. Mater. 2003, 15, 1020–1028. [Google Scholar] [CrossRef]
- Karvonen, L.; Saynatjoki, A.; Chen, Y.; Jussila, H.; Ronn, J.; Ruoho, M.; Alasaarela, T.; Kujala, S.; Norwood, R.A.; Peyghambarian, N.; et al. Enhancement of the third-order optical nonlinearity in ZnO/Al2O3 nanolaminates fabricated by atomic layer deposition. Appl. Phys. Lett. 2013, 103, 031903. [Google Scholar] [CrossRef]
- Abou Chaaya, A.; Viter, R.; Bechelany, M.; Alute, Z.; Erts, D.; Zalesskaya, A.; Kovalevskis, K.; Rouessac, V.; Smyntyna, V.; Miele, P. Evolution of microstructure and related optical properties of ZnO grown by atomic layer deposition. Beilstein J. Nanotechnol. 2013, 4, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Oelkers, A.B.; Toivola, R.; Johnson, D.C.; Elam, J.W.; George, S.M. X-ray reflectivity characterization of ZnO/Al2O3 multilayers prepared by atomic layer deposition. Chem. Mater. 2002, 14, 2276–2282. [Google Scholar] [CrossRef]
- Choi, D.W.; Kim, S.J.; Lee, J.H.; Chung, K.B.; Park, J.S. A study of thin film encapsulation on polymer substrate using low temperature hybrid ZnO/Al2O3 layers atomic layer deposition. Curr. Appl. Phys. 2012, 12, 192. [Google Scholar] [CrossRef]
- Martinez-Castelo, J.R.; Lopez, J.; Dominguez, D.; Murillo, E.; Machorro, R.; Borbon-Nunez, H.A.; Fernandez-Alvarez, I.; Arias, A.; Curiel, M.; Nedev, N.; et al. Structural and electrical characterization of multilayer Al2O3/ZnO nanolaminates grown by atomic layer deposition. Mater. Sci. Semicon. Proc. 2017, 71, 290–295. [Google Scholar] [CrossRef]
- Homola, T.; Bursikova, V.; Ivanova, T.V.; Soucek, P.; Maydannik, P.S.; Cameron, D.C.; Lackner, J.M. Mechanical properties of atomic layer deposited Al2O3/ZnO nanolaminates. Surf. Coat. Technol. 2015, 284, 198–205. [Google Scholar] [CrossRef]
- Yang, F.; Brede, J.; Ablat, H.; Abadia, M.; Zhang, L.B.; Rogero, C.; Elliott, S.D.; Knez, M. Reversible and Irreversible Reactions of Trimethylaluminum with Common Organic Functional Groups as a Model for Molecular Layer Deposition and Vapor Phase Infiltration. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Parsons, G.N.; Atanasov, S.E.; Dandley, E.C.; Devine, C.K.; Gong, B.; Jur, J.S.; Lee, K.; Oldham, C.J.; Peng, Q.; Spagnola, J.C.; et al. Mechanisms and reactions during atomic layer deposition on polymers. Coord. Chem. Rev. 2013, 257, 3323–3331. [Google Scholar] [CrossRef]
- Spagnola, J.C.; Gong, B.; Arvidson, S.A.; Jur, J.S.; Khan, S.A.; Parsons, G.N. Surface and sub-surface reactions during low temperature aluminium oxide atomic layer deposition on fiber-forming polymers. J. Mater. Chem. 2010, 20, 4213–4222. [Google Scholar] [CrossRef]
- Sun, Y.J.; Padbury, R.P.; Akyildiz, H.I.; Goertz, M.P.; Palmer, J.A.; Jur, J.S. Influence of Subsurface Hybrid Material Growth on the Mechanical Properties of Atomic Layer Deposited Thin Films on Polymers. Chem. Vap. Depos. 2013, 19, 134–141. [Google Scholar] [CrossRef]
- Lee, S.M.; Pippel, E.; Knez, M. Metal Infiltration into Biomaterials by ALD and CVD: A Comparative Study. Chemphyschem 2011, 12, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Carcia, P.F.; McLean, R.S.; Groner, M.D.; Dameron, A.A.; George, S.M. Gas diffusion ultrabarriers on polymer substrates using Al2O3 atomic layer deposition and SiN plasma-enhanced chemical vapor deposition. J. Appl. Phys. 2009, 106, 023533. [Google Scholar] [CrossRef]
- Carcia, P.F.; McLean, R.S.; Reilly, M.H. Permeation measurements and modeling of highly defective Al2O3 thin films grown by atomic layer deposition on polymers. Appl. Phys. Lett. 2010, 97, 221901. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Mustonen, T.; Iiskola, E.; Karppinen, M. Atomic layer deposited aluminum oxide barrier coatings for packaging materials. Thin Solid Films 2010, 518, 2654–2658. [Google Scholar] [CrossRef]
- Kim, H.G.; Lee, J.G.; Kim, S.S. Surface modification of polymeric substrates to enhance the barrier properties of an Al2O3 layer formed by PEALD process. Org. Electron. 2017, 50, 239–246. [Google Scholar] [CrossRef]
- Nehm, F.; Klumbies, H.; Richter, C.; Singh, A.; Schroeder, U.; Mikolajick, T.; Monch, T.; Hossbach, C.; Albert, M.; Bartha, J.W.; et al. Breakdown and Protection of ALD Moisture Barrier Thin Films. ACS Appl. Mater. Interfaces 2015, 7, 22121–22127. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.W.; Chang, R.P.H. Atomic layer-controlled growth of transparent conducting ZnO on plastic substrates. Mater. Chem. Phys. 1999, 58, 132–138. [Google Scholar] [CrossRef]
- Ruoho, M.; Juntunen, T.; Alasaarela, T.; Pudas, M.; Tittonen, I. Transparent, Flexible, and Passive Thermal Touch Panel. Adv. Mater. Technol.-US 2016, 1. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Hsu, C.C.; Tseng, M.H.; Shyue, J.J.; Tsai, F.Y. Stable and High-Performance Flexible ZnO Thin-Film Transistors by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2015, 7, 22610–22617. [Google Scholar] [CrossRef]
- Luka, G.; Witkowski, B.S.; Wachnicki, L.; Jakiela, R.; Virt, I.S.; Andrzejczuk, M.; Lewandowska, M.; Godlewski, M. Electrical and mechanical stability of aluminum-doped ZnO films grown on flexible substrates by atomic layer deposition. Mater. Sci. Eng. B-Adv. 2014, 186, 15–20. [Google Scholar] [CrossRef]
- Bulusu, A.; Behm, H.; Sadeghi-Tohidi, F.; Bahre, H.; Baumert, E.; Samet, D.; Hopmann, C.; Winter, J.; Pierron, O.; Graham, S. 29.3: Invited Paper: The Mechanical Reliability of Flexible ALD Barrier Films. SID Symp. Dig. Techn. Pap. 2013, 44, 361–364. [Google Scholar] [CrossRef]
- Roth, K.M.; Roberts, K.G.; Hyde, G.K. Effect of Weave Geometry on Surface Energy Modification of Textile Materials via Atomic Layer Deposition. Text. Res. J. 2010, 80, 1970–1981. [Google Scholar] [CrossRef]
- Agrawal, D.C.; Raj, R. Measurement of the ultimate shear strength of a metal-ceramic interface. Acta Metall. 1989, 37, 1265–1270. [Google Scholar] [CrossRef]
- Taylor, A.A.; Cordill, M.J.; Dehm, G. On the limits of the interfacial yield model for fragmentation testing of brittle films on polymer substrates. Philos. Mag. 2012, 92, 3363–3380. [Google Scholar] [CrossRef]
- Thouless, M.D.; Li, Z.; Douville, N.J.; Takayama, S. Periodic cracking of films supported on compliant substrates. J. Mech. Phys. Solids 2011, 59, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Tyson, W.R. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–350. [Google Scholar] [CrossRef]
- Leterrier, Y.; Boogh, L.; Andersons, J.; Manson, J.A.E. Adhesion of silicon oxide layers on poly(ethylene terephthalate). I: Effect of substrate properties on coating’s fragmentation process. J. Polym. Sci. Part B-Polym. Phys. 1997, 35, 1449–1461. [Google Scholar] [CrossRef]
- Taylor, A.A.; Edlmayr, V.; Cordill, M.J.; Dehm, G. The effect of film thickness variations in periodic cracking: Analysis and experiments. Surf. Coat. Technol. 2011, 206, 1830–1836. [Google Scholar] [CrossRef]
- Meyer, J.; Gorrn, P.; Bertram, F.; Hamwi, S.; Winkler, T.; Johannes, H.H.; Weimann, T.; Hinze, P.; Riedl, T.; Kowalsky, W. Al2O3/ZrO2 Nanolaminates as Ultrahigh Gas-Diffusion Barriers-A Strategy for Reliable Encapsulation of Organic Electronics. Adv.Mater. 2009, 21, 1845–1849. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vaha-Nissi, M.; Nikkola, J.; Harlin, A.; Karppinen, M. Thin Al2O3 barrier coatings onto temperature-sensitive packaging materials by atomic layer deposition. Surf. Coat. Technol. 2011, 205, 5088–5092. [Google Scholar] [CrossRef]
- Lei, W.W.; Li, X.C.; Chen, Q.; Wang, Z.D. Plasma-Assisted ALD of an Al2O3 Permeation Barrier Layer on Plastic. Plasma Sci. Technol. 2012, 14, 129–133. [Google Scholar] [CrossRef]
- Vaha-Nissi, M.; Sundberg, P.; Kauppi, E.; Hirvikorpi, T.; Sievanen, J.; Sood, A.; Karppinen, M.; Harlin, A. Barrier properties of Al2O3 and alucone coatings and nanolaminates on flexible biopolymer films. Thin Solid Films 2012, 520, 6780–6785. [Google Scholar] [CrossRef]
- Gebhard, M.; Mitschker, F.; Hoppe, C.; Aghaee, M.; Rogalla, D.; Creatore, M.; Grundmeier, G.; Awakowicz, P.; Devi, A. A combinatorial approach to enhance barrier properties of thin films on polymers: Seeding and capping of PECVD thin films by PEALD. Plasma Process. Polym. 2018, 15, 1700209. [Google Scholar] [CrossRef]
- Guerra-Nunez, C.; Dobeli, M.; Michler, J.; Utke, I. Reaction and Growth Mechanisms in Al2O3 deposited via Atomic Layer Deposition: Elucidating the Hydrogen Source. Chem. Mater. 2017, 29, 8690–8703. [Google Scholar] [CrossRef]
- Griffith, A.A.; Eng, M., VI. The phenomena of rupture and flow in solids. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1921, 221, 163–198. [Google Scholar] [CrossRef]
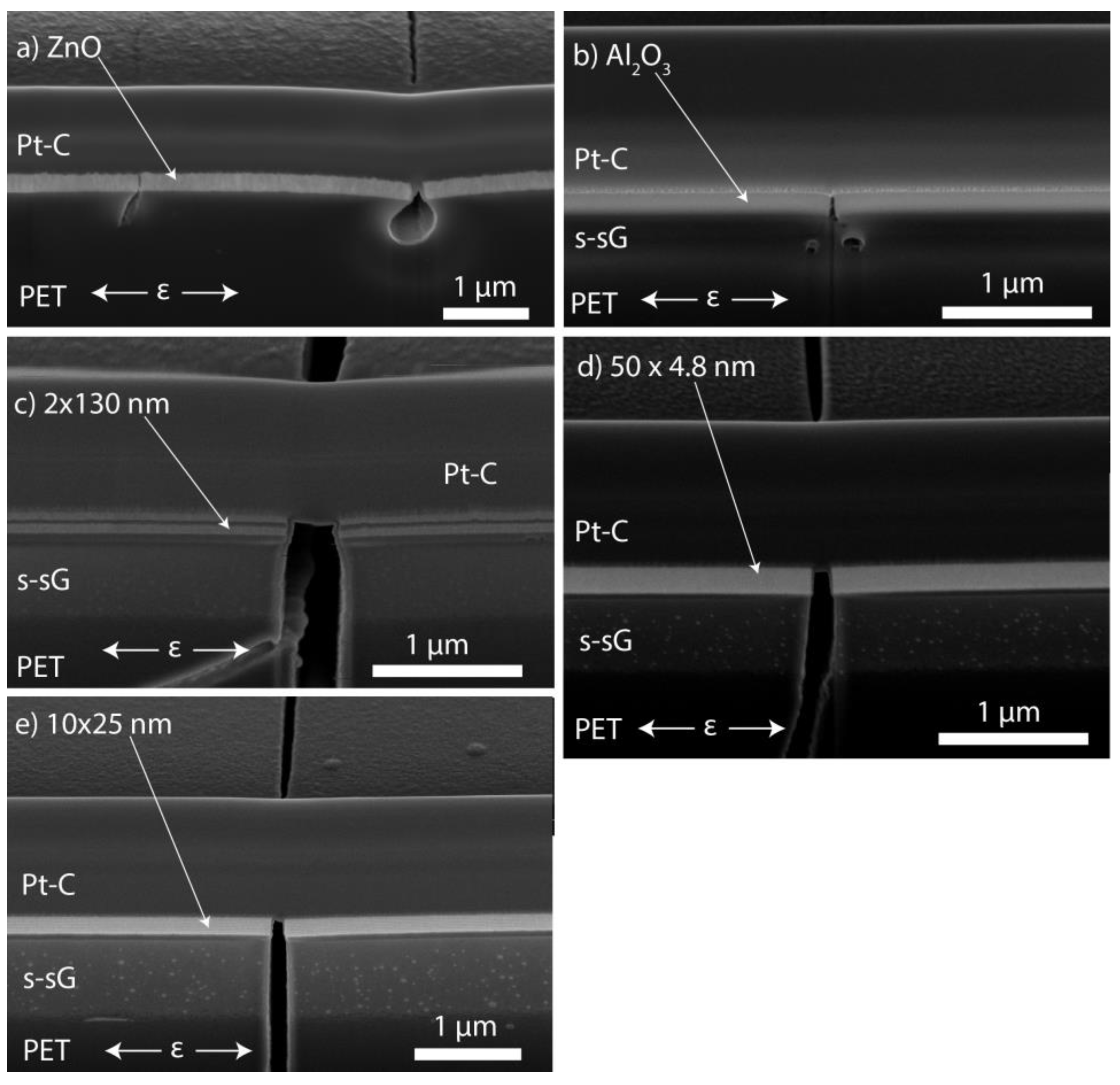
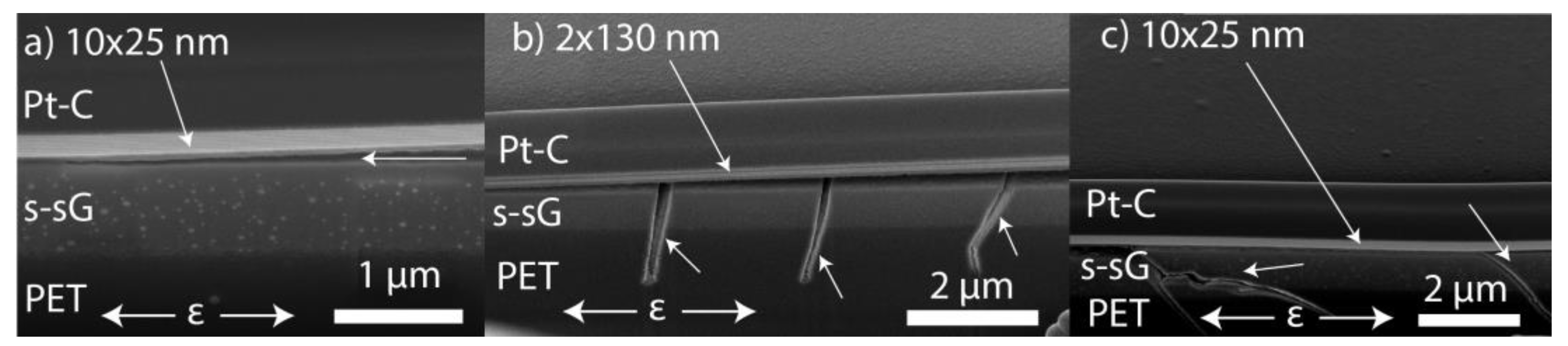
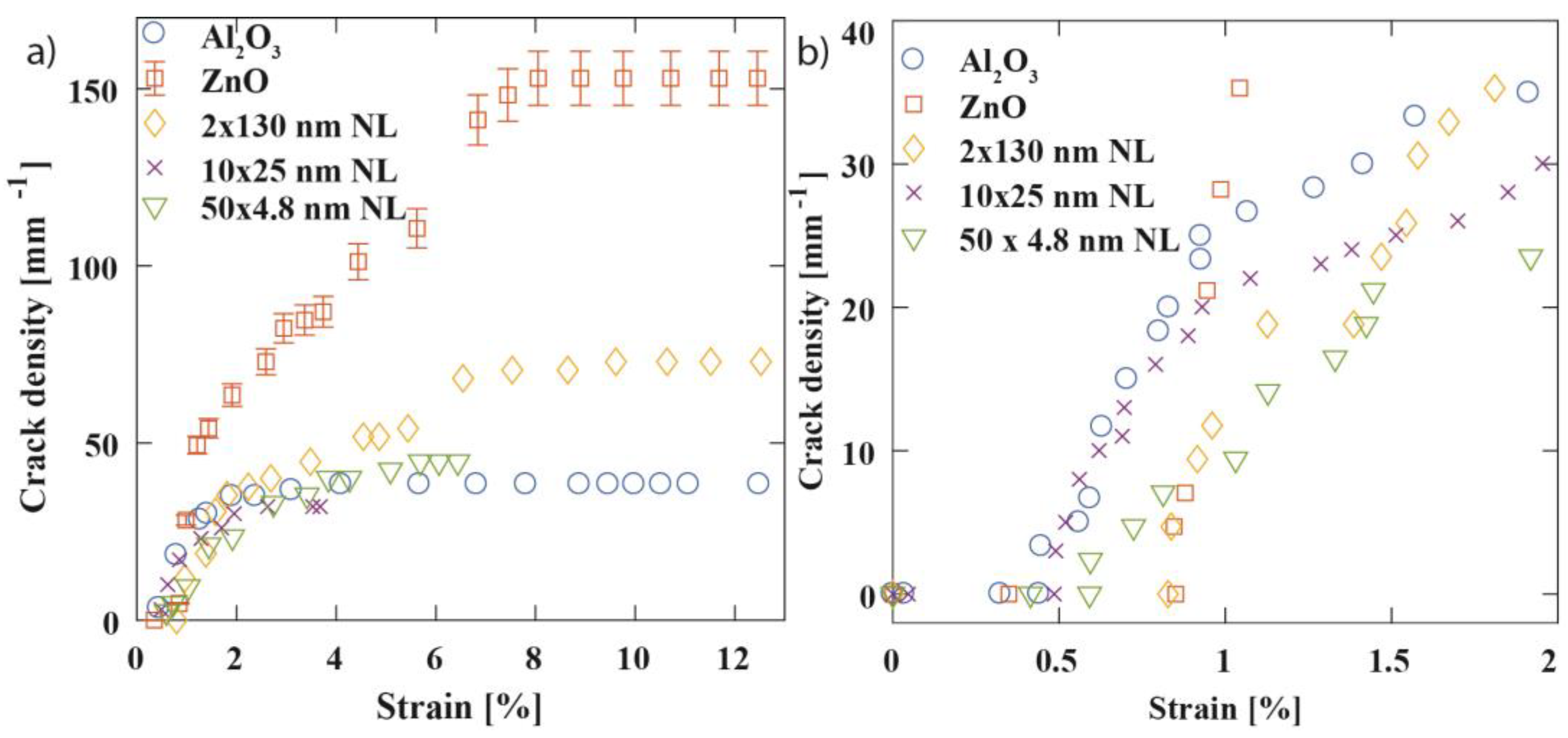
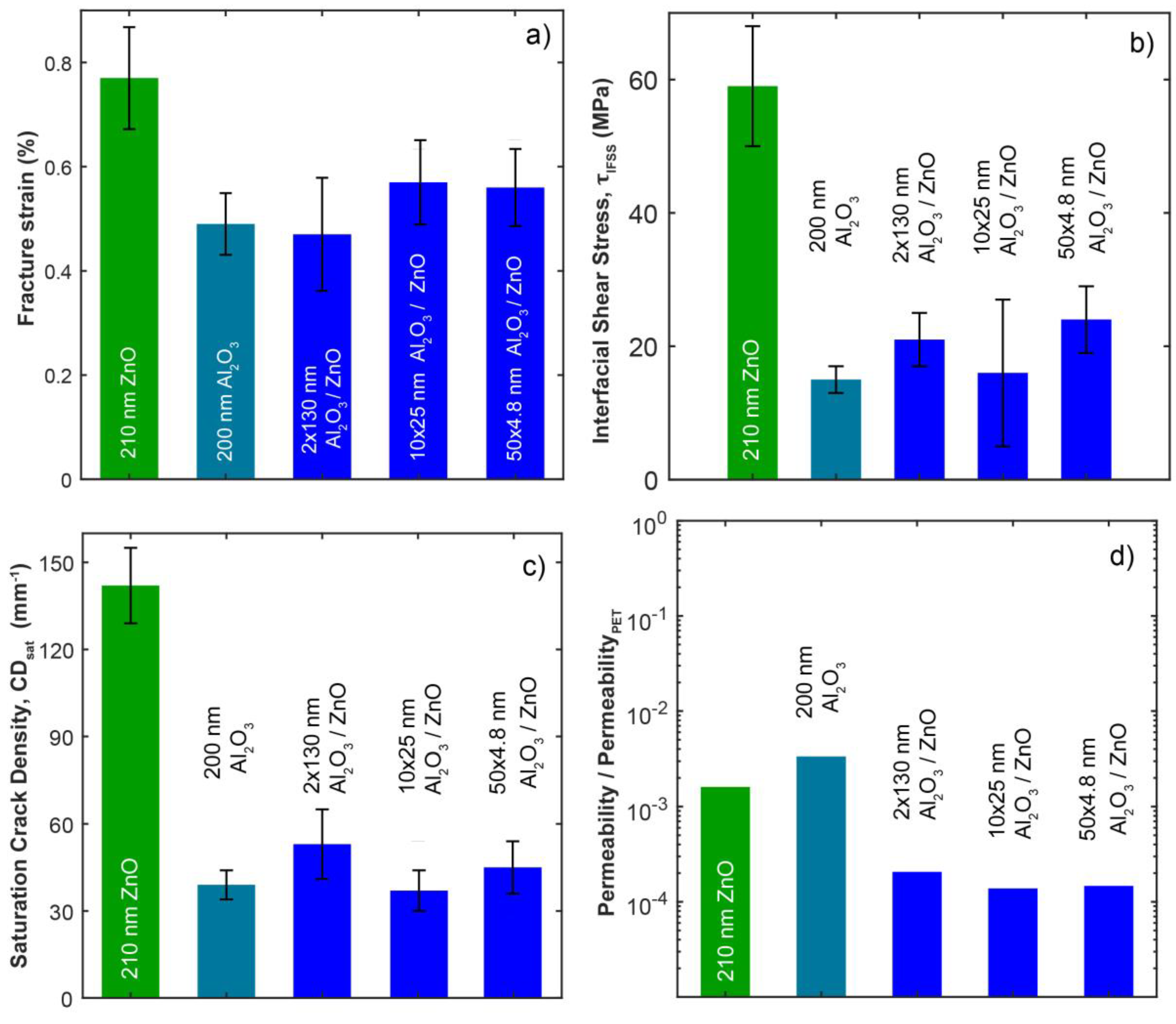
| Sample | Grain Size (nm) | Fracture Strain, εf (%) | Saturation Crack Density, CDsat (mm−1) | Cohesive Strength (MPa) | Interfacial Shear Stress, τIFSS (MPa) | Layer Thickness, h (nm) | Sub- surface Growth, Thickness (nm) | Young’s Modulus, Ef (GPa) | Bend Radii (mm) | O2 Permeability (Barrer) | O2 Permeation Rate (cm3 m−2 day−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number × Thickness of Bilayers | |||||||||||
| ZnO | 14 | 0.77 ± 0.1 | 142 ± 13 | 1460 ± 280 | 59 ± 9 | 210 | - | 145 | 11.4 | 1.02E−06 | 1.10E−01 |
| Al2O3 | A | 0.49 ± 0.06 | 39 ± 5 | 1460 ± 330 | 15 ± 2 | 200 | 300 | 164 | 17.9 | 2.13E−06 | 2.30E−01 |
| 2 × 130 nm | 19 | 0.47 ± 0.11 | 53 ± 12 | 1100 ± 376 | 21 ± 4 | 260 | 670–820 | 152 | 18.6 | 1.31E−07 | 1.40E−02 |
| 10 × 25 nm | 11 | 0.57 ± 0.08 | 37 ± 7 | 1450 ± 850 | 16 ± 11 | 250 | 880–1200 | 146 | 15.6 | 8.74E−08 | 9.40E−03 |
| 50 × 4.8 nm | 4 | 0.56 ± 0.07 | 45 ± 9 | 1670 ± 450 | 24 ± 5 | 240 | 630 | 141 | 15.4 | 9.31E−08 | 1.00E−02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chawla, V.; Ruoho, M.; Weber, M.; Chaaya, A.A.; Taylor, A.A.; Charmette, C.; Miele, P.; Bechelany, M.; Michler, J.; Utke, I. Fracture Mechanics and Oxygen Gas Barrier Properties of Al2O3/ZnO Nanolaminates on PET Deposited by Atomic Layer Deposition. Nanomaterials 2019, 9, 88. https://doi.org/10.3390/nano9010088
Chawla V, Ruoho M, Weber M, Chaaya AA, Taylor AA, Charmette C, Miele P, Bechelany M, Michler J, Utke I. Fracture Mechanics and Oxygen Gas Barrier Properties of Al2O3/ZnO Nanolaminates on PET Deposited by Atomic Layer Deposition. Nanomaterials. 2019; 9(1):88. https://doi.org/10.3390/nano9010088
Chicago/Turabian StyleChawla, Vipin, Mikko Ruoho, Matthieu Weber, Adib Abou Chaaya, Aidan A. Taylor, Christophe Charmette, Philippe Miele, Mikhael Bechelany, Johann Michler, and Ivo Utke. 2019. "Fracture Mechanics and Oxygen Gas Barrier Properties of Al2O3/ZnO Nanolaminates on PET Deposited by Atomic Layer Deposition" Nanomaterials 9, no. 1: 88. https://doi.org/10.3390/nano9010088
APA StyleChawla, V., Ruoho, M., Weber, M., Chaaya, A. A., Taylor, A. A., Charmette, C., Miele, P., Bechelany, M., Michler, J., & Utke, I. (2019). Fracture Mechanics and Oxygen Gas Barrier Properties of Al2O3/ZnO Nanolaminates on PET Deposited by Atomic Layer Deposition. Nanomaterials, 9(1), 88. https://doi.org/10.3390/nano9010088








