Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice
Abstract
1. Introduction
2. Experimental Section
2.1. Film Preparation
2.2. Sample Characterization
3. Results and Discussion
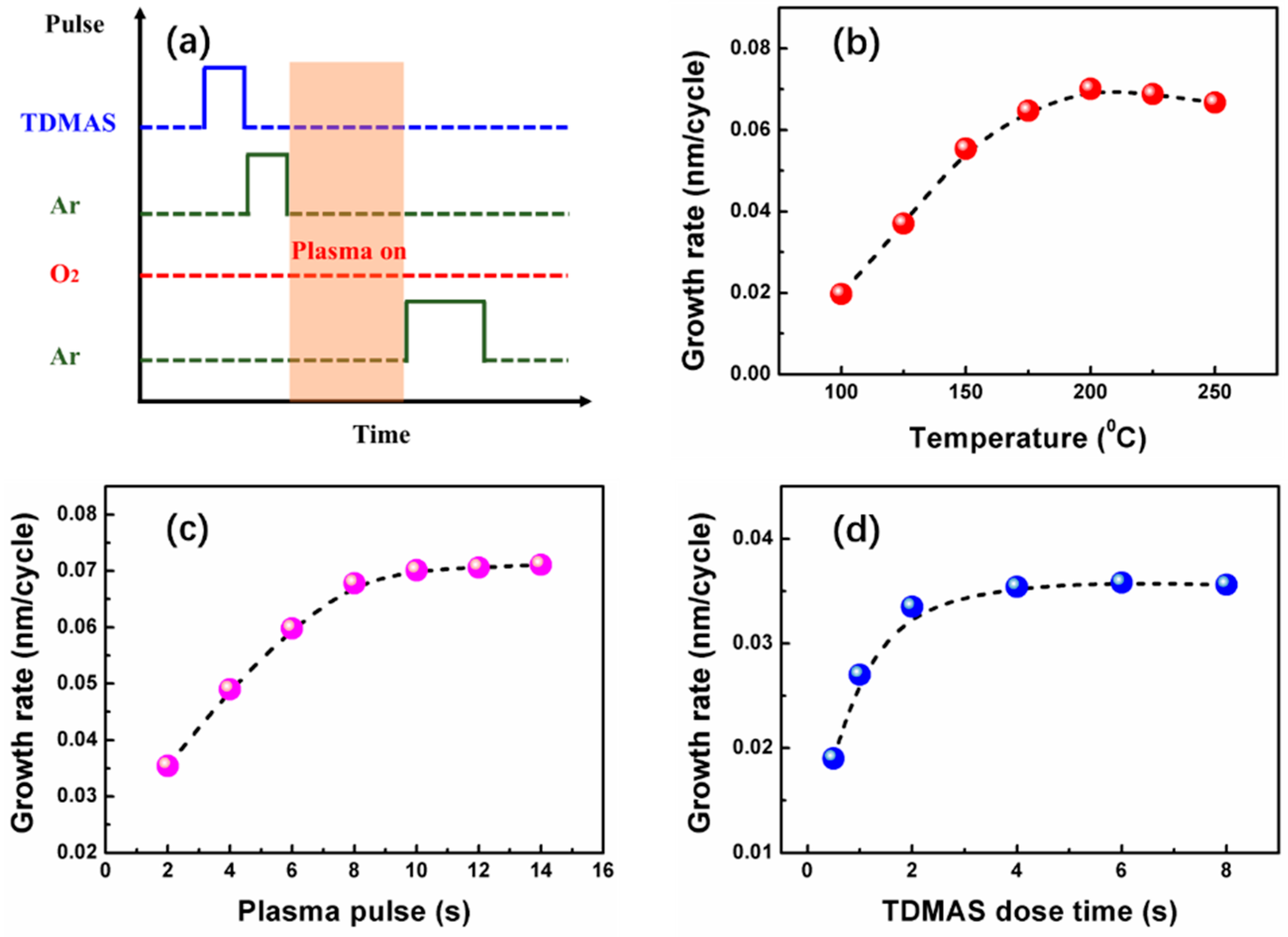
3.1. Film Fabrication and Growth Rate Experiments
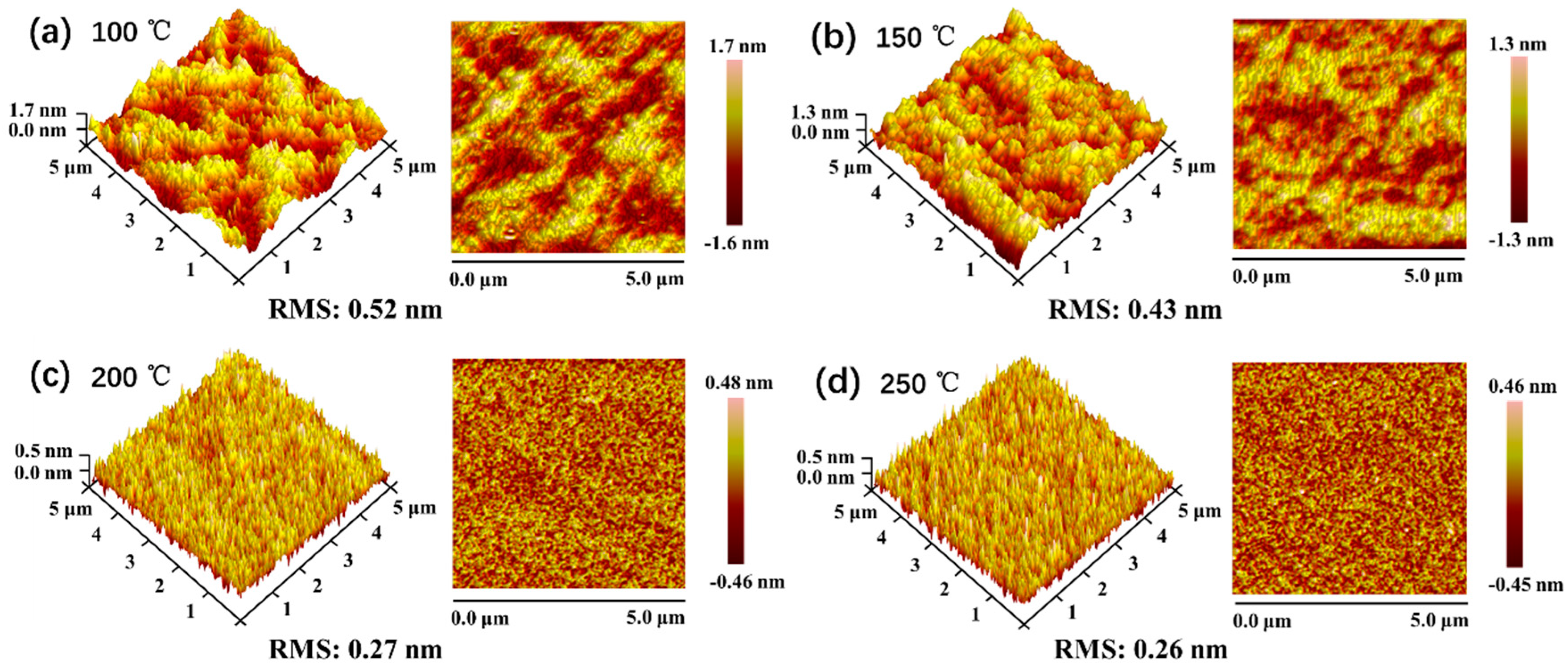
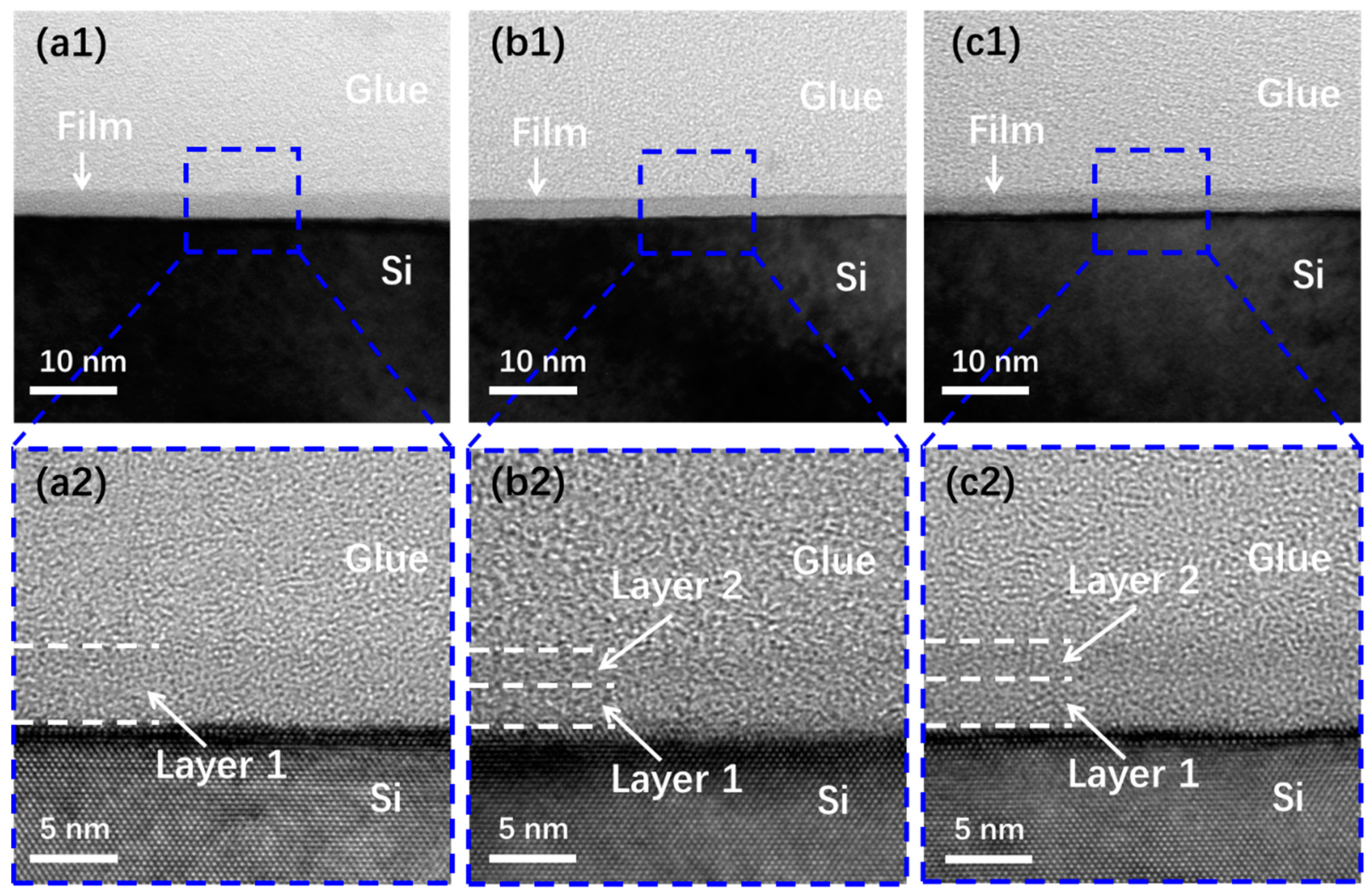
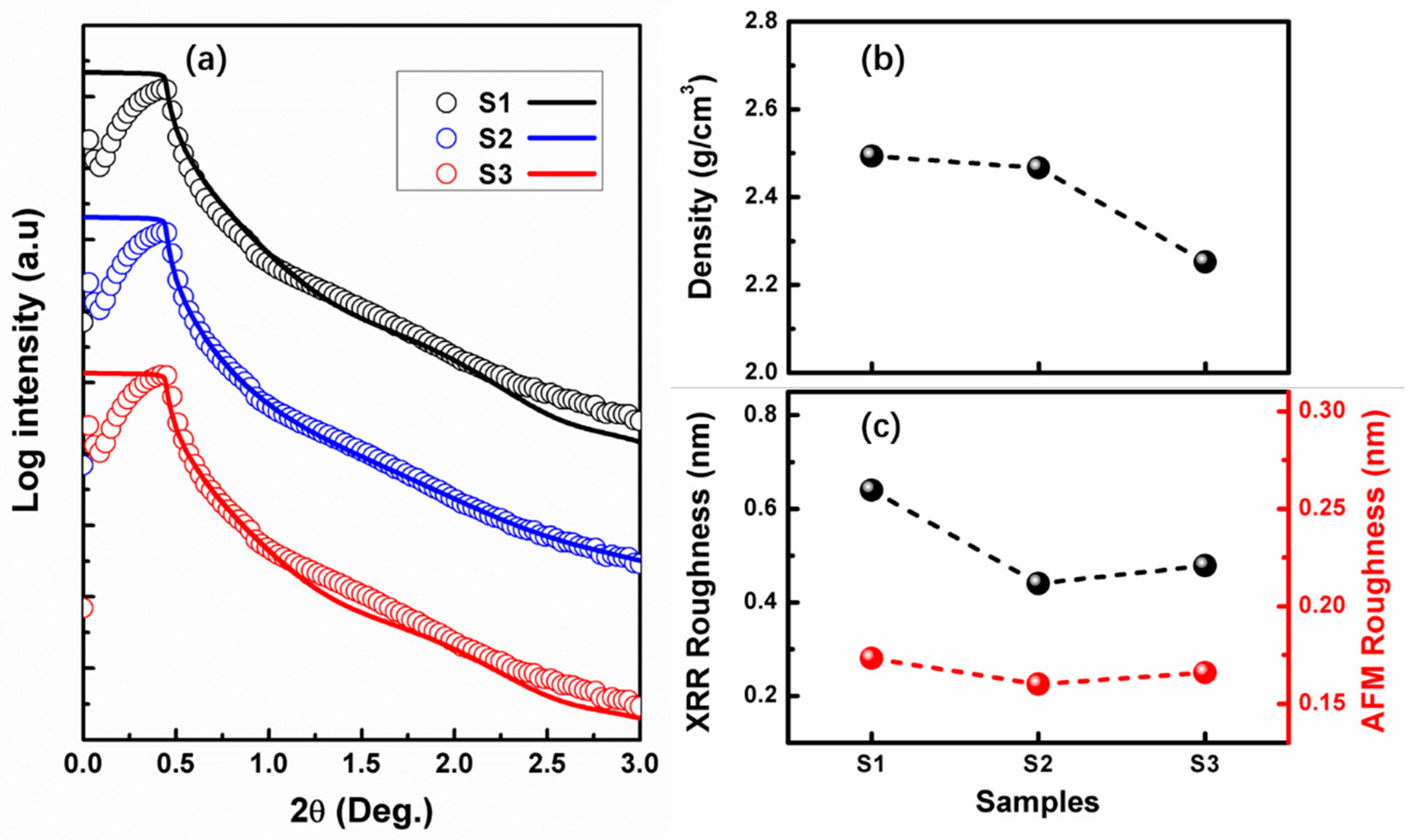
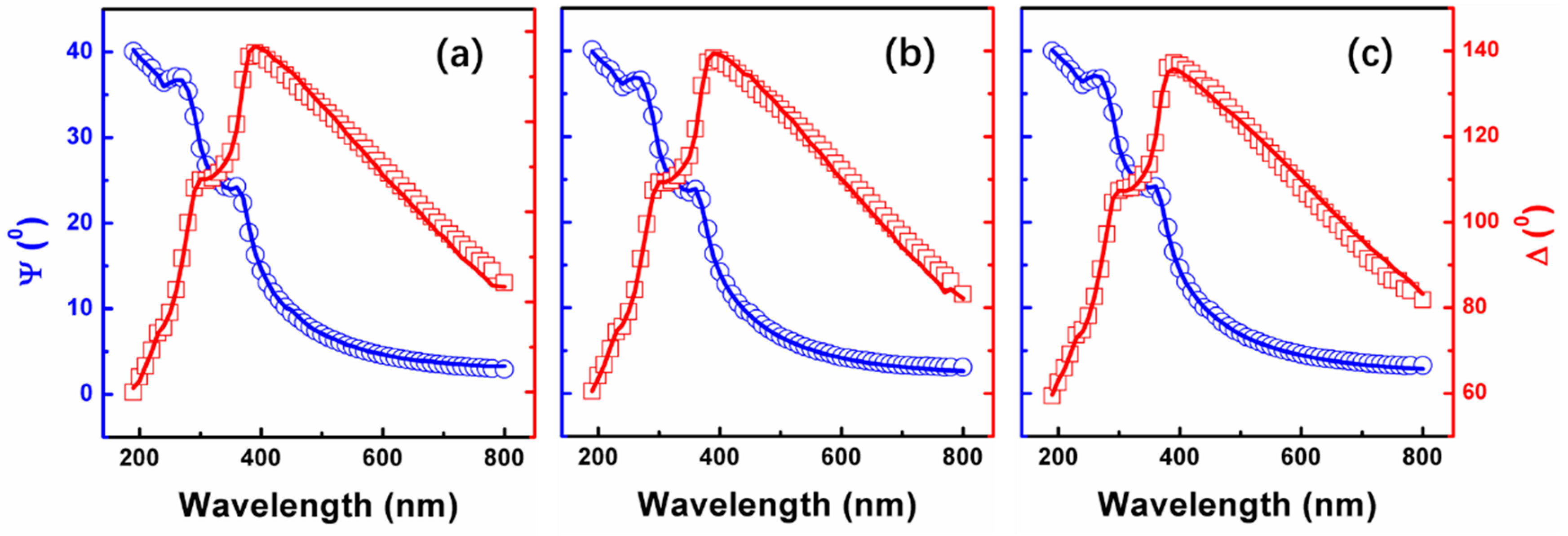
3.2. Properties of SiOx Film with Different Stoichiometry
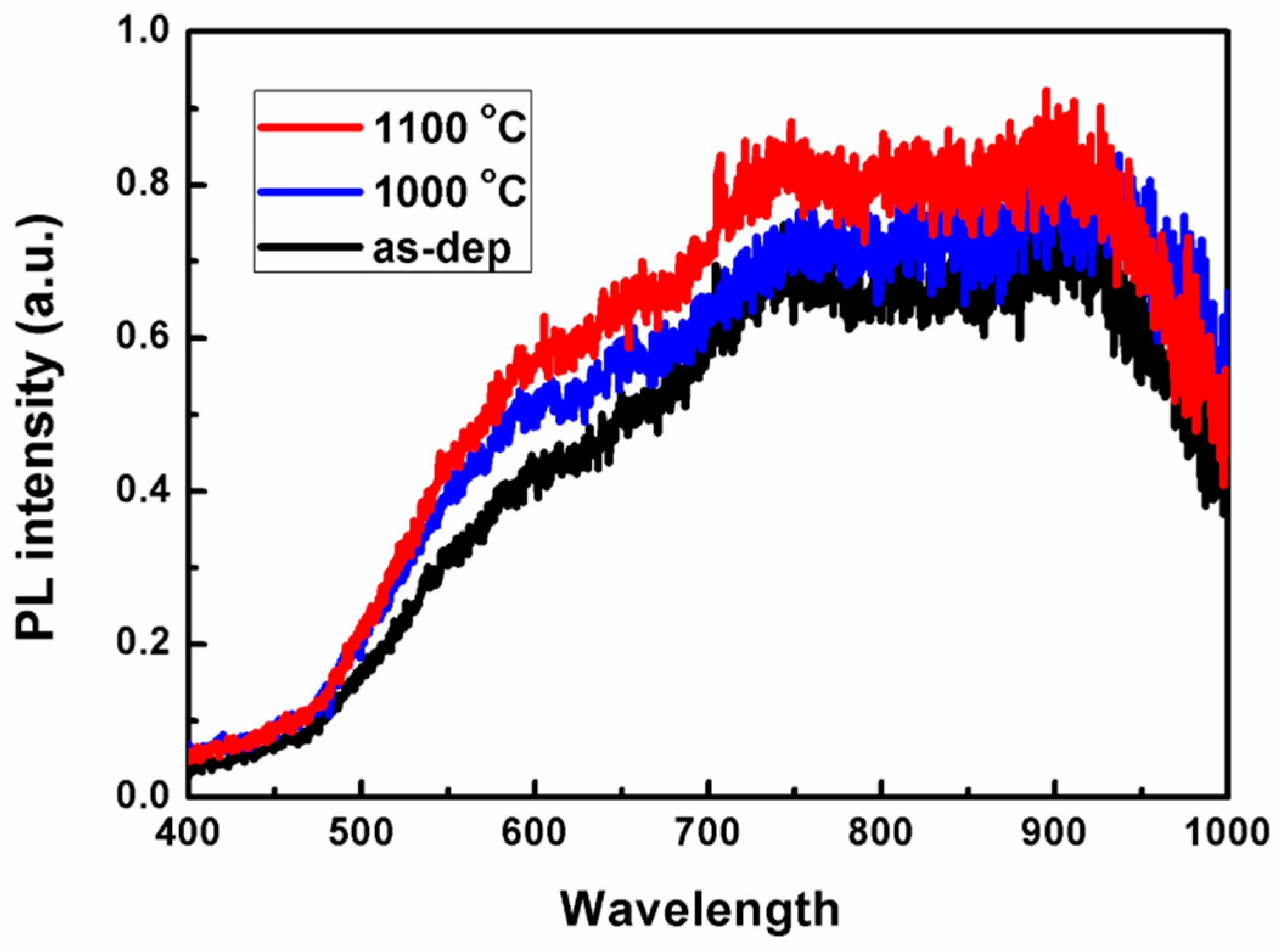
3.3. Photoluminescence Properties of SiO1.6/SiO2 Super-Lattice
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heitmann, J.; Scholz, R.; Schmidt, M.; Zacharias, M. Size controlled nc-Si synthesis by SiO/SiO2 superlattices. J. Cryst. Solids 2002, 299, 1075–1078. [Google Scholar] [CrossRef]
- Huh, C.; Kim, B.K.; Park, B.J.; Jang, E.H.; Kim, S.H. Enhancement in electron transport and light emission efficiency of a Si nanocrystal light-emitting diode by a SiCN/SiC superlattice structure. Nanoscale Res. Lett. 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhigunov, D.M.; Sarikov, A.; Chesnokov, Y.M.; Vasiliev, A.L.; Zakharov, N.; Kashkarov, P.K. Thickness and temperature depending intermixing of SiOx/SiO2 and SiOxNy/SiO2 superlattices: Experimental observation and thermodynamic modeling. Appl. Phys. Lett. 2016, 108, 223102. [Google Scholar] [CrossRef]
- Canham, L.T. Silicon quantum wire array fabrication by electrochemical and chemical dissolution of wafers. Appl. Phys. Lett. 1990, 57, 1046–1048. [Google Scholar] [CrossRef]
- Kanemitsu, Y. Luminescence properties of nanometer-sized Si crystallites: Core and surface states. Phys. Rev. B 1994, 49, 16845–16848. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Chen, W.-H.; Hong, F.C.-N. Visible electroluminescence from silicon nanocrystals embedded in amorphous silicon nitride matrix. Appl. Phys. Lett. 2005, 86, 193506. [Google Scholar] [CrossRef]
- Di Bartolomeo, A.; Rücker, H.; Schley, P.; Fox, A.; Lischke, S.; Na, K.-Y. A single-poly EEPROM cell for embedded memory applications. Solid State Electron. 2009, 53, 644–648. [Google Scholar] [CrossRef]
- Tiwari, S.; Rana, F.; Hanafi, H.; Hartstein, A.; Crabbé, E.F.; Chan, K. A silicon nanocrystals based memory. Appl. Phys. Lett. 1996, 68, 1377–1379. [Google Scholar] [CrossRef]
- Zacharias, M.; Heitmann, J.; Scholz, R.; Kahler, U.; Schmidt, M.; Bläsing, J. Size-controlled highly luminescent silicon nanocrystals: A SiO/SiO2 superlattice approach. Appl. Phys. Lett. 2002, 80, 661–663. [Google Scholar] [CrossRef]
- Gutsch, S.; Hiller, D.; Laube, J.; Zacharias, M.; Kubel, C. Observing the morphology of single-layered embedded silicon nanocrystals by using temperature-stable TEM membranes. Beilstein J. Nanotechnol. 2015, 6, 964–970. [Google Scholar] [CrossRef]
- Yi, L.; Scholz, R.; Zacharias, M. Size and density control of Si-nanocrystals realized by SiOx/SiO2 superlattice. J. Lumin. 2007, 122–123, 750–752. [Google Scholar] [CrossRef]
- Godefroo, S.; Hayne, M.; Jivanescu, M.; Stesmans, A.; Zacharias, M.; Lebedev, O.I.; Van Tendeloo, G.; Moshchalkov, V.V. Classification and control of the origin of photoluminescence from Si nanocrystals. Nat. Nanotechnol. 2008, 3, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Hiller, D.; Jivanescu, M.; Stesmans, A.; Zacharias, M. Pb(0) centers at the Si-nanocrystal/SiO2 interface as the dominant photoluminescence quenching defect. J. Appl. Phys. 2010, 107, 084309. [Google Scholar] [CrossRef]
- Konig, D.; Gutsch, S.; Gnaser, H.; Wahl, M.; Kopnarski, M.; Gottlicher, J.; Steininger, R.; Zacharias, M.; Hiller, D. Location and Electronic Nature of Phosphorus in the Si Nanocrystal-SiO2 System. Sci. Rep. 2015, 5, 9702. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, L.V.; Nguyen, P.H.; Karner, V.L.; D’Ortenzio, R.; Chaudhary, S.; Mokry, C.R.; Simpson, P.J. Si quantum dots in silicon nitride: Quantum confinement and defects. J. Appl. Phys. 2015, 118, 224302. [Google Scholar] [CrossRef]
- Hiller, D.; Zelenina, A.; Gutsch, S.; Dyakov, S.A.; López-Conesa, L.; López-Vidrier, J.; Estradé, S.; Peiró, F.; Garrido, B.; Valenta, J.; et al. Absence of quantum confinement effects in the photoluminescence of Si3N4–embedded Si nanocrystals. J. Appl. Phys. 2014, 115, 204301. [Google Scholar] [CrossRef]
- Suwa, Y.; Saito, S. Intrinsic optical gain of ultrathin silicon quantum wells from first-principles calculations. Phys. Rev. B 2009, 79, 233308. [Google Scholar] [CrossRef]
- Hartel, A.M.; Hiller, D.; Gutsch, S.; Löper, P.; Estradé, S.; Peiró, F.; Garrido, B.; Zacharias, M. Formation of size-controlled silicon nanocrystals in plasma enhanced chemical vapor deposition grown SiOxNy/SiO2 superlattices. Thin Solid Films 2011, 520, 121–125. [Google Scholar] [CrossRef]
- Liao, W.; Zeng, X.; Yao, W.; Wen, X. Photoluminescence and carrier transport mechanisms of silicon-rich silicon nitride light emitting device. Appl. Surf. Sci. 2015, 351, 1053–1059. [Google Scholar] [CrossRef]
- Janz, S.; Löper, P.; Schnabel, M. Silicon nanocrystals produced by solid phase crystallisation of superlattices for photovoltaic applications. Mater. Sci. Eng. B-Solid 2013, 178, 542–550. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Kepaptsoglou, D.M.; Ramasse, Q.M.; Sunding, M.F.; Vestland, L.O.; Finstad, T.G.; Olsen, A. Impact of oxygen bonding on the atomic structure and photoluminescence properties of Si-rich silicon nitride thin films. J. Appl. Phys. 2012, 112, 073514. [Google Scholar] [CrossRef]
- Di, D.; Xu, H.; Perez-Wurfl, I.; Green, M.A.; Conibeer, G. Optical characterisation of silicon nanocrystals embedded in SiO2/Si3N4 hybrid matrix for third generation photovoltaics. Nanoscale Res. Lett. 2011, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.F.; Hsiao, C.Y.; Su, K.W. Enhanced white photoluminescence in silicon-rich oxide/SiO2 superlattices by low-energy ion-beam treatment. Opt. Express 2013, 21, 15888–15895. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sundararaman, R.; Tiwari, S.; Lee, J.-W. Charge Trapping Devices Using a Bilayer Oxide Structure. J. Nanosci. Nanotechnol. 2012, 12, 423–427. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, R.; Rechmann, J.; Aghaee, M.; Rogalla, D.; Becker, H.W.; Creatore, M.; Wieck, A.D.; Devi, A. Low temperature growth of gallium oxide thin films via plasma enhanced atomic layer deposition. Dalton Trans. 2017, 46, 16551–16561. [Google Scholar] [CrossRef] [PubMed]
- Biyikli, N.; Haider, A. Atomic layer deposition: An enabling technology for the growth of functional nanoscale semiconductors. Semicond. Sci. Technol. 2017, 32, 093002. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Goto, H.; Shibahara, K.; Yokoyama, S. Atomic layer controlled deposition of silicon nitride with self-limiting mechanism. Appl. Phys. Lett. 1996, 68, 3257–3259. [Google Scholar] [CrossRef]
- Knoops, H.C.; Braeken, E.M.; de Peuter, K.; Potts, S.E.; Haukka, S.; Pore, V.; Kessels, W.M. Atomic Layer Deposition of Silicon Nitride from Bis(tert-butylamino)silane and N2 Plasma. ACS Appl. Mater. Interfaces 2015, 7, 19857–19862. [Google Scholar] [CrossRef]
- Kim, Y.; Provine, J.; Walch, S.P.; Park, J.; Phuthong, W.; Dadlani, A.L.; Kim, H.J.; Schindler, P.; Kim, K.; Prinz, F.B. Plasma-Enhanced Atomic Layer Deposition of SiN-AlN Composites for Ultra Low Wet Etch Rates in Hydrofluoric Acid. ACS Appl. Mater. Interfaces 2016, 8, 17599–17605. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. Oxygen Plasma and Humidity Dependent Surface Analysis of Silicon, Silicon Dioxide and Glass for Direct Wafer Bonding. ECS J. Solid State Sci. Technol. 2013, 2, P515–P523. [Google Scholar] [CrossRef]
- Laube, J.; Gutsch, S.; Hiller, D.; Bruns, M.; Kübel, C.; Weiss, C.; Zacharias, M. Formation of size controlled silicon nanocrystals in nitrogen free silicon dioxide matrix prepared by plasma enhanced chemical vapor deposition. J. Appl. Phys. 2014, 116, 223501. [Google Scholar] [CrossRef]
- Yang, D.Q.; Gillet, J.N.; Meunier, M.; Sacher, E. Room temperature oxidation kinetics of Si nanoparticles in air, determined by X-ray photoelectron spectroscopy. J. Appl. Phys. 2005, 97, 024303. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lu, H.L.; Chen, J.X.; Zhang, F.; Ji, X.M.; Liu, W.J.; Yang, X.F.; Zhang, D.W. Low-Temperature One-Step Growth of AlON Thin Films with Homogenous Nitrogen-Doping Profile by Plasma-Enhanced Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2017, 9, 38662–38669. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fronk, M.; Geng, Y.; Chen, L.; Sun, Q.Q.; Gordan, O.D.; Zhou, P.; Zahn, D.R.; Zhang, D.W. Optical properties and bandgap evolution of ALD HfSiOx films. Nanoscale Res. Lett. 2015, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Wemple, S.H.; DiDomenico, M. Behavior of the Electronic Dielectric Constant in Covalent and Ionic Materials. Phys. Rev. B 1971, 3, 1338–1351. [Google Scholar] [CrossRef]
- Wemple, S.H. Refractive-Index Behavior of Amorphous Semiconductors and Glasses. Phys. Rev. B 1973, 7, 3767–3777. [Google Scholar] [CrossRef]
- Gonzalez-Leal, J.M.; Marquez, E.; Bernal-Oliva, A.M.; Ruiz-Perez, J.J.; Jimenez-Garay, R. Derivation of the optical constants of thermally-evaporated uniform films of binary chalcogenide glasses using only their reflection spectra. Thin Solid Films 1998, 317, 223–227. [Google Scholar] [CrossRef]
- Márquez, E.; Ramírez-malo, J.B.; Villares, P.; Jiménez-Garay, R.; Swanepoel, R. Optical characterization of wedge-shaped thin films of amorphous arsenic trisulphide based only on their shrunk transmission spectra. Thin Solid Films 1995, 254, 83–91. [Google Scholar] [CrossRef]
- Tanaka, K. Optical properties and photoinduced changes in amorphous AsxS1001-x films. Thin Solid Films 1980, 66, 271–279. [Google Scholar] [CrossRef]
- Zhang, F.; Saito, K.; Tanaka, T.; Nishio, M.; Arita, M.; Guo, Q. Wide bandgap engineering of (AlGa)2O3 films. Appl. Phys. Lett. 2014, 105, 162107. [Google Scholar] [CrossRef]
- Kamimura, T.; Sasaki, K.; Hoi Wong, M.; Krishnamurthy, D.; Kuramata, A.; Masui, T.; Yamakoshi, S.; Higashiwaki, M. Band alignment and electrical properties of Al2O3/β-Ga2O3 heterojunctions. Appl. Phys. Lett. 2014, 104, 192104. [Google Scholar] [CrossRef]
- Nichols, M.T.; Li, W.; Pei, D.; Antonelli, G.A.; Lin, Q.; Banna, S.; Nishi, Y.; Shohet, J.L. Measurement of bandgap energies in low-k organosilicates. J. Appl. Phys. 2014, 115, 094105. [Google Scholar] [CrossRef]
- Mardare, D.; Hones, P. Optical dispersion analysis of TiO2 thin films based on variable-angle spectroscopic ellipsometry measurements. Mater. Sci. Eng. B 1999, 68, 42–47. [Google Scholar] [CrossRef]
- Yi, L.X.; Heitmann, J.; Scholz, R.; Zacharias, M. Si rings, Si clusters, and Si nanocrystals—Different states of ultrathin SiOx layers. Appl. Phys. Lett. 2002, 81, 4248–4250. [Google Scholar] [CrossRef]
- Yi, L.X.; Heitmann, J.; Scholz, R.; Zacharias, M. Phase separation of thin SiO layers in amorphous SiO/SiO2 superlattices during annealing. J. Phys. Condens. Matter 2003, 15, S2887–S2895. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.B.; Ren, Y.; Zhu, J.; Zhao, Y.Y.; Lu, M. A multilayered approach of Si/SiO to promote carrier transport in electroluminescence of Si nanocrystals. Nanoscale Res. Lett. 2012, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.; Muller, F.; Yi, L.X.; Zacharias, M.; Kovalev, D.; Eichhorn, F. Excitons in Si nanocrystals: Confinement and migration effects. Phys. Rev. B 2004, 69, 195309. [Google Scholar] [CrossRef]
- López-Vidrier, J.; Hernández, S.; Hiller, D.; Gutsch, S.; López-Conesa, L.; Estradé, S.; Peiró, F.; Zacharias, M.; Garrido, B. Annealing temperature and barrier thickness effect on the structural and optical properties of silicon nanocrystals/SiO2 superlattices. J. Appl. Phys. 2014, 116, 133505. [Google Scholar] [CrossRef]
- Kahler, U.; Hofmeister, H. Visible light emission from Si nanocrystalline composites via reactive evaporation of SiO. Opt. Mater. 2001, 17, 83–86. [Google Scholar] [CrossRef]
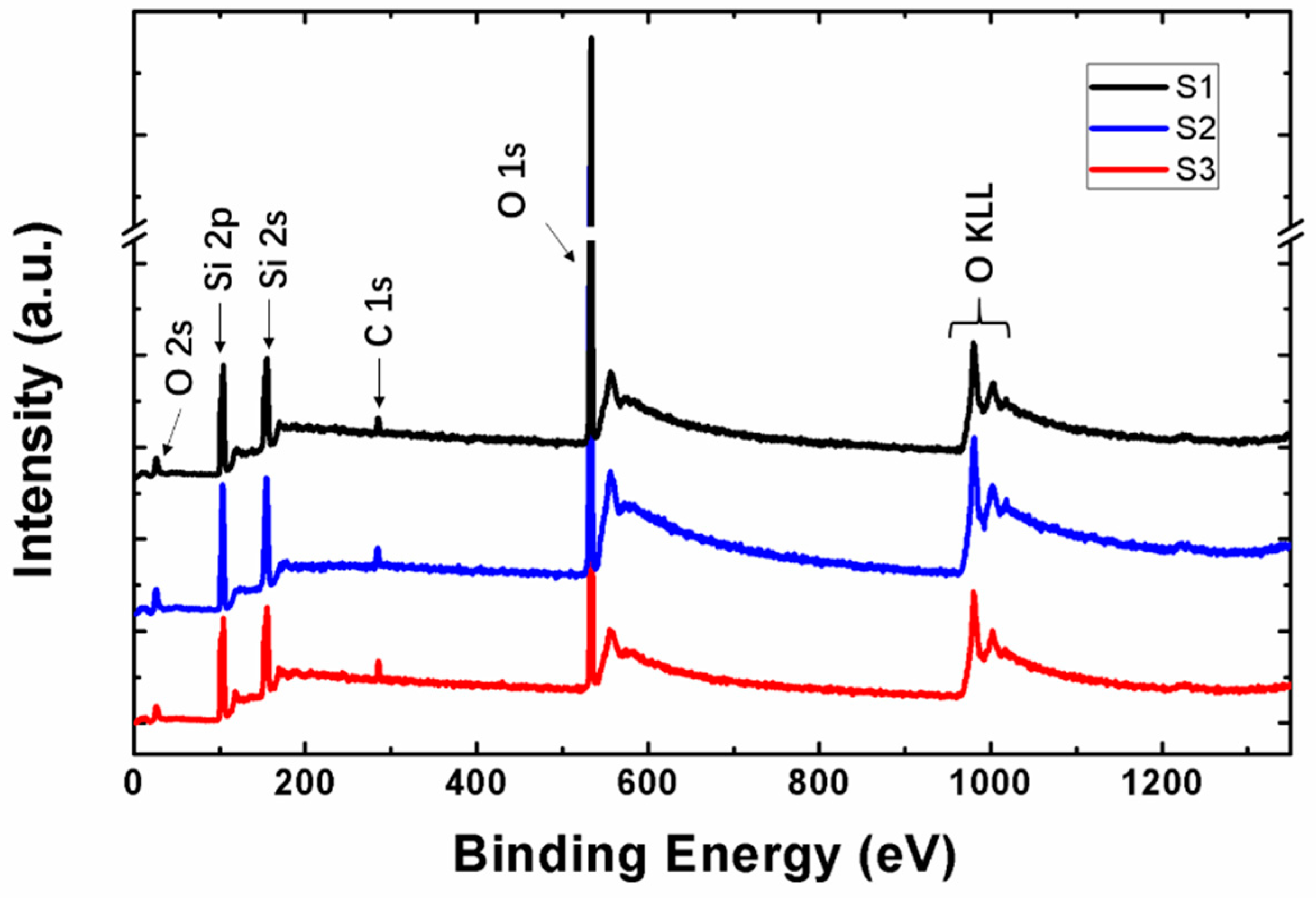

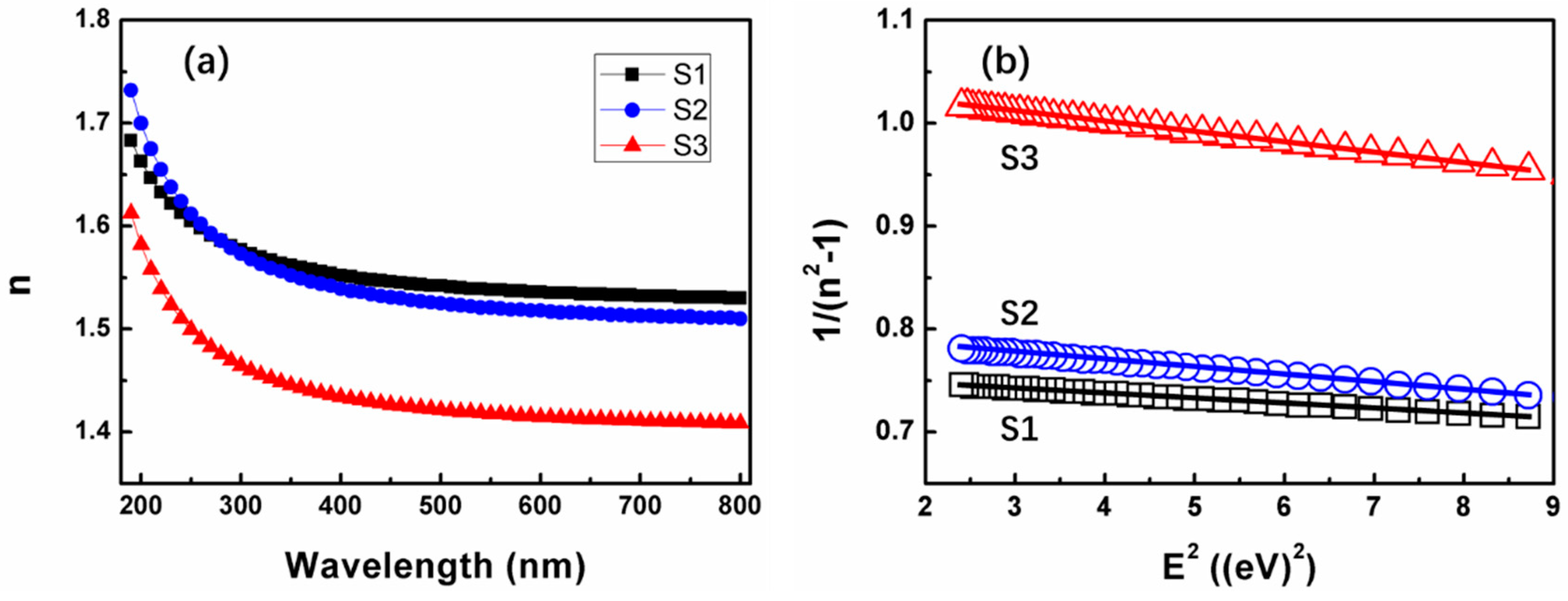
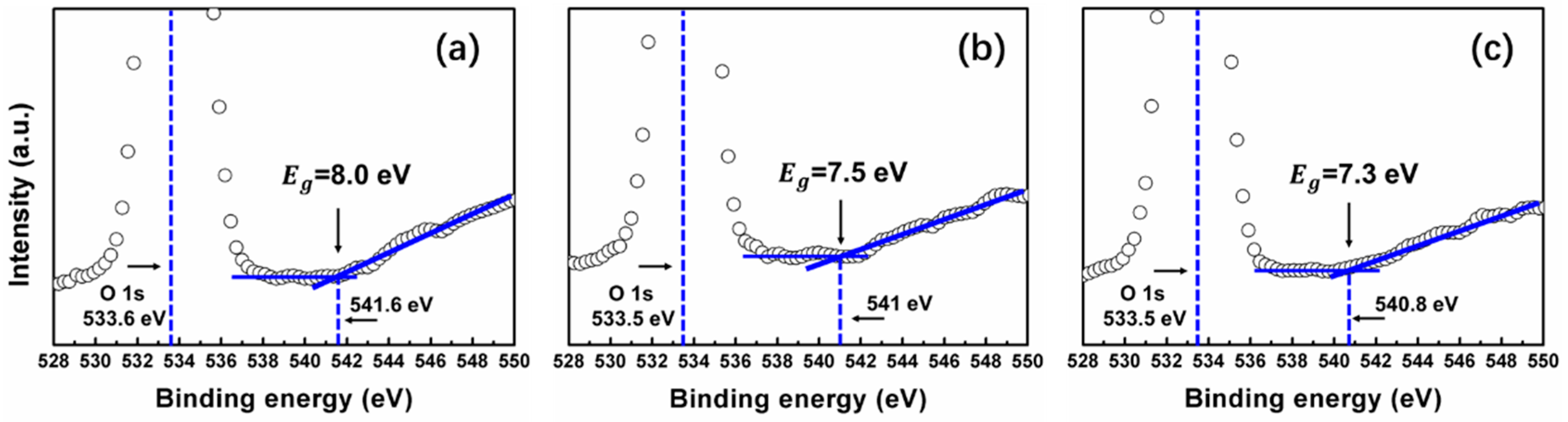
| Sample | E0/Ed | (E0Ed)−1 | E0 | Ed | Eg | Eg (by O 1s) | n(0) | n/(632.8 nm) |
|---|---|---|---|---|---|---|---|---|
| S1 (SiO2) | 0.757 | 0.0048 | 12.52 | 16.54 | 8.3 | 8.0 | 1.52 | 1.54 |
| S2 (SiO1.8) | 0.821 | 0.0074 | 10.53 | 12.83 | 7.0 | 7.5 | 1.49 | 1.51 |
| S3 (SiO1.6) | 1.062 | 0.0099 | 10.37 | 9.75 | 6.9 | 7.3 | 1.39 | 1.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.-P.; Yang, J.-H.; Yang, J.-G.; Zhu, L.-Y.; Huang, W.; Yuan, G.-J.; Feng, J.-J.; Jen, T.-C.; Lu, H.-L. Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice. Nanomaterials 2019, 9, 55. https://doi.org/10.3390/nano9010055
Ma H-P, Yang J-H, Yang J-G, Zhu L-Y, Huang W, Yuan G-J, Feng J-J, Jen T-C, Lu H-L. Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice. Nanomaterials. 2019; 9(1):55. https://doi.org/10.3390/nano9010055
Chicago/Turabian StyleMa, Hong-Ping, Jia-He Yang, Jian-Guo Yang, Li-Yuan Zhu, Wei Huang, Guang-Jie Yuan, Ji-Jun Feng, Tien-Chien Jen, and Hong-Liang Lu. 2019. "Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice" Nanomaterials 9, no. 1: 55. https://doi.org/10.3390/nano9010055
APA StyleMa, H.-P., Yang, J.-H., Yang, J.-G., Zhu, L.-Y., Huang, W., Yuan, G.-J., Feng, J.-J., Jen, T.-C., & Lu, H.-L. (2019). Systematic Study of the SiOx Film with Different Stoichiometry by Plasma-Enhanced Atomic Layer Deposition and Its Application in SiOx/SiO2 Super-Lattice. Nanomaterials, 9(1), 55. https://doi.org/10.3390/nano9010055









