Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol
Abstract
:1. Introduction
2. Results and Discussion
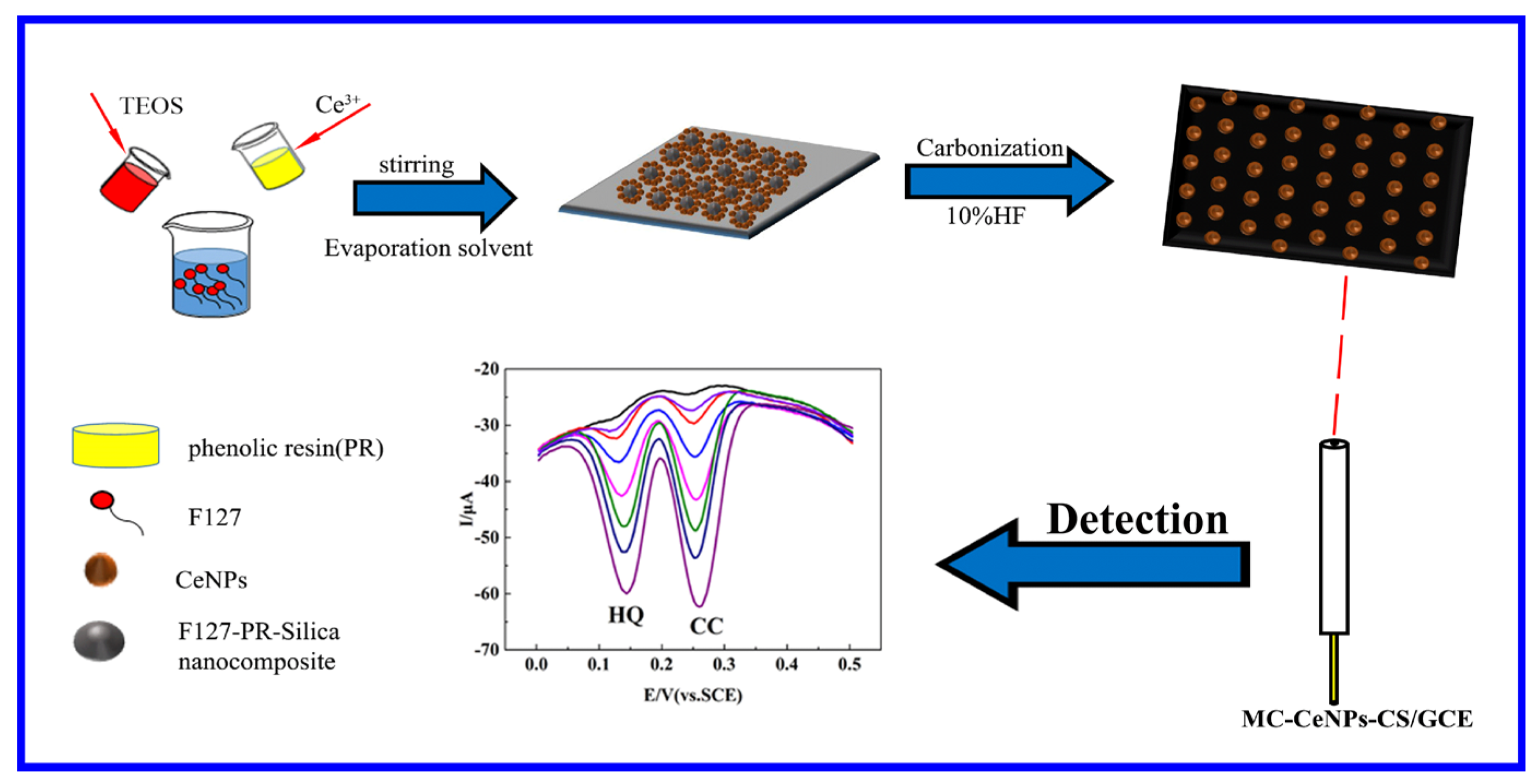
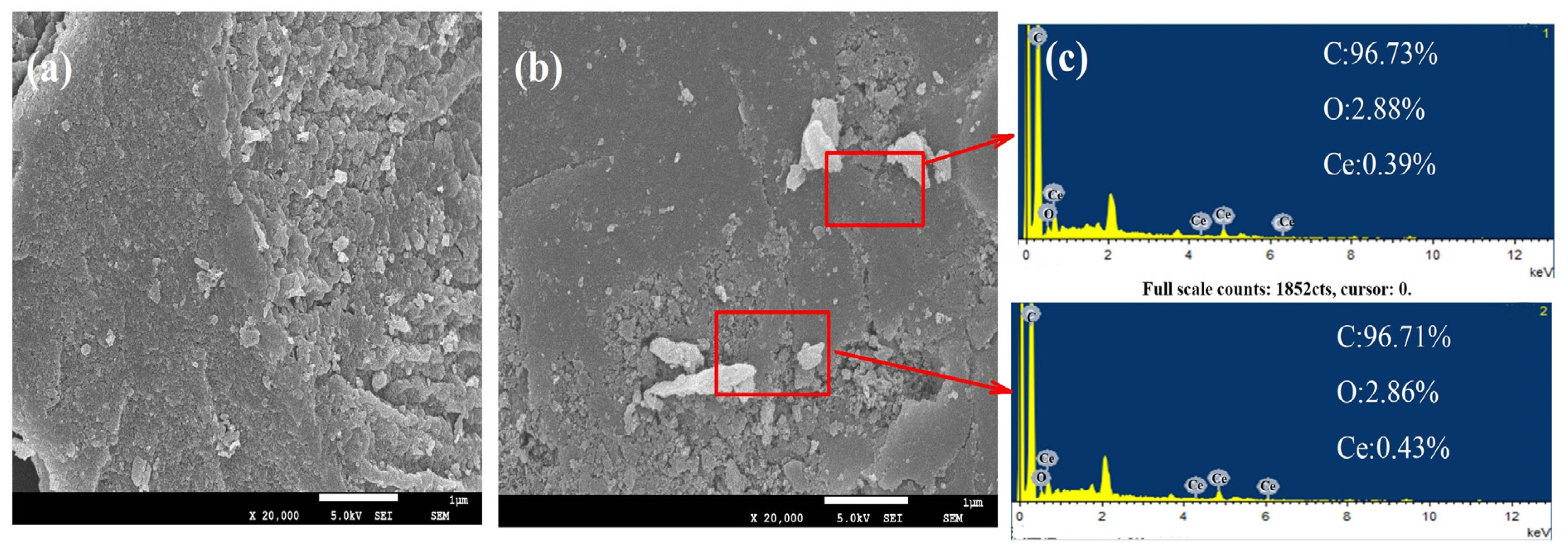
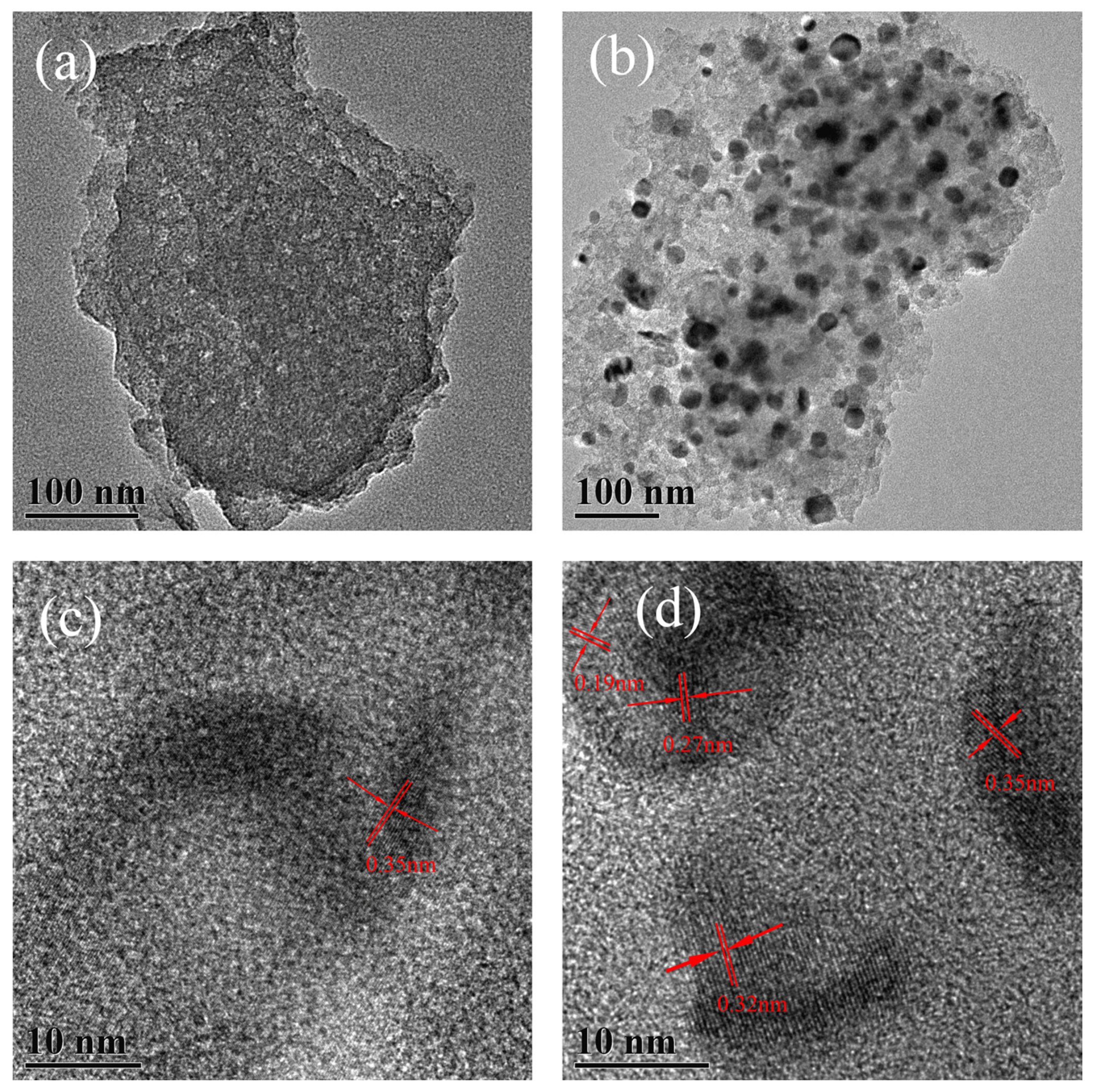
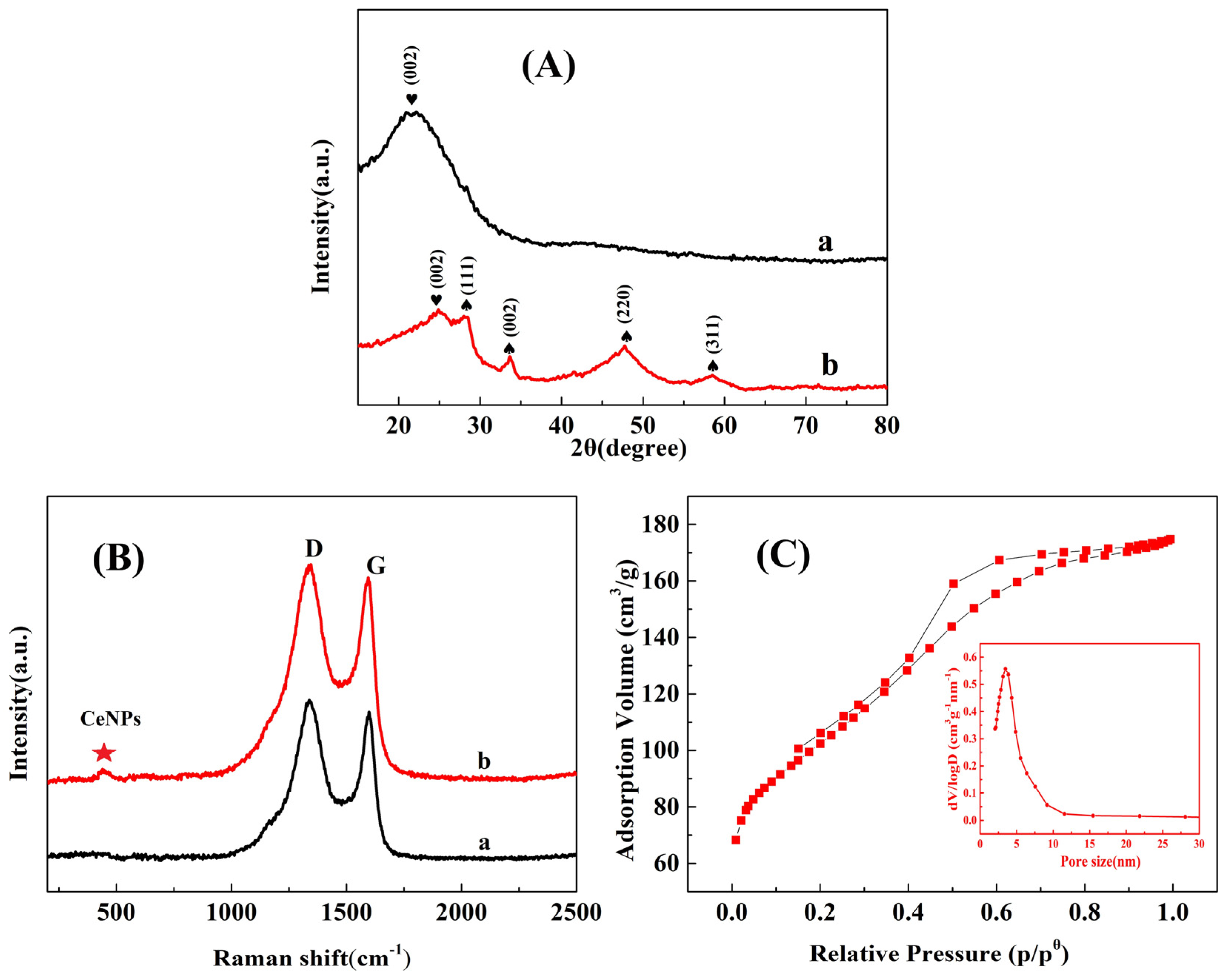
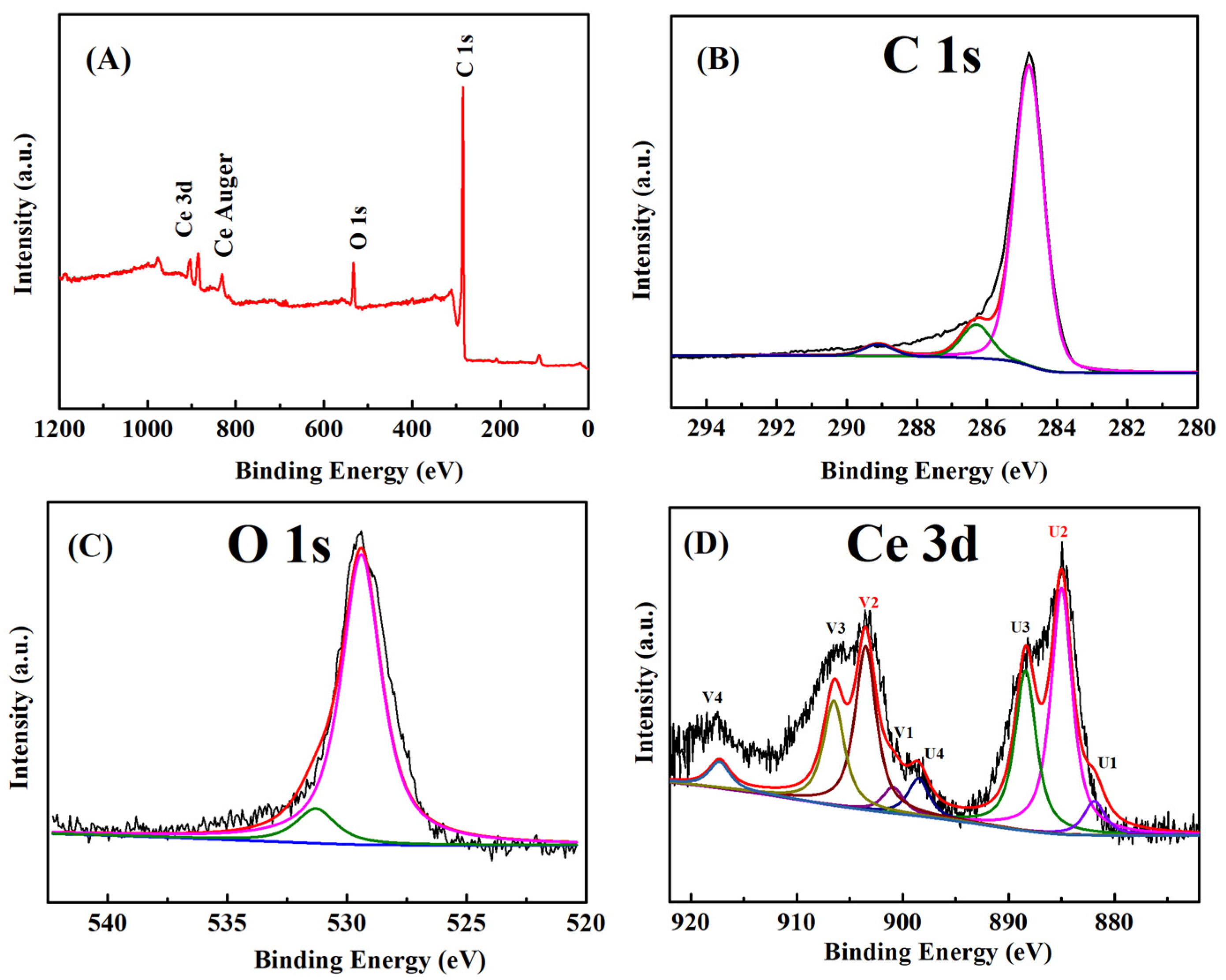
2.1. Characterization of MC–CeNPs
2.2. Electrochemical Behavior of the Modified Electrodes
2.3. Electrochemical Behaviors of HQ and CC
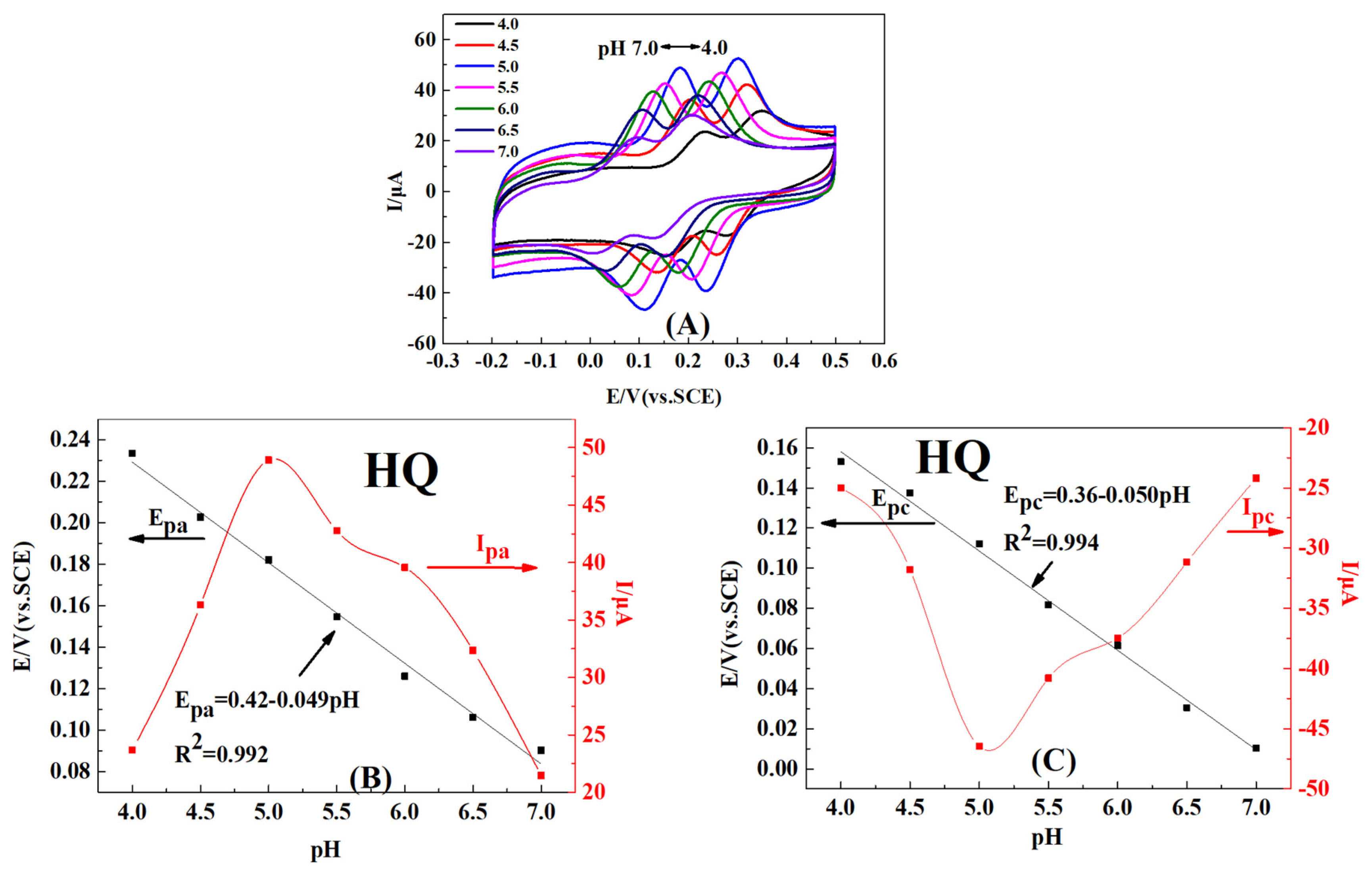
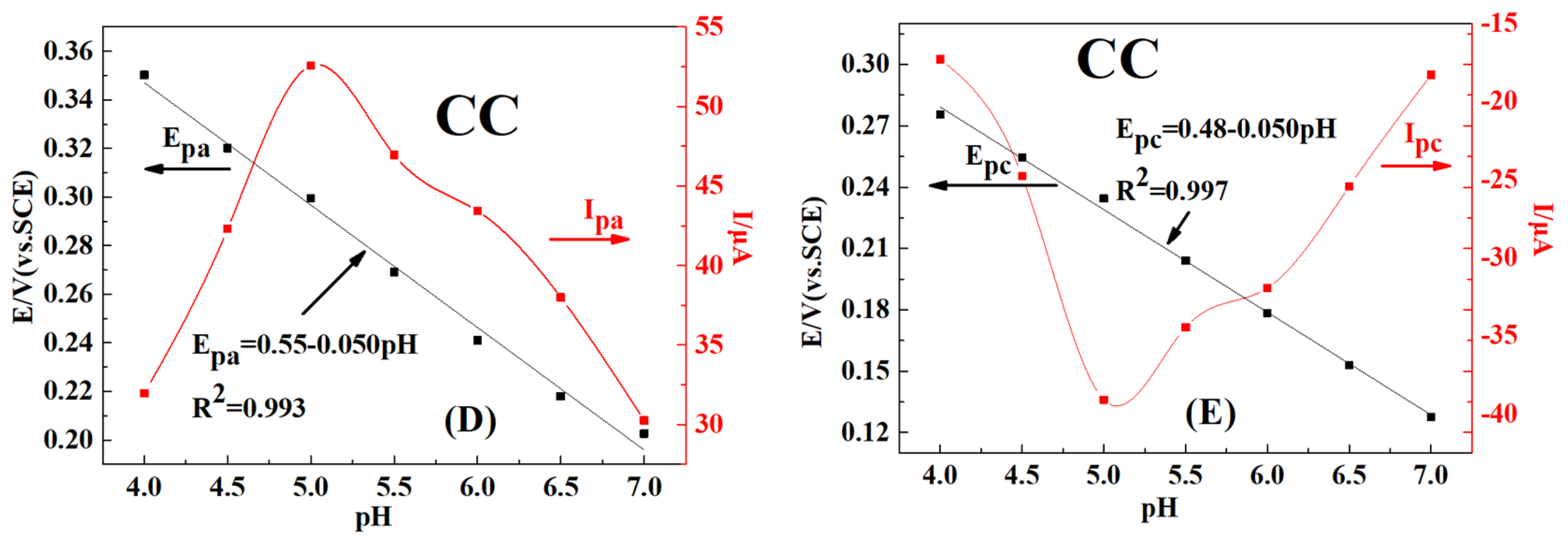
2.4. Effect of pH
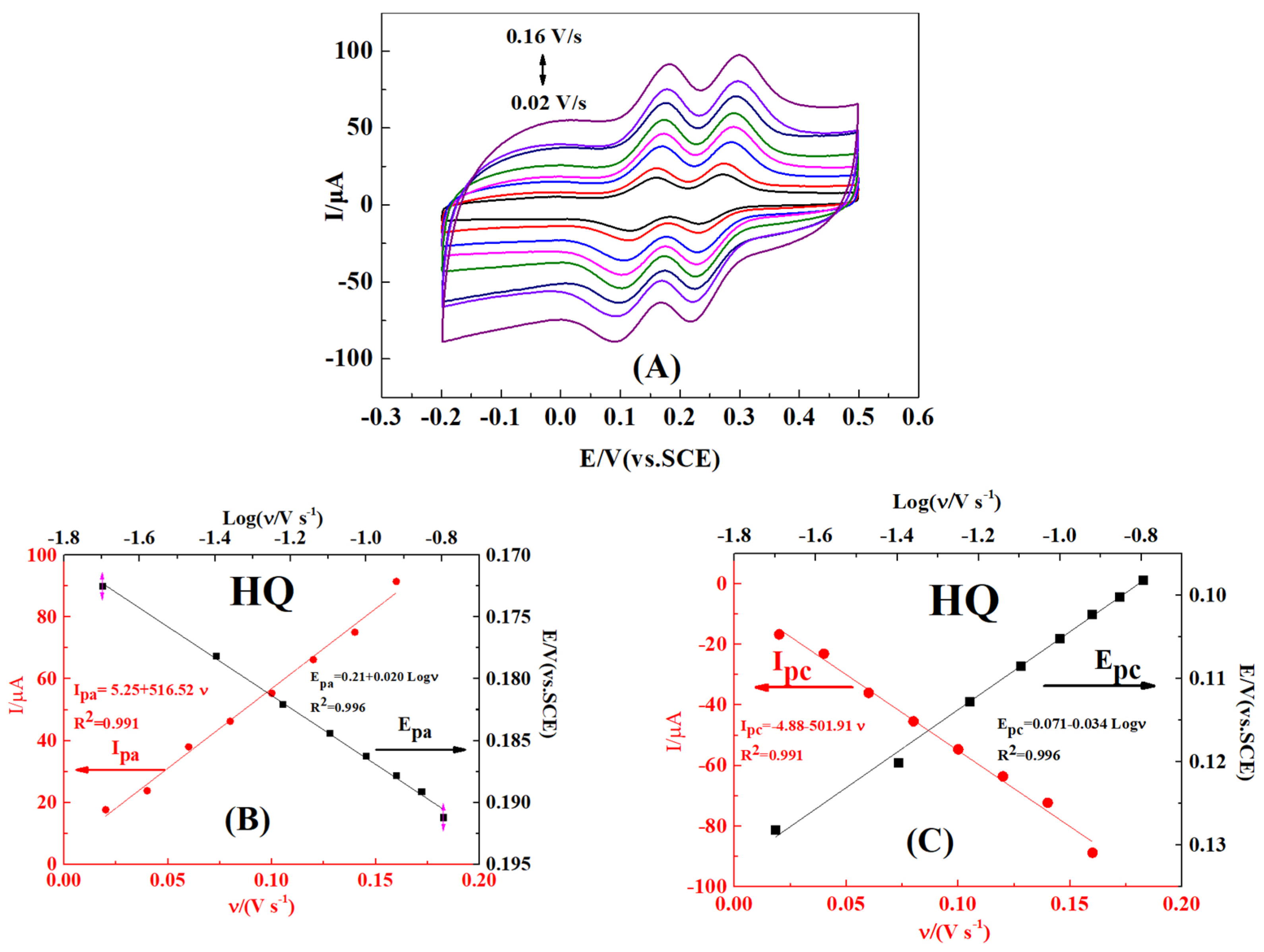
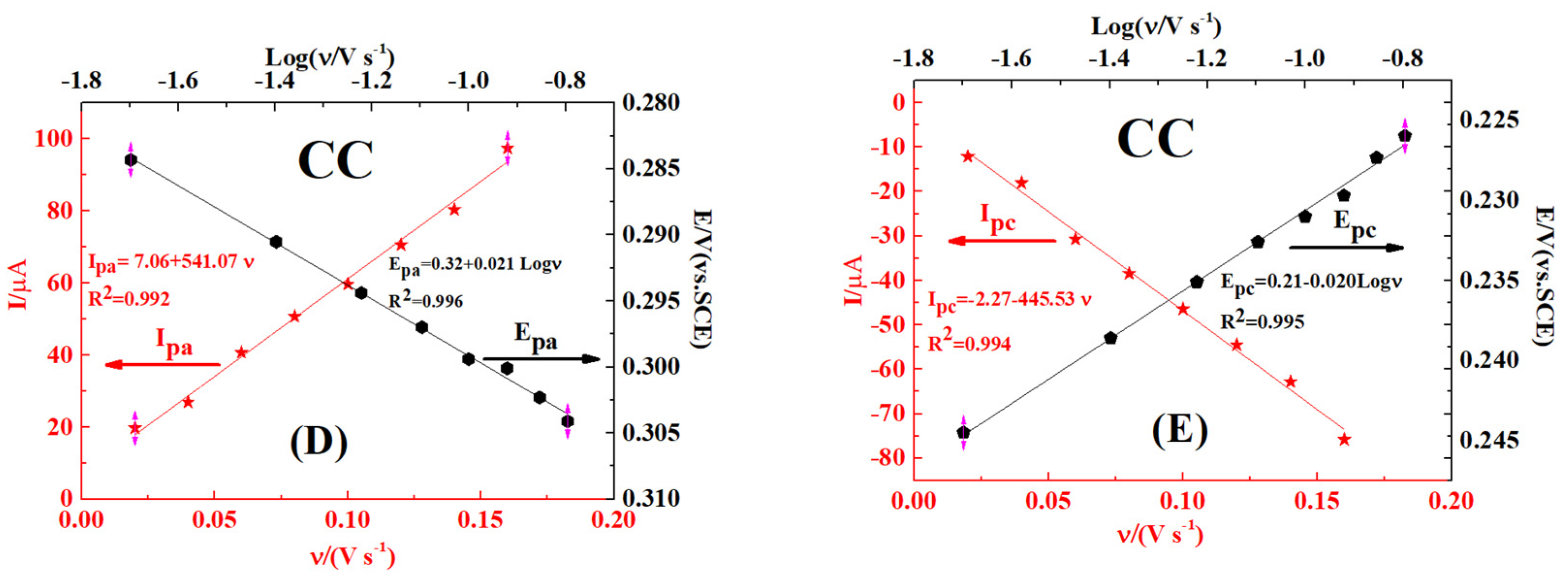
2.5. Effect of Scan Rate
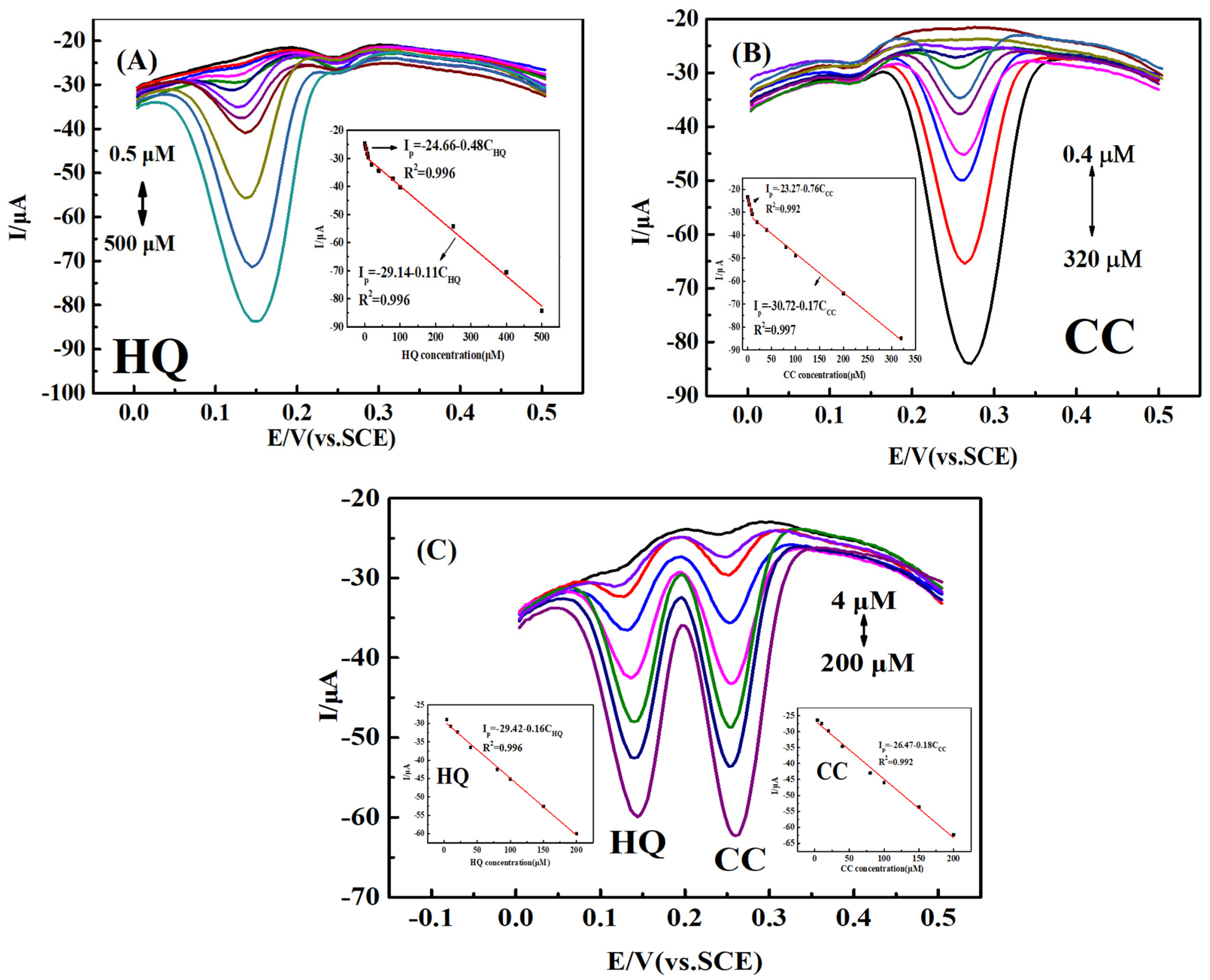
2.6. Simultaneous Determination of HQ and CC
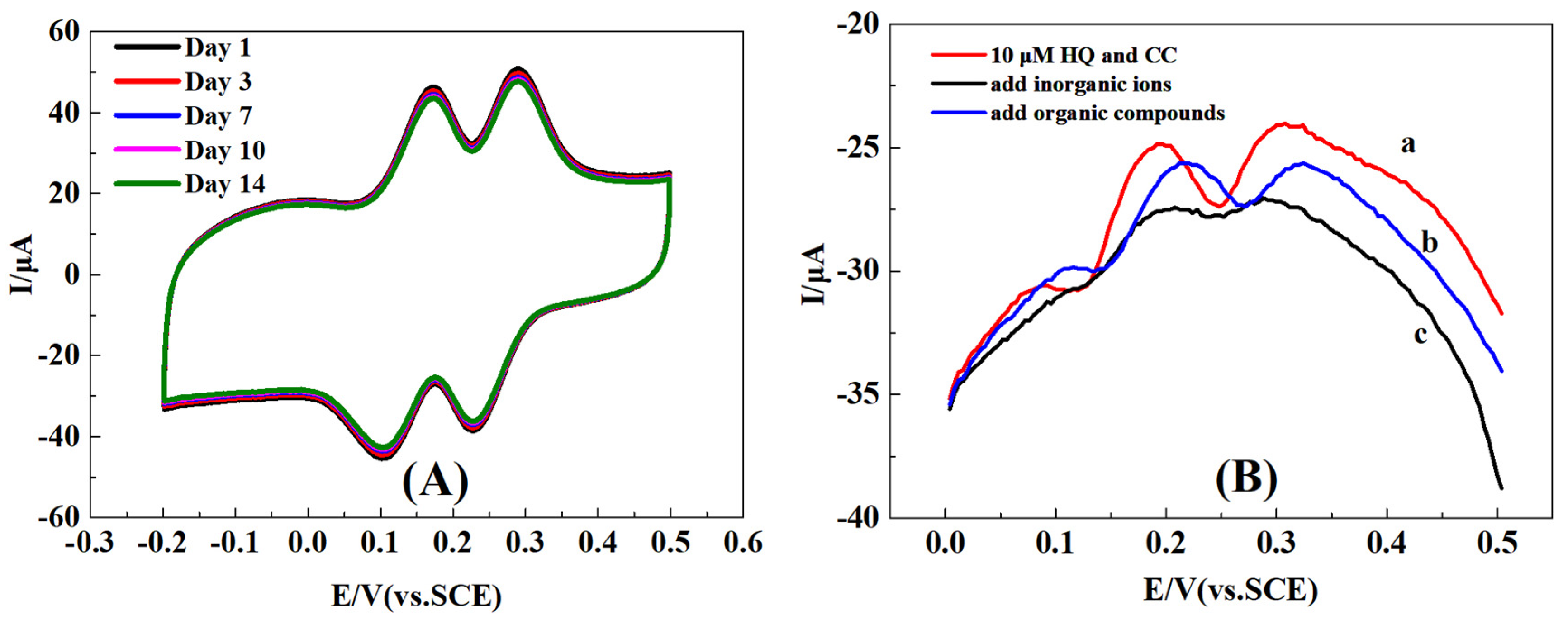
2.7. Reproducibility, Stability, and Selectively
2.8. Real Sample Analysis
3. Experimental
3.1. Materials and Reagents
3.2. Synthesis of the MC–CeNPs Nanocomposite
3.3. Preparation of Modified Electrode
3.4. Characterization
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, H.; Huang, T.; Chen, D.; Li, S.; Yu, H.; Li, Y.; Song, Q. Copper nanoparticles modified nitrogen doped reduced graphene oxide 3-D superstructure for simultaneous determination of dihydroxybenzene isomers. Sens. Actuators B Chem. 2017, 249, 405–413. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Min, X.; Cheng, D.; Zhou, J.; Li, Y.; Xie, Z.; Liu, P.; Cai, W.; Zhang, C. Determination of catechol and hydroquinone with high sensitivity using MOF–graphene composites modified electrode. J. Electroanal. Chem. 2017, 789, 114–122. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, J.; Chen, P.; Liu, Y.; Hou, H.; You, T. Simultaneous determination of catechol and hydroquinone using electrospun carbon nanofibers modified electrode. Sens. Actuators B Chem. 2012, 163, 179–185. [Google Scholar] [CrossRef]
- Pistonesi, M.F.; Di Nezio, M.S.; Centurion, M.E.; Palomeque, M.E.; Lista, A.G.; Fernandez Band, B.S. Determination of phenol, resorcinol and hydroquinone in air samples by synchronous fluorescence using partial least–squares (PLS). Talanta 2006, 69, 1265–1268. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Highly sensitive electrochemical sensor for simultaneous determination of dihydroxybenzene isomers based on Co doped SnO2 nanoparticles. RSC Adv. 2016, 6, 68211–68219. [Google Scholar] [CrossRef]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct Analysis of Phenol, Catechol and Hydroquinone in Human Urine by Coupled–Column HPLC with Fluorimetric Detection. Chromatographia 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Zhang, X.; Zhou, S.; Zhang, W.; Liu, X.; Zhao, P. Simultaneous Determination of Hydroquinone, Catechol and Resorcinol with High Selectivity Based on Hollow Nitrogen–Doped Mesoporous Carbon Spheres Decorated Graphene. J. Electrochem. Soc. 2018, 165, B212–B219. [Google Scholar] [CrossRef]
- Mao, H.; Liu, M.; Cao, Z.; Ji, C.; Sun, Y.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.-M. Poly(4-vinylphenylboronic acid) functionalized polypyrrole/graphene oxide nanosheets for simultaneous electrochemical determination of catechol and hydroquinone. Appl. Surf. Sci. 2017, 420, 594–605. [Google Scholar] [CrossRef]
- Liu, M.; Guan, Q.; Liu, S. Nitrogen-doped hollow carbon spheres for electrochemical detection of heavy metal ions. Ionics 2017, 24, 2783–2793. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Z.; Wan, Q.; Wang, Z.; Wu, K.; Yang, N. High–Performance Hydrazine Sensor Based on Graphene Nano Platelets Supported Metal Nanoparticles. Electroanal 2016, 28, 126–132. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, L.; Liu, S. Development of gold-doped carbon foams as a sensitive electrochemical sensor for simultaneous determination of Pb (II) and Cu (II). Chem. Eng. J. 2016, 284, 650–656. [Google Scholar] [CrossRef]
- Zhou, X.; He, Z.; Lian, Q.; Li, Z.; Jiang, H.; Lu, X. Simultaneous determination of dihydroxybenzene isomers based on graphene–graphene oxide nanocomposite modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 193, 198–204. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, S.; Qu, J. Simultaneous determination of hydroquinone and catechol using a glassy carbon electrode modified with Au@Pd loaded on reduced graphene oxide. Anal. Methods 2018, 10, 1331–1338. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Daneshi, M.; Nami-Ana, F.; Behbahani, M.; Bagheri, A. Simultaneous determination of hydroquinone and catechol at gold nanoparticles mesoporous silica modified carbon paste electrode. J. Hazard. Mater. 2016, 318, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, L.; Dorraji, P.S.; Keshmiri, Y.S.S.; Hamtak, M. Electrochemical Sensor Based on Nanocomposite of Multi–Walled Carbon Nanotubes/TiO2 Nanoparticles in Chitosan Matrix for Simultaneous and Separate Determination of Dihydroxybenzene Isomers. J. Electrochem. Soc. 2018, 165, B202–B211. [Google Scholar] [CrossRef]
- Alshahrani, L.A.; Liu, L.; Sathishkumar, P.; Nan, J.; Gu, F.L. Copper oxide and carbon nano-fragments modified glassy carbon electrode as selective electrochemical sensor for simultaneous determination of catechol and hydroquinone in real-life water samples. J. Electroanal. Chem. 2018, 815, 68–75. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ramezani, M.; Darroudi, M. Bio–sensing applications of cerium oxide nanoparticles: Advantages and disadvantages. Biosens. Bioelectron. 2017, 96, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Li, W.; Cao, Z.; Xu, J.; Li, X.; Zhang, X. Mesoporous Cu2O-CeO2 composite nanospheres with enhanced catalytic activity for 4-nitrophenol reduction. Appl. Surf. Sci. 2018, 430, 420–429. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, H.; Wang, Q.; Cao, H.; Xue, J. Carbon Encapsulated FeCu4 Alloy Nanoparticles Modified Glass Carbon Electrode to Promote the Degradation for P-Nitrophenol. Integr. Ferroelectr. 2013, 147, 97–102. [Google Scholar] [CrossRef]
- He, F.; Luo, J.; Liu, S. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Jin, S.; Shen, X. Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid–functionalized mesoporous carbon catalyst. Fuel 2017, 192, 102–107. [Google Scholar] [CrossRef]
- Qin, Y.-H.; Xiong, Z.-Y.; Ma, J.; Yang, L.; Wu, Z.; Feng, W.; Wang, T.-L.; Wang, W.-G.; Wang, C.-W. Enhanced electrocatalytic activity and stability of Pd nanoparticles supported on TiO2-modified nitrogen-doped carbon for ethanol oxidation in alkaline media. Int. J. Hydrogen Energy 2017, 42, 1103–1112. [Google Scholar] [CrossRef]
- Xu, J.L.; Wang, S.G.; Deng, Q.R.; Liu, Y.; Zhu, J.L.; Xu, C.B.; Wang, J.H. High–performance Pt/CNTs catalysts via hydrogen plasma for methanol electrooxidation. Nano 2014, 9, 1450018. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, D.; Liu, M.; Duan, H.; Wang, Z.; Lv, Y.; Xiong, W.; Zhu, Q.; Li, L.; Chai, X.; Gan, L. Cooking carbon with protic salt: Nitrogen and sulfur self-doped porous carbon nanosheets for supercapacitors. Chem. Eng. J. 2018, 347, 233–242. [Google Scholar] [CrossRef]
- Jiang, L.; Yao, M.; Liu, B.; Li, Q.; Liu, R.; Lv, H.; Lu, S.; Gong, C.; Zou, B.; Cui, T.; et al. Controlled Synthesis of CeO2/Graphene Nanocomposites with Highly Enhanced Optical and Catalytic Properties. J. Phys. Chem. C 2012, 116, 11741–11745. [Google Scholar] [CrossRef]
- Ansari, S.; Ansari, M.S.; Devnani, H.; Satsangee, S.P.; Jain, R. CeO2/g–C3N4 nanocomposite: A perspective for electrochemical sensing of anti–depressant drug. Sens. Actuators B Chem. 2018, 273, 1226–1236. [Google Scholar] [CrossRef]
- Üğe, A.; Koyuncu Zeybek, D.; Zeybek, B. An electrochemical sensor for sensitive detection of dopamine based on MWCNTs/CeO2 -PEDOT composite. J. Electroanal. Chem. 2018, 813, 134–142. [Google Scholar] [CrossRef]
- Xi, J.; Wang, Q.; Liu, J.; Huan, L.; He, Z.; Qiu, Y.; Zhang, J.; Tang, C.; Xiao, J.; Wang, S. N,P-dual-doped multilayer graphene as an efficient carbocatalyst for nitroarene reduction: A mechanistic study of metal-free catalysis. J. Catal. 2018, 359, 233–241. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Wan, Q.; Li, Y.; Yang, N. Expanded graphite and carbon nanotube supported palladium nanoparticles for electrocatalytic oxidation of liquid fuels. Carbon 2018, 131, 111–119. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, H.; Yuan, Q.; Shen, H.; Zhu, X.; Liu, Y.; Gan, W. N-Doped Ordered Mesoporous Carbon Originated from a Green Biological Dye for Electrochemical Sensing and High-Pressure CO2 Storage. ACS Appl. Mater. Interfaces 2016, 8, 918–926. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, S.; Ding, Y.; Yang, X.; Guo, K.; Zhao, J.T. Two-dimensional mesoporous ZnCo2O4 nanosheets as a novel electrocatalyst for detection of o-nitrophenol and p-nitrophenol. Biosens. Bioelectron. 2018, 112, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Li, H.; Li, M.; Li, C.; Wu, X.; Yang, B. Synthesis of a ZnO nanorod/CVD graphene composite for simultaneous sensing of dihydroxybenzene isomers. Carbon 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Tian, Q.; Jiang, S.; Zhang, Y.; Wu, Y. Electrocatalytic performances of phosphorus doped carbon supported Pd towards formic acid oxidation. Electrochim. Acta 2016, 213, 21–30. [Google Scholar] [CrossRef]
- Du, X.; Jiang, D.; Chen, S.; Dai, L.; Zhou, L.; Hao, N.; You, T.; Mao, H.; Wang, K. CeO2 nanocrystallines ensemble-on-nitrogen-doped graphene nanocomposites: One-pot, rapid synthesis and excellent electrocatalytic activity for enzymatic biosensing. Biosens. Bioelectron. 2015, 89, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, L.; Liu, H.; Shi, Z.; Tan, Y.; Wu, C.; Liu, Y.; Wang, J.; Zhang, S. One-step synthesis of three–dimensional interconnected porous carbon and their modified electrode for simultaneous determination of hydroquinone and catechol. Sens. Actuators B Chem. 2018, 267, 302–311. [Google Scholar] [CrossRef]
- Subbiah, D.K.; Kulandaisamy, A.J.; George, R.B.; Shankar, P.; Mani, G.K.; Jayanth Babu, K.; Rayappan, J.B.B. Nano ceria as xylene sensor—Role of cerium precursor. J. Alloys Compd. 2018, 753, 771–780. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Song, J.Y.; Lee, Y.S.; Hwang, S.K.; Kim, H.J.; Jun, Y.S.; Huh, Y.S.; Han, Y.K. Salt–templated three-dimensional porous carbon for electrochemical determination of gallic acid. Biosens. Bioelectron. 2018, 117, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, Z.; Sun, H.; Chen, F.; Xu, X.; Wu, Z.; Li, P.; Ge, W. Enhanced Photocatalytic H2 Production on Three–Dimensional Porous CeO2/Carbon Nanostructure. ACS Sustain. Chem. Eng. 2018, 6, 9691–9698. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, R.; Lv, Y.; Deng, Y.; Elzatahrya, A.A.; Zhao, D. Nitrogen–doped ordered mesoporous carbons based on cyanamide as the dopant for supercapacitor. Carbon 2015, 84, 335–346. [Google Scholar] [CrossRef]
- Ali, A.; Abdiryim, T.; Huang, X.; Jamal, R.; Simayi, R. Hollow, Spherical, Poly(3,4-ethylenedioxythiophene)-Bearing Methanethiol as a Gold Stabilizer for High-Efficiency Electrochemical Sensors. J. Electrochem. Soc. 2018, 165, B335–B343. [Google Scholar] [CrossRef]
- Kaur, B.; Srivastava, R.; Satpati, B. Highly Efficient CeO2 Decorated Nano-ZSM-5 Catalyst for Electrochemical Oxidation of Methanol. ACS Catal. 2016, 6, 2654–2663. [Google Scholar] [CrossRef]
- Meng, F.; Miao, H.; Shi, J.; Hu, Z.; Li, G.; Ding, Y. The synthesis of carbon/cerium oxide composites clusters with the assistance of the glucaminium-based surfactant and their electrochemical performance in the glucose monitoring. J. Alloys Compd. 2017, 713, 125–131. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Yang, W.; Dong, P.; Hao, A. Self-assembly of graphitic carbon nitride nanosheets-carbon nanotube composite for electrochemical simultaneous determination of catechol and hydroquinone. Electrochim. Acta 2015, 176, 28–35. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, W.; Lin, X.; Liu, B.; Zhang, J.; Ye, J.; Ye, J. Facile one-step fabrication of a novel 3D honeycomb-like bismuth nanoparticles decorated N-doped carbon nanosheet frameworks: Ultrasensitive electrochemical sensing of heavy metal ions. Electrochim. Acta 2018, 266, 94–102. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangavelu, K.; Chen, S.M.; Velusamy, V.; Chen, T.W.; Kannan, R.S. Preparation and characterization of a novel hybrid hydrogel composite of chitin stabilized graphite: Application for selective and simultaneous electrochemical detection of dihydroxybenzene isomers in water. J. Electroanal. Chem. 2017, 785, 40–47. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, L.; Luo, L.; Yuan, Q.; Qin, D.; Hu, G.; Gan, W. Nitrogen, Sulfur Dual–Doped Mesoporous Carbon Modified Glassy Carbon Electrode for Simultaneous Determination of Hydroquinone and Catechol. J. Electrochem. Soc. 2016, 163, B617–B623. [Google Scholar] [CrossRef]
- Deng, M.; Lin, S.; Bo, X.; Guo, L. Simultaneous and sensitive electrochemical detection of dihydroxybenzene isomers with UiO-66 metal-organic framework/mesoporous carbon. Talanta 2017, 174, 527–538. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef]
- Li, Z.; Yue, Y.; Hao, Y.; Feng, S.; Zhou, X. A glassy carbon electrode modified with cerium phosphate nanotubes for the simultaneous determination of hydroquinone, catechol and resorcinol. Mikrochim. Acta 2018, 185, 215. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, W.; Zheng, J.; Shi, J.; Lin, Z.; Zhong, L.; Cai, G.; Wei, C.; Zhang, H.; Hao, A. One step synthesis cadmium sulphide/reduced graphene oxide sandwiched film modified electrode for simultaneous electrochemical determination of hydroquinone, catechol and resorcinol. RSC Adv. 2015, 5, 18615–18621. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Q.; Zhou, Y.; Ma, Q.; Liu, T.; Zhu, L.; Ai, S. Electrochemical behavior of catechol, resorcinol and hydroquinone at graphene–chitosan composite film modified glassy carbon electrode and their simultaneous determination in water samples. Electrochim. Acta 2011, 56, 2748–2753. [Google Scholar] [CrossRef]
- Gao, J.; Fang, J.; Ju, X.; Zhu, W.; Lin, X.; Zhang, S.; Ma, C.; Song, H. Hierarchical Self-Assembly of Cyclodextrin and Dimethylamino-Substituted Arylene-Ethynylene on N-doped Graphene for Synergistically Enhanced Electrochemical Sensing of Dihydroxybenzene Isomers. ACS Appl. Mater. Interfaces 2017, 9, 38802–38813. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Rao, D.; Sheng, Q.; Zheng, J. Simultaneous voltammetric determination of hydroquinone and catechol by using a glassy carbon electrode modified with carboxy-functionalized carbon nanotubes in a chitosan matrix and decorated with gold nanoparticles. Microchim. Acta 2017, 184, 3591–3601. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Yang, L.; Yan, S.; Wang, M.; Cheng, D.; Chen, Q.; Dong, Y.; Liu, P.; Cai, W.; et al. The Cu-MOF-199/single-walled carbon nanotubes modified electrode for simultaneous determination of hydroquinone and catechol with extended linear ranges and lower detection limits. Anal. Chim. Acta 2015, 899, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, C.; Thirumalraj, B.; Chen, S.M.; Veerakumar, P.; Lin, K.C. Voltammetric determination of catechol and hydroquinone using nitrogen-doped multiwalled carbon nanotubes modified with nickel nanoparticles. Mikrochim. Acta 2018, 185, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, Y.; Qu, J.; Qu, J.; Li, S. Selective sensing of hydroquinone and catechol based on multiwalled carbon nanotubes/polydopamine/gold nanoparticles composites. Sens. Actuators B Chem. 2016, 223, 501–508. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Qiu, W.; Guo, H.; Gao, F. Covalent Immobilization of Cu3(btc)2 at Chitosan-Electroreduced Graphene Oxide Hybrid Film and Its Application for Simultaneous Detection of Dihydroxybenzene Isomers. J. Phys. Chem. C 2016, 120, 9794–9803. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Raja, N.; Chen, S.-M.; Sukanya, R.; Thirumalraj, B. Chitosan Stabilized Multi–Walled Carbon Nanotubes for Electrochemical Determination of Dihydroxybenzene Isomers. J. Electrochem. Soc. 2017, 164, H958–H966. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, J.; Yue, S.; Zhang, L.; Wang, Z.; Guo, P.; Liu, Q. Nickel oxide/carbon nanotube nanocomposites prepared by atomic layer deposition for electrochemical sensing of hydroquinone and catechol. J. Electroanal. Chem. 2018, 808, 245–251. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Zhou, L.; You, T. Simultaneous electrochemical determination of dihydroxybenzene isomers using electrospun nitrogen-doped carbon nanofiber film electrode. Sens. Actuators B Chem. 2016, 224, 568–576. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, J.H.; Sun, X.; Su, Z.B.; Xing, H.T.; Hu, S.R.; Weng, W.; Guo, H.X.; Wu, W.B.; He, Y.S. One-pot hydrothermal synthesis carbon nanocages-reduced graphene oxide composites for simultaneous electrochemical detection of catechol and hydroquinone. Sens. Actuators B Chem. 2015, 212, 165–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Luo, B.; Wang, L. Boron-doped graphene as high-performance electrocatalyst for the simultaneously electrochemical determination of hydroquinone and catechol. Electrochim. Acta 2015, 156, 228–234. [Google Scholar] [CrossRef]
- Zhang, H.; Bo, X.; Guo, L. Electrochemical preparation of porous graphene and its electrochemical application in the simultaneous determination of hydroquinone, catechol, and resorcinol. Sens. Actuators B Chem. 2015, 220, 919–926. [Google Scholar] [CrossRef]
- Fan, J.; Ran, X.; Ren, Y.; Wang, C.; Yang, J.; Teng, W.; Zou, L.; Sun, Y.; Lu, B.; Deng, Y.; et al. Ordered Mesoporous Carbonaceous Materials with Tunable Surface Property for Enrichment of Hexachlorobenzene. Langmuir 2016, 32, 9922–9929. [Google Scholar] [CrossRef] [PubMed]


| Electrode | Linear Range (µM) | Detection Limit (µM) | Ref. | ||
|---|---|---|---|---|---|
| HQ | CC | HQ | CC | ||
| c–MWCNTs a/CTS b/Au/GCE | 0.5–1500 | 5–900 | 0.17 | 0.89 | [53] |
| Cu–MOF199 c/SWCNTs d/GCE | 0.1–1453 | 0.1–1150 | 0.08 | 0.1 | [54] |
| Ni/N–MWCNT e | 0.1–301 | 0.1–301 | 0.01 | 0.01 | [55] |
| CS/MWCNTs/PDA f/AuNPs g/GCE | 0.1–10 | 0.1–10 | 0.035 | 0.047 | [56] |
| Cu3(btc)2 h/CS–ERGO i | 5.0–400 | 2.0–200 | 0.017 | 0.069 | [57] |
| CS/f–MWCNT j | 0.09–171.4 | 0.09–155.4 | 0.027 | 0.029 | [58] |
| NiO/CNT/GCE | 10–500 | 10–400 | 2.5 | 2.5 | [59] |
| NCNF k–GCE | 1–400 | 1–400 | 0.3 | 0.4 | [60] |
| CNCs l–RGO/GCE | 1–400 | 1–300 | 0.87 | 0.40 | [61] |
| BG m | 5–100 | 1–75 | 0.3 | 0.2 | [62] |
| P–rGO n | 5–90 | 5–120 | 0.08 | 0.18 | [63] |
| MC–CeNPs–CS/GCE | 0.5–10, | 0.4–10, | 0.24 | 0.13 | This work |
| 10–500 | 10–320 | ||||
| Sample | Added( μM) | Found( μM) | Recovery (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| HQ | CC | HQ | CC | HQ | CC | HQ | CC | |
| Tap water | 0 | 0 | 0 | 0 | – | – | – | – |
| 10 | 10 | 9.9 | 9.5 | 99.0 | 98.6 | 2.3 | 1.2 | |
| 50 | 50 | 51.6 | 51.7 | 103.2 | 103.4 | 3.6 | 2.7 | |
| 100 | 100 | 102.7 | 100.7 | 102.7 | 100.7 | 2.8 | 3.4 | |
| 0 | 0 | 0 | 0 | – | – | – | – | |
| Lake water | 10 | 10 | 10.1 | 9.8 | 101.0 | 98.0 | 1.4 | 3.6 |
| 50 | 50 | 49.8 | 50.6 | 99.6 | 101.2 | 3.1 | 2.9 | |
| 100 | 100 | 98.5 | 99.2 | 98.5 | 99.2 | 1.9 | 2.6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, F.; Yu, D.; Yu, J.; Ding, Y. Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol. Nanomaterials 2019, 9, 54. https://doi.org/10.3390/nano9010054
Liu D, Li F, Yu D, Yu J, Ding Y. Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol. Nanomaterials. 2019; 9(1):54. https://doi.org/10.3390/nano9010054
Chicago/Turabian StyleLiu, Dong, Fan Li, Dezhong Yu, Junxia Yu, and Yigang Ding. 2019. "Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol" Nanomaterials 9, no. 1: 54. https://doi.org/10.3390/nano9010054
APA StyleLiu, D., Li, F., Yu, D., Yu, J., & Ding, Y. (2019). Mesoporous Carbon and Ceria Nanoparticles Composite Modified Electrode for the Simultaneous Determination of Hydroquinone and Catechol. Nanomaterials, 9(1), 54. https://doi.org/10.3390/nano9010054




