Green Synthesis of AgNPs from Celtis africana: Biological and Catalytic Insights
Abstract
1. Introduction
2. Materials and Methods
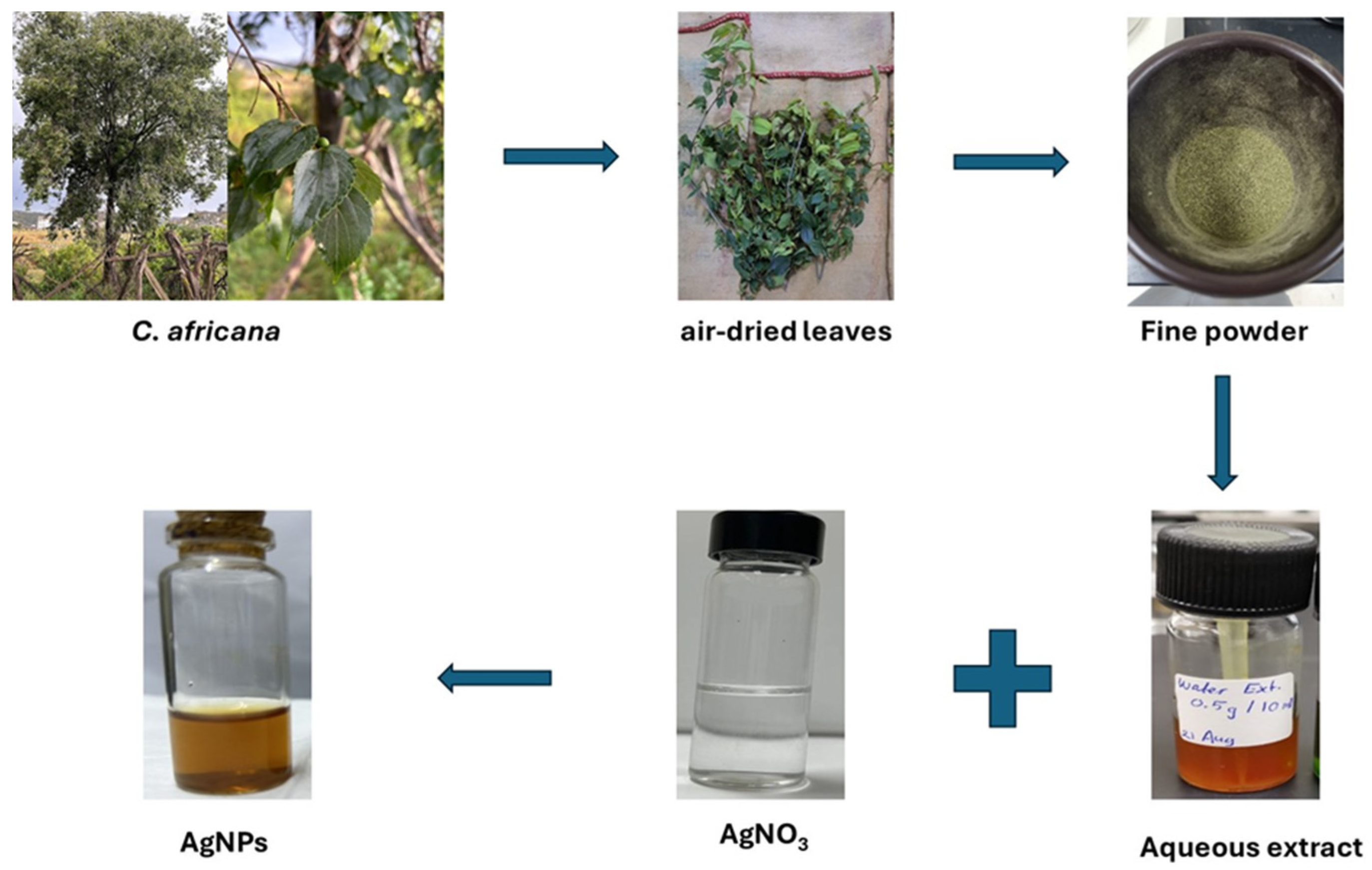
2.1. Collection and Identification of Celtis africana Leaves
2.2. Preparation of Aqueous Celtis africana Leaf Extract
2.3. Synthesis of AgNPs
2.4. Structural and Morphological Characterization
2.5. Antimicrobial Activity
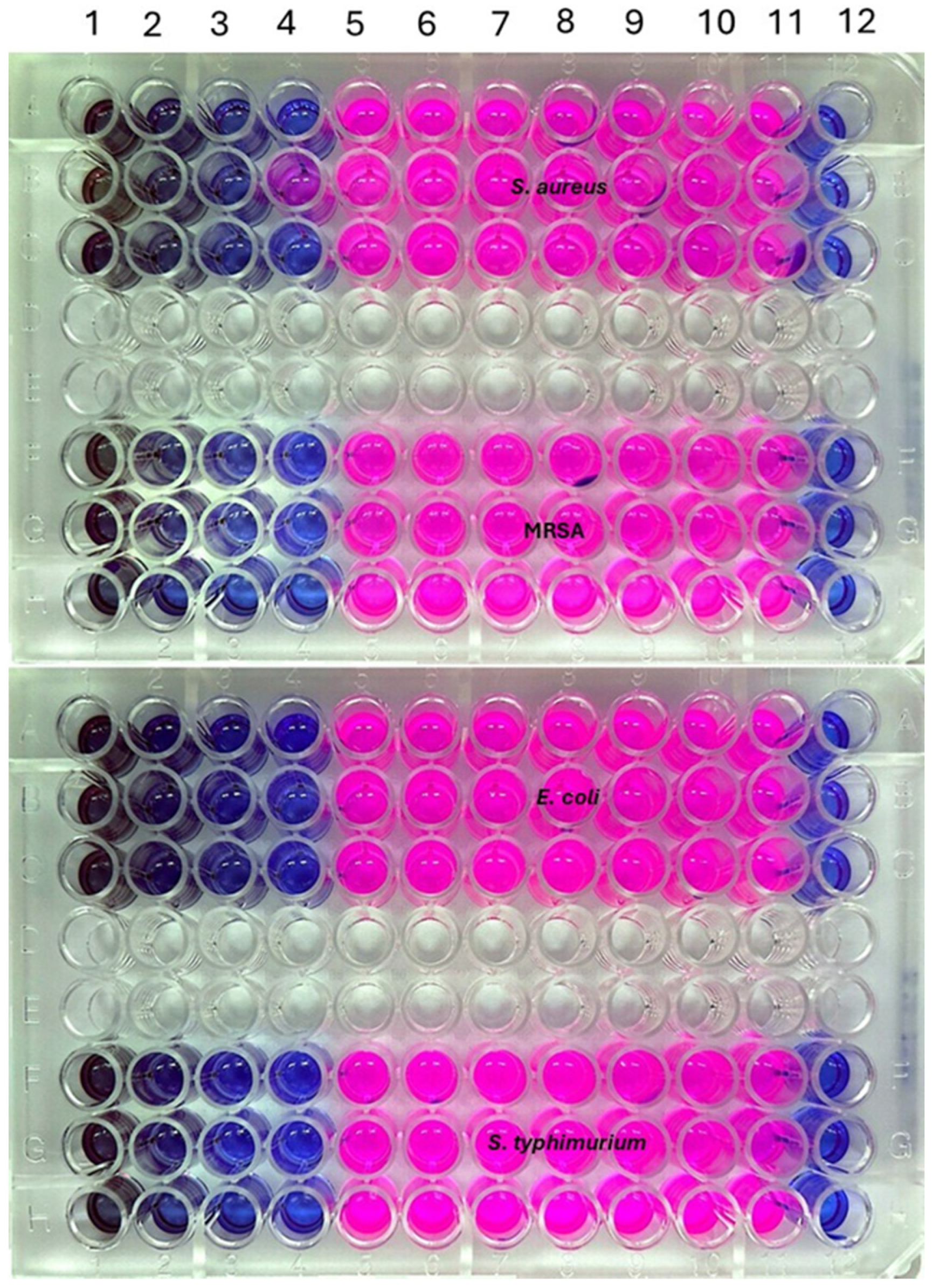
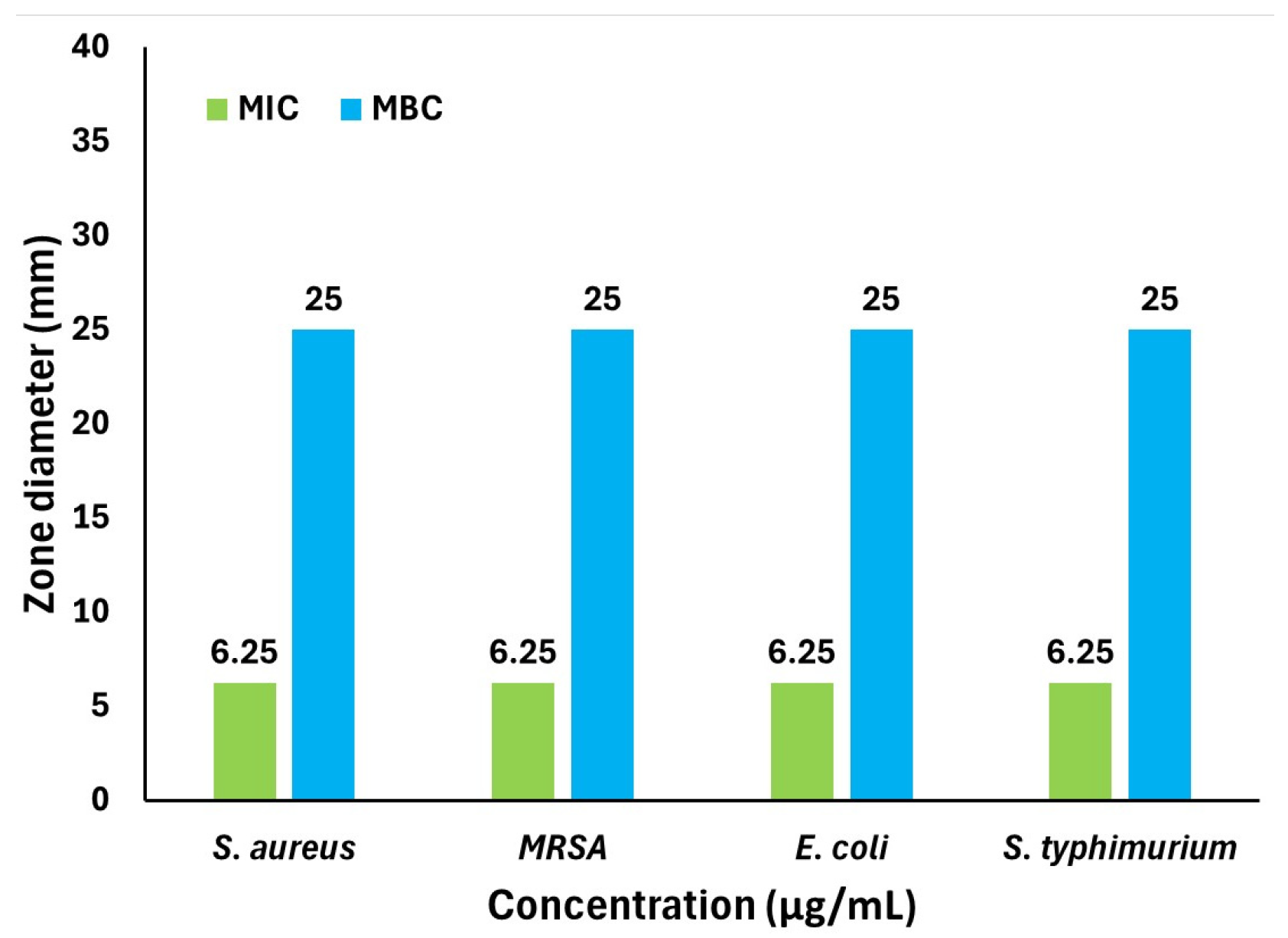
2.6. MIC and MBC
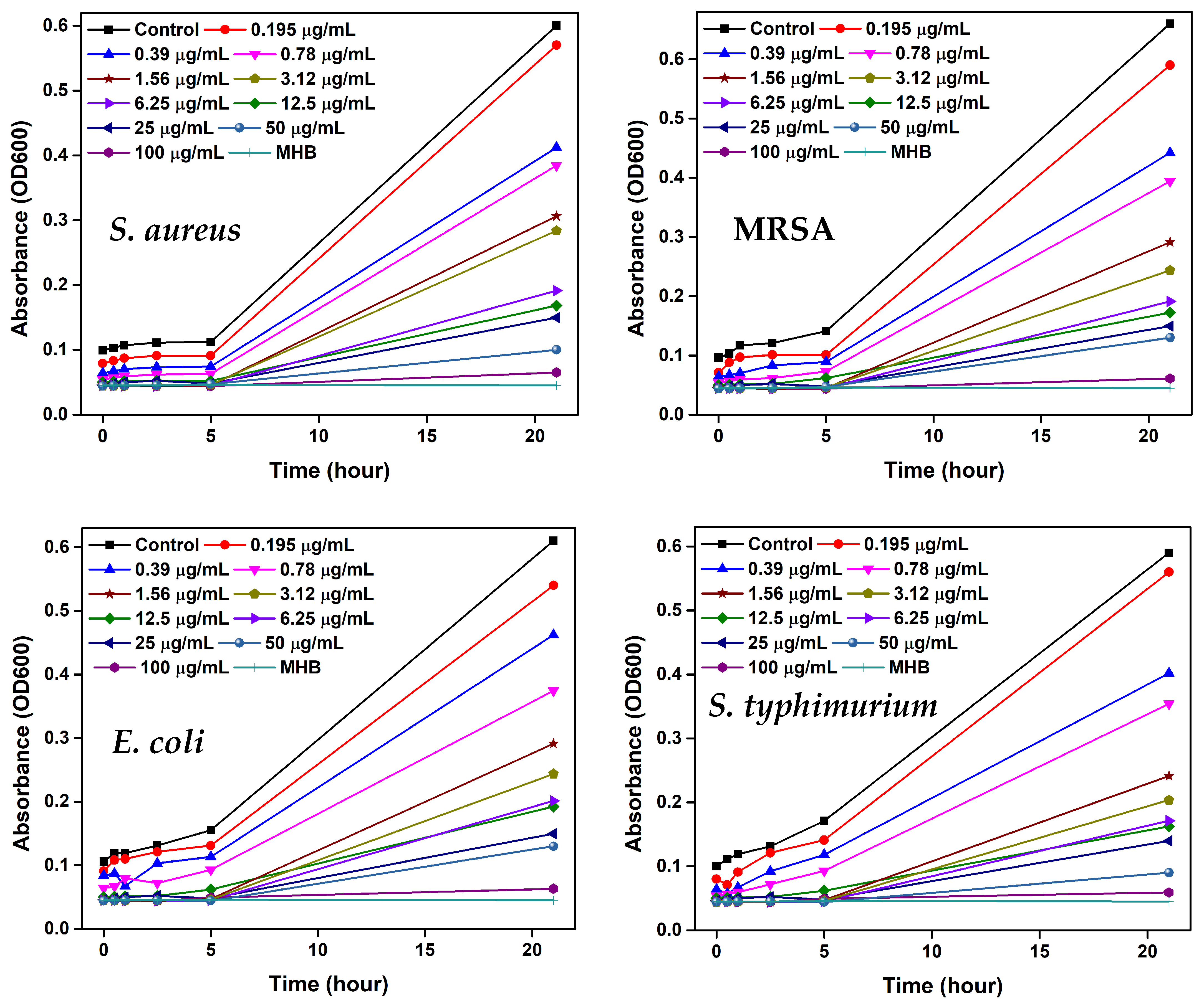
2.7. Bacterial Growth Behavior
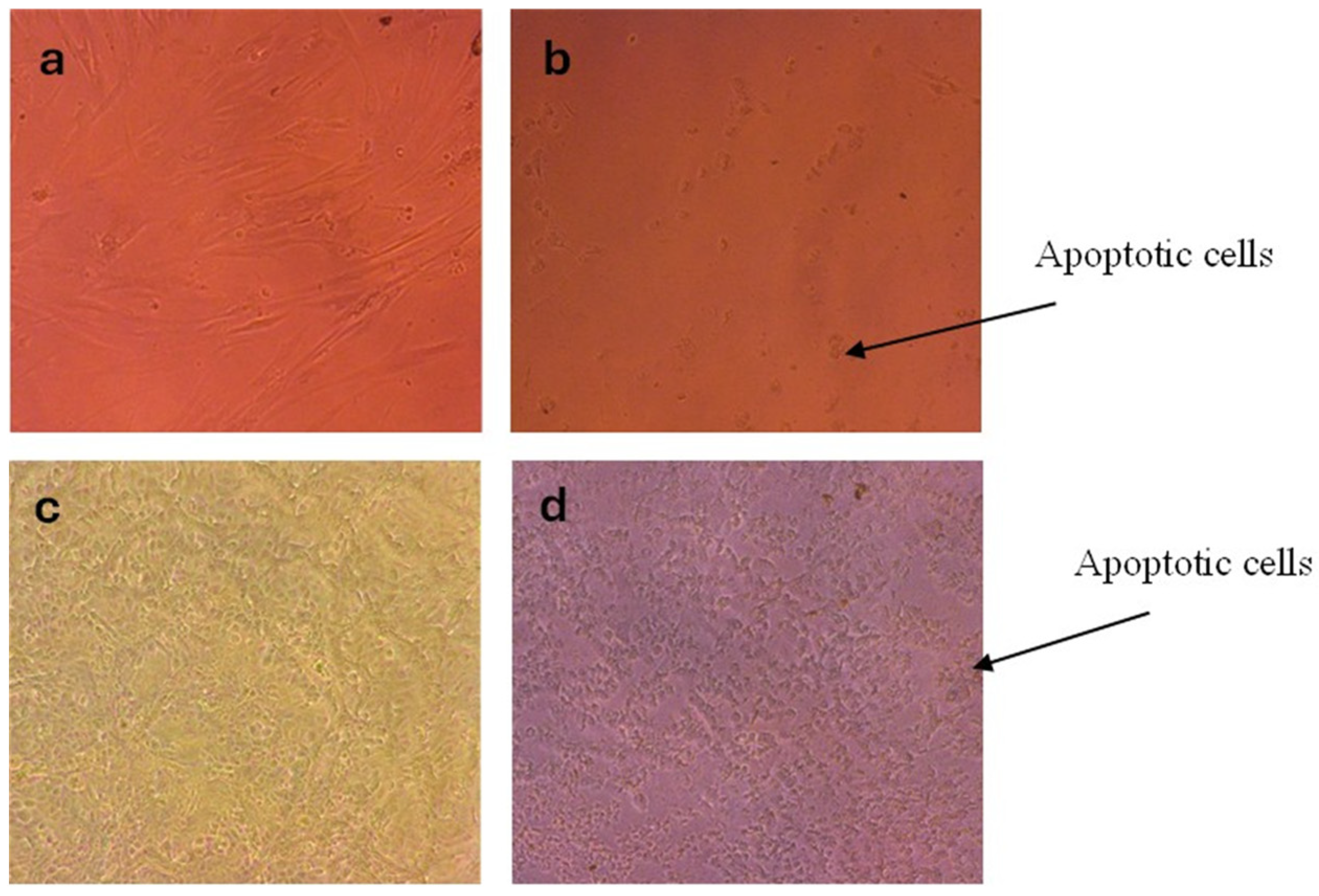
2.8. Cytotoxicity Assay
2.9. Antioxidant Efficacy
2.10. Catalytic Activity
2.11. Statistical Analysis
3. Results and Discussion
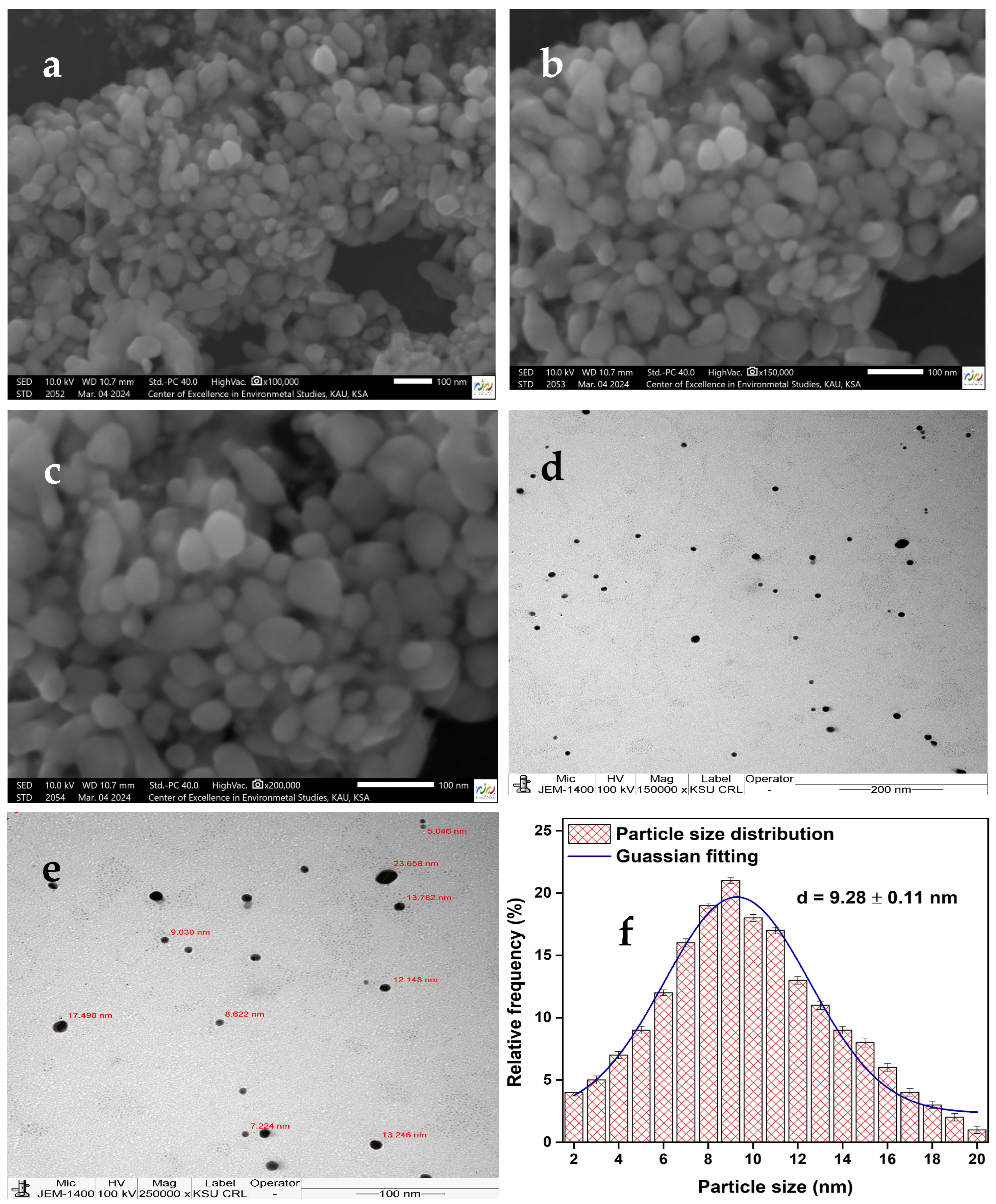
3.1. SEM Analysis
3.2. TEM Particle Size Distribution
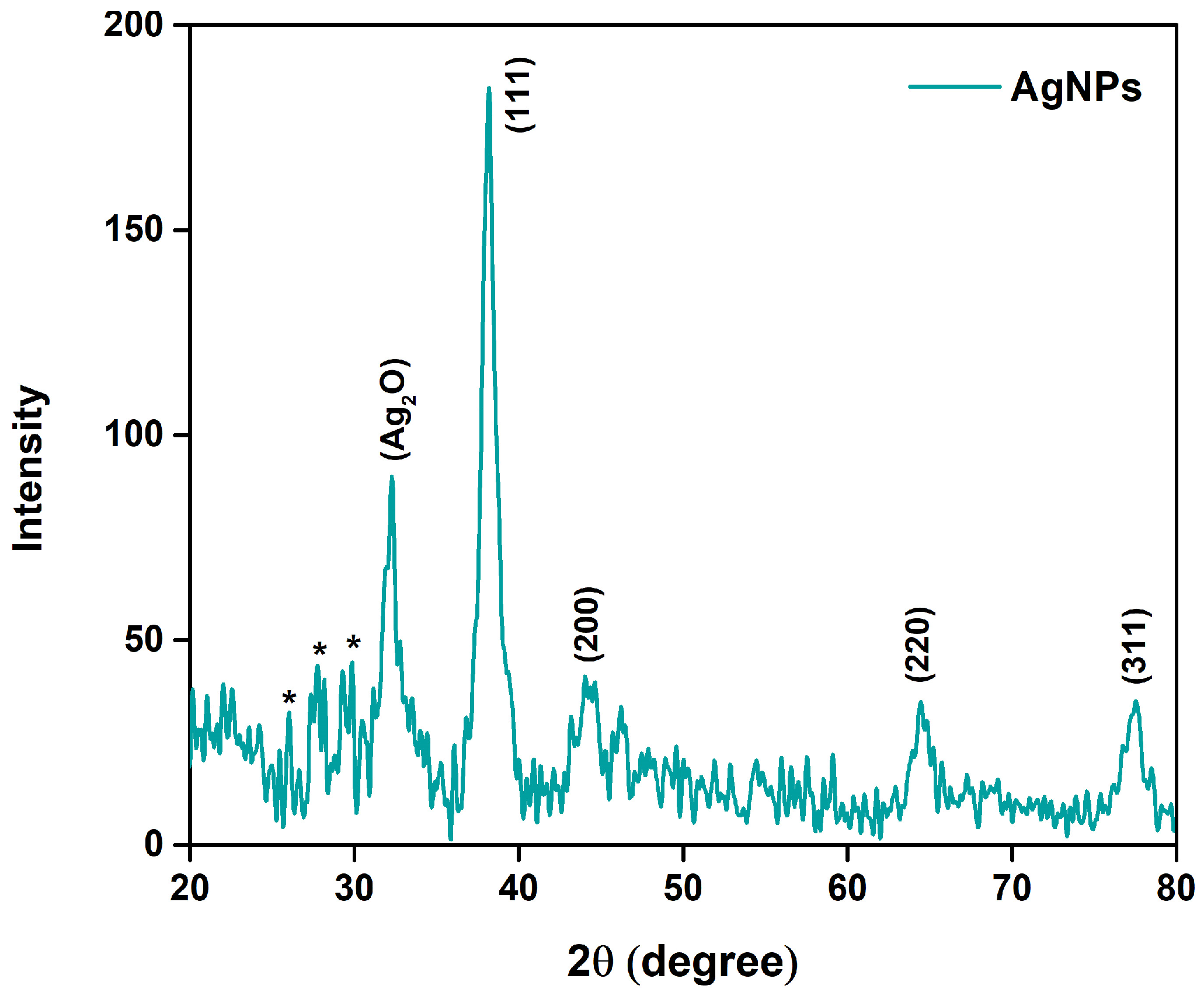
3.3. XRD Analysis
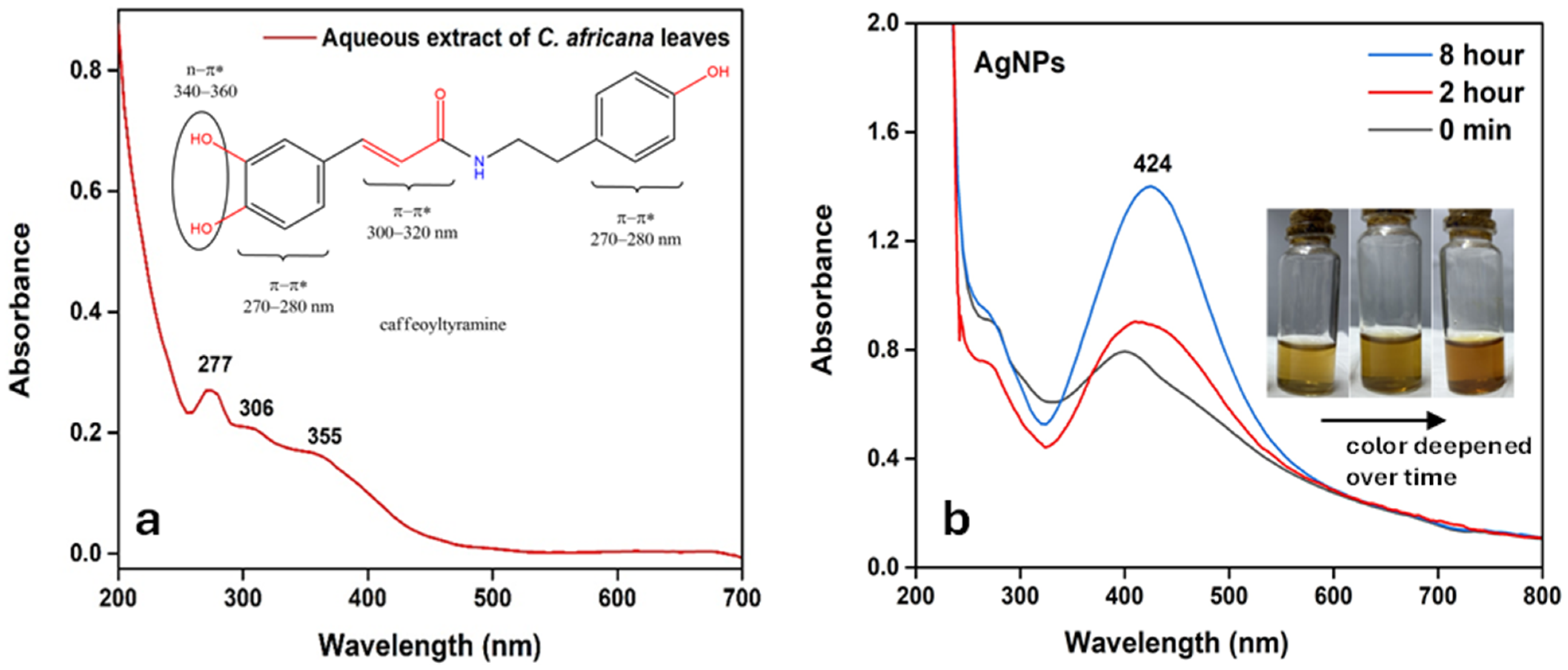
3.4. UV–Visible Spectroscopy of Plant Extract and AgNPs
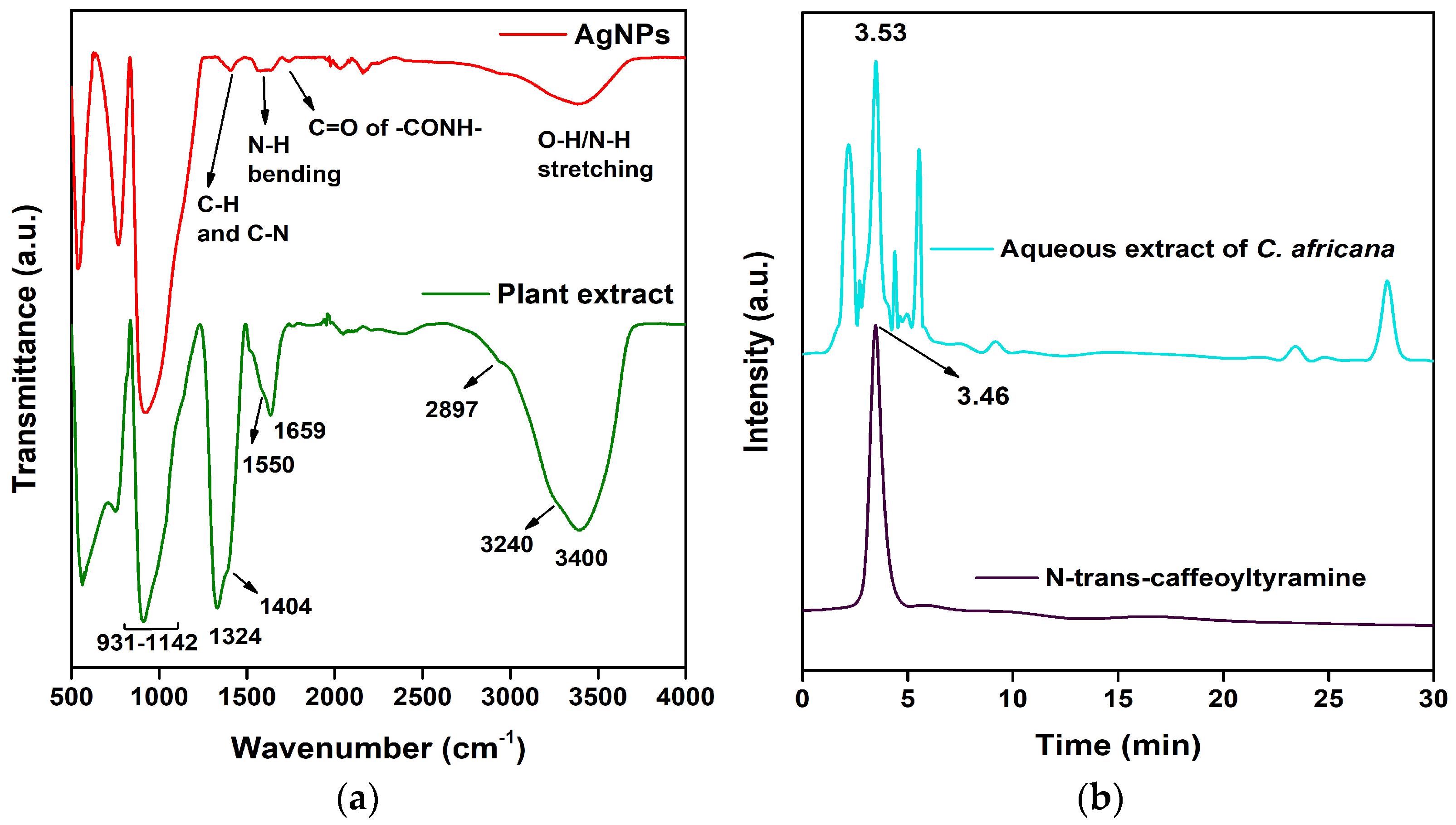
3.5. FTIR Spectral Analysis
3.6. HPLC Analysis of Celtis africana Leaf Extract
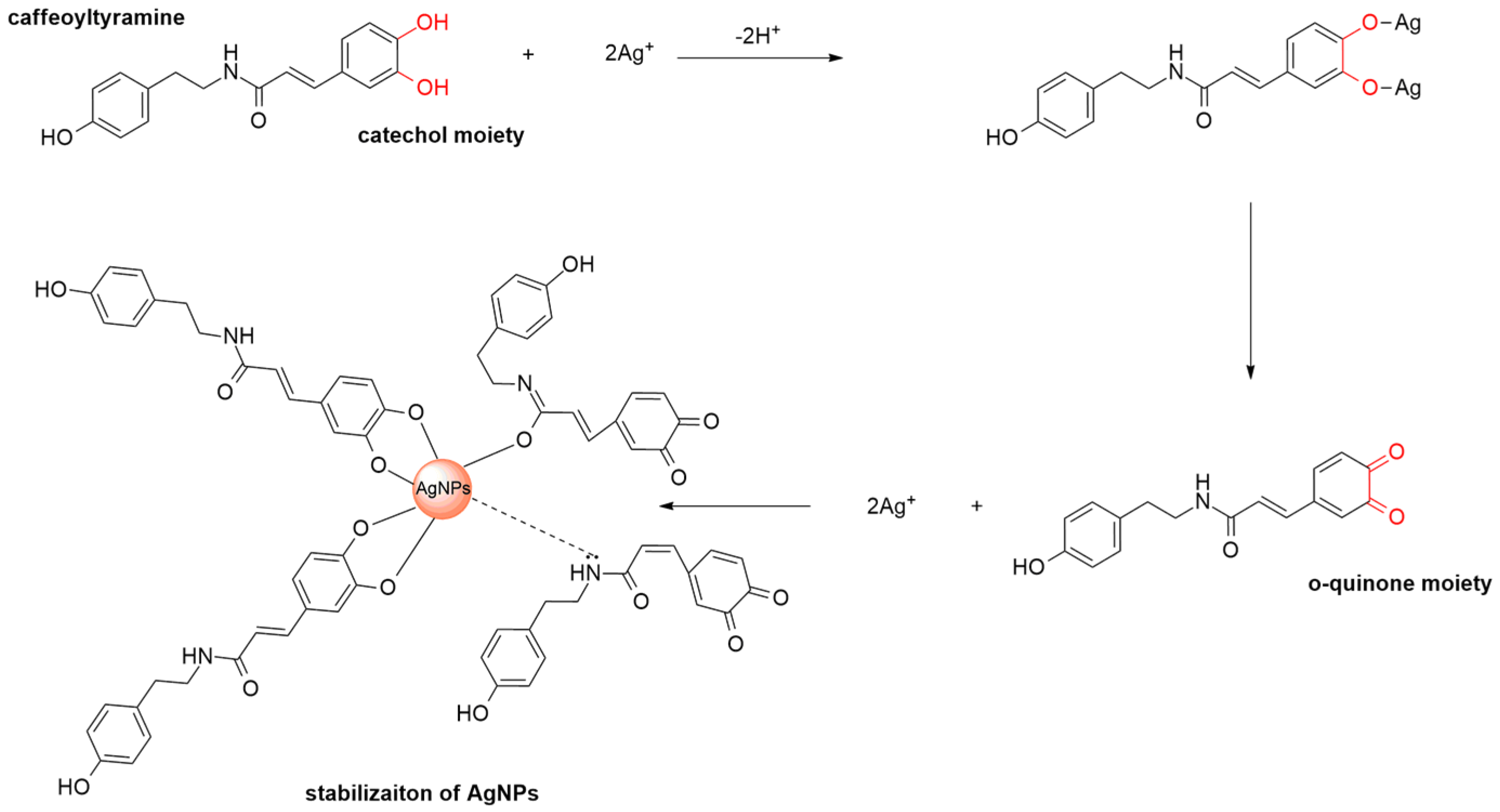
3.7. Synthesis Mechanism of AgNPs
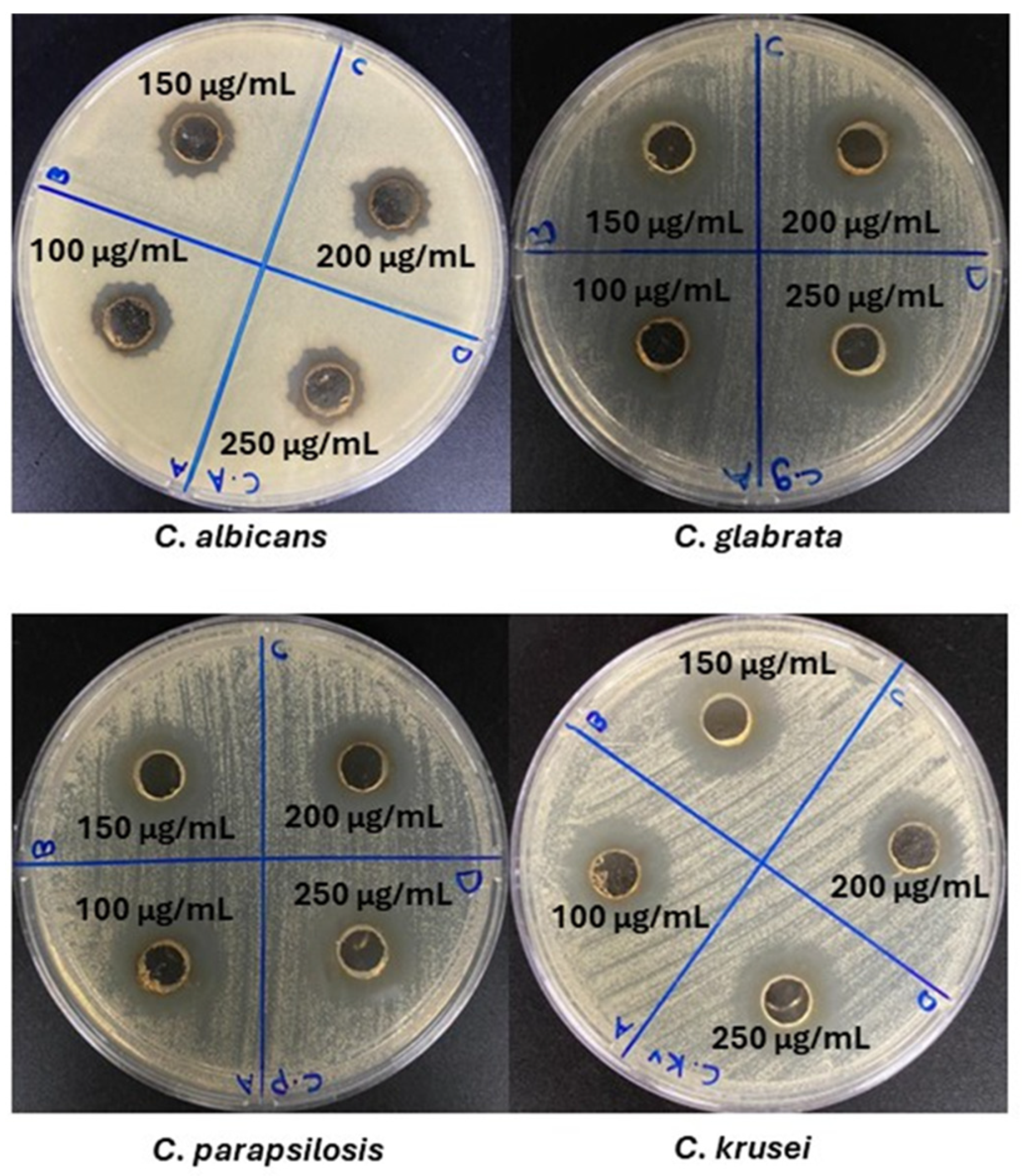
3.8. Antimicrobial Activity Evaluation
3.9. Determination of MIC and MBC of AgNPs
3.10. Bacterial Growth Kinetics
3.11. Proposed Antimicrobial Mechanism of AgNPs
3.12. Cytotoxic Potential of AgNPs from Celtis africana
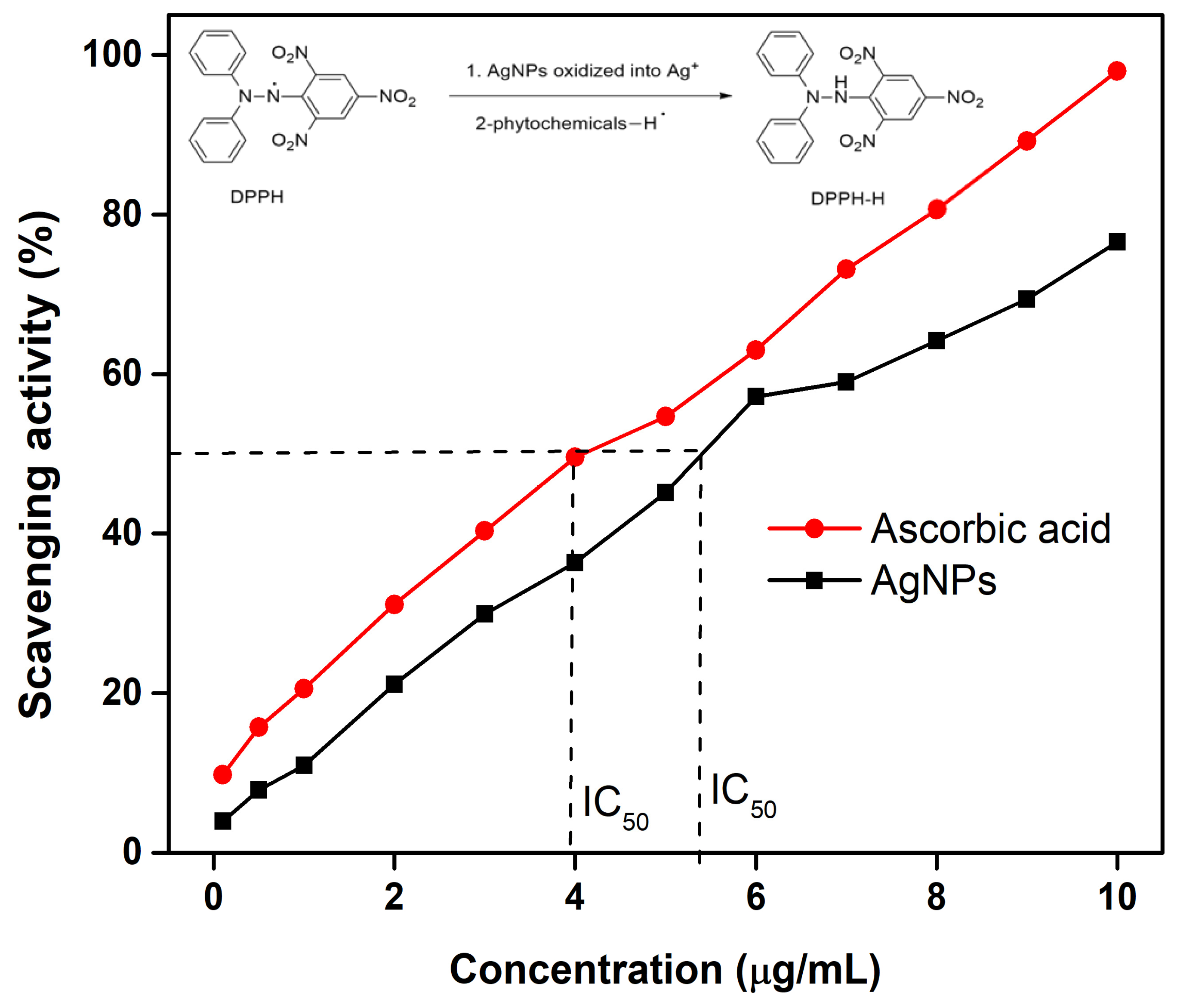
3.13. Antioxidant Activity
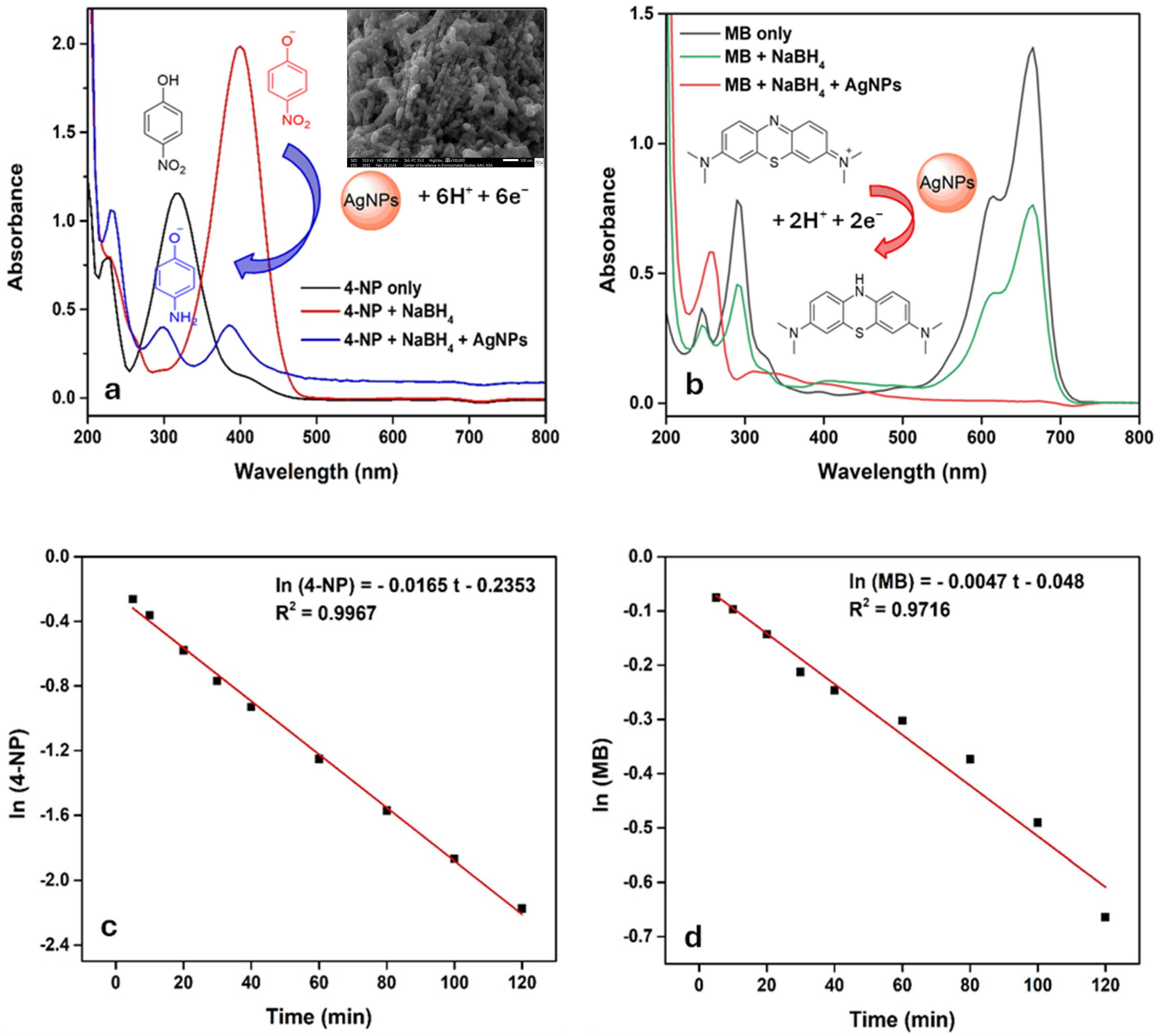
3.14. Catalytic Activity of AgNPs
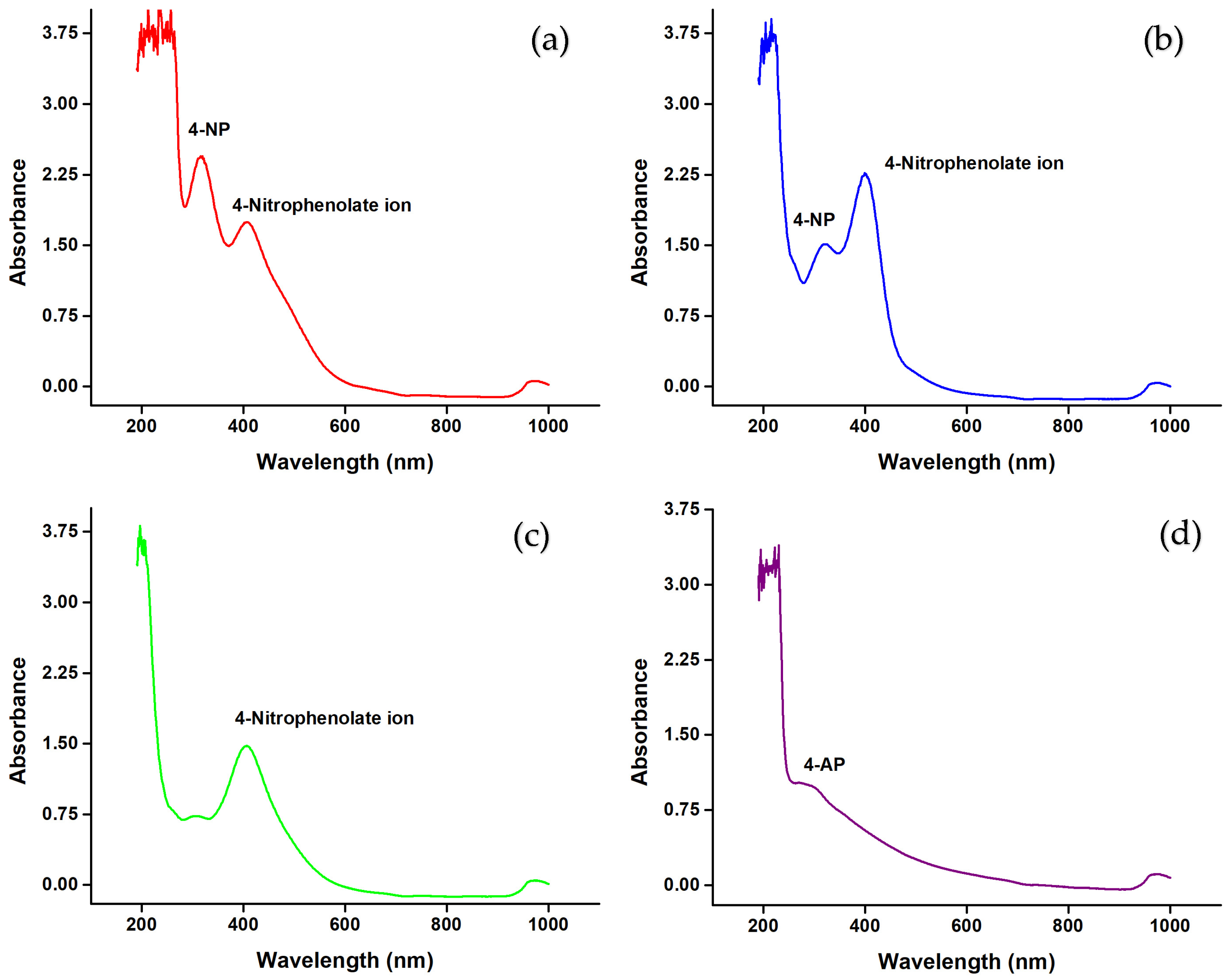
3.15. Catalytic Activity of AgNPs in Industrial Wastewater
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaifi, M.M.; Al-Khulaidi, A.W.; Alaklabi, A.; Al-Gifri, A.N.; Al-Faify, E.A. New record of vascular plant for the flora of Saudi Arabia: Celtis toka (Forssk.) Hepper and Wood, Cannabaceae. Int. J. Curr. Res. Biosci. Plant Biol. 2021, 8, 1. [Google Scholar] [CrossRef]
- Laryea, M.K.; Borquaye, L.S. Antimalarial, antioxidant, and toxicological evaluation of extracts of Celtis africana, Grosseria vignei, Physalis micrantha, and Stachytarpheta angustifolia. Biochem. Res. Int. 2021, 2021, 9971857. [Google Scholar] [CrossRef]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Bioactive phenolic amides from Celtis africana. Molecules 2012, 17, 2675. [Google Scholar] [CrossRef]
- Aati, H.Y.; Perveen, S.; Orfali, R.; Al-Taweel, A.M.; Aati, S.; Wanner, J.; Khan, A.; Mehmood, R. Chemical composition and antimicrobial activity of the essential oils of Artemisia absinthium, Artemisia scoparia, and Artemisia sieberi grown in Saudi Arabia. Arab. J. Chem. 2020, 13, 8209. [Google Scholar] [CrossRef]
- Abada, E.; Mashriaq, A.; Modafer, Y.; Al-Abboud, M.A.; Ell-Shabasy, A. Review green synthesis of silver nanoparticles by using plant extracts and their antimicrobial activity. Saudi J. Biol. Sci. 2024, 31, 103877. [Google Scholar] [CrossRef]
- Adhikari, A.; Lamichhane, L.; Adhikari, A.; Gyawali, G.; Acharya, D.; Baral, E.R.; Chhetri, K. Green Synthesis of Silver Nanoparticles Using Artemisia vulgaris Extract and Its Application toward Catalytic and Metal-Sensing Activity. Inorganics 2022, 10, 113. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Hikal, W.M.; Mahmoud, A.A. Biological activity of Moringa peregrina: A review. Am. J. Food Sci. Health 2017, 3, 83. [Google Scholar]
- El-Sherbiny, G.M.; Alluqmani, A.J.; Elesehemy IAKalaba, M.H. Antibacterial, antioxidant, cytotoxicity, and phytochemical screening of Moringa oleifera leaves. Sci. Rep. 2024, 14, 30485. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Balusamy, S.R.; Sukweenadhi, J.; Nag, S.; Mubarakali, D.; Farh, M.E.; Vijay, H.; Rahimi, S. A comprehensive review on Moringa oleifera nanoparticles: Importance of polyphenols in nanoparticle synthesis, nanoparticle efficacy and their applications. J. Nanobiotechnol. 2024, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Alrashidi, M.; Derawi, D.; Salimon, J.; Yusoff, M.F. The effects of different extraction solvents on the yield and antioxidant properties of Nigella sativa oil from Saudi Arabia. J. Taibah Univ. Sci. 2022, 16, 330. [Google Scholar] [CrossRef]
- Boke, E.; Saygi, K.; Erenler, R.; Kacmaz, B.; Ergene, A. Nigella sativa seed-mediated green biosynthesis of silver nanoparticles and antimicrobial activity. Rev. Mex. Fiscia 2024, 7, 051001. [Google Scholar] [CrossRef]
- Anwar, Y.; Ullah, I.; Al Johny, B.O.; Al-Shehri, A.M.G.; Bakhsh, E.M.; Ul-Islam, M.; Asiri, A.M.; Kamal, T. Nigella sativa L. seeds extract assisted synthesis of silver nanoparticles and their antibacterial and catalytic performance. Appl. Nanosci. 2022, 12, 3185. [Google Scholar] [CrossRef]
- Khan, A.N.; Aldowairy, N.N.A.; Alorfi, H.S.S.; Aslam, M.; Bawazir, W.A.; Hameed, A.; Soomro, M.T. Excellent antimicrobial, antioxidant, and catalytic activities of medicinal plant aqueous leaf extract derived silver nanoparticles. Processes 2022, 10, 1949. [Google Scholar] [CrossRef]
- Alorfi, H.; Alomari, M.; Bawakid, N.; Khan, A.; Althagbi, H.; Alsebaii, N.; Soomro, M. Green synthesis of AgNPs using Forsskaolea tenacissima: Sustainable nanotechnology for antimicrobial, antioxidant, and catalytic activities. Front. Nanotechnol. 2025, 7, 1587084. [Google Scholar] [CrossRef]
- Zulfikar, T.; Siregar, T.N.; Rozaliyani, A.; Sutriana, A. Antimicrobial Potential of Calotropis gigantea Leaf Against Klebsiella pneumoniae in Ventilator-Associated Pneumonia. J. Hum. Earth Future 2024, 5, 456. [Google Scholar] [CrossRef]
- Fatimah, S.; Sarto, S.; Fahrurrozi, M.; Budhijanto, B. Synthesis and Characterization of Hybridfiber from Gelatin Modified by PVACOS Using Coaxial Electrospinning Techniques as an Advanced Medical Textile Material. Emerg. Sci. J. 2024, 8, 716. [Google Scholar] [CrossRef]
- Sitio, R.; Akmal, M.; Marlina, M.; Gholib, G. Investigating Ethanolic Extract from Acehnese Lime (Citrus aurantifolia) Peel as Potential Anti-Hypercholesterolemia Agent. J. Hum. Earth Future 2024, 5, 348. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm producing methicillin-resistant Staphylococcus aureus (MRSA) infections in humans: Clinical implications and management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Archambaud, C.; Nunez, N.; da Silva, R.A.; Kline, K.A.; Serror, P. Enterococcus faecalis: An overlooked cell invader. Microbiol. Mol. Biol. Rev. 2024, 88, e00069-24. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary tract infections caused by uropathogenic Escherichia coli: Mechanisms of infection and treatment options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, S.; Zhang, L. Mechanisms of antibiotic resistance and developments in therapeutic strategies to combat Klebsiella pneumoniae infection. Infect. Drug Resist. 2024, 17, 1107. [Google Scholar] [CrossRef]
- Galán, J.E. Salmonella Typhimurium and inflammation: A pathogen-centric affair. Nat. Rev. Microbiol. 2021, 19, 716. [Google Scholar] [CrossRef]
- Abban, M.K.; Ayerakwa, E.A.; Mosi, L.; Isawumi, A. The burden of hospital acquired infections and antimicrobial resistance. Heliyon 2023, 9, e25561. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Steixner, S. The changing epidemiology of fungal infections. Mol. Asp. Med. 2023, 94, 101215. [Google Scholar] [CrossRef] [PubMed]
- Macias-Paz, I.U.; Pérez-Hernández, S.; Tavera-Tapia, A.; Luna-Arias, J.P.; Guerra-Cárdenas, J.E.; Reyna-Beltrán, E. Candida albicans the main opportunistic pathogenic fungus in humans. Rev. Argent. Microbiol. 2023, 55, 189. [Google Scholar] [CrossRef] [PubMed]
- Katsipoulaki, M.; Stappers, M.H.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A. Candida albicans and Candida glabrata: Global priority pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e00021. [Google Scholar] [CrossRef]
- Govrins, M.; Lass-Flörl, C. Candida parapsilosis complex in the clinical setting. Nat. Rev. Microbiol. 2024, 22, 46. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L.; Rodrigues, C.F. Overview on the infections related to rare Candida species. Pathogens 2022, 11, 963. [Google Scholar] [CrossRef]
- Tao, L.; Chen, X.; Sun, J.; Wu, C. Silver nanoparticles achieve cytotoxicity against breast cancer by regulating long-chain noncoding RNA XLOC_006390-mediated pathway. Toxicol. Res. 2021, 10, 123. [Google Scholar] [CrossRef]
- Kiran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Khan, M.F.; Khan, M.A. Plant-derived metal nanoparticles (PDMNPs): Synthesis, characterization, and oxidative stress-mediated therapeutic actions. Future Pharmacol. 2023, 3, 252. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver nanoparticles (AgNPs): Comprehensive insights into bio/synthesis, key influencing factors, multifaceted applications, and toxicity–A 2024 update. ACS Omega 2025, 10, 7549. [Google Scholar] [CrossRef]
- Singh, J.; Gupta, P.; Das, A. Dyes from textile industry wastewater as emerging contaminants in agricultural fields. In Sustainable Agriculture Reviews; Singh, K.V., Singh, R., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 50, p. 109. [Google Scholar]
- Boulkhessaim, S.; Gacem, A.; Khan, S.H.; Amari, A.; Yadav, V.K.; Harharah, H.N.; Elkhaleefa, A.M.; Yadav, K.K.; Rather, S.; Ahn, H.-J.; et al. Emerging trends in the remediation of persistent organic pollutants using nanomaterials and related processes: A review. Nanomaterials 2022, 12, 2148. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, A.; Singh, P.; Mishra, V.K. Organic Pollutants in Groundwater Resource. In Groundwater Geochemistry: Pollution and Remediation Methods; Madhav, S., Singh, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 139. [Google Scholar]
- Rani, G.; Bala, A.; Ahlawat, R.; Nunach, A.; Chahar, S. Recent advances in synthesis of AgNPs and their role in degradation of organic dyes. Comments Inorg. Chem. 2025, 45, 1. [Google Scholar] [CrossRef]
- Doan, V.D.; Nguyen, N.V.; Nguyen, T.L.H.; Tran, V.A.; Le, V.T. High-efficient reduction of methylene blue and 4-nitrophenol by silver nanoparticles embedded in magnetic graphene oxide. Environ. Sci. Pollut. Res. 2023, 30, 71543. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Gholami, T.; Seifi, H.; Dawi, E.A.; Said, E.A.; Hamoody, A.H.M.; Altimari, U.S.; Salavati-Niasari, M. Green synthesis of nanomaterials by using plant extracts as reducing and capping agents. Environ. Sci. Pollut. Res. 2024, 31, 24768. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Godeto, Y.G.; Ayele, A.; Ahmed, I.N.; Husen, A.; Bachheti, R.K. Medicinal Plant-Based Metabolites in Nanoparticles Synthesis and Their Cutting-Edge Applications: An Overview. In Secondary Metabolites from Medicinal Plants, 1st ed.; Bachheti, R.K., Bachheti, A., Eds.; CRC Press: Boca Raton, FL, USA, 2023; p. 1. [Google Scholar]
- Velgosova, O.; Dolinská, S.; Podolská, H.; Mačák, L.; Čižmárová, E. Impact of plant extract phytochemicals on the synthesis of silver nanoparticles. Materials 2024, 17, 2252. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, S.; Bouzid, G. Exploring the role of different phytochemicals on the morphological variations of metal and metal oxide nanomaterials for biomedical application. Interactions 2024, 245, 234. [Google Scholar] [CrossRef]
- Sharma, N.K.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; Almasoud, N.; Bhattarai, A. Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 2022, 7, 27004. [Google Scholar] [CrossRef]
- Alam, M. Analyses of biosynthesized silver nanoparticles produced from strawberry fruit pomace extracts in terms of biocompatibility, cytotoxicity, antioxidant ability, photodegradation, and in-silico studies. J. King Saud Univ. Sci. 2022, 34, 102327. [Google Scholar] [CrossRef]
- Ali, E.; Abu-Hussien, S.H.; Hesham, E.; Ahmed, S.; Mostafa, H.; Gamal, A.; El-Sayed, S.M.; Hemdan, B.; Bakry, A.; Ebeed, N.M.; et al. Compatibility and antimicrobial activity of silver nanoparticles synthesized using Lycopersicon esculentum peels. AMB Express 2024, 14, 120. [Google Scholar] [CrossRef]
- Keskin, S.Y.; Avci, A.; Kurnia, H.F.F. Analyses of phytochemical compounds in the flowers and leaves of Spiraea japonica var. fortunei using UV-VIS, FTIR, and LC-MS techniques. Heliyon 2024, 10, e25496. [Google Scholar]
- Nashikkar, R.; Baokar, S.; Ghodke, D.; Patil, R. The role of UV-visible spectroscopy for phenolic compounds quantification in winemaking. Asian J. Pharm. Anal. 2024, 14, 261. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, H.H.; Lee, I.S.; Moon, Y.H.; Woo, E.R. A new phenolic amide from Lycium chinense Miller. Arch. Pharmacal Res. 2002, 25, 433. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wu, P.; Li, L.; Yu, L.; Ruan, B.; Gong, B.; Zhu, N. Cr(VI) reduction and Cr(III) immobilization by resting cells of Pseudomonas aeruginosa CCTCC AB93066: Spectroscopic, microscopic, and mass balance analysis. Environ. Sci. Pollut. Res. 2017, 24, 5949. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Yi, M.; Ge, J.; Yin, G.; Li, X. Preparation and physicochemical properties of blend films of feather keratin and poly(vinyl alcohol) compatibilized by tris(hydroxymethyl) aminomethane. Polymers 2018, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Wongsa, P.; Phatikulrungsun, P.; Prathumthong, S. FT-IR characteristics, phenolic profiles and inhibitory potential against digestive enzymes of 25 herbal infusions. Sci. Rep. 2022, 12, 6631. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, E.O. Green silver nanoparticles: An antibacterial mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Alzhrani, F.E.; Gull, M.; Khan, A.N.; Aslam, M.; Bawazir, W.A.; Bataweel, N.M.; Al-Hejin, A.M.; Hameed, A.; Soomro, M.T. Malic acid as an organic linker for attaching Ag NPs to Fe3O4 nanoclusters: Synergistic enhancement of antimicrobial and antioxidant activities. Next Mater. 2025, 8, 100542. [Google Scholar] [CrossRef]
- Gong, L.; Li, J.; Jin, R.; Li, M.; Peng, J.; Zhu, J. Dual-Functional AgNPs/Magnetic Coal Fly Ash Composite for Wastewater Disinfection and Azo Dye Removal. Molecules 2025, 30, 3255. [Google Scholar] [CrossRef] [PubMed]
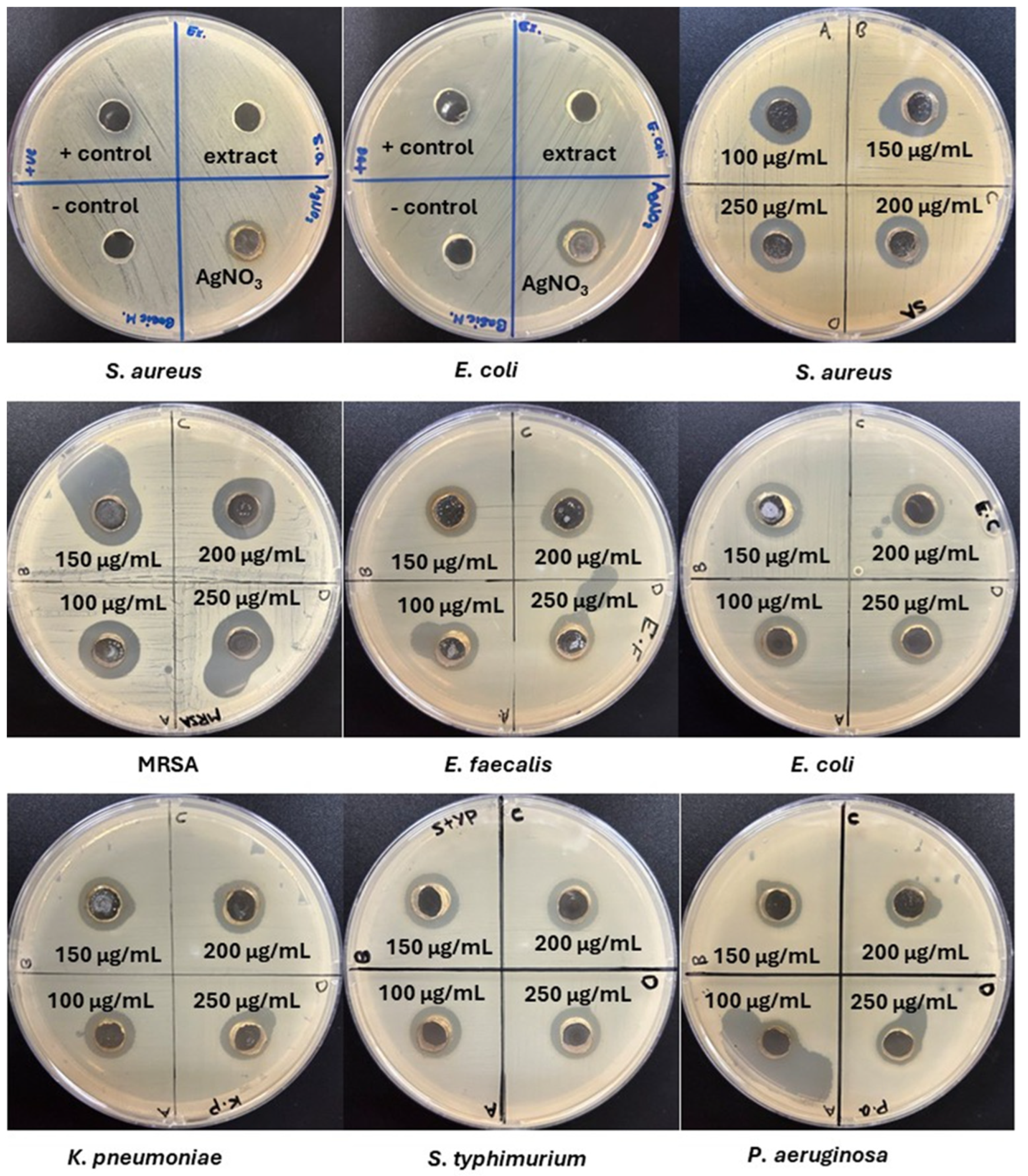
| Microorganism | Aqueous Extract | AgNPs | AgNO3 | Tetracycline | |||
|---|---|---|---|---|---|---|---|
| µg/mL | |||||||
| 100 | 150 | 200 | 250 | 100 | 40 | ||
| S. aureus | - | 16 ± 0.13 a | 16 ± 0.12 a | 15 ± 0.12 c | 16 ± 0.11 a | 11 ± 0.12 h | 13 ± 0.12 d |
| MRSA | - | 17 ± 0.1 b | 17 ± 0.1 b | 17 ± 0.11 b | 17 ± 0.12 b | 12 ± 0.12 f | 16 ± 0.11 a |
| E. faecalis | - | 15 ± 0.13 c | 17 ± 0.11 b | 17 ± 0.11 b | 17 ± 0.11 b | 11 ± 0.11 h | 12 ± 0.13 f |
| E. coli | - | 15 ± 0.11 c | 15 ± 0.12 c | 14 ± 0.12 e | 14 ± 0.13 e | 13 ± 0.13 d | 10 ± 0.11 g |
| K. pneumoniae | - | 15 ± 0.11 c | 15 ± 0.12 c | 14 ± 0.11 e | 14 ± 0.14 e | 12 ± 0.13 f | 14 ± 0.14 e |
| S. typhimurium | - | 15 ± 0.11 c | 15 ± 0.11 c | 14 ± 0.11 e | 15 ± 0.11 c | 12 ± 0.12 f | 16 ± 0.13 a |
| P. aeruginosa | - | 13 ± 0.11 d | 13 ± 0.11 d | 13 ± 0.11 d | 12 ± 0.11 f | 12 ± 0.12 f | 17 ± 0.11 b |
| Microorganism | AgNPs | Tetracycline |
|---|---|---|
| IC50 (µg/mL) | ||
| S. aureus | 42.3 | 19.1 |
| MRSA | 35 | 16.42 |
| E. faecalis | 33 | 21.2 |
| E. coli | 40 | 23 |
| K. pneumoniae | 41 | 18.4 |
| S. typhimurium | 37.8 | 15.9 |
| P. aeruginosa | 46.1 | 14.7 |
| Microorganism | Aqueous Extract | AgNPs | AgNO3 | Fluconazole | |||
|---|---|---|---|---|---|---|---|
| µg/mL | |||||||
| 100 | 150 | 200 | 250 | 100 | 25 | ||
| C. albicans | - | 15 ± 0.11 a | 15 ± 0.11 a | 13 ± 0.11 c | 15 ± 0.12 a | 15 ± 0.11 a | 20 ± 0.12 b |
| C. glabrata | - | 20 ± 0.1 b | 24 ± 0.1 d | 20 ± 0.12 b | 21 ± 0.11 e | 14 ± 0.13 f | 19 ± 0.11 g |
| C. parapsilosis | - | 20 ± 0.11 b | 20 ± 0.12 b | 20 ± 0.11 b | 20 ± 0.11 b | 14 ± 0.11 f | 17 ± 0.13 h |
| C. krusei | - | 21 ± 0.11 e | 20 ± 0.11 b | 19 ± 0.11 g | 21 ± 0.13 e | 14 ± 0.11 f | 14 ± 0.11 f |
| Microorganism | Celtis africana | Artemisia vulgaris | Moringa oleifera | Nigella sativa |
|---|---|---|---|---|
| S. aureus | Strong | strong | Strong | Strong |
| MRSA | Strong | Moderate | Moderate | strong |
| E. faecalis | Strong | Not specified | Not specified | Strong |
| E. coli | Moderate | Strong | Very strong | Moderate |
| K. pneumoniae | Moderate | Moderate | Moderate | Not specified |
| S. typhimurium | Moderate | Moderate | Not specified | Moderate |
| P. aeruginosa | Moderate | Moderate | Strong | Moderate |
| C. albicans | Moderate | strong | Moderate | Moderate |
| C. glabrata | Very strong | Not specified | Not specified | Not specified |
| C. parapsilosis | Very strong | Not specified | Not specified | Not specified |
| C. krusei | Very strong | Not specified | Not specified | Not specified |
| Plant Source | AgNPs | |||
|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | Antioxidant (IC50, (µg/mL) | Rate Constant (s−1) | |
| Celtis Africana | 6.25 | 25 | 5.41 | 0.0165 (4-NP) 0.0047 (MB) |
| Artemisia vulgaris [4,5,6] | 250 | - | 11.4 | 0.0121 (4-NP) |
| Moringa oleifera [7,8,9] | 14–24 | - | 16.4 | 0.00438 (MB) |
| Nigella sativa [10,11,12] | 15–30 | 50 | - | 0.00152 (4-NP) 0.007 (MB) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.N. Green Synthesis of AgNPs from Celtis africana: Biological and Catalytic Insights. Nanomaterials 2025, 15, 1821. https://doi.org/10.3390/nano15231821
Khan AN. Green Synthesis of AgNPs from Celtis africana: Biological and Catalytic Insights. Nanomaterials. 2025; 15(23):1821. https://doi.org/10.3390/nano15231821
Chicago/Turabian StyleKhan, Amna N. 2025. "Green Synthesis of AgNPs from Celtis africana: Biological and Catalytic Insights" Nanomaterials 15, no. 23: 1821. https://doi.org/10.3390/nano15231821
APA StyleKhan, A. N. (2025). Green Synthesis of AgNPs from Celtis africana: Biological and Catalytic Insights. Nanomaterials, 15(23), 1821. https://doi.org/10.3390/nano15231821








