Investigation on the Mass Distribution and Chemical Compositions of Various Ionic Liquids-Extracted Coal Fragments and Their Effects on the Electrochemical Performance of Coal-Derived Carbon Nanofibers (CCNFs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations of Coal Precursors
2.3. CCNFs Fabrication
2.4. Characterizations of CCNFs
2.5. Electrochemical Measurements
3. Results
3.1. Chemical Properties of ILs-Derived Coal Precursors
3.2. Surface Properties of CCNFs
3.3. Electrochemical Measurements
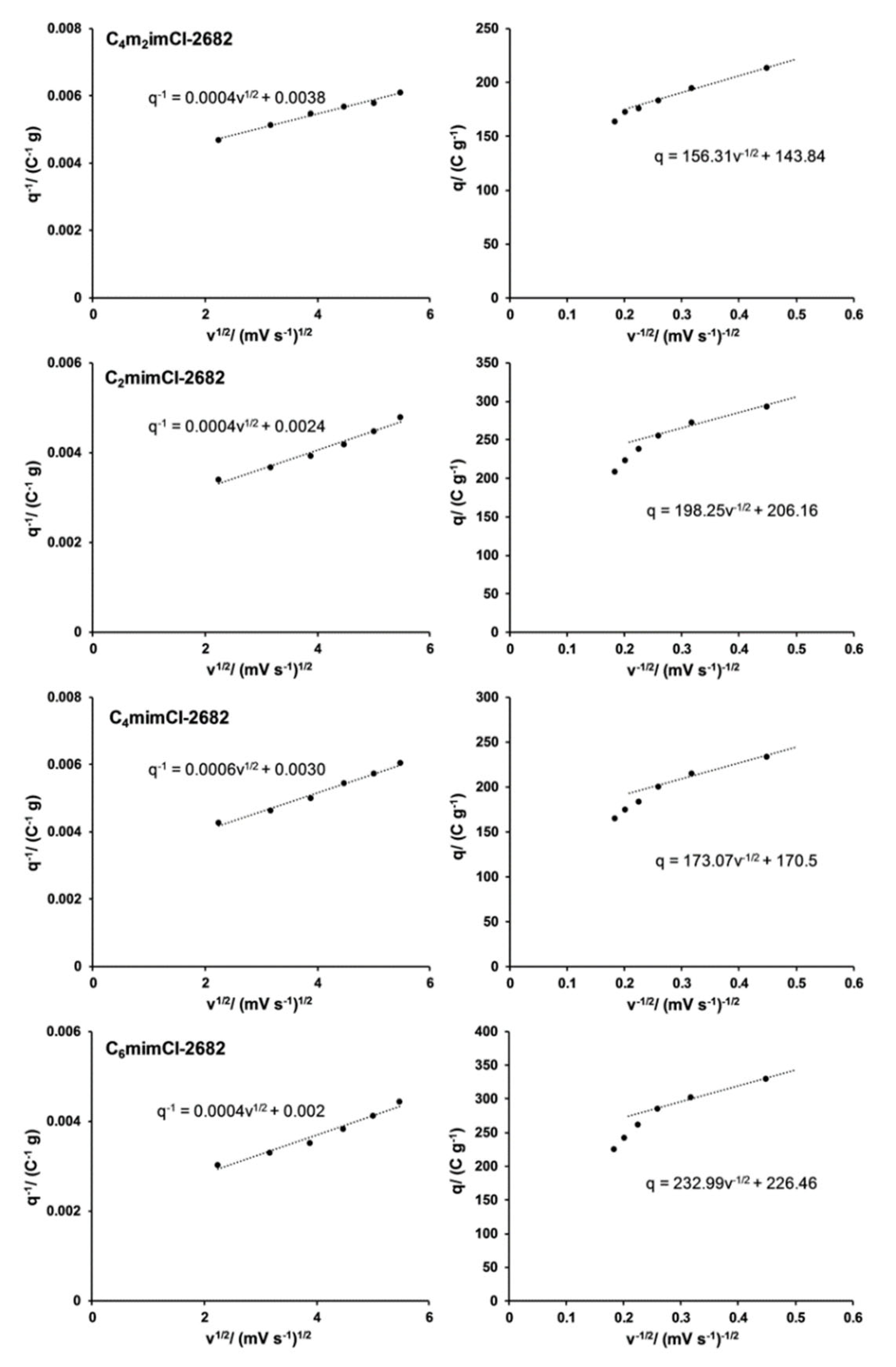
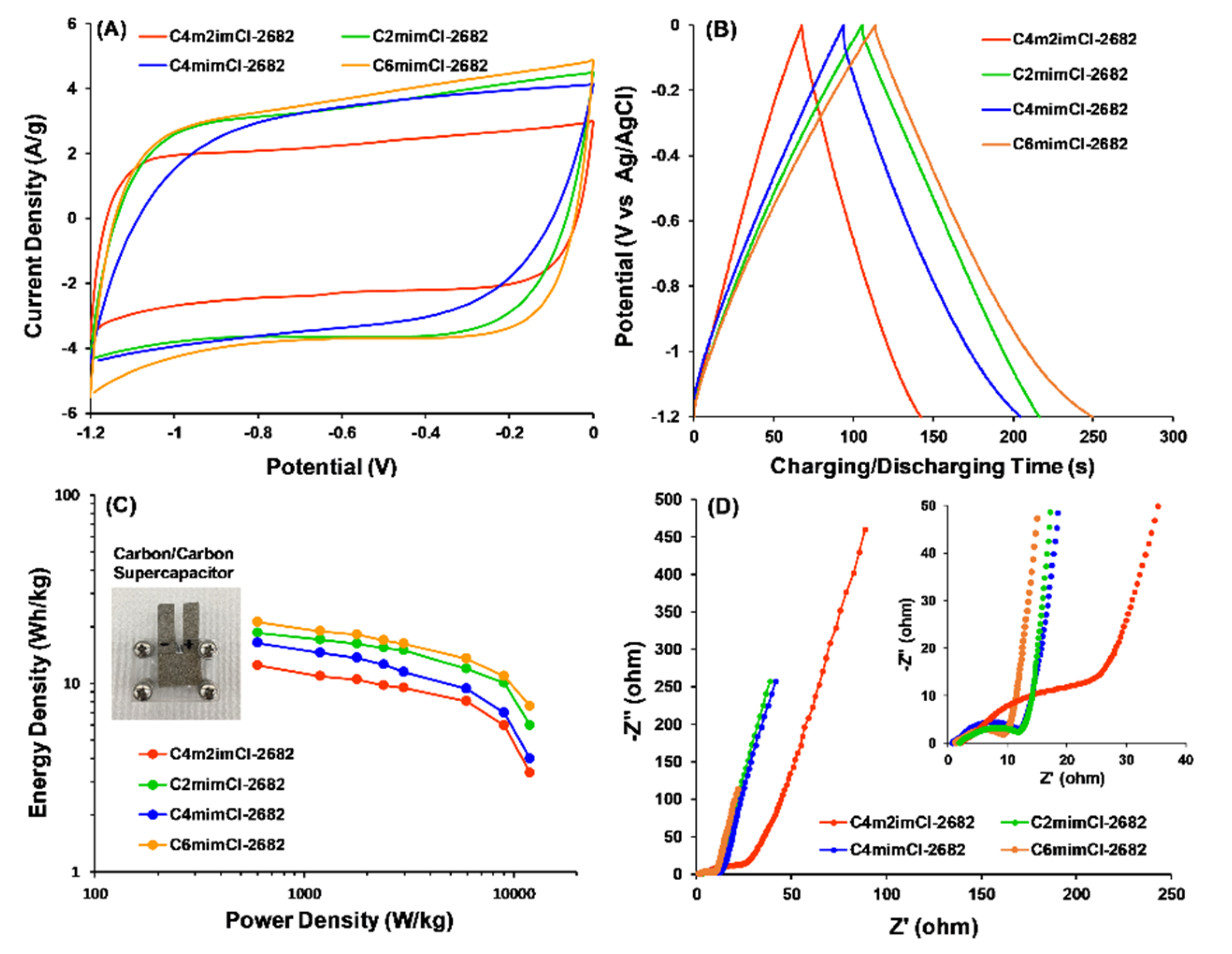
3.3.1. CCNFs Derived from ILs-Extracted 2682 Coal Fragment
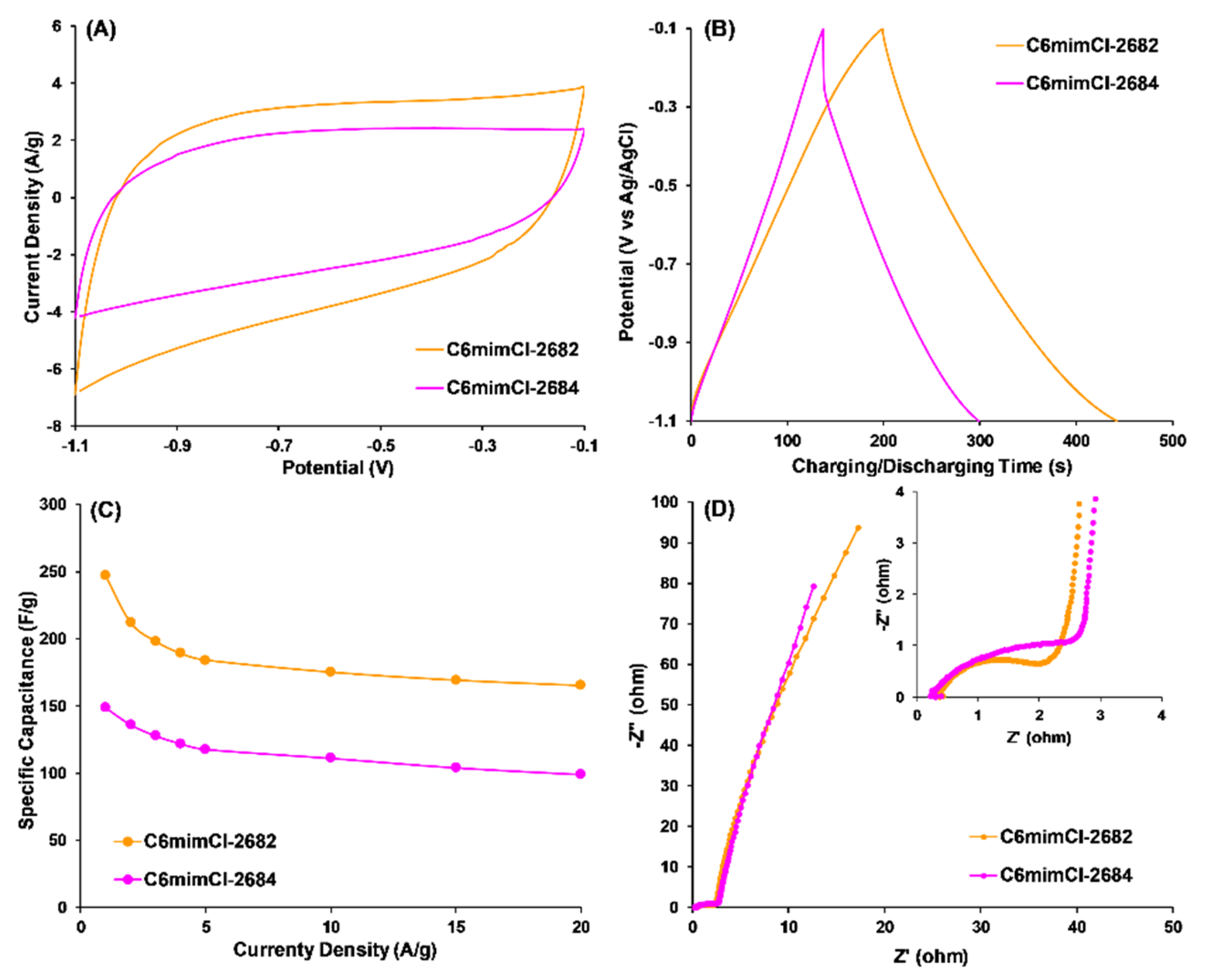
3.3.2. CCNFs Derived from an ILs-Extracted 2684 Coal Precursor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Kang, F.; Tarascon, J.; Kim, J.-K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Mao, X.; Hatton, T.; Rutledge, G. A Review of Electrospun Carbon Fibers as Electrode Materials for Energy Storage. Curr. Org. Chem. 2013, 17, 1390–1401. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Fu, S.; Liu, Y.; Shao, C. Polyacrylonitrile and carbon nanofibers with controllable nanoporous structures by electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.; Xu, H.; Xiong, X.; Jiang, Y.; Zou, F.; Hu, X.; Xin, Y.; Zhang, Z.; Huang, Y. Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2497–2504. [Google Scholar] [CrossRef]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon fibers: Precursors, manufacturing, and properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ooi, C.-H.; Othman, R.; Yeoh, F.-Y. Activated Carbon Fiber-the Hybrid of Carbon Fiber and Activated Carbon. Rev. Adv. Mater. Sci. 2014, 36, 118–136. [Google Scholar]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P.; Ruoff, R.S. Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Lee, J.K.; An, K.W.; Ju, J.B.; Cho, B.W.; Cho, W.I.; Park, D.; Yun, K.S. Electrochemical properties of PAN-based carbon fibers as anodes for rechargeable lithium ion batteries. Carbon 2001, 39, 1299–1305. [Google Scholar] [CrossRef]
- Arshad, S.N.; Naraghi, M.; Chasiotis, I. Strong carbon nanofibers from electrospun polyacrylonitrile. Carbon 2011, 49, 1710–1719. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, J.; Xie, Z.; Wang, X.; Lin, T. Preparation, structure and supercapacitance of bonded carbon nanofiber electrode materials. Carbon 2011, 49, 2380–2388. [Google Scholar] [CrossRef]
- Qian, W.; Yu, D.G.; Li, Y.; Liao, Y.Z.; Wang, X.; Wang, L. Dual drug release electrospun core-shell nanofibers with tunable dose in the second phase. Int. J. Mol. Sci. 2014, 15, 774–786. [Google Scholar] [CrossRef]
- Kim, C.; Cho, Y.J.; Yun, W.Y.; Ngoc, B.T.N.; Yang, K.S.; Chang, D.R.; Lee, J.W.; Kojima, M.; Kim, Y.A.; Endo, M. Fabrications and structural characterization of ultra-fine carbon fibres by electrospinning of polymer blends. Solid State Commun. 2007, 142, 20–23. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, Y.I.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Endo, M.; Lee, J.-W. Synthesis and characterization of porous carbon nanofibers with hollow cores through the thermal treatment of electrospun copolymeric nanofiber webs. Small 2007, 3, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Wang, H.; Jin, X.; Niu, H.; Wang, H.; Zhou, H.; Lin, T. High Performance Supercapacitor Electrode Materials from Electrospun Carbon Nanofibers in Situ Activated by High Decomposition Temperature Polymer. ACS Appl. Energy Mater. 2018, 1, 431–439. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kang, F. Coaxial carbon nanofibers/MnO2nanocomposites as freestanding electrodes for high-performance electrochemical capacitors. Electrochim. Acta 2011, 56, 9240–9247. [Google Scholar] [CrossRef]
- Cakici, M.; Reddy, K.R.; Alonso-Marroquin, F. Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem. Eng. J. 2017, 309, 151–158. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Yang, W.D.; Lee, K.C.; Huang, C.M. An effective electrodeposition mode for porous MnO2/Ni foam composite for asymmetric supercapacitors. Materials 2016, 9, 246. [Google Scholar] [CrossRef]
- Iqbal, N.; Wang, X.; Babar, A.A.; Zainab, G.; Yu, J.; Ding, B. Flexible Fe3O4@carbon nanofibers hierarchically assembled with MnO2particles for high-performance supercapacitor electrodes. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Béguin, F. Tuning carbon materials for supercapacitors by direct pyrolysis of seaweeds. Adv. Funct. Mater. 2009, 19, 1032–1039. [Google Scholar] [CrossRef]
- Béguin, F.; Szostak, K.; Lota, G.; Frackowiak, E. A self-supporting electrode for supercapacitors prepared by one-step pyrolysis of carbon nanotube/polyacrylonitrile blends. Adv. Mater. 2005, 17, 2380–2384. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Leroux, F.; Béguin, F. A high-performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer. Adv. Mater. 2006, 18, 1877–1882. [Google Scholar] [CrossRef]
- Jurewicz, K.; Babeł, K.; Pietrzak, R.; Delpeux, S.; Wachowska, H. Capacitance properties of multi-walled carbon nanotubes modified by activation and ammoxidation. Carbon 2006, 44, 2368–2375. [Google Scholar] [CrossRef]
- Xu, B.; Duan, H.; Chu, M.; Cao, G.; Yang, Y. Facile synthesis of nitrogen-doped porous carbon for supercapacitors. J. Mater. Chem. A 2013, 1, 4565. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Park, S.G.; Shanmugam, S. Three-dimensional hierarchical nitrogen-doped arch and hollow nanocarbons: Morphological influences on supercapacitor applications. J. Mater. Chem. A 2015, 3, 16242–16250. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Gavrilov, N.; Pašti, I.A.; Vujković, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G.; Mentus, S.V. High-performance charge storage by N-containing nanostructured carbon derived from polyaniline. Carbon 2012, 50, 3915–3927. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Li, W.; Xin, L.; Xu, X.; Liu, Q.; Zhang, M.; Ding, S.; Zaho, M.; Lou, X. Facile synthesis of three-dimensional structured carbon fiber-NiCo2O4-Ni(OH)2 high-performance electrode for pseudocapacitors. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Ding, Y.; Feng, S.; Wang, Z.L.; Liu, M. Nickel-cobalt hydroxide nanosheets coated on NiCo2O4 nanowires grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2013, 13, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Hyun, B.G.; Son, H.J.; Ji, S.; Jang, J.; Hur, S.H.; Park, J.U. Multi-dimensional carbon nanofibers for supercapacitor electrodes. J. Electroceram. 2017, 38, 43–50. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Wang, L.; Liu, L.; Sun, Z.; Wang, S.; Zhao, H.; Kong, L.; Jia, D. Coal-Based Hierarchical Porous Carbon Synthesized with a Soluble Salt Self-Assembly-Assisted Method for High Performance Supercapacitors and Li-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 3255–3263. [Google Scholar] [CrossRef]
- Qu, W.H.; Guo, Y.B.; Shen, W.Z.; Li, W.C. Using Asphaltene Supermolecules Derived from Coal for the Preparation of Efficient Carbon Electrodes for Supercapacitors. J. Phys. Chem. C 2016, 120, 15105–15113. [Google Scholar] [CrossRef]
- Guo, M.; Guo, J.; Jia, D.; Zhao, H.; Sun, Z.; Song, X.; Li, Y. Coal Derived Porous Carbon Fibers with Tunable Internal Channels for Flexible Electrodes and Organic Matter Absorption. J. Mater. Chem. A 2015, 3, 21178–21184. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wang, L.X.; Jia, D.Z.; Xia, W.; Li, J.; Guo, Z.P. Coal based activated carbon nanofibers prepared by electrospinning. J. Mater. Chem. A 2014, 2, 9338–9344. [Google Scholar] [CrossRef]
- Tan, S.; Kraus, T.J.; Li-Oakey, K.D. Understanding the supercapacitor properties of electrospun carbon nanofibers from Powder River Basin coal. Fuel 2019, 245, 148–159. [Google Scholar] [CrossRef]
- Tan, S.; Li-Oakey, K.D. Effect of Structural Orientation on the Performance of Supercapacitor Electrodes from Electrospun Coal-Derived Carbon Nanofibers (CCNFs). J. Electrochem. Soc. 2019, 166, A3294. [Google Scholar] [CrossRef]
- He, W.; Liu, Z.; Liu, Q.; Shi, L.; Shi, X.; Wu, J.; Guo, X. Behavior of radicals during solvent extraction of three low rank bituminous coals. Fuel Process Technol. 2017, 156, 221–227. [Google Scholar] [CrossRef]
- Duber, S.; Wipcckowski, A.B. Effects of Organic Solvents on the EPR Spectrum of Coal. Fuel 1984, 63, 1641–1644. [Google Scholar] [CrossRef]
- Shui, H.; Wang, Z.; Gao, J. Examination of the role of CS2 in the CS2/NMP mixed solvents to coal extraction. Fuel Process Technol. 2006, 87, 185–190. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Sakanishi, K.; Nakazato, T.; Tao, H.; Takanohashi, T.; Takarada, T.; Saito, I. Investigation of the remaining major and trace elements in clean coal generated by organic solvent extraction. Fuel 2005, 84, 1487–1493. [Google Scholar] [CrossRef]
- Shui, H.; Wang, Z.; Wang, G. Effect of hydrothermal treatment on the extraction of coal in the CS2/NMP mixed solvent. Fuel 2006, 85, 1798–1802. [Google Scholar] [CrossRef]
- Painter, P.; Cetiner, R.; Pulati, N.; Sobkowiak, M.; Mathews, J. Dispersion of Liquefaction Catalysts in Coal Using Ionic Liquids. Energy Fuels 2010, 24, 3086–3092. [Google Scholar] [CrossRef]
- Painter, P.; Pulati, N.; Cetiner, R.; Sobkowiak, M.; Mitchell, G.; Mathews, J. Dissolution and Dispersion of Coal in Ionic Liquids. Energy Fuels 2010, 24, 1848–1853. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Weber, C.C.; Gräsvik, J.; Welton, T.; Brandt, A.; Hallett, J.P. Mechanistic insights into lignin depolymerisation in acidic ionic liquids. Green Chem. 2016, 18, 5456–5465. [Google Scholar] [CrossRef]
- George, A.; Brandt, A.; Tran, K.; Zahari, S.M.N.S.; Klein-Marcuschamer, D.; Sun, N.; Sathitsuksanoh, N.; Shi, J.; Stavila, V.; Parthasarathi, R.; et al. Design of low-cost ionic liquids for lignocellulosic biomass pretreatment. Green Chem. 2015, 17, 1728–1734. [Google Scholar] [CrossRef]
- De Gregorio, G.F.; Prado, R.; Vriamont, C.; Erdocia, X.; Labidi, J.; Hallett, J.P.; Welton, T. Oxidative depolymerization of lignin using a novel polyoxometalate-protic ionic liquid system. ACS Sustain. Chem. Eng. 2016, 4, 6031–6036. [Google Scholar] [CrossRef]
- Tan, S.; Helling, M.R.; Basile, F.; Li-Oakey, K.D. Systematic Study of Ionic Liquids Based Coal Extraction: Selectivity in Extract Molecular Weights and Targeted Functional Groups. Energy Fuels 2020, 34, 4554–4564. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, J.; Welton, T.; Armstrong, D.W. Characterizing Ionic Liquids on the Basis of Multiple Solvation Interactions. J. Am. Chem. Soc. 2002, 124, 14247–14254. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.A.; Kirchner, B.; Welton, T. Characterising the electronic structure of ionic liquids: An examination of the 1-butyl-3-methylimidazolium chloride ion pair. Chemistry 2006, 12, 6762–6775. [Google Scholar] [CrossRef] [PubMed]
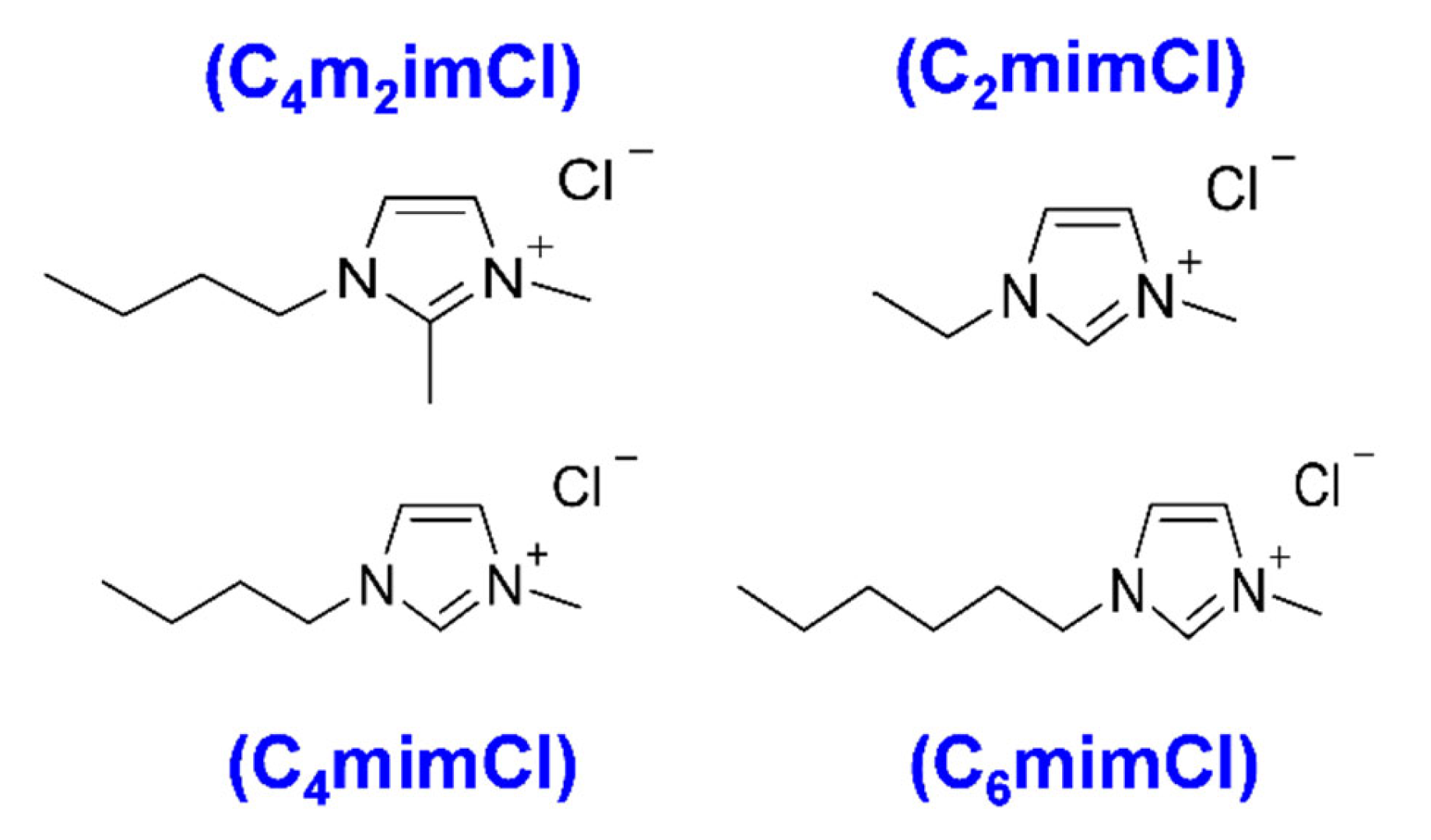
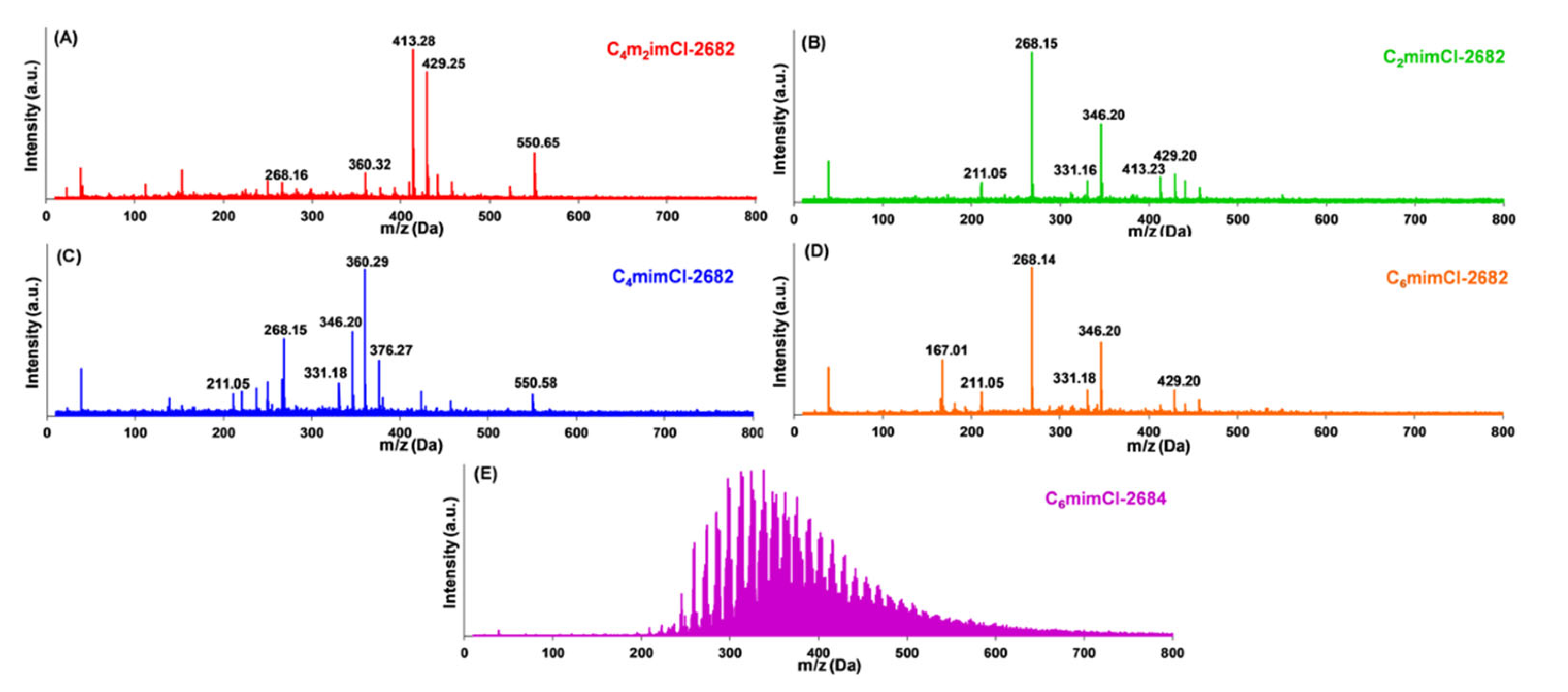
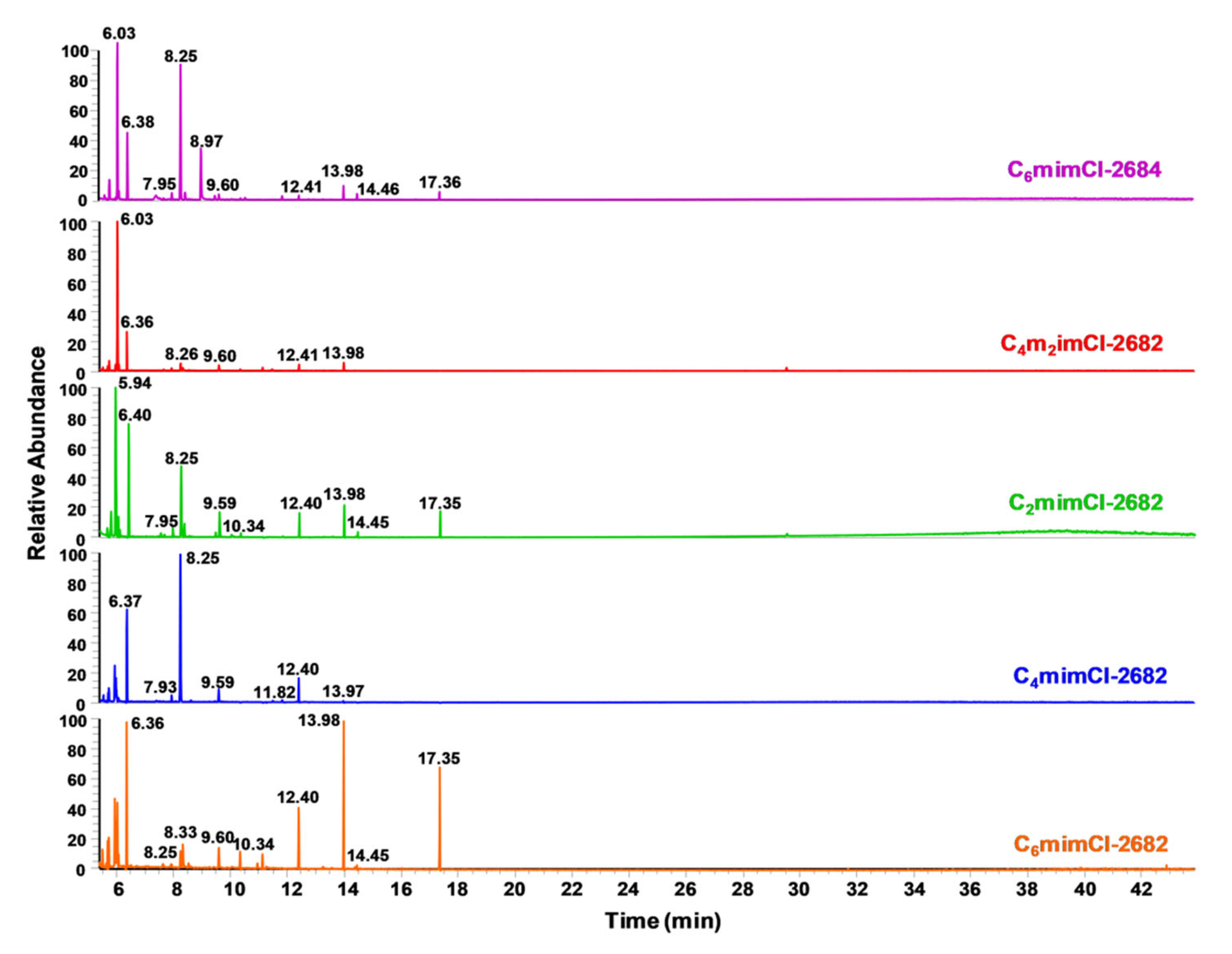

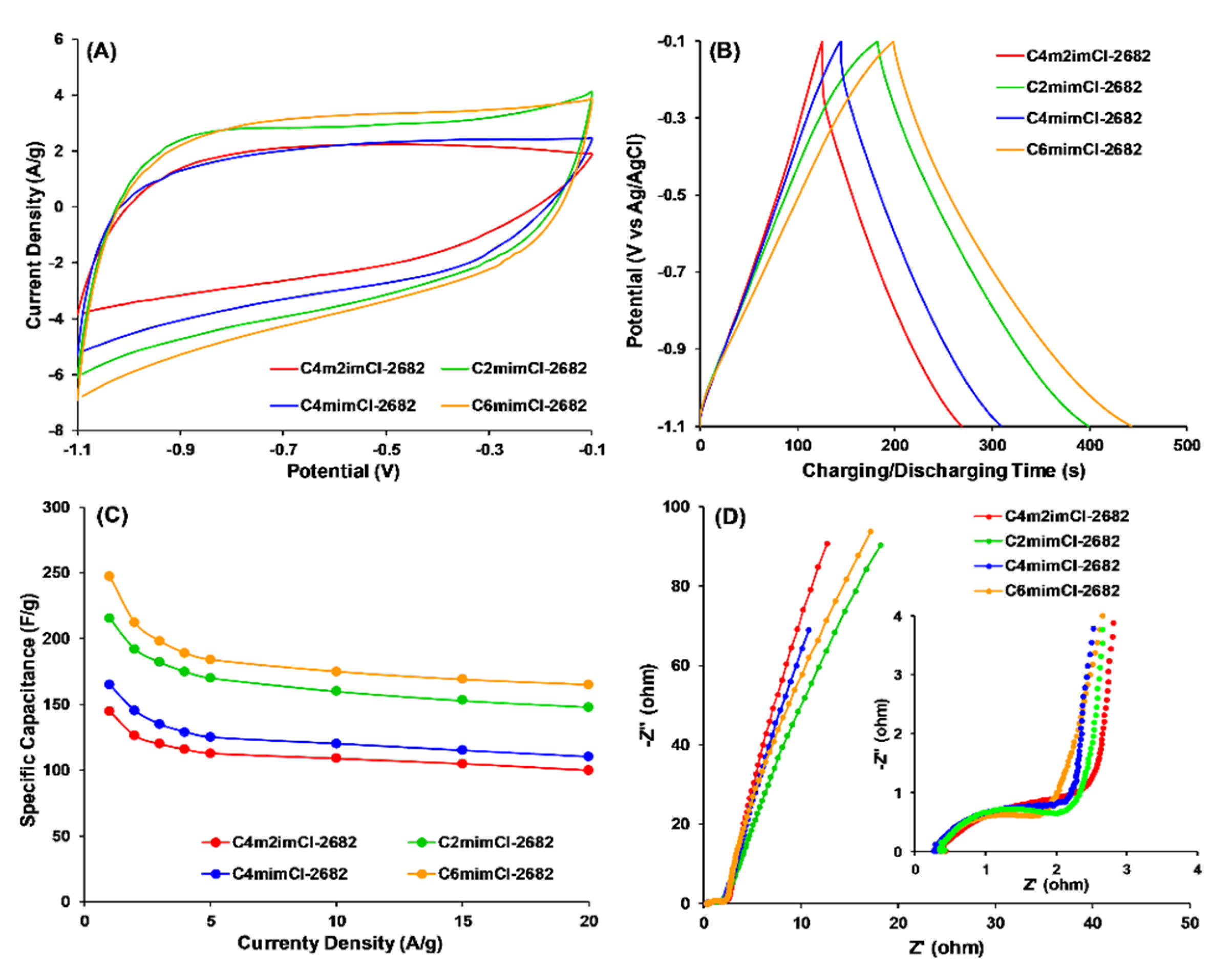

| CCNFs | High-Resolution Scan | Survey Scan | ||||
|---|---|---|---|---|---|---|
| O 1s% | O% | N% | C% | |||
| OI | OII | OIII | ||||
| C4m2imCl-2682 | 18.9 | 55.4 | 25.7 | 1.9 | 4.0 | 94.1 |
| C2mimCl-2682 | 29.5 | 47.5 | 23.0 | 4.1 | 4.1 | 91.8 |
| C4mimCl-2682 | 23.8 | 51.8 | 24.5 | 2.6 | 4.4 | 93.0 |
| C6mimCl-2682 | 38.1 | 47.2 | 14.7 | 4.2 | 5.4 | 90.4 |
| C6mimCl-2684 | 24.3 | 51.0 | 24.7 | 2.6 | 3.7 | 93.7 |
| CCNFs | Rsum (Ω) | Rs (Ω) | Rct (Ω) | Rp (Ω) |
|---|---|---|---|---|
| C4m2im-2682 | 3.985 | 0.24 | 3.70 | 0.045 |
| C2mim-2682 | 2.597 | 0.26 | 2.30 | 0.037 |
| C4mim-2682 | 3.092 | 0.24 | 2.80 | 0.052 |
| C6mim-2682 | 2.275 | 0.26 | 1.98 | 0.035 |
| C6mim-2684 | 3.828 | 0.24 | 3.50 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Kraus, T.J.; Helling, M.R.; Mignon, R.K.; Basile, F.; Li-Oakey, K.D. Investigation on the Mass Distribution and Chemical Compositions of Various Ionic Liquids-Extracted Coal Fragments and Their Effects on the Electrochemical Performance of Coal-Derived Carbon Nanofibers (CCNFs). Nanomaterials 2021, 11, 664. https://doi.org/10.3390/nano11030664
Tan S, Kraus TJ, Helling MR, Mignon RK, Basile F, Li-Oakey KD. Investigation on the Mass Distribution and Chemical Compositions of Various Ionic Liquids-Extracted Coal Fragments and Their Effects on the Electrochemical Performance of Coal-Derived Carbon Nanofibers (CCNFs). Nanomaterials. 2021; 11(3):664. https://doi.org/10.3390/nano11030664
Chicago/Turabian StyleTan, Shuai, Theodore John Kraus, Mitchell Ross Helling, Rudolph Kurtzer Mignon, Franco Basile, and Katie Dongmei Li-Oakey. 2021. "Investigation on the Mass Distribution and Chemical Compositions of Various Ionic Liquids-Extracted Coal Fragments and Their Effects on the Electrochemical Performance of Coal-Derived Carbon Nanofibers (CCNFs)" Nanomaterials 11, no. 3: 664. https://doi.org/10.3390/nano11030664
APA StyleTan, S., Kraus, T. J., Helling, M. R., Mignon, R. K., Basile, F., & Li-Oakey, K. D. (2021). Investigation on the Mass Distribution and Chemical Compositions of Various Ionic Liquids-Extracted Coal Fragments and Their Effects on the Electrochemical Performance of Coal-Derived Carbon Nanofibers (CCNFs). Nanomaterials, 11(3), 664. https://doi.org/10.3390/nano11030664







