Cyclodextrin-Based Functional Glyconanomaterials
Abstract
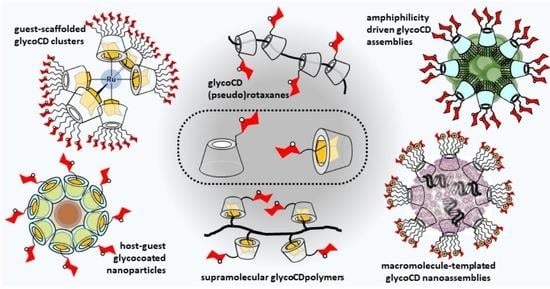
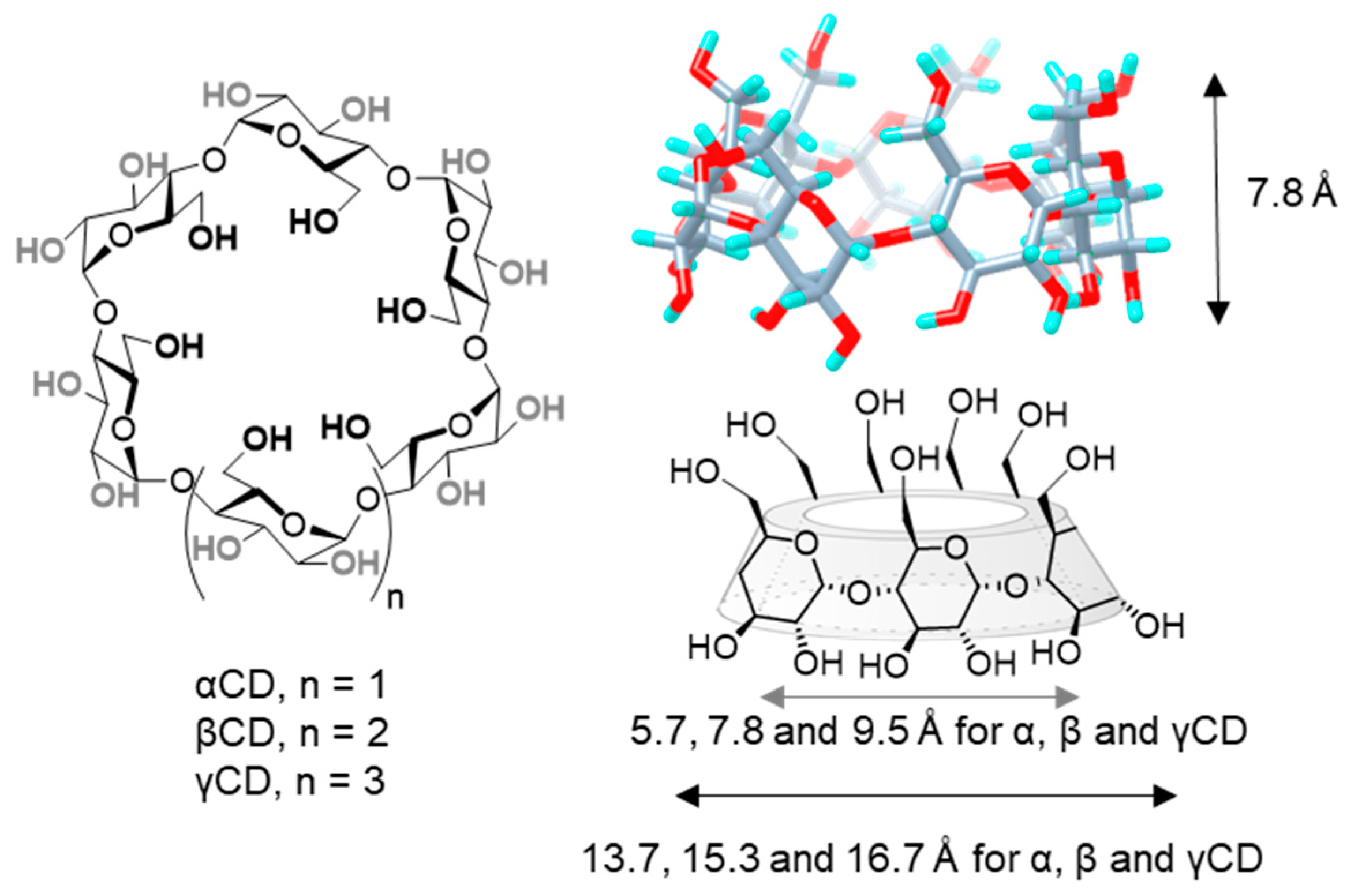
1. Introduction
2. Multitopic Guest-Driven Clusterization of GlycoCDs
3. Amphiphilicity as an Action Principle for GlycoCD Self-Assembly
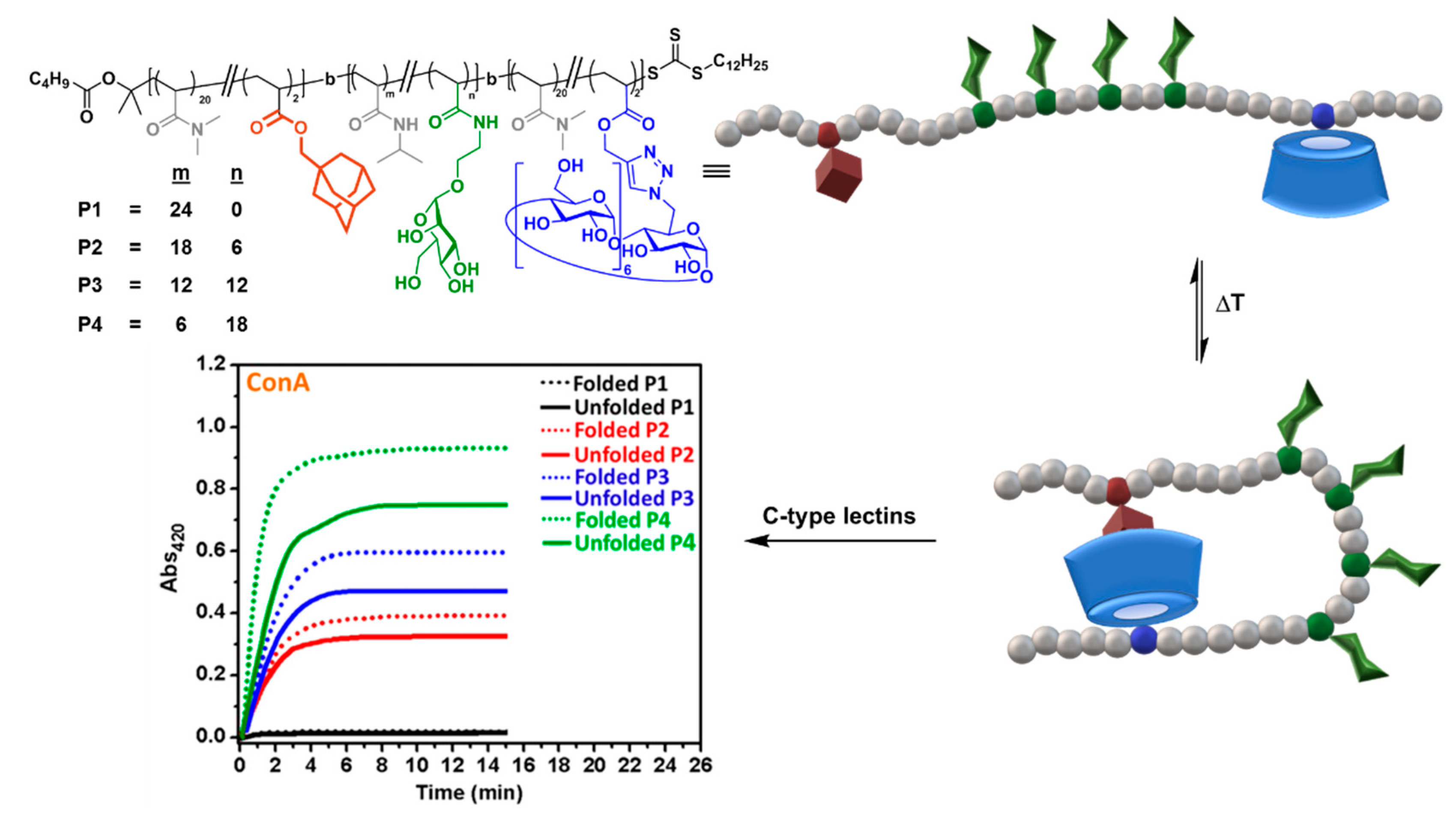
4. Tailoring Glycopolymer Topology by CD Inclusion-Promoted Macromolecular Folding and Self-Assembly
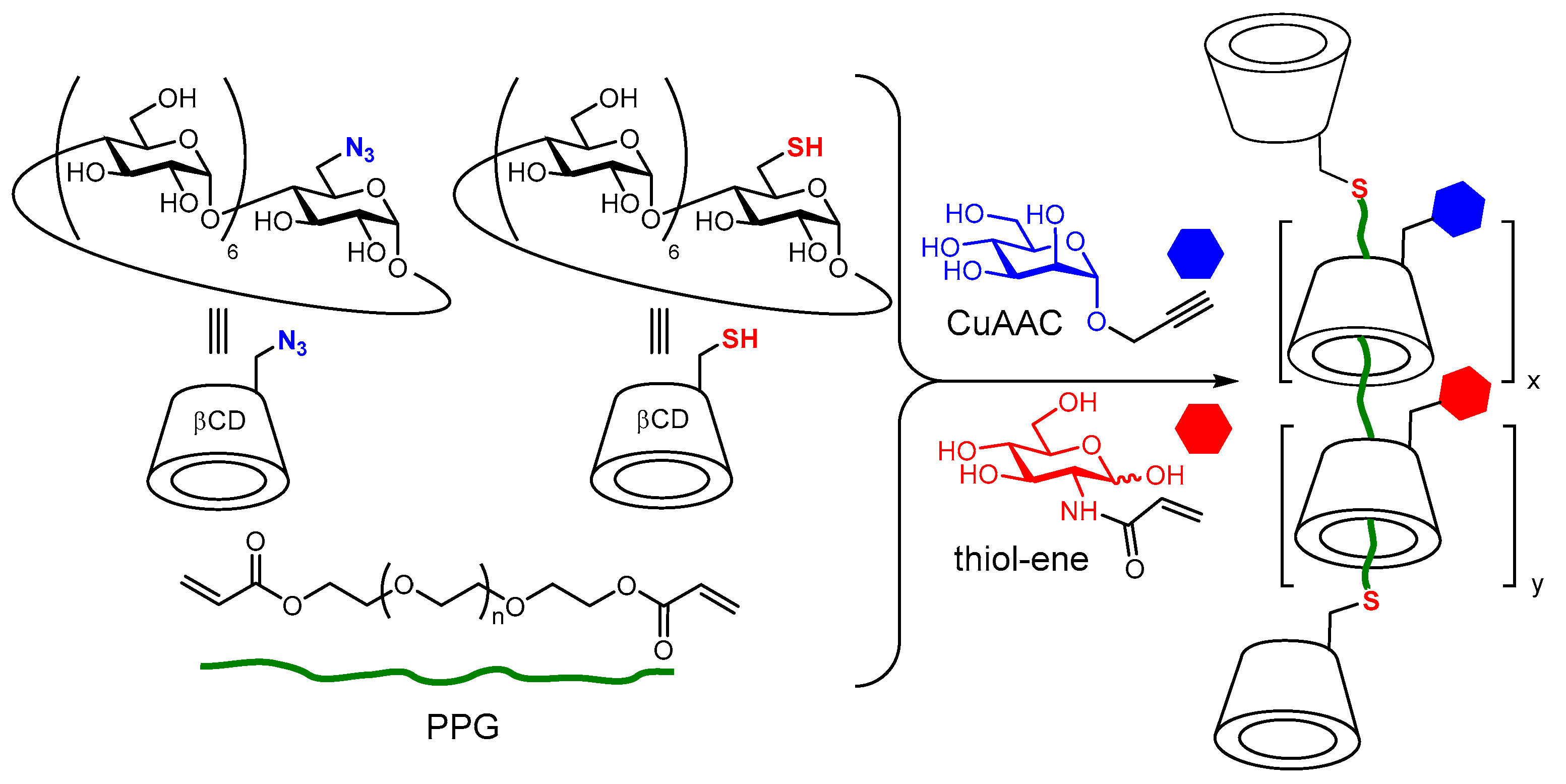
5. GlycoCD Rotaxanation and Self-Aggregation Strategies
6. Biomacromolecule-Templated Formation of Functional GlycoCD Nanoassemblies
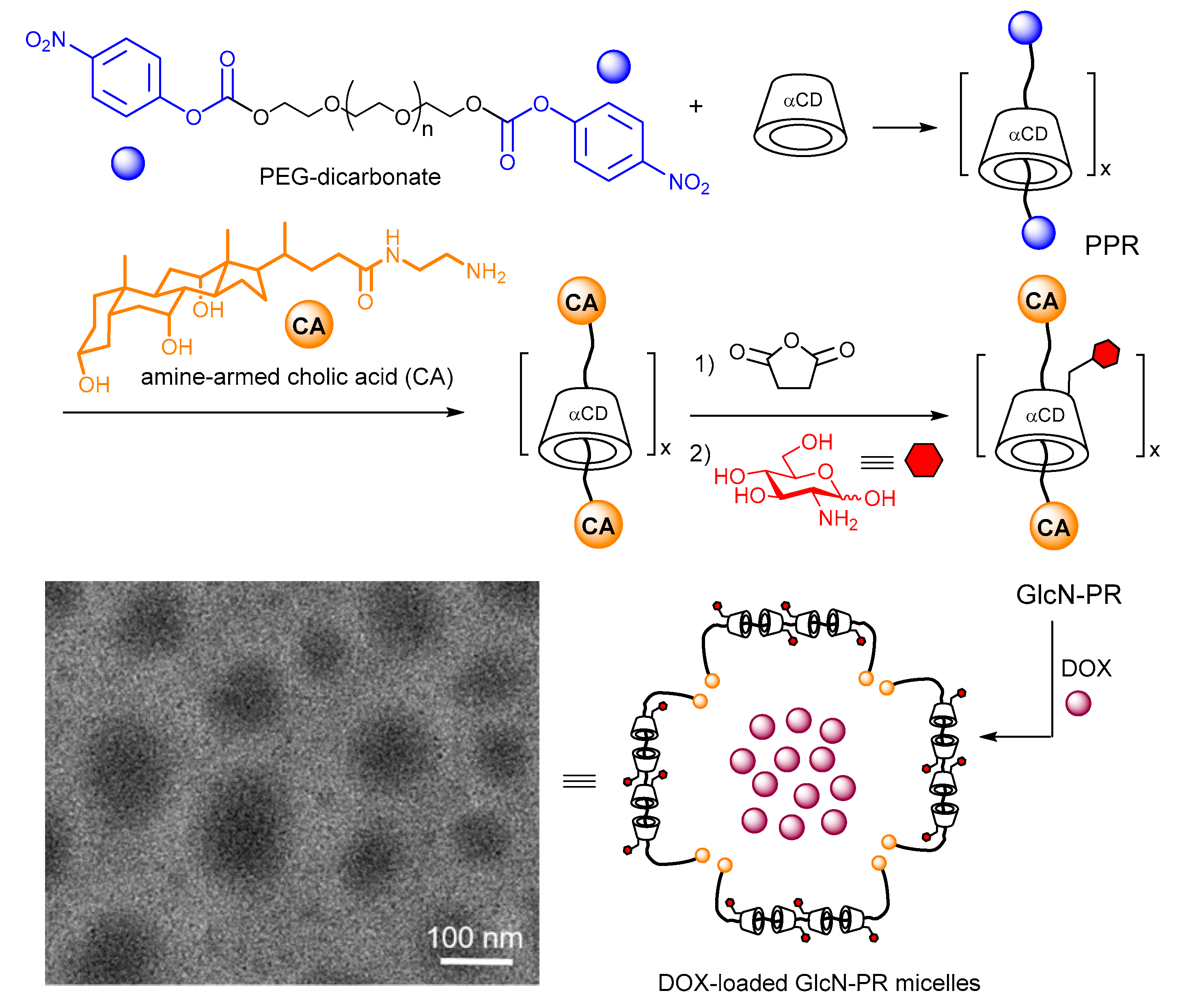
7. Host–Guest Mediated Glyco-Coating of Self-Assembled CD Nanoparticles
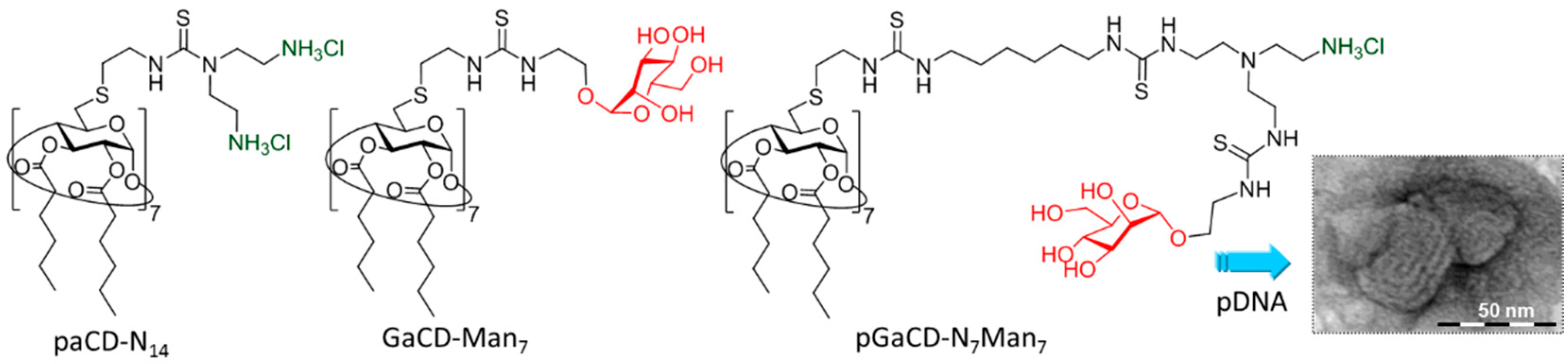
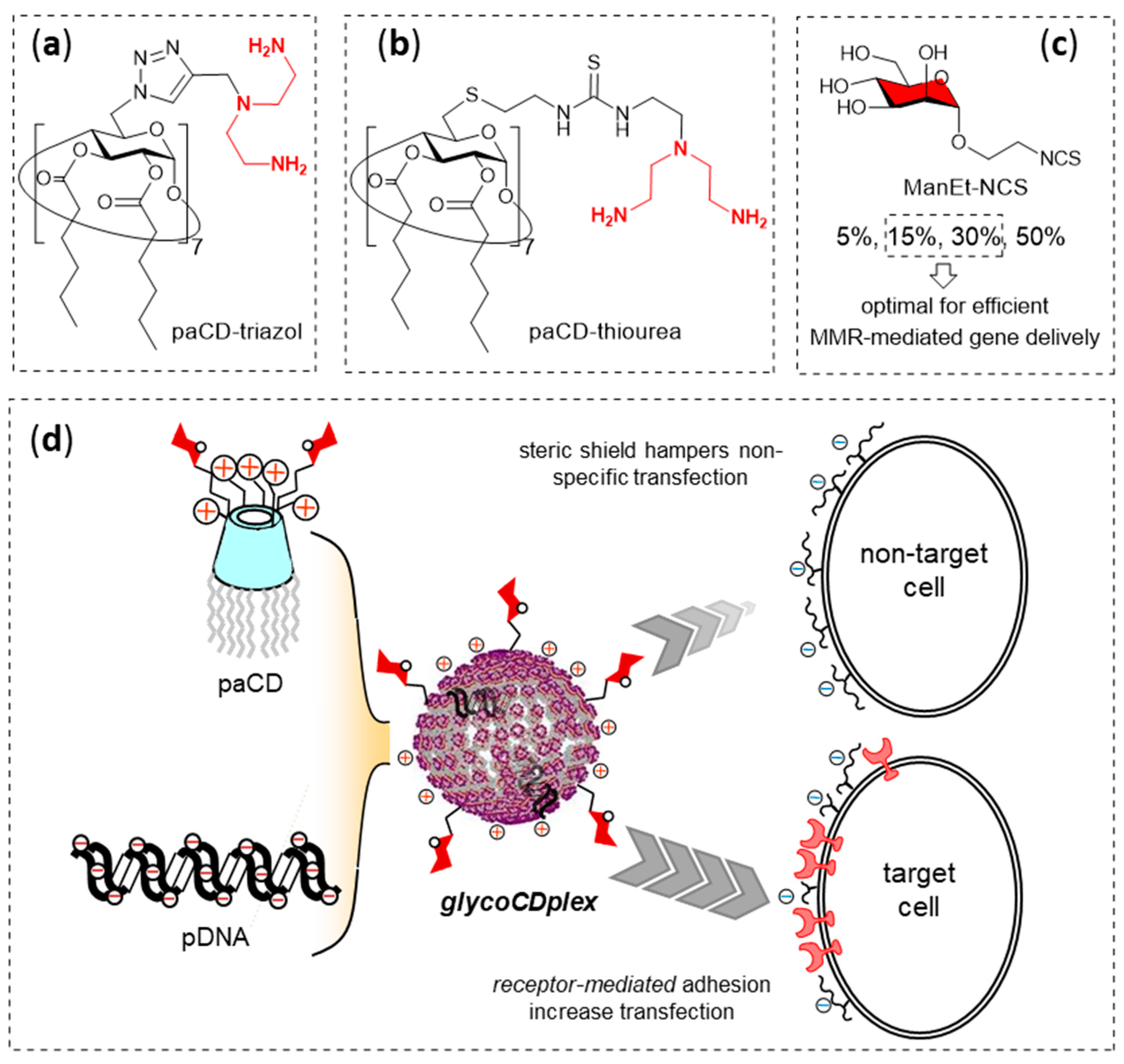
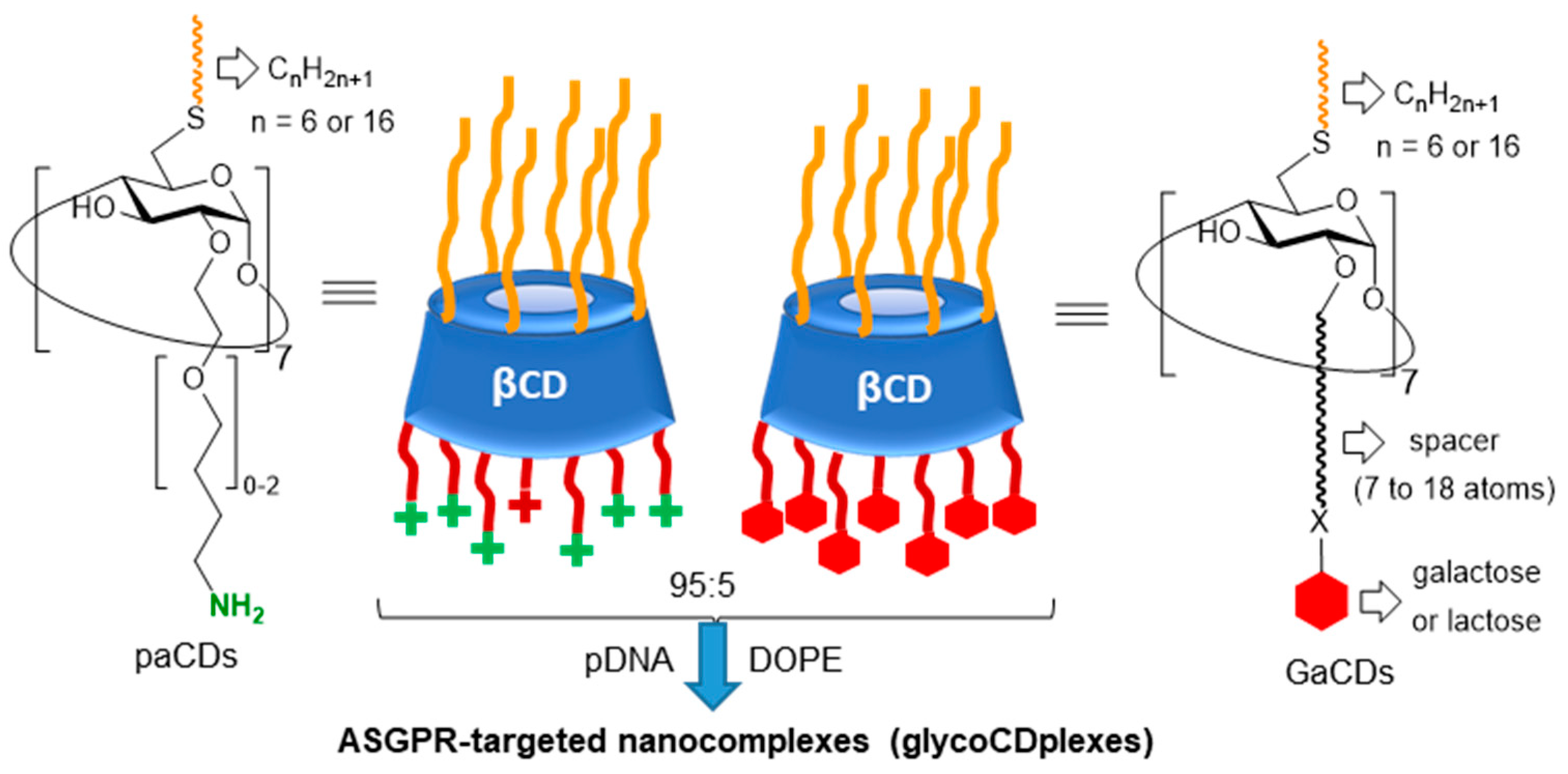
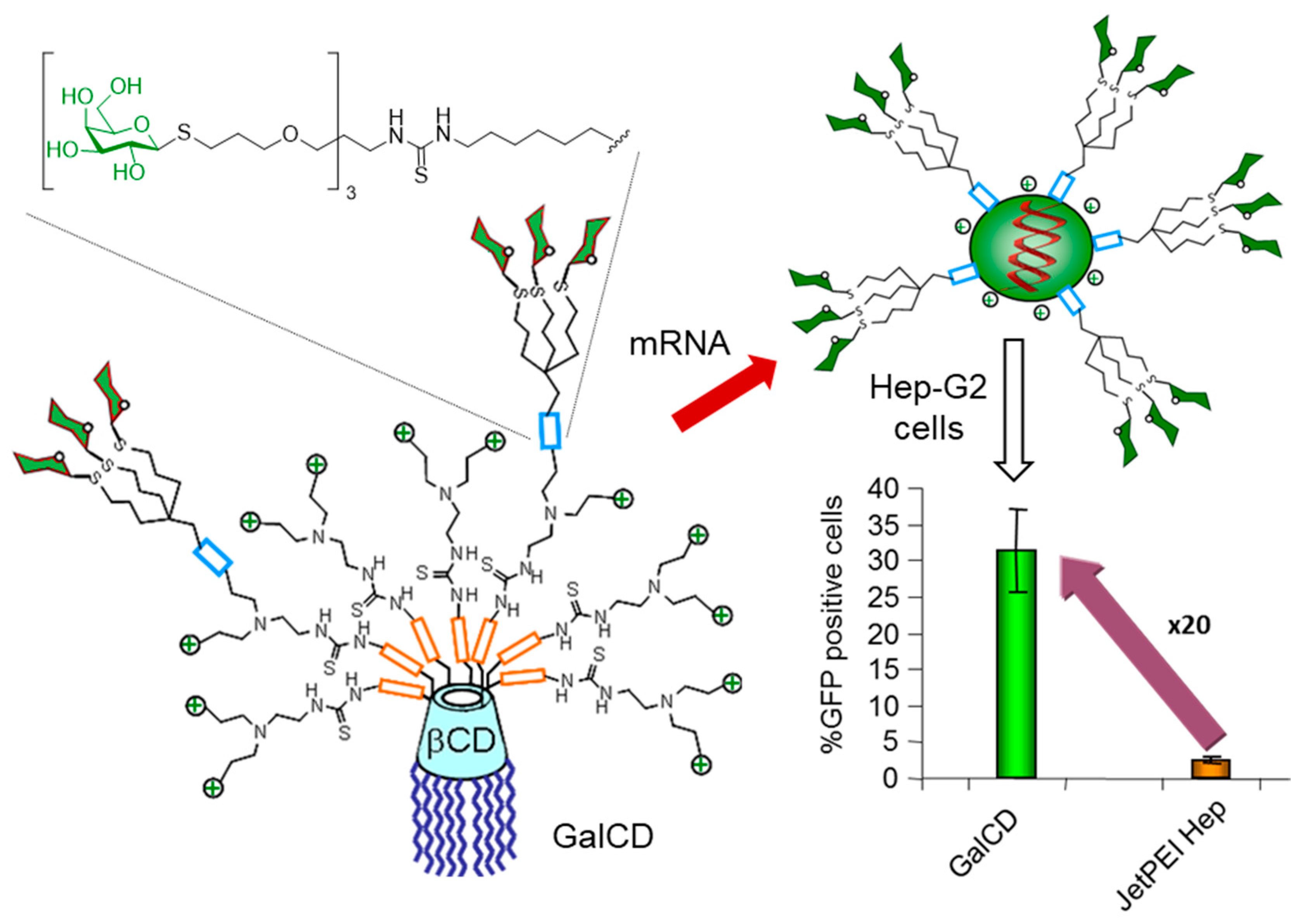
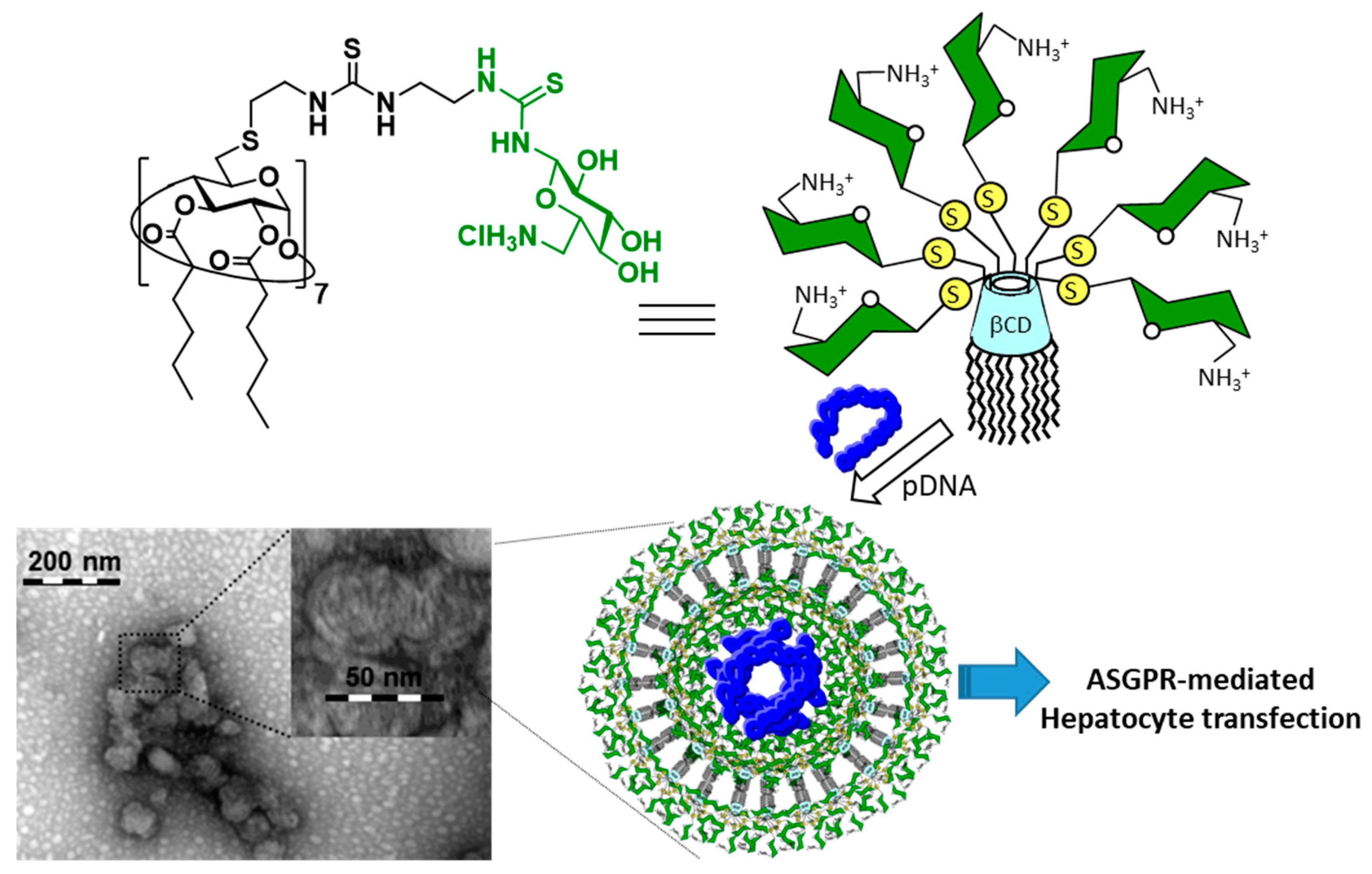
8. Functional Glyconanoparticles through CD-Mediated Glyco-Coating Strategies
9. Covalent Strategies to CD-Appended Glyconanomaterials
10. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; García Fernández, J.M.; Benito, J.M. Cyclodextrins for pharmacological and biomedical applications. In Supramolecular Systems in Biomedical Fields; Schneider, H.-J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; Chapter 5; pp. 94–139. [Google Scholar]
- Fakayode, S.O.; Lowry, M.; Fletcher, K.A.; Huang, X.; Powe, A.M.; Warner, I.M. Cyclodextrins host-guest chemistry in analytical and environmental chemistry. Curr. Anal. Chem. 2007, 3, 171–181. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and noncovalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, A.; Garcia-Fandino, R. The Lord of the nanorings: Cyclodextrins and the battle against SARS-CoV-2. J. Pharm. Sci. 2020, 588, 119689. [Google Scholar]
- García Fernández, J.M.; Ortiz Mellet, C.; Defaye, J. Glyconanocavities: Cyclodextrins and beyond. J. Incl. Phenom. Macrocyl. Chem. 2006, 56, 149–159. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Yu, X.; Wang, C.-L.; Sun, H.-J.; Hsieh, I.-F.; Li, Y.; Dong, X.-H.; Yue, K.; Van Horn, R.; Cheng, S.Z.D. Molecular nanoparticles are unique elements for macromolecular science: From “nanoatoms” to giant molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Yin, G.-Z.; Zhang, W.-B.; Cheng, S.Z.D. Giant molecules: Where chemistry, physics, and bio-science meet. Sci. China Chem. 2017, 60, 338–352. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; de Rossi, R.H. Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-H.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, 1806158. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. The classification and application of cyclodextrin polymers: A review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Shelley, H.; Babu, R.J. Role of cyclodextrins in nanoparticle-based drug delivery systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Gim, S.; Zhu, Y.; Seeberger, P.; Delbianco, M. Carbohydrate-based nanomaterials for biomedical applications. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1558. [Google Scholar] [CrossRef]
- Ortiz-Mellet, C.; Benito, J.M.; García Fernández, J.M.; Law, H.; Chmurski, K.; Defaye, J.; O’Sullivan, M.L.; Caro, H.N. Cyclodextrin-scaffolded glycoclusters. Chem. Eur. J. 1998, 4, 2523–2531. [Google Scholar] [CrossRef]
- Vargas-Berenguel, A.; Ortega-Caballero, F.; Casas-Solvas, J.M. Supramolecular chemistry of carbohydrate clusters with cores having guest binding abilities. Mini-Rev. Org. Chem. 2007, 4, 1–14. [Google Scholar] [CrossRef]
- Martínez, A.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate–protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Shiao, T.C. Glyconanosynthons as powerful scaffolds and building blocks for the rapid construction of multifaceted, dense and chiral dendrimers. Chem. Soc. Rev. 2015, 44, 3924–3941. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Godula, K. Nanoscale materials for probing the biological functions of the glycocalyx. Glycobiology 2016, 26, 797–803. [Google Scholar] [CrossRef]
- Zhan, W.; Wei, T.; Yu, Q.; Chen, H. Fabrication of supramolecular bioactive surfaces via β-cyclodextrin based host−guest interactions. ACS Appl. Mater. Interfaces 2018, 10, 36585–36601. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.-H.; Zhang, X.-H.; Tan, L.; Liu, C.-J. A highly efficient bactericidal surface based on the co-capture function and photodynamic sterilization. J. Mater. Chem. B 2018, 6, 6831–6841. [Google Scholar] [CrossRef]
- Zhan, W.; Wei, T.; Cao, L.; Hu, C.; Qu, Y.; Yu, Q.; Chen, H. Supramolecular platform with switchable multivalent affinity: Photo-reversible capture and release of bacteria. ACS Appl. Mater. Interfaces 2017, 9, 3505–3513. [Google Scholar] [CrossRef]
- Qu, Y.; Wei, T.; Zhan, W.; Hu, C.; Cao, L.; Yu, Q.; Chen, H. A reusable supramolecular platform for the specific capture and release of proteins and bacteria. J. Mater. Chem. B 2017, 5, 444–453. [Google Scholar] [CrossRef]
- Gade, M.; Paul, A.; Alex, C.; Choudhury, D.; Thulasiram, H.V.; Kikkeri, R. Supramolecular scaffolds on glass slides as sugar based rewritable sensors for bacteria. Chem. Commun. 2015, 51, 6346–6349. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-protein interactions: Basis of glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Jayaraman, N. Multivalent ligand presentation as a central concept to study intricate carbohydrate–protein interactions. Chem. Soc. Rev. 2009, 38, 3463–3483. [Google Scholar] [CrossRef]
- Cecioni, S.; Imberty, A.; Vidal, S. Glycomimetics versus multivalent glycoconjugates for the design of high affinity lectin ligands. Chem. Rev. 2015, 115, 525–561. [Google Scholar] [CrossRef]
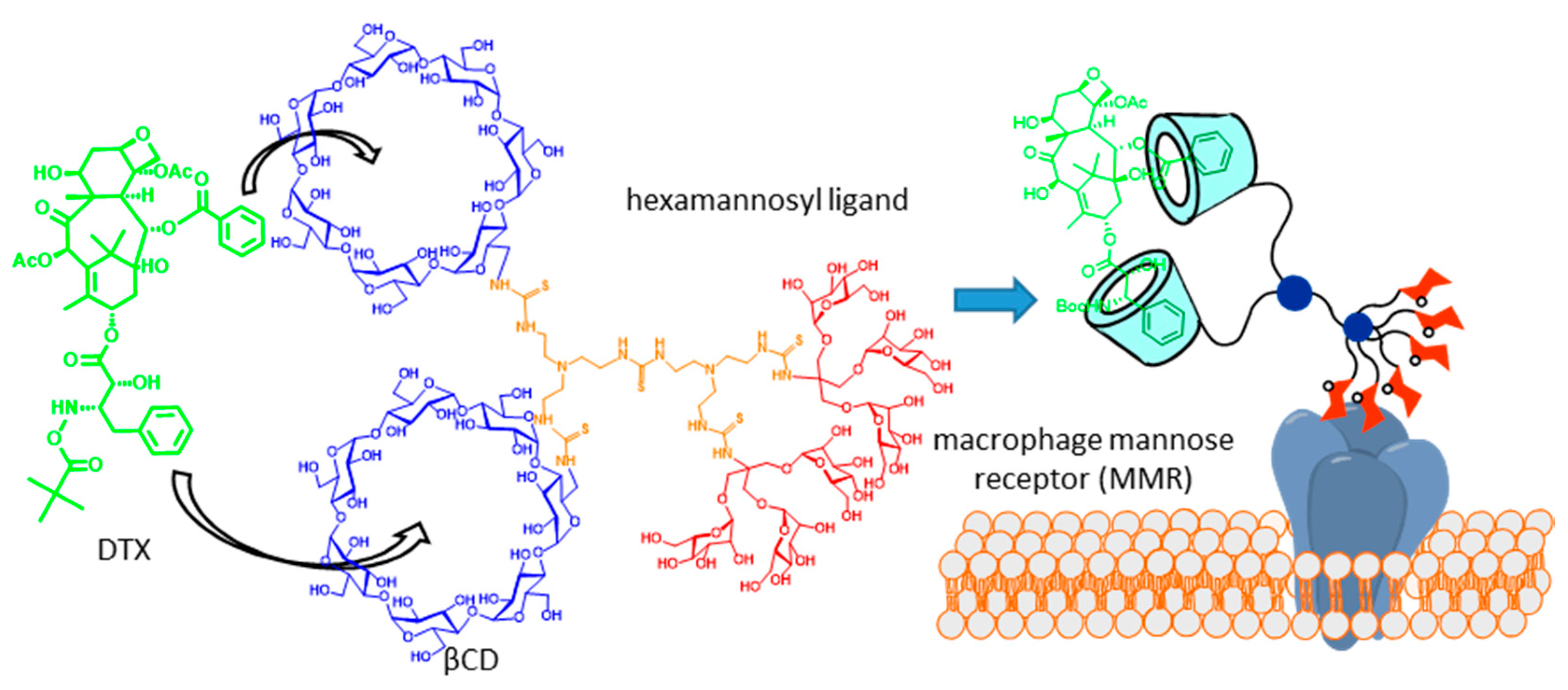
- Benito, J.M.; Gómez-García, M.; Ortiz Mellet, C.; Baussanne, I.; Defaye, J.; García Fernández, J.M. Optimizing saccharide-directed molecular delivery to biological receptors: Design, synthesis, and biological evaluation of glycodendrimer-cyclodextrin conjugates. J. Am. Chem. Soc. 2004, 126, 10355–10363. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Barner-Kowollik, C. Dynamic macromolecular material design—The versatility of cyclodextrin-based host–guest chemistry. Angew. Chem. Int. Ed. 2017, 56, 8350–8369. [Google Scholar] [CrossRef]
- Grünstein, D.; Maglinao, M.; Kikkeri, R.; Collot, M.; Barylyuk, K.; Lepenies, B.; Kamena, F.; Zenobi, R.; Seeberger, P.H. Hexameric supramolecular scaffold orients carbohydrates to sense bacteria. J. Am. Chem. Soc. 2011, 133, 13957–13966. [Google Scholar] [CrossRef]
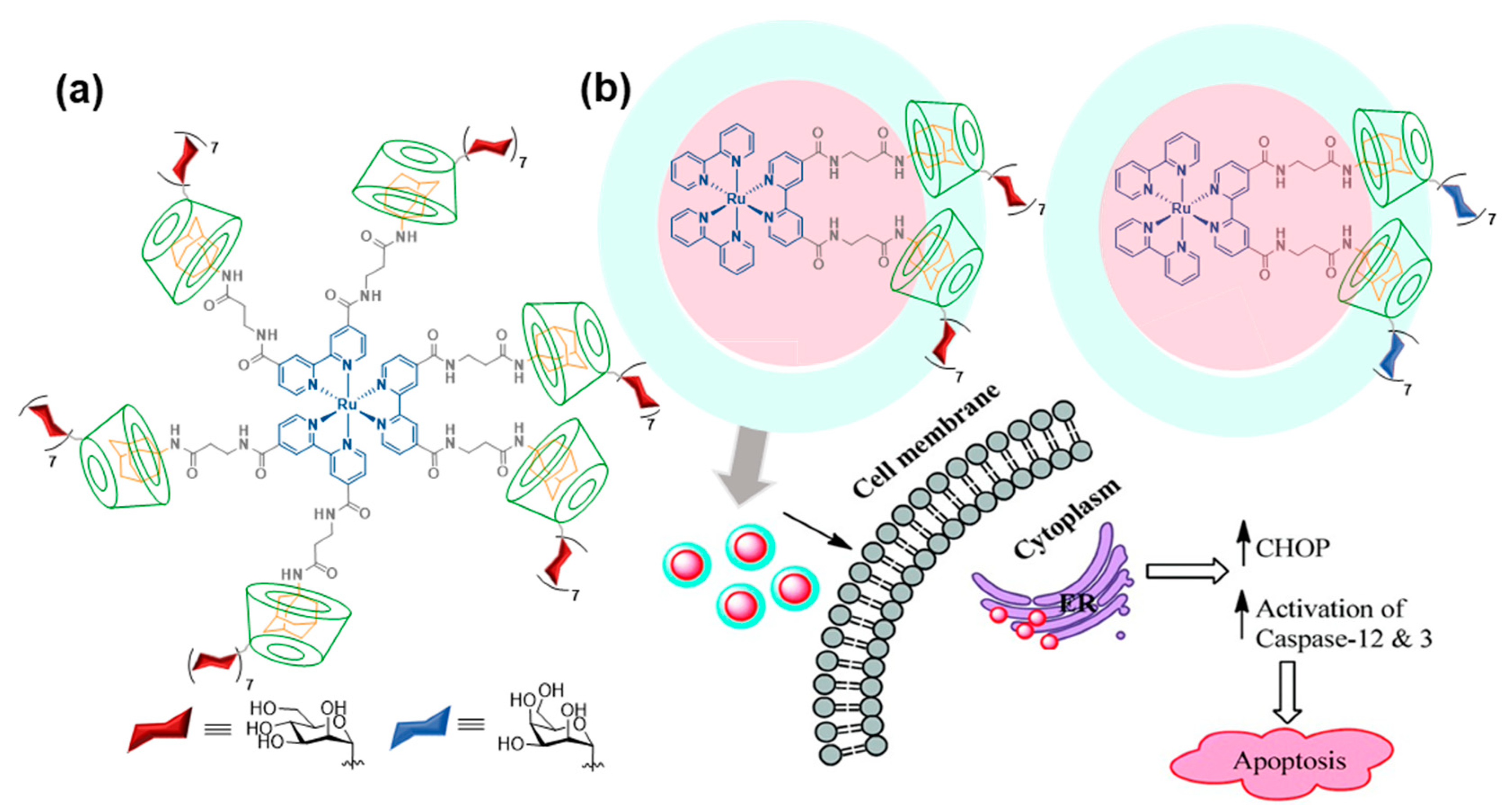
- Bavireddi, H.; Vasudeva Murthy, R.; Gade, M.; Sangabathuni, S.; Madhukar Chaudhary, P.; Alex, C.; Lepenies, B.; Kikkeri, R. Understanding carbohydrate–protein interactions using homologous supramolecular chiral Ru(II)-glyconanoclusters. Nanoscale 2016, 8, 19696–19702. [Google Scholar] [CrossRef]
- Bavireddi, H.; Vasudeva Murthy, R.; Gade, M.; Sangabathuni, S.; Kikkeri, R. Supramolecular metalloglycodendrimers selectively modulate lectin binding and delivery of Ru(II) complexes into mammalian cells. Org. Biomol. Chem. 2016, 14, 10816–10821. [Google Scholar] [CrossRef]
- Bavireddi, H.; Bharatez, P.; Kikkeri, R. Use of Boolean and fuzzy logics in lactose glycocluster research. Chem. Commun. 2013, 49, 9185–9187. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Wang, X.-J.; Xu, J.-L.; Ye, Z.; Wang, S.; Seeberger, P.H.; Yin, J. Targeted photodynamic killing of breast cancer cells employing heptamannosylated β-cyclodextrin-mediated nanoparticle formation of an adamantane-functionalized BODIPY photosensitizer. ACS Appl. Mater. Interfaces 2016, 8, 33405–33411. [Google Scholar] [CrossRef]
- Kang, Y.; Tang, X.; Cai, Z.; Zhang, X. Supra-amphiphiles for functional assemblies. Adv. Funct. Mater. 2016, 26, 8920–8931. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Q.; Bi, Y.; Chen, X.; Liu, L.; Ruan, C.; Zhao, Z.; Jiang, C. Supramolecular amphiphiles based on cyclodextrin and hydrophobic drugs. J. Mater. Chem. B 2017, 5, 2644–2654. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.P.; Song, X.; Zhuo, L.; Bu, H.; Tian, W. Photo- and pH- dual-responsive β-cyclodextrin-based supramolecular prodrug complex self-assemblies for programmed drug delivery. Chem. Asian J. 2018, 13, 3903–3911. [Google Scholar] [CrossRef]
- Yang, T.; Du, G.; Cui, Y.; Yu, R.; Hua, C.; Tian, W.; Zhang, Y. pH-sensitive doxorubicin-loaded polymeric nanocomplex based on β-cyclodextrin for liver cancer-targeted therapy. Int. J. Nanomed. 2019, 14, 1997–2010. [Google Scholar] [CrossRef]
- Varan, G.; Benito, J.M.; Ortiz Mellet, C.; Bilensoy, E. Development of polycationic amphiphilic cyclodextrin nanoparticles for anticancer drug delivery. Beilstein J. Nanotechnol. 2017, 8, 1457–1467. [Google Scholar] [CrossRef]
- Méndez-Ardoy, A.; Gomez-Garcia, M.; Geze, A.; Putaux, J.L.; Wouessidjewe, D.; Ortiz Mellet, C.; Defaye, J.; Garcia Fernandez, J.M.; Benito, J.M. Monodisperse nanoparticles from self-assembling amphiphilic cyclodextrins: Modulable tools for the encapsulation and controlled release of pharmaceuticals. Med. Chem. 2012, 8, 524–532. [Google Scholar] [CrossRef]
- Sallas, F.; Darcy, R. Amphiphilic cyclodextrins—Advances in synthesis and supramolecular chemistry. Eur. J. Org. Chem. 2008, 957–969. [Google Scholar] [CrossRef]
- Sallas, F.; Niikura, K.; Nishimura, S.-I. A practical synthesis of amphiphilic cyclodextrins fully substituted with sugar residues on the primary face. Chem. Commun. 2004, 596–597. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Forde, D.; Garozzo, D.; Malvagna, P.; Ravoo, B.J.; Darcy, R. Multivalent binding of galactosylated cyclodextrin vesicles to lectin. Org. Biomol. Chem. 2004, 2, 957–960. [Google Scholar] [CrossRef]
- Micali, N.; Villari, V.; Mazzaglia, A.; Monsú Scolaro, L.; Valerio, A.; Rencurosi, A.; Lay, L. Cyclodextrin nanoaggregates and their assembly with protein: A spectroscopic investigation. Nanotechnology 2006, 17, 3239–3244. [Google Scholar] [CrossRef]
- Mazzaglia, A.; Valerio, A.; Villari, V.; Rencurosi, A.; Lay, L.; Spadaro, S.; Monsú Scolaro, L.; Micali, N. Probing specific protein recognition by size-controlled glycosylated cyclodextrin nanoassemblies. New J. Chem. 2006, 30, 1662–1668. [Google Scholar] [CrossRef]
- McNicholas, S.; Rencurosi, A.; Lay, L.; Mazzaglia, A.; Sturiale, L.; Perez, M.; Darcy, R. Amphiphilic N-glycosyl-thiocarbamoyl cyclodextrins: synthesis, self-assembly, and fluorimetry of recognition by Lens culinaris lectin. Biomacromolecules 2007, 8, 1851–1857. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-targeted drug delivery with mannose-functionalized nanoparticles self-assembled from amphiphilic β-cyclodextrins. Chem. Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Méndez-Ardoy, A.; García Fernández, J.M. Click multivalent glycomaterials: Glycoclusters, glycodendrimers, glycopolymers, hybrid glycomaterials, and glycosurfaces. In Click Chemistry in Glycoscience: New Developments and Strategies; Witczak, Z.J., Bielski, R., Eds.; Wiley: Hoboken, NJ, USA, 2013; Chapter 6; pp. 143–182. [Google Scholar]
- Wei, P.; Ye, Z.; Cao, S.; Bai, S.; Seeberger, P.H.; Yin, J.; Hu, J. Combination therapy with amphotericin B and doxorubicin encapsulated in mannosylated nanomicelles for visceral leishmaniasis. Coll. Surf. A 2020, 598, 124804. [Google Scholar] [CrossRef]
- Fenyvesi, É. Cyclodextrin polymers in the pharmaceutical industry. J. Incl. Phenom. 1988, 6, 537–545. [Google Scholar] [CrossRef]
- Simoes, S.M.N.; Rey-Rico, A.; Concheiro, A.; Alvarez-Lorenzo, C. Supramolecular cyclodextrin-based drug nanocarriers. Chem. Commun. 2015, 51, 6275–6289. [Google Scholar] [CrossRef]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Cakir, N.; Hizal, G.; Becer, C.R. Supramolecular glycopolymers with thermoresponsive self-assembly and lectin binding. Polym. Chem. 2015, 6, 6623–6631. [Google Scholar] [CrossRef]
- Seidi, F.; Shamsabadi, A.A.; Amini, M.; Shabanian, M.; Crespy, D. Functional materials generated by allying cyclodextrin-based supramolecular chemistry with living polymerization. Polym. Chem. 2019, 10, 3674–3711. [Google Scholar] [CrossRef]
- Yilmaz, G.; Uzunova, V.; Napier, R.; Becer, C.R. Single-chain glycopolymer folding via host−guest interactions and its unprecedented effect on DC-SIGN binding. Biomacromolecules 2018, 19, 3040–3047. [Google Scholar] [CrossRef]
- Nelson, A.; Belitsky, J.M.; Vidal, S.; Joiner, C.S.; Baum, L.G.; Stoddart, J.F. A self-assembled multivalent pseudopolyrotaxane for binding galectin-1. J. Am. Chem. Soc. 2004, 126, 11914–11922. [Google Scholar] [CrossRef] [PubMed]
- Yui, N.; Ooya, T. Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials. Chem. Eur. J. 2006, 12, 6730–6737. [Google Scholar] [CrossRef] [PubMed]
- Nishide, J.-I.; Kashiwao, K.; Kitauchi, K.; Sasabe, H. Lectin recognition characteristics of carbohydrate-polyrotaxane beads. Mol. Cryst. Liq. Cryst. 2010, 519, 108–114. [Google Scholar] [CrossRef]
- Chwalek, M.; Auzély, R.; Fort, S. Synthesis and biological evaluation of multivalent carbohydrate ligands obtained by click assembly of pseudo-rotaxanes. Org. Biomol. Chem. 2009, 7, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gao, J.; Luo, Y.; Wei, T.; Dong, Y.; Chen, G.; Chen, H. One-pot multicomponent synthesis of glycopolymers through a combination of host-guest interaction, thiol-ene, and copper-catalyzed click reaction in water. Macromol. Rapid Commun. 2017, 38, 1700434. [Google Scholar] [CrossRef] [PubMed]
- Gómez-García, M.; Benito, J.M.; Rodríguez-Lucena, D.; Yu, J.; Chmurski, K.; Ortiz Mellet, C.; Gutiérrez Gallego, R.; Maestre, A.; Defaye, J.; Garcıía Fernández, J.M. Probing secondary carbohydrate-protein interactions with highly dense cyclodextrin-centered heteroglycoclusters: the heterocluster effect. J. Am. Chem. Soc. 2005, 127, 7970–7971. [Google Scholar] [CrossRef]
- Jiménez Blanco, J.L.; Ortiz Mellet, C.; García Fernández, J.M. Multivalency in heterogeneous glycoenvironments: Hetero-glycoclusters, -glycopolymers and -glycoassemblies. Chem. Soc. Rev. 2013, 42, 4518–4531. [Google Scholar] [CrossRef]
- Müller, C.; Despras, G.; Lindhorst, T.K. Organizing multivalency in carbohydrate recognition. Chem. Soc. Rev. 2016, 45, 3275–3302. [Google Scholar]
- Ortiz Mellet, C.; Nierengarten, J.-F.; García Fernández, J.M. Multivalency as an action principle in multimodal lectin recognition and glycosidase inhibition: A paradigm shift driven by carbon-based Glyconanomaterials. J. Mater. Chem. B 2017, 5, 6428–6436. [Google Scholar] [CrossRef]
- García-Moreno, M.I.; Ortega-Caballero, F.; Rísquez-Cuadro, R.; Ortiz Mellet, C.; García Fernández, J.M. The impact of heteromultivalency in lectin recognition and glycosidase inhibition: An integrated mechanistic study. Chem. Eur. J. 2017, 23, 6295–6304. [Google Scholar] [CrossRef]
- Rísquez-Cuadro, R.; Matsumoto, R.; Ortega-Caballero, F.; Nanba, E.; Higaki, K.; García Fernández, J.M.; Ortiz Mellet, C. Pharmacological chaperones for the treatment of α-mannosidosis. J. Med. Chem. 2019, 62, 5832–5843. [Google Scholar] [CrossRef] [PubMed]
- González-Cuesta, M.; Ortiz Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, J.; Jia, Y.-G.; Wang, J.; Mo, L.; Chen, X.; Qi, D.; Chen, Y.; Ren, L. Glycopolymers made from polyrotaxanes terminated with bile acids: Preparation, self-assembly, and targeting delivery. Macromol. Biosci. 2019, 19, 1800478. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, A.; Miyauchi, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Formation of supramolecular isomers; poly[2]rotaxane and supramolecular assembly. Chem. Commun. 2008, 4, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Colesnic, D.; de Beaumais, S.A.; Pembouong, G.; Portier, F.; Queijo, A.A.; Vázquez Tato, J.; Zhang, Y.; Ménand, M.; Bouteiller, L.; et al. Cyclodextrin-adamantane conjugates, self-inclusion and aggregation versus supramolecular polymer formation. Org. Chem. Front. 2014, 1, 703–706. [Google Scholar] [CrossRef]
- Evenou, P.; Rossignol, J.; Pembouong, G.; Gothland, A.; Colesnic, D.; Barbeyron, R.; Rudiuk, S.; Marcelin, A.-G.; Ménand, M.; Baigl, D.; et al. Bridging β-cyclodextrin prevents self-Inclusion, promotes supramolecular polymerization, and promotes cooperative interaction with nucleic acids. Angew. Chem. Int. Ed. 2018, 57, 7753–7758. [Google Scholar] [CrossRef]
- Cutrone, G.; Benkovics, G.; Malanga, M.; Casas-Solvas, J.M.; Fenyvesi, É.; Sortino, S.; García-Fuentes, L.; Vargas-Berenguel, A. Mannoside and 1,2-mannobioside β-cyclodextrin-scaffolded NO-photodonors for targeting antibiotic resistant bacteria. Carbohydr. Polym. 2018, 199, 649–660. [Google Scholar] [CrossRef]
- Wang, L.; Gong, C.; Yuan, X.; Wei, G. Controlling the self-assembly of biomolecules into functional nanomaterials through internal interactions and external stimulations: A review. Nanomaterials 2019, 9, 285. [Google Scholar] [CrossRef]
- Wei, K.; Li, J.; Chen, G.; Jiang, M. Dual molecular recognition leading to a protein−polymer conjugate and further self-assembly. ACS Macro Lett. 2013, 2, 278–283. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Nonviral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Lostalé-Seijo, I.; Montenegro, J. Synthetic materials at the forefront of gene delivery. Nat. Rev. Chem. 2018, 2, 258–277. [Google Scholar] [CrossRef]
- Ortiz Mellet, C.; Benito, J.M.; García Fernández, J.M. Preorganized, macromolecular, gene-delivery systems. Chem. Eur. J. 2010, 16, 6728–6742. [Google Scholar] [CrossRef]
- Jiménez Blanco, J.L.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M. Molecular nanoparticle-based gene delivery systems. J. Drug Deliv. Sci. Technol. 2017, 42, 18–37. [Google Scholar] [CrossRef]
- Geng, W.-C.; Huang, Q.; Xu, Z.; Wang, R.; Guo, D.-S. Gene delivery based on macrocyclic amphiphiles. Theranostics 2019, 9, 3094–3106. [Google Scholar] [CrossRef]
- Carbajo-Gordillo, A.I.; Jiménez Blanco, J.L.; Benito, J.M.; Lana, H.; Marcelo, G.; Di Giorgio, C.; Przybylski, C.; Hinou, H.; Ceña, V.; Ortiz Mellet, C.; et al. Click Synthesis of size and shape-tunable star polymers with functional macrocyclic cores for synergistic DNA complexation and delivery. Biomacromolecules 2020, 21, 5173–5188. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Hu, X.-Y.; Guo, D.-S. Biomedical applications of calixarenes: State-of-the-art and perspectives. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Illescas, B.M.; Pérez-Sánchez, A.; Mallo, A.; Martín-Domenech, Á.; Rodríguez-Crespo, I.; Martín, N. Multivalent cationic dendrofullerenes for gene transfer: Synthesis and DNA complexation. J. Chem. B 2020, 8, 4505–4515. [Google Scholar] [CrossRef]
- Carbajo-Gordillo, A.I.; Rodríguez-Lavado, J.; Jiménez Blanco, J.L.; Benito, J.M.; Di Giorgio, C.; Vélaz, I.; Tros de Ilarduya, C.; Ortiz Mellet, C.; García Fernández, J.M. Trehalose-based Siamese twin amphiphiles with tunable self-assembling, DNA nanocomplexing and gene delivery properties. Chem. Commun. 2019, 55, 8227–8230. [Google Scholar] [CrossRef]
- Gasparello, J.; Manicardi, A.; Casnati, A.; Corradini, R.; Gambari, R.; Finotti, A.; Sansone, F. Efficient cell penetration and delivery of peptide nucleic acids by an argininocalix[4]arene. Sci. Rep. 2019, 9, 3036. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Okamoto, K.; Harano, K.; Noiri, E.; Nakamura, E. Hierarchical assembly of siRNA with tetraamino fullerene in physiological conditions for efficient internalization into cells and knockdown. ACS Appl. Mater. Interfaces 2018, 10, 19347–19354. [Google Scholar] [CrossRef]
- Manzanares, D.; Araya-Durán, I.; Gallego-Yerga, L.; Játiva, P.; Márquez-Miranda, V.; Canan, J.; Jiménez Blanco, J.L.; Ortiz Mellet, C.; González-Nilo, F.D.; García Fernández, J.M.; et al. Molecular determinants for cyclooligosaccharide-based nanoparticle mediated effective siRNA transfection. Nanomedicine 2017, 12, 1607–1621. [Google Scholar] [CrossRef]
- Jiménez Blanco, J.L.; Ortega-Caballero, F.; Blanco-Fernández, L.; Carmona, T.; Marcelo, G.; Martínez-Negro, M.; Aicart, E.; Junquera, E.; Mendicuti, F.; Tros de Ilarduya, C.; et al. Trehalose-based Janus cyclooligosaccharides: The “click” synthesis and DNA-directed assembly into pH-sensitive transfectious nanoparticles. Chem. Commun. 2016, 52, 10117–10120. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Mellet, C.; García Fernández, J.M.; Benito, J.M. Cyclodextrin-based gene delivery systems. Chem. Soc. Rev. 2011, 40, 1586–1608. [Google Scholar] [CrossRef] [PubMed]
- García Fernández, J.M.; Benito, J.M.; Ortiz Mellet, C. Cyclodextrin-scaffolded glycotransporters for gene delivery. Pure Appl. Chem. 2013, 85, 1825–1845. [Google Scholar] [CrossRef]
- Lai, W.-F. Cyclodextrins in non-viral gene delivery. Biomaterials 2014, 35, 401–411. [Google Scholar] [CrossRef]
- Przybylski, C.; Benito, J.M.; Bonnet, V.; Ortiz Mellet, C.; García Fernández, J.M. Deciphering of polycationic carbohydrate based non-viral gene delivery agents by ESI-LTQ-Orbitrap using CID/HCD pairwise tandem mass spectrometry. RSC Adv. 2016, 6, 78803–78817. [Google Scholar] [CrossRef]
- Przybylski, C.; Benito, J.M.; Bonnet, V.; Ortiz Mellet, C.; García Fernández, J.M. Toward a suitable structural analysis of gene delivery carrier based on polycationic carbohydrates by electron transfer dissociation tandem mass spectrometry. Anal. Chim. Acta 2016, 948, 62–72. [Google Scholar] [CrossRef][Green Version]
- Przybylski, C.; Benito, J.M.; Bonnet, V.; Ortiz Mellet, C.; García Fernández, J.M. Revealing cooperative binding of polycationic cyclodextrins with DNA oligomers by capillary electrophoresis coupled to mass spectrometry. Anal. Chim. Acta 2018, 1002, 70–81. [Google Scholar] [CrossRef]
- Latxague, L.; Gaubert, A.; Barthélémy, P. Recent advances in the chemistry of glycoconjugate amphiphiles. Molecules 2018, 23, 89. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, Y.-L.; Li, Z.; Loh, X.J. Cyclodextrin-based sustained gene release systems: A supramolecular solution towards clinical applications. Mater. Chem. Front. 2019, 3, 181–192. [Google Scholar] [CrossRef]
- Díaz-Moscoso, A.; Patricia Balbuena, P.; Gómez-Garcia, M.; Ortiz Mellet, C.; Benito, J.M.; Le Gourriérec, L.; Di Giorgio, C.; Vierling, P.; Mazzaglia, A.; Micali, N.; et al. Rational design of cationic cyclooligosaccharides as efficient gene delivery systems. Chem. Commun. 2008, 17, 2001–2003. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Caballero, F.; Ortiz Mellet, C.; Le Gourriérec, L.; Guilloteau, N.; Di Giorgio, C.; Vierling, P.; Defaye, J.; García Fernández, J.M. Tailoring β-cyclodextrin for DNA complexation and delivery by homogeneous functionalization at the secondary face. Org. Lett. 2008, 10, 5143–5146. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Sallas, F.; Rai, D.K.; Ogier, J.; Darcy, R. Poly-6-cationic amphiphilic cyclodextrins designed for gene delivery. Org. Biomol. Chem. 2009, 7, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Moscoso, A.; Vercauteren, D.; Rejman, J.; Benito, J.M.; Ortiz Mellet, C.; De Smedt, S.C.; García Fernández, J.M. Insights in cellular uptake mechanisms of pDNA-policationic amphiphilic cyclodextrin nanoparticles (CDplexes). J. Control. Release 2010, 143, 318–325. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.J.; Guoa, J.; Byrne, C.; Darcy, R.; O’Driscoll, C.M. Mechanistic studies on the uptake and intracellular trafficking of novel cyclodextrin transfection complexes by intestinal epithelial cells. Int. J. Pharm. 2011, 413, 174–183. [Google Scholar] [CrossRef]
- Méndez-Ardoy, A.; Urbiola, K.; Aranda, C.; Ortiz Mellet, C.; García Fernández, J.M.; Tros de Ilarduya, C. Polycationic amphiphilic cyclodextrin-based nanoparticles for therapeutic dene Delivery. Nanomedicine 2011, 6, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, C.; Martínez, A.; Jiménez Blanco, J.L.; Di Giorgio, C.; Vierling, P.; Ortiz Mellet, C.; Defaye, J.; García Fernández, J.M. Polycationic amphiphilic cyclodextrins as gene vectors: Effect of the macrocyclic ring size on the DNA complexing and delivery properties. Org. Biomol. Chem. 2012, 10, 5570–5581. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Ogier, J.; Desgranges, S.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. A click chemistry route to 2-functionalised PEGylated and cationic β-cyclodextrins: Co-formulation opportunities for siRNA delivery. Org. Biomol. Chem. 2012, 10, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Doyle, D.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Characterisation of cationic amphiphilic cyclodextrins for neuronal delivery of siRNA: Effect of reversing primary and secondary face modifications. Eur. J. Pharm. Sci. 2012, 47, 896–903. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Godinho, B.M.D.C.; Ogier, J.; Devocelle, M.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Click-modified cyclodextrins as nonviral vectors for neuronal siRNA delivery. ACS Chem. Neurosci. 2012, 3, 744–752. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Ogier, J.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. Cationic and PEGylated amphiphilic cyclodextrins: Co-formulation opportunities for neuronal siRNA delivery. PLoS ONE 2013, 8, e66413. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, M.J.; O’Mahony, A.M.; Byrne, C.; Darcy, R.; O’Driscoll, C.M. Gastrointestinal gene delivery by cyclodextrins—In vitro quantification of extracellular barriers. Int. J. Pharm. 2013, 456, 390–399. [Google Scholar]
- Martínez, A.; Bienvenu, C.; Jiménez Blanco, J.L.; Vierling, P.; Ortiz Mellet, C.; García Fernández, J.M.; Di Giorgio, C. Amphiphilic oligoethyleneimine-β-cyclodextrin “click” clusters for enhanced DNA delivery. J. Org. Chem. 2013, 78, 8143–8148. [Google Scholar] [CrossRef]
- Villari, V.; Mazzaglia, A.; Darcy, R.; O’Driscoll, C.M.; Micali, N. Nanostructures of cationic amphiphilic cyclodextrin complexes with DNA. Biomacromolecules 2013, 14, 811–817. [Google Scholar] [CrossRef]
- Godinho, B.M.D.C.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Self-assembling modified β-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: Focus on Huntington’s disease. Mol. Pharm. 2013, 10, 640–649. [Google Scholar] [CrossRef]
- McCarthy, J.; O’Neill, M.J.; Bourre, L.; Walsh, D.; Quinlan, A.; Hurley, G.; Ogier, J.; Shanahan, F.; Melgar, S.; Darcy, R.; et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. J. Control. Release 2013, 168, 28–34. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, A.M.; Cronin, M.F.; McMahon, A.; Evans, J.C.; Daly, K.; Darcy, R.; O’Driscoll, C.M. Biophysical and structural characterisation of nucleic acid complexes with modified cyclodextrins using circular dichroism. J. Pharm. Sci. 2014, 103, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Hibbitts, A.; O’Mahony, A.M.; Forde, E.; Nolan, L.; Ogier, J.; Desgranges, S.; Darcy, R.; MacLoughlin, R.; O’Driscoll, C.M.; Cryan, S.A. Early-stage development of novel cyclodextrin siRNA nanocomplexes allows for successful postnebulization transfection of bronchial epithelial cells. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Lomazzi, M.; Franceschi, V.; Sansone, F.; Ortiz Mellet, C.; Donofrio, G.; Casnati, A.; García Fernández, J.M. Cyclodextrin- and calixarene-based polycationic amphiphiles as gene delivery systems: A structure-activity relationship study. Org. Biomol. Chem. 2015, 13, 1708–1723. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; McCarthy, J.; Torres-Fuentes, C.; Cryan, J.F.; Ogier, J.; Darcy, R.; Watson, R.W.; O’Driscoll, C.M. Cyclodextrin mediated delivery of NF-kB and SRF siRNA reduces the invasion potential of prostate cancer cells in vitro. Gene Ther. 2015, 22, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Guo, J.; Tierney, E.G.; Curtin, C.M.; Malhotra, M.; Darcy, R.; O’Brien, F.J.; O’Driscoll, C.M. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials 2015, 66, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Pflueger, I.; Charrat, C.; Ortiz Mellet, C.; García Fernández, J.M.; Di Giorgio, C.; Benito, J.M. Cyclodextrin-Based Facial Amphiphiles: Assessing the impact of the hydrophilic-lipophilic balance in the self-assembly, DNA complexation and gene delivery capabilities. Org. Biomol. Chem. 2016, 14, 10037–10049. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Guo, J.; Raftery, R.M.; Mencía Castaño, I.; Curtin, C.M.; Gooding, M.; Darcy, R.; O’ Brien, F.J.; O’ Driscoll, C.M. Nanoparticle-mediated siRNA delivery assessed in a 3D co-culture model simulating prostate cancer bone metastasis. Int. J. Pharm. 2016, 511, 1058–1069. [Google Scholar] [CrossRef]
- Remaut, K.; De Clercq, E.; Andries, V.; Rombouts, K.; Van Gils, V.; Cicchelero, V.; Vandenbussche, I.; Van Praet, S.; Benito, J.M.; García Fernández, J.M.; et al. Aerosolized non-viral nucleic acid delivery in the vaginal tract of pigs. Pharm. Res. 2016, 3, 384–394. [Google Scholar] [CrossRef]
- Minnaert, A.-K.; Dakwar, G.R.; Benito, J.M.; García Fernández, J.M.; Ceelen, W.; De Smedt, S.C.; Remaut, K. High-Pressure Nebulization as Application Route for the Peritoneal Administration of siRNA Complexes. Macromol. Biosci. 2017, 17, 1700024. [Google Scholar]
- Martínez-Negro, M.; Caracciolo, G.; Palchetti, S.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Laganà, A.; Ortiz Mellet, C.; Benito, J.M.; García Fernández, J.M.; et al. Biophysics and protein corona analysis of Janus cyclodextrin-DNA nanocomplexes. Efficient cellular transfection on cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1737–1749. [Google Scholar] [CrossRef]
- Gallego-Yerga, L.; Benito, J.M.; Blanco-Fernández, L.; Martínez-Negro, M.; Vélaz, I.; Aicart, E.; Junquera, E.; Ortiz Mellet, C.; Tros de Ilarduya, C.; García Fernández, J.M. Plasmid-templated control of DNA–cyclodextrin nanoparticle morphology through molecular vector design for effective gene delivery. Chem. Eur. J. 2018, 24, 3825–3835. [Google Scholar] [CrossRef]
- Singh, R.P.; Hidalgo, T.; Cazade, P.-A.; Darcy, R.; Cronin, M.F.; Dorin, I.; O’Driscoll, C.M.; Thompson, D. Self-assembled cationic β-cyclodextrin nanostructures for siRNA delivery. Mol. Pharmaceutics 2019, 16, 1358–1366. [Google Scholar] [CrossRef]
- Martín-Moreno, A.; Jiménez Blanco, J.L.; Mosher, J.; Swanson, D.R.; García Fernández, J.M.; Sharma, A.; Ceña, V.; Muñoz-Fernández, M.A. Nanoparticle-delivered HIV peptides to dendritic cells a promising approach to generate a therapeutic vaccine. Pharmaceutics 2020, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Blanco-Fernández, L.; Urbiola, K.; Carmona, T.; Marcelo, G.; Benito, J.M.; Mendicuti, F.; Tros de Ilarduya, C.; Ortiz Mellet, C.; García Fernández, J.M. Host-guest mediated DNA templation of polycationic supramolecules for hierarchical nanocondensation and the delivery of gene material. Chem. Eur. J. 2015, 21, 12093–12104. [Google Scholar] [CrossRef] [PubMed]
- Balbuena, P.; Lesur, D.; González Álvarez, M.J.; Mendicuti, F.; Ortiz Mellet, C.; García Fernández, J.M. One-pot regioselective synthesis of 2I,3I-O-(o-xylylene)-capped cyclomaltooligosaccharides: Tailoring the topology and supramolecular properties of cyclodextrins. Chem Commun. 2007, 31, 3270–3272. [Google Scholar] [CrossRef] [PubMed]
- González-Álvarez, M.J.; Balbuena, P.; Ortiz Mellet, C.; García Fernández, J.M.; Mendicuti, F. Study of the conformational and self-aggregation properties of 2I,3I-O-(o-Xylylene)-per-O-Me-α- and -β-cyclodextrins by fluorescence and molecular modeling. J. Phys. Chem. B 2008, 112, 13717–13729. [Google Scholar] [CrossRef]
- Gonzalez-Álvarez, M.J.; Vicente, J.; Ortiz Mellet, C.; García Fernández, J.M.; Mendicuti, F. Thermodynamics of the dimer formation of 2I,3I-O-(o-xylylene)-per-O-Me-γ-cyclodextrin: Fluorescence, molecular mechanics and molecular dynamics. J. Fluoresc. 2009, 19, 975–988. [Google Scholar] [CrossRef]
- González-Álvarez, M.J.; Méndez-Ardoy, A.; Benito, J.M.; García Fernández, J.M.; Mendicuti, F. Self-association of a naphthalene-capped-β-cyclodextrin through cooperative strong hydrophobic interactions. J. Photochem. Photobiol. A 2011, 223, 25–36. [Google Scholar] [CrossRef]
- González-Álvarez, M.J.; Mayordomo, N.; Gallego-Yerga, L.; Ortiz Mellet, C.; Mendicuti, F. Improving inclusion capabilities of permethylated cyclodextrins by appending a cap-like aromatic moiety. Tetrahedron 2012, 68, 2961–2972. [Google Scholar] [CrossRef]
- González-Álvarez, M.J.; Benito, J.M.; García Fernández, J.M.; Ortiz Mellet, C.; Mendicuti, F. Influence of the macroring size on the self-association thermodynamics of cyclodextrins with a double-linked naphthalene at the secondary face. J. Phys. Chem. B 2013, 117, 5472–5485. [Google Scholar] [CrossRef]
- Balbuena, P.; Gonçalves-Pereira, R.; Jiménez Blanco, J.L.; García-Moreno, M.I.; Lesur, D.; Ortiz Mellet, C.; García Fernández, J.M. o-Xylylene protecting group in carbohydrate chemistry: Application to the regioselective protection of a single vic-diol segment in cyclodextrins. J. Org. Chem. 2013, 78, 1390–1403. [Google Scholar] [CrossRef]
- Gallego-Yerga, L.; González-Álvarez, M.J.; Mayordomo, N.; Santoyo-González, F.; Benito, J.M.; Ortiz Mellet, C.; Mendicuti, F.; García Fernández, J.M. Dynamic self-assembly of polycationic clusters based on cyclodextrins for pH-Sensitive DNA nanocondensation and delivery by component design. Chem. Eur. J. 2014, 22, 6622–6627. [Google Scholar] [CrossRef]
- Neva, T.; Carmona, T.; Benito, J.M.; Przybylski, C.; Ortiz Mellet, C.; Mendicuti, F.; García Fernández, J.M. Xylylene clips for the topology-guided control of the inclusion and self-assembling properties of cyclodextrins. J. Org. Chem. 2018, 83, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Neva, T.; Carmona, T.; Benito, J.M.; Przybylski, C.; Ortiz Mellet, C.; Mendicuti, F.; García Fernández, J.M. Dynamic control of the self-assembling properties of cyclodextrins by the interplay of aromatic and host-guest interactions. Front. Chem. 2019, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Neva, T.; Ortiz Mellet, C.; García Fernández, J.M.; Benito, J.M. Multiply–linked cyclodextrin–aromatic hybrids: Caps, hinges and clips. J. Carbohydr. Chem. 2019, 38, 470–493. [Google Scholar] [CrossRef]
- Neva, T.; Carbajo-Gordillo, A.I.; Benito, J.M.; Lana, H.; Marcelo, G.; Ortiz Mellet, C.; Tros de Ilarduya, C.; Mendicuti, F.; García Fernández, J.M. Tuning the topological landscape of DNA-cyclodextrin nanocomplexes by molecular design. Chem. Eur. J. 2020, 26, 15259–15269. [Google Scholar] [CrossRef]
- Guo, J.; Ogier, J.R.; Desgranges, S.; Darcy, R.; O’Driscoll, C. Anisamide-targeted cyclodextrin nanoparticles for siRNA delivery to prostate tumours in mice. Biomaterials 2012, 33, 7775–7784. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Desgranges, S.; Ogier, J.; Quinlan, A.; Devocelle, M.; Darcy, R.; Cryan, J.F.; O’Driscoll, C.M. In vitro investigations of the efficacy of cyclodextrin-siRNA complexes modified with lipid-PEG-octaarginine: Towards a formulation strategy for non-viral neuronal siRNA delivery. Pharm. Res. 2013, 30, 1086–1098. [Google Scholar] [CrossRef]
- Aranda, C.; Urbiola, K.; Méndez Ardoy, A.; García Fernández, J.M.; Ortiz Mellet, C.; Tros de Ilarduya, C. Targeted gene delivery by new folate–polycationic amphiphilic cyclodextrin–DNA nanocomplexes in vitro and in vivo. Eur. J. Pharm. Biopharm. 2013, 85, 390–397. [Google Scholar] [CrossRef]
- Gooding, M.; Malhotra, M.; McCarthy, D.J.; Godinho, B.M.D.C.; Cryan, J.F.; Darcy, R.; O’Driscoll, C.M. Synthesis and characterization of rabies virus glycoprotein tagged amphiphilic cyclodextrins for siRNA delivery in human glioblastoma cells: In vitro analysis. Eur. J. Pharm. Sci. 2015, 71, 80–92. [Google Scholar] [CrossRef]
- Malhotra, M.; Gooding, M.; Sallas, F.; Evans, J.C.; Fitzgerald, K.A.; Evans, J.C.; Darcy, R.; O’Driscoll, C.M. A novel, anisamide-targeted cyclodextrin nanoformulation for siRNA delivery to prostate cancer cells expressing the sigma-1 receptor. Int. J. Pharm. 2016, 499, 131–145. [Google Scholar]
- Malhotra, M.; Guo, J.; Guo, J.F.; O’Shea, J.P.; Hanrahan, K.; O’Neill, A.; Landry, W.D.; Griffin, B.T.; Darcy, R.; Watson, R.W.; et al. Folate-targeted amphiphilic cyclodextrin.siRNA nanoparticles for prostate cancer therapy exhibit PSMA mediated uptake, therapeutic gene silencing in vitro and prolonged circulation in vivo. Nanomedicine Nanotechnol. Biol. Med. 2016, 12, 2341–2351. [Google Scholar]
- Evans, J.C.; Malhotra, M.; Fitzgerald, K.A.; Guo, J.; Cronin, M.F.; Fitzgerald, K.A.; Cronin, M.F.; Curtin, C.M.; O’Brien, F.J.; Darcy, R.; et al. Formulation and evaluation of anisamide-targeted amphiphilic cyclodextrin nanoparticles to promote therapeutic gene silencing in a 3D prostate cancer bone metastases model. Mol. Pharmaceutics 2017, 14, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Russell, E.G.; Darcy, R.; Cotter, T.G.; McKenna, S.L.; Cahill, M.R.; O’Driscoll, C.M. Antibody-targeted cyclodextrin-based nanoparticles for siRNA delivery in the treatment of acute myeloid leukemia: Physicochemical characteristics, in vitro mechanistic studies, and ex vivo patient derived therapeutic efficacy. Mol. Pharmaceutics 2017, 14, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.C.; Malhotra, M.; Sweeney, K.; Darcy, R.; Nelson, C.C.; Hollier, B.G.; O’Driscoll, C.M. Folate-targeted amphiphilic cyclodextrin nanoparticles incorporating a fusogenic peptide deliver therapeutic siRNA and inhibit the invasive capacity of 3D prostate cancer tumours. Int. J. Pharm. 2017, 532, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Moscoso, A.; Guilloteau, N.; Bienvenu, C.; Mendez-Ardoy, A.; Jiménez Blanco, J.L.; Benito, J.M.; Le Gourriérec, L.; Di Giorgio, C.; Vierling, P.; Defaye, J.; et al. Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery. Biomaterials 2011, 32, 7263–7273. [Google Scholar] [CrossRef]
- Cendret, V.; François-Heude, M.; Méndez-Ardoy, A.; Moreau, V.; García Fernández, J.M.; Djedaïni-Pilard, F. Design and synthesis of a ‘‘click’’ high-mannose oligosaccharide mimic emulating Man8 binding affinity towards Con A. Chem. Commun. 2012, 48, 3733–3735. [Google Scholar] [CrossRef]
- Rodríguez-Lavado, J.; de La Mata, M.; Jiménez-Blanco, J.L.; García-Moreno, M.I.; Benito, J.M.; Díaz-Quintana, A.; Sánchez-Alcázar, J.A.; Higaki, K.; Nanba, E.; Ohno, K.; et al. Targeted delivery of pharmacological chaperones for Gaucher disease to macrophages by a mannosylated cyclodextrin carrier. Org. Biomol. Chem. 2014, 12, 2289–2301. [Google Scholar] [CrossRef]
- François-Heude, M.; Méndez-Ardoy, A.; Cendret, V.; Lafite, P.; Daniellou, R.; Ortiz Mellet, C.; García Fernández, J.M.; Moreau, V.; Djedaïni-Pilard, F. Synthesis of high-mannose oligosaccharide analogues through click chemistry: True functional mimics of their natural counterparts against lectins? Chem. Eur. J. 2015, 21, 1978–1991. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Goyard, D.; Nanba, E.; Higaki, K.; García Fernández, J.M.; Renaudet, O.; Ortiz Mellet, C. Multivalent glycoligands with lectin/enzyme dual specificity: Self-deliverable glycosidase regulators. Chem. Commun. 2019, 55, 12845–12848. [Google Scholar] [CrossRef]
- Gadekar, A.; Bhowmick, S.; Pandit, A. A glycotherapeutic approach to functionalize biomaterial-based systems. Adv. Funct. Mater. 2020, 1910031. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Application of glycosylation in targeted drug delivery. Eur. J. Med. Chem. 2019, 182, 111612. [Google Scholar] [CrossRef]
- Kawakami, S.; Hashida, M. Glycosylation-mediated targeting of carriers. J. Control. Release 2014, 190, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ardoy, A.; Díaz-Moscoso, A.; Ortiz Mellet, C.; Di Giorgio, C.; Vierling, P.; Benito, J.M.; García Fernández, J.M. Harmonized tuning of nucleic acid and lectin binding properties with multivalent cyclodextrins for macrophage-selective gene delivery. RSC Adv. 2015, 5, 76464–76471. [Google Scholar] [CrossRef]
- McMahon, A.; O’Neill, M.J.; Gomez, E.; Donohue, R.; Forde, D.; Darcy, R.; O’Driscoll, C.M. Targeted gene delivery to hepatocytes with galactosylated amphiphilic cyclodextrins. J. Pharm. Pharmacol. 2012, 64, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Donohue, R.; Mazzaglia, A.; Ravoo, B.J.; Darcy, R. Cationic β-cyclodextrin bilayer vesicles. Chem. Commun. 2002, 7, 2864–2865. [Google Scholar] [CrossRef]
- Symens, N.; Méndez-Ardoy, A.; Díaz-Moscoso, A.; Sánchez-Fernández, E.; Remaut, K.; Demeester, J.; García Fernández, J.M.; De Smedt, S.C.; Rejman, J. Efficient transfection of hepatocytes mediated by mRNA complexed to galactosylated cyclodextrins. Bioconjugate Chem. 2012, 23, 1276–1289. [Google Scholar] [CrossRef]
- Baussanne, I.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M.; Law, H.; Defaye, J. Synthesis and comparative lectin-binding affinity of mannosyl-coated β-cyclodextrin-dendrimer constructs. Chem. Commun. 2000, 1489–1490. [Google Scholar] [CrossRef]
- Smiljanic, N.; Moreau, V.; Yockot, D.; Benito, J.M.; García Fernández, J.M.; Djedaïni-Pilard, F. Supramolecular control of oligosaccharide-protein interactions: Switchable and tunable ligands for concanavalin A based on β-cyclodextrin. Angew. Chem. Int. Ed. 2006, 45, 5465–5468. [Google Scholar] [CrossRef]
- Aguilar-Moncayo, E.M.; Guilloteau, N.; Bienvenu, C.; Jiménez Blanco, J.L.; Di Giorgio, C.; Vierling, P.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-scaffolded amphiphilic aminoglucoside clusters: Self-assembling and gene delivery capabilities. New J. Chem. 2014, 38, 5215–5225. [Google Scholar] [CrossRef]
- Guilloteau, N.; Bienvenu, C.; Charrat, C.; Jiménez Blanco, J.L.; Díaz-Moscoso, A.; Ortiz Mellet, C.; García Fernández, J.M.; Vierling, P.; Di Giorgio, C. Cell uptake mechanisms of glycosylated cationic pDNA–cyclodextrin nanoparticles. RSC Adv. 2015, 5, 29135–29144. [Google Scholar] [CrossRef]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with α-cyclodextrin. Bioconjugate Chem. 2002, 13, 1211–1219. [Google Scholar] [CrossRef]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. In vitro and in vivo gene transfer by an optimized α-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjugate Chem. 2003, 14, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Mori, Y.; Jono, H.; Ando, Y.; Arima, H. Design and evaluation of polyamidoamine dendrimer conjugate with PEG, α-cyclodextrin and lactose as a novel hepatocyte-selective gene carrier in vitro and in vivo. J. Drug Target. 2013, 21, 487–496. [Google Scholar] [CrossRef]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Tanaka, T.; Ohyama, A.; Sato, N.; Higashi, T.; Arima, H. Design and Evaluation of Thioalkylated Mannose-Modified Dendrimer (G3)/α-cyclodextrin conjugates as antigen-presenting cell-selective siRNA carriers. AAPS J. 2014, 16, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
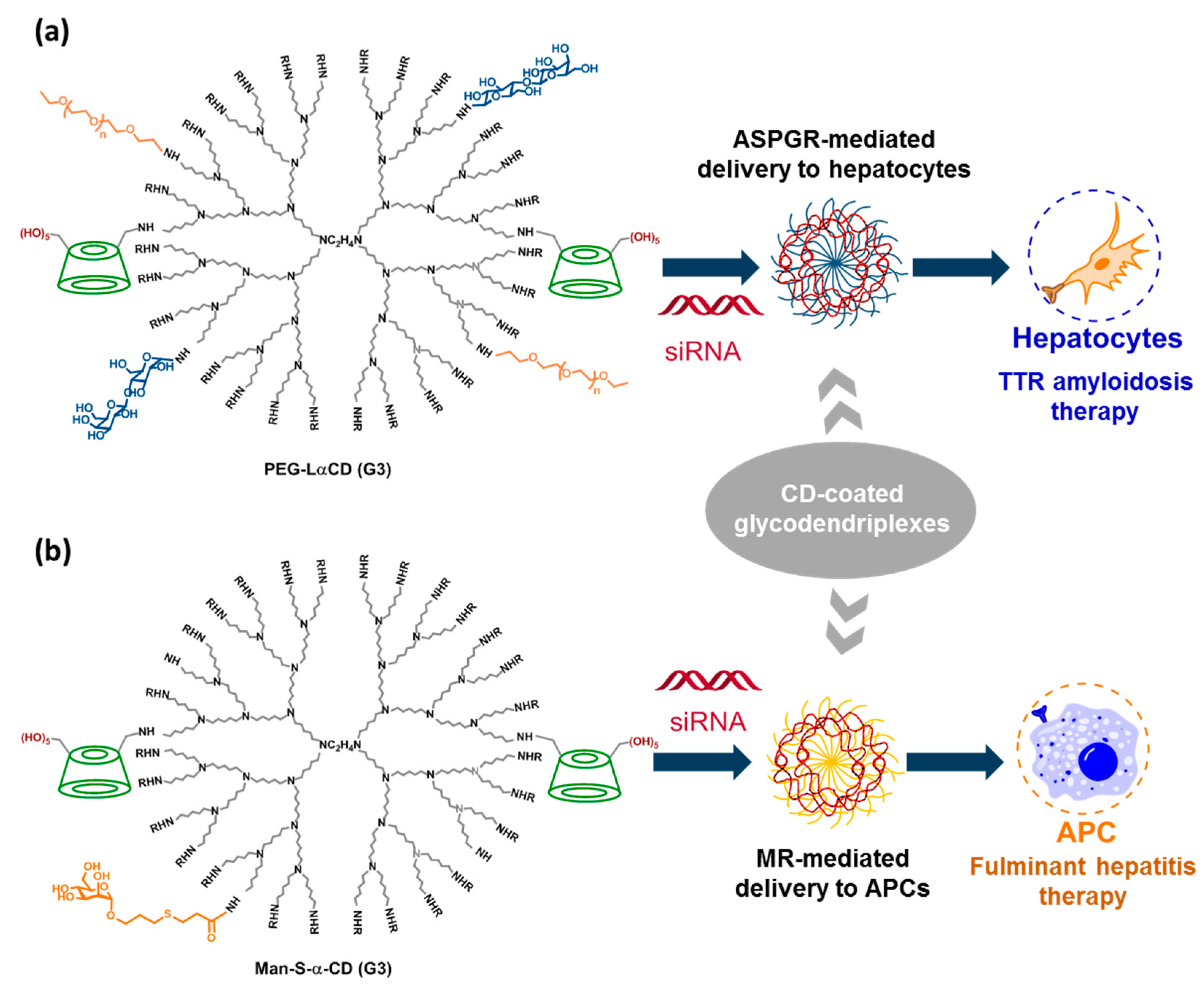
- Hayashi, Y.; Higashi, T.; Motoyama, K.; Hiroumi Jono, H.; Yukio Ando, Y.; Onodera, R.; Arima, H. Hepatocyte-targeted delivery of siRNA polyplex with PEG-modified lactosylated dendrimer/cyclodextrin conjugates for transthyretin-related amyloidosis therapy. Biol. Pharm. Bull. 2019, 42, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, K.; Mitsuyasu, R.; Akao, C.; Hashim, I.I.A.; Sato, N.; Tanaka, T.; Higashi, T.; Arima, H. Potential use of thioalkylated mannose-modified dendrimer (G3)/α-cyclodextrin conjugate as an NF-κB siRNA carrier for the treatment of fulminant hepatitis. Mol. Pharmaucetics 2015, 12, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, J.; Stuart, M.C.A.; Ravoo, B.J. Sugar-decorated sugar vesicles: Lectin-carbohydrate recognition at the surface of cyclodextrin vesicles. Chem. Eur. J. 2010, 16, 2790–2796. [Google Scholar] [CrossRef]
- Vico, R.V.; Voskuhl, J.; Ravoo, B.J. Multivalent interaction of cyclodextrin vesicles, carbohydrate guests, and lectins: A kinetic investigation. Langmuir 2011, 27, 1391–1397. [Google Scholar] [CrossRef]
- Ravoo, B.J.; Darcy, R. Cyclodextrin bilayer vesicles. Angew. Chem. Int. Ed. 2000, 39, 4324–4326. [Google Scholar] [CrossRef]
- Falvey, P.; Lim, C.W.; Darcy, R.; Revermann, T.; Karst, U.; Giesbers, M.; Marcelis, A.T.M.; Lazar, A.; Coleman, A.W.; Reinhoudt, D.N.; et al. Bilayer vesicles of amphiphilic cyclodextrins: Host membranes that recognize guest molecules. Chem. Eur. J. 2005, 11, 1171–1180. [Google Scholar] [CrossRef]
- de Vries, W.C.; Tesch, M.; Studer, A.; Ravoo, B.J. Molecular recognition and immobilization of ligand-conjugated redox-responsive polymer nanocontainers. ACS Appl. Mater. Interfaces 2017, 9, 41760–41766. [Google Scholar] [CrossRef]
- Schibilla, F.; Voskuhl, J.; Fokina, N.A.; Jeremy, E.P.; Dahl, J.E.P.; Schreiner, P.R.; Ravoo, B.J. Host-guest complexes of cyclodextrins and nanodiamonds as a strong non-covalent binding motif for self-sssembled nanomaterials. Chem. Eur. J. 2017, 23, 16059–16065. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, S.; Chu, C.-W.; Kumari, S.; Silberreis, K.; Böttcher, C.; Dernedde, J.; Ravoo, B.J.; Haag, R. A toolbox approach for multivalent presentation of ligand–receptor recognition on a supramolecular scaffold. J. Mater. Chem. B 2018, 6, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
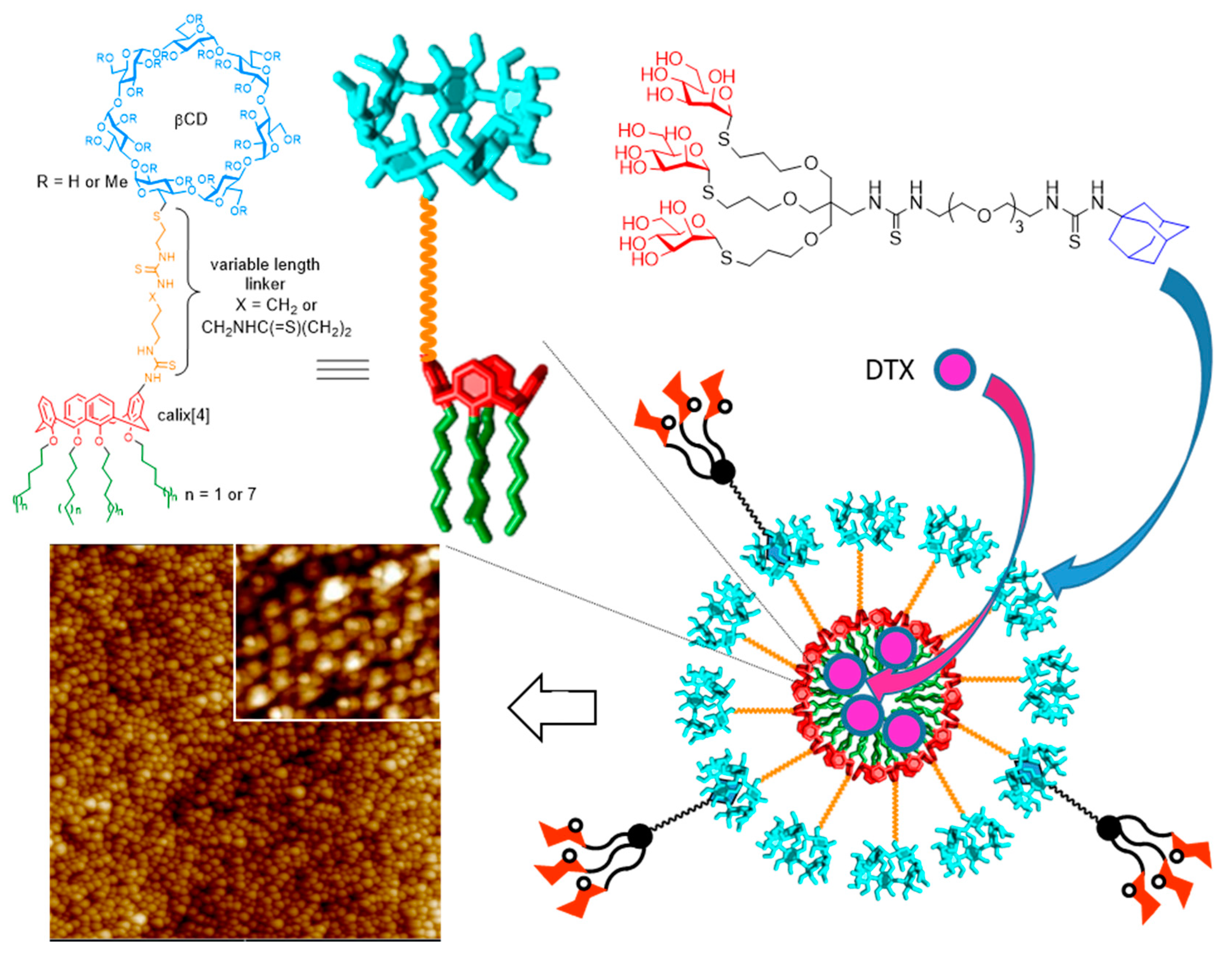
- Gallego-Yerga, L.; de la Torre, C.; Sansone, F.; Casnati, F.; Ortiz Mellet, C.; García Fernández, J.M.; Ceña, V. Synthesis, self-assembly and anticancer drug encapsulation and delivery properties of cyclodextrin-based giant amphiphiles. Carbohydr. Polym. 2021, 252, 117135. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Posadas, I.; de la Torre, C.; Ruiz-Almansa, J.; Sansone, F.; Ortiz Mellet, C.; Casnati, A.; García Fernández, J.M.; Ceña, V. Docetaxel-loaded nanoparticles assembled from β- cyclodextrin/calixarene giant surfactants: Physicochemical properties and cytotoxic effect in prostate cancer and glioblastoma cells. Front. Pharmacol. 2017, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Yerga, L.; Lomazzi, M.; Sansone, F.; Ortiz Mellet, C.; Casnati, A.; García Fernandez, J.M. Glycoligand-targeted core-shell nanospheres with tunable drug release profiles from calixarene-cyclodextrin heterodimers. Chem. Commun. 2014, 50, 7440–7443. [Google Scholar] [CrossRef]
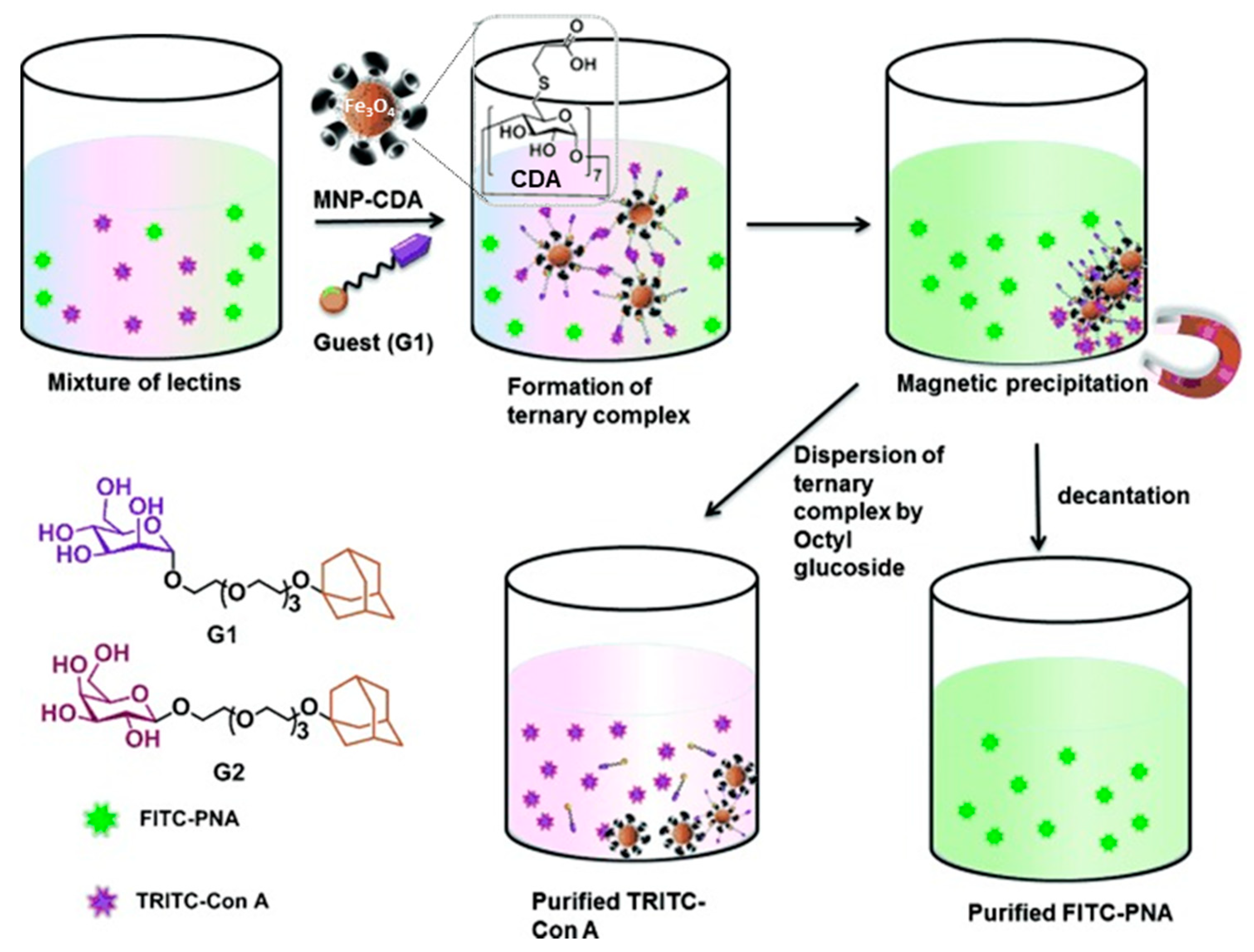
- Samanta, A.; Ravoo, B.J. Magnetic separation of proteins by a self-assembled supramolecular ternary complex. Angew. Chem. Int. Ed. 2014, 53, 12946–12950. [Google Scholar] [CrossRef]
- Hu, C.; Wu, J.; Wei, T.; Zhan, W.; Qu, Y.; Pan, Y.; Yu, Q.; Chen, H. A supramolecular approach for versatile biofunctionalization of magnetic nanoparticles. J. Mater. Chem. B 2018, 6, 2198–2203. [Google Scholar] [CrossRef]
- Oz, Y.; Abdouni, Y.; Yilmaz, G.; Becer, C.R.; Sanyal, A. Magnetic glyconanoparticles for selective lectin separation and purification. Polym. Chem. 2019, 10, 3351–3361. [Google Scholar] [CrossRef]
- Bavireddi, H.; Kikkeri, R. Glyco-β-cyclodextrin capped quantum dots: Synthesis, cytotoxicity and optical detection of carbohydrate–protein interactions. Analyst 2012, 137, 5123–5127. [Google Scholar] [CrossRef]
- Oikonomou, M.; Wang, J.; Carvalho, J.J.; Velders, A.H. Ternary supramolecular quantum-dot network flocculation for selective lectin detection. Nano Res. 2016, 9, 1904–1912. [Google Scholar] [CrossRef]
- Luo, G.-F.; Chen, W.-H.; Lei, Q.; Qiu, W.-X.; Liu, Y.-X.; Cheng, Y.-J.; Zhang, X.-Z. A triple-collaborative strategy for high-performance tumor therapy by multifunctional mesoporous silica-coated gold nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Qi, Z.; Bharate, P.; Lai, C.-H.; Ziem, B.; Böttcher, C.; Schulz, A.; Beckert, F.; Hatting, B.; Mülhaupt, R.; Seeberger, P.H.; et al. Multivalency at interfaces: Supramolecular carbohydrate-functionalized graphene derivatives for bacterial capture, release, and disinfection. Nano Lett. 2015, 15, 6051–6057. [Google Scholar] [CrossRef] [PubMed]
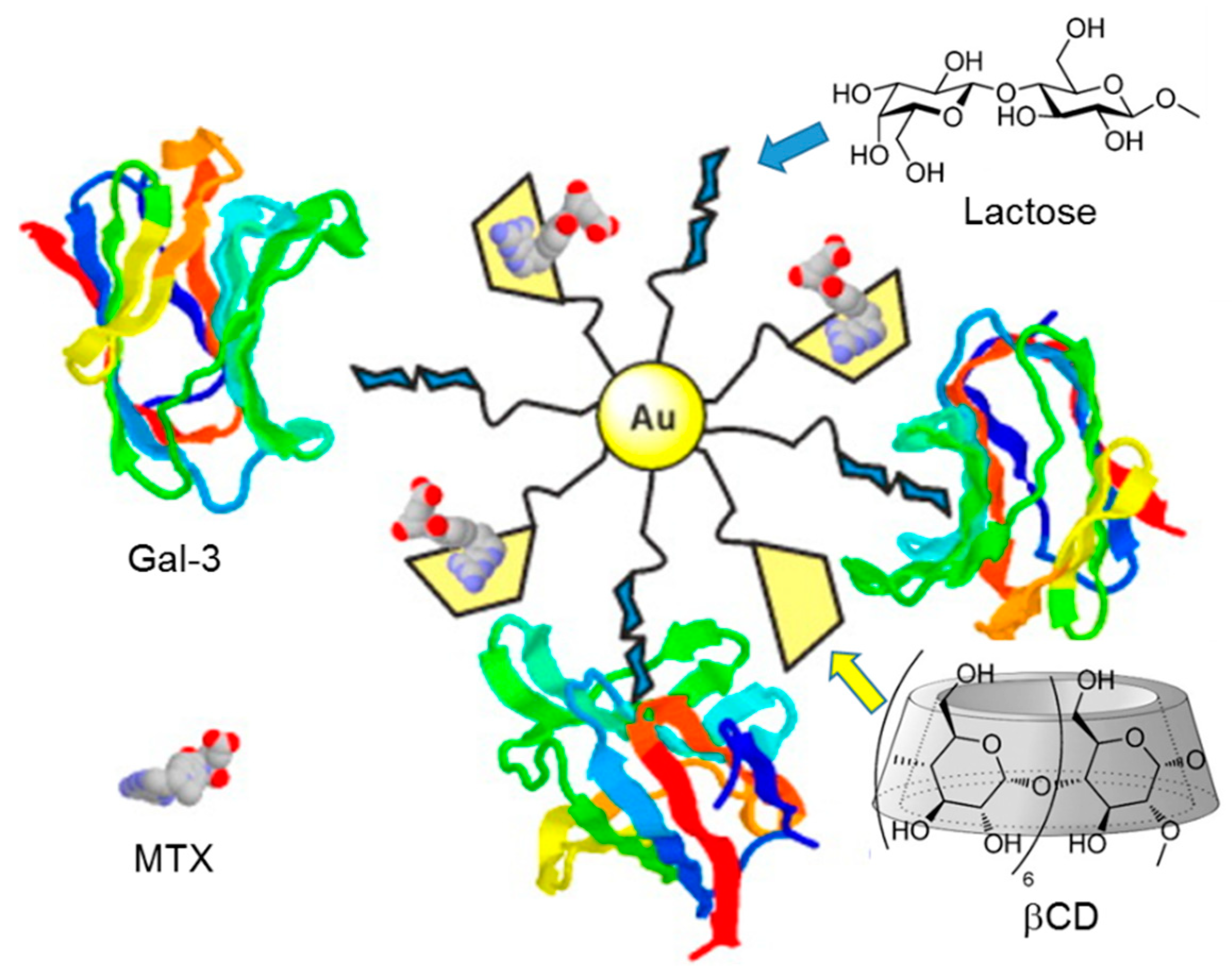
- Aykaç, A.; Martos-Maldonado, M.C.; Casas-Solvas, J.M.; Quesada-Soriano, I.; García-Maroto, F.; García-Fuentes, L.; Vargas-Berenguel, A. β-Cyclodextrin-bearing gold glyconanoparticles for the development of site specific drug delivery systems. Langmuir 2014, 30, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Lo Meo, P.; Noto, R.; Cavallaro, G.; Milioto, S.; Lazzara, G. Functionalized halloysite multivalent glycocluster as a new drug delivery system. J. Mater. Chem. B 2014, 2, 7732–7738. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Riela, S.; Baiamonte, C.; Jiménez Blanco, J.L.; Giordano, C.; Lo Meo, P.; Milioto, S.; Noto, R.; Parisi, F.; Pizzolanti, G.; et al. Dual drug-loaded halloysite hybrid-based glycocluster for sustained release of hydrophobic molecules. RSC Adv. 2016, 6, 87935–87944. [Google Scholar] [CrossRef]
- Cutrone, G.; Li, X.; Casas-Solvas, J.M.; Menendez-Miranda, M.; Qiu, J.; Benkovics, G.; Constantin, D.; Malanga, M.; Moreira-Alvarez, B.; Costa-Fernandez, J.M.; et al. Design of engineered cyclodextrin derivatives for spontaneous coating of highly porous metal-organic framework nanoparticles in aqueous media. Nanomaterials 2019, 9, 1103. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Ferreira, M.; Léger, B.; Bricout, H.; Tilloy, S.; Monflier, E. Catalysis in cyclodextrin-based unconventional reaction media: Recent developments and future opportunities. ACS Sustainable Chem. Eng. 2017, 5, 3598–3606. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, G.; Chamoreau, L.-M.; Zhang, Y.; Mouriès-Mansuy, V.; Fensterbank, L.; Bistri-Aslanoff, O.; Sylvain Roland, S.; Sollogoub, M. Permethylated NHC-capped α- and β-cyclodextrins (ICyDMe) regioselective and enantioselective gold-catalysis in pure water. Chem. Eur. J. 2020, in press. [Google Scholar] [CrossRef]
- Morillo, E.; Madrid, F.; Lara-Moreno, A.; Villaverde, J. Soil bioremediation by cyclodextrins. A review. Int. J. Pharm. 2020, 591, 119943. [Google Scholar] [CrossRef]
- Muñoz, J.; Crivillers, N.; Ravoo, B.J.; Mas-Torrent, M. Cyclodextrin-based superparamagnetic host vesicles as ultrasensitive nanobiocarriers for electrosensing. Nanoscale 2020, 12, 9884–9889. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Yang, J.-M.; Jin, X.-Y.; Cong, H.; Ge, Q.-M.; Liu, M.; Tao, Z. Recent development of supramolecular sensors constructed by hybridization of organic macrocycles with nanomaterials. Curr. Org. Chem. 2020, 24, 265–290. [Google Scholar] [CrossRef]
- Casulli, M.A.; Taurino, I.; Carrara, S.; Hayashita, T. Integration methods of cyclodextrins on gold and carbon electrodes for electrochemical sensors. C J. Carbon Res. 2019, 5, 78. [Google Scholar] [CrossRef]
- Luo, H.; Chen, L.-X.; Ge, Q.-M.; Liu, M.; Tao, Z.; Zhou, Y.-H.; Cong, H. Applications of macrocyclic compounds for electrochemical sensors to improve selectivity and sensitivity. J. Incl. Phenom. Macrocycl. Chem. 2019, 95, 171–198. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. Metallocyclodextrins in medicinal chemistry. Future Med. Chem. 2018, 10, 663–677. [Google Scholar] [CrossRef]
- Guillen-Poza, P.A.; Sánchez-Fernández, E.M.; Artigas, G.; García Fernández, J.M.; Hinou, H.; Ortiz Mellet, C.; Nishimura, S.-I.; Garcia-Martin, F. Amplified detection of breast cancer autoantibodies using MUC1-based Tn antigen mimics. J. Med. Chem. 2020, 63, 8524–8533. [Google Scholar] [CrossRef]
- Yao, X.; Mu, J.; Zeng, L.; Lin, J.; Nie, Z.; Jiang, X.; Huang, P. Stimuli-responsive cyclodextrin-based nanoplatforms for cancer treatment and theranostics. Mater. Horiz. 2019, 6, 846–870. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. Host-guest chemistry in supramolecular theranostics. Theranostics 2019, 9, 3041–3074. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Bermejo, I.A.; Navo, C.D.; Castro-López, J.; Guerreiro, A.; Jiménez-Moreno, E.; Sánchez Fernández, E.M.; García-Martín, F.; Hinou, H.; Nishimura, S.-I.; García Fernández, J.M.; et al. Synthesis, conformational analysis and in vivo assays of an anti-cancer vaccine that features an unnatural antigen based on an sp2-iminosugar fragment. Chem. Sci. 2020, 11, 3996–4006. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Abhay Pandit, A.; Rochev, Y.A. Recent advances in host–guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- García-Moreno, M.I.; de la Mata, M.; Sánchez-Fernández, E.M.; Juan, M.; Benito, J.M.; Díaz-Quintana, A.; Fustero, S.; Nanba, E.; Higaki, K.; Sánchez-Alcázar, J.A.; et al. Fluorinated chaperone_β-cyclodextrin formulations for β-glucocerebrosidase activity enhancement in neuronopathic Gaucher disease. J. Med. Chem. 2017, 60, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
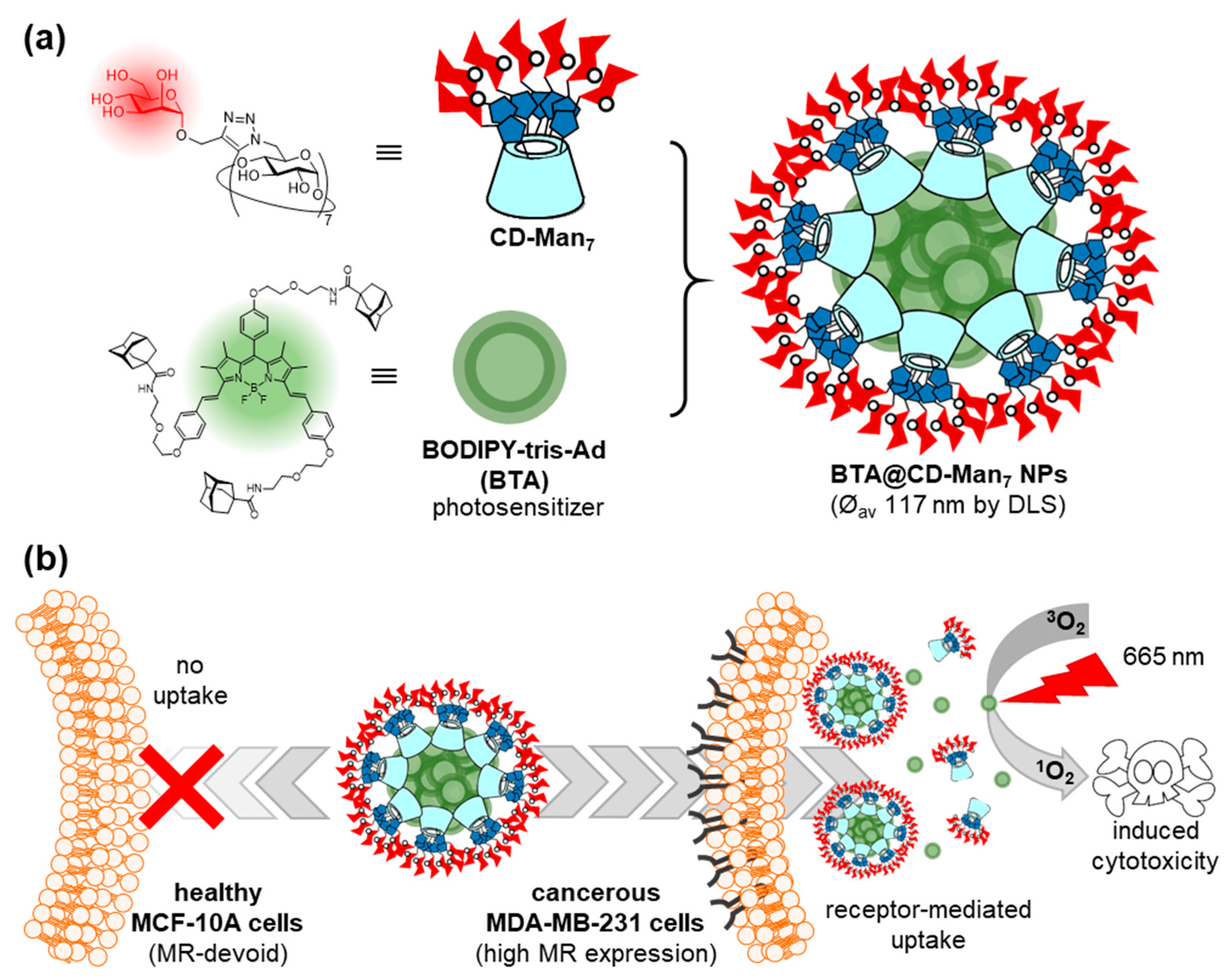
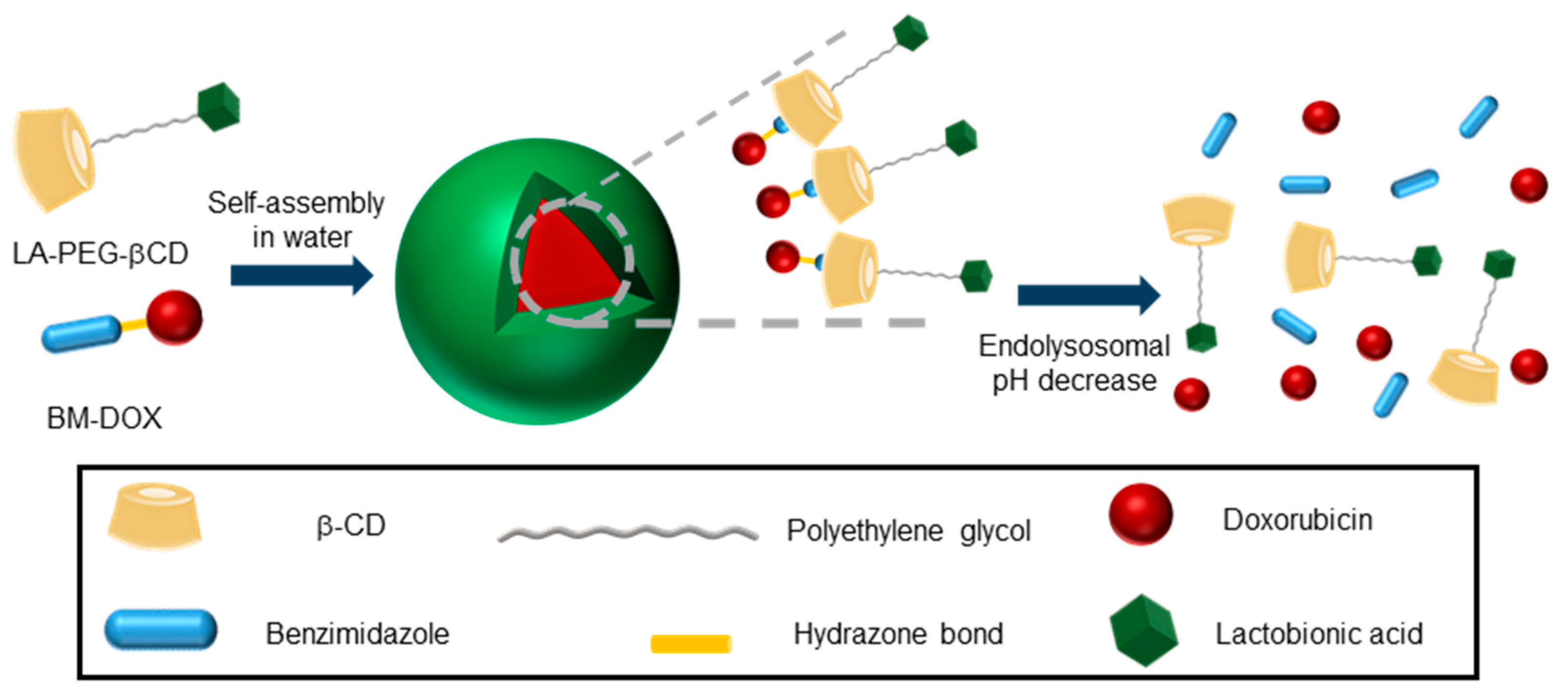
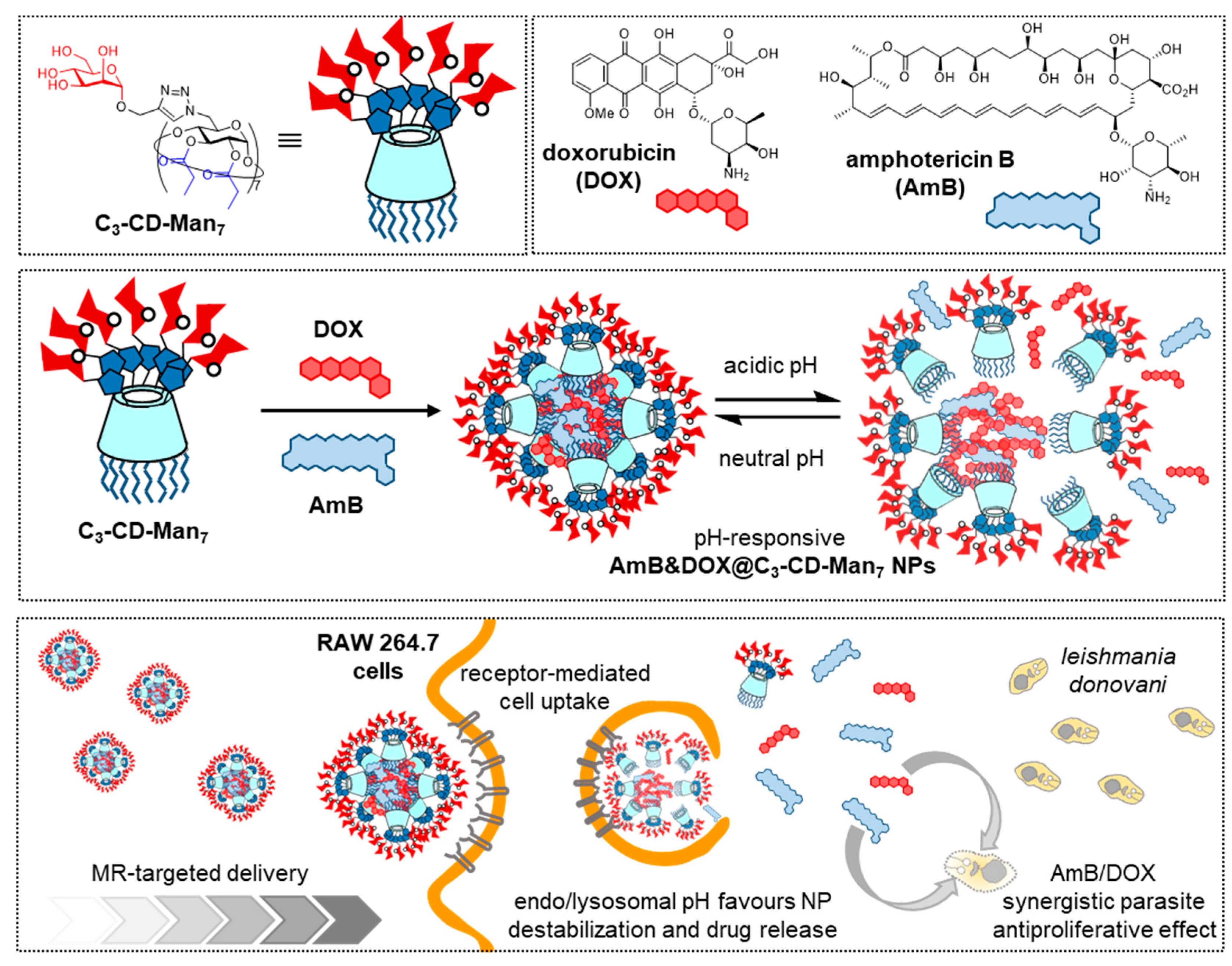
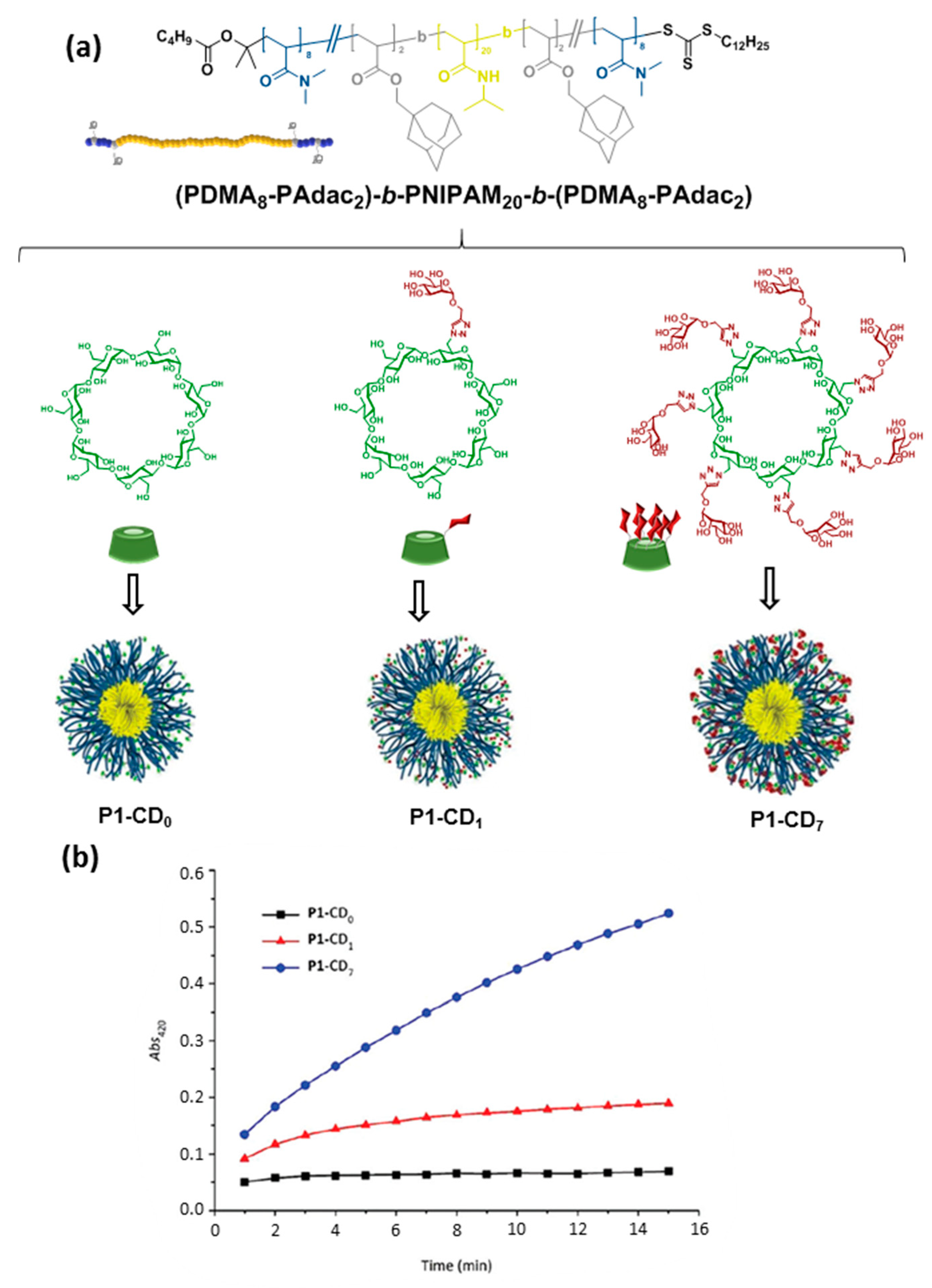



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Barbarroja, G.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-Based Functional Glyconanomaterials. Nanomaterials 2020, 10, 2517. https://doi.org/10.3390/nano10122517
Rivero-Barbarroja G, Benito JM, Ortiz Mellet C, García Fernández JM. Cyclodextrin-Based Functional Glyconanomaterials. Nanomaterials. 2020; 10(12):2517. https://doi.org/10.3390/nano10122517
Chicago/Turabian StyleRivero-Barbarroja, Gonzalo, Juan Manuel Benito, Carmen Ortiz Mellet, and José Manuel García Fernández. 2020. "Cyclodextrin-Based Functional Glyconanomaterials" Nanomaterials 10, no. 12: 2517. https://doi.org/10.3390/nano10122517
APA StyleRivero-Barbarroja, G., Benito, J. M., Ortiz Mellet, C., & García Fernández, J. M. (2020). Cyclodextrin-Based Functional Glyconanomaterials. Nanomaterials, 10(12), 2517. https://doi.org/10.3390/nano10122517