Dependence of the Abundance of Reed Glass-Winged Cicadas (Pentastiridius leporinus (Linnaeus, 1761)) on Weather and Climate in the Upper Rhine Valley, Southwest Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographical Framework
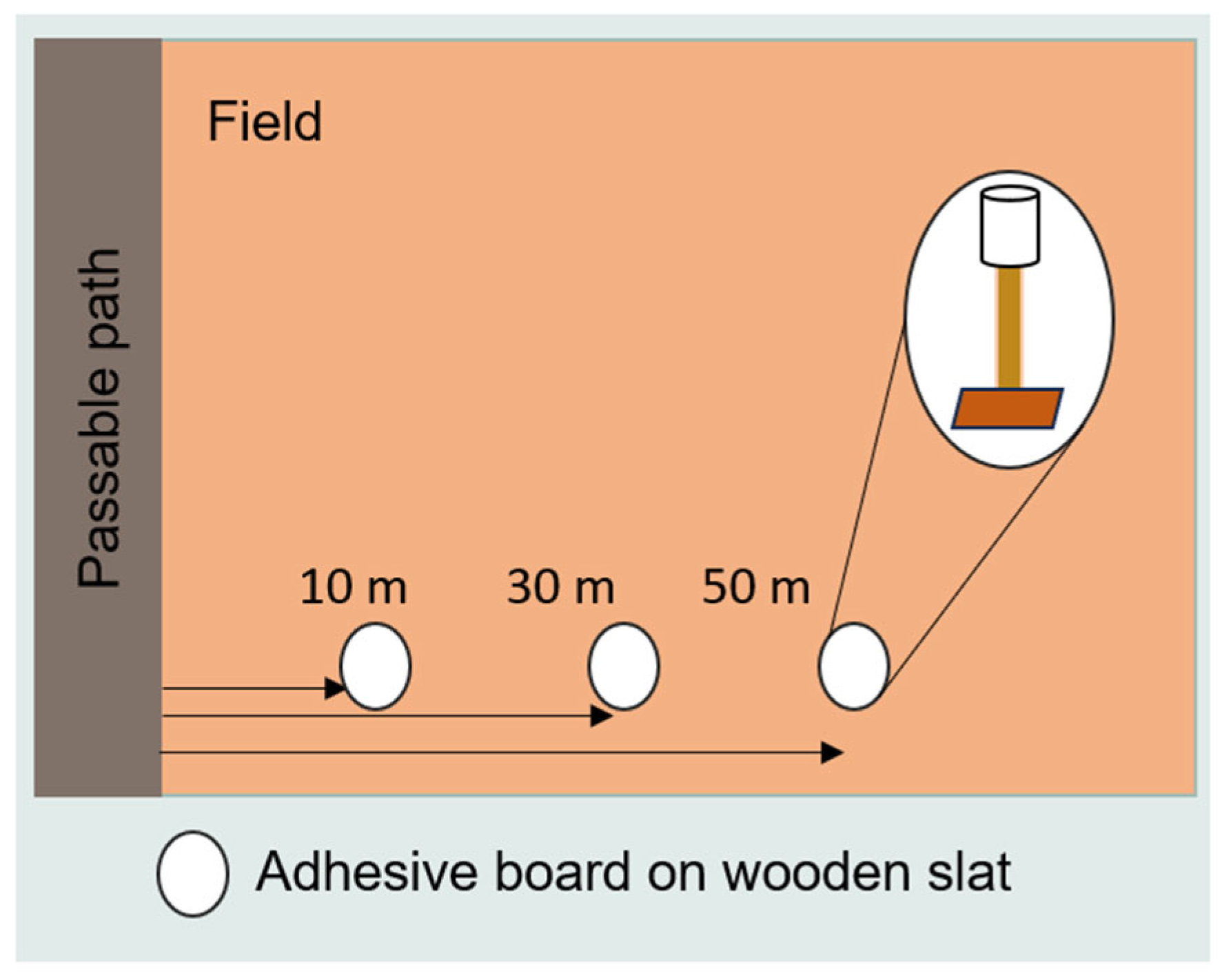
2.2. Data Collection (Cicada Field Samples)
2.3. Data Preparation
2.4. Statistical Models
2.4.1. Model 1
2.4.2. Model 2
2.4.3. Model Validation
2.4.4. Projections
3. Results
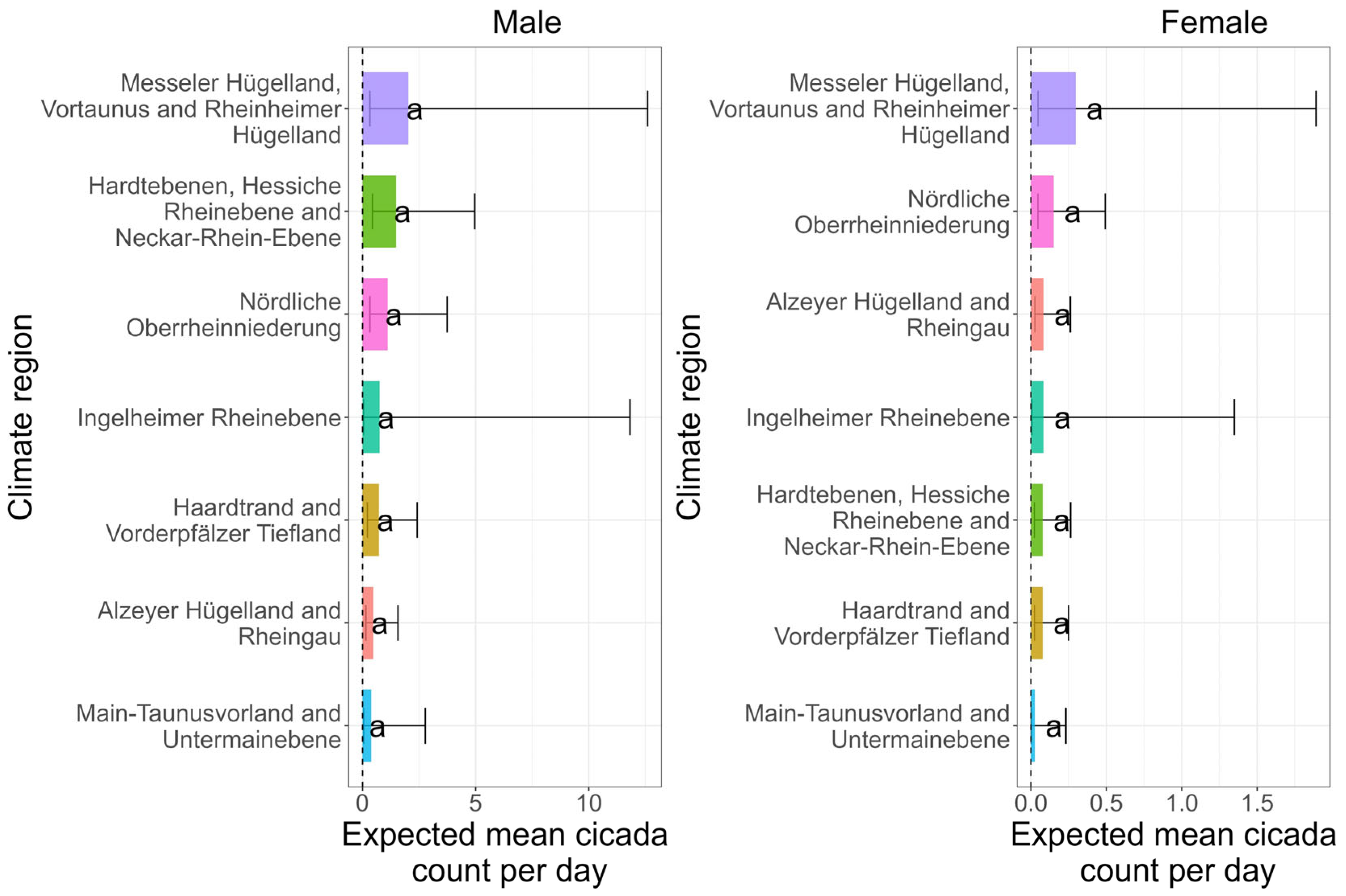
3.1. Does Cicada Abundance Differ Between Climate Regions?
3.2. To What Extent Do Mean Temperature and Precipitation of Climate Regions Influence Cicada Populations?
3.2.1. Impact of Long-Term Averages (1991–2020) on Cicadas
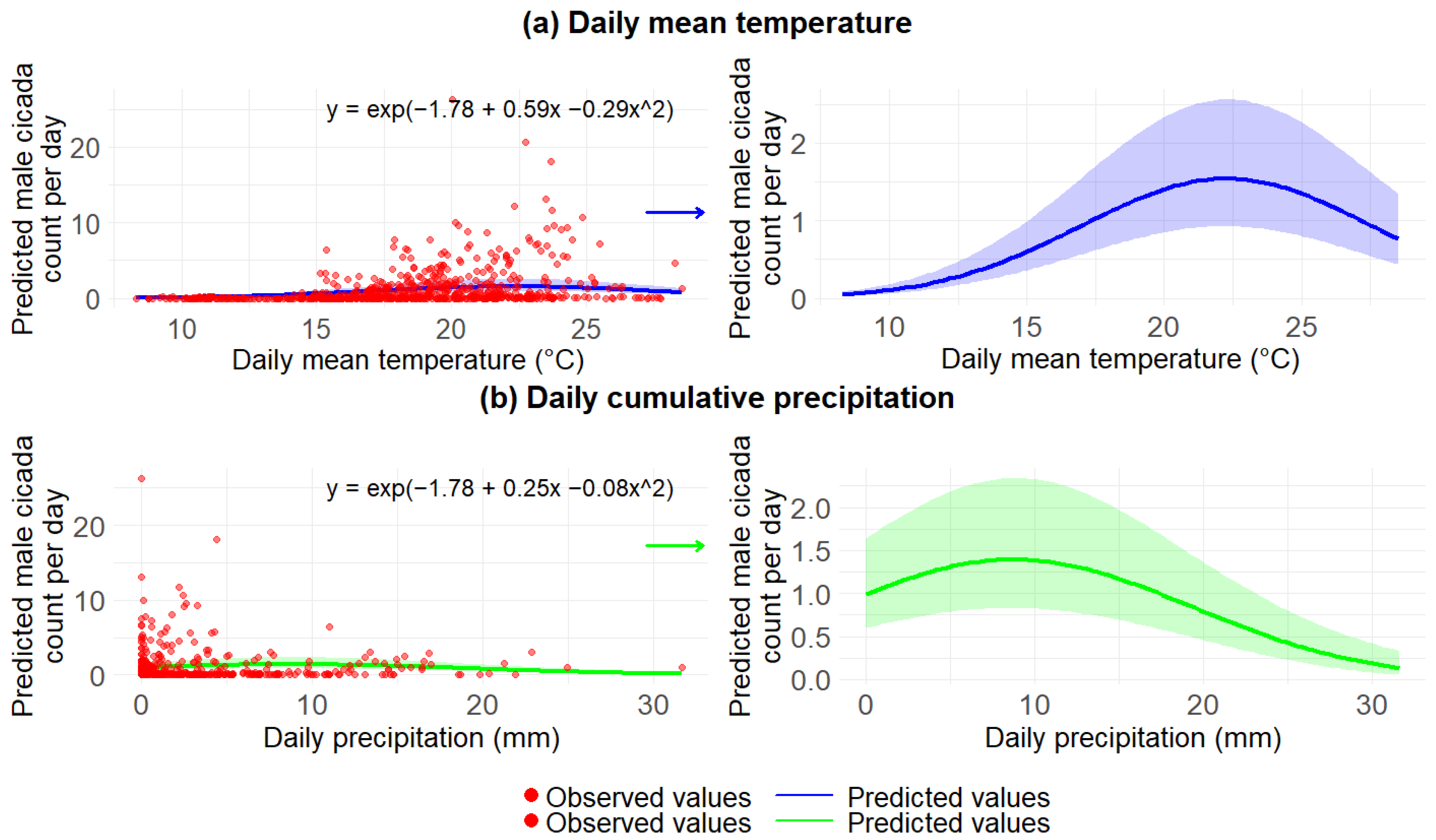
3.2.2. Impact of Daily Weather on Male Cicadas During 2020–2023
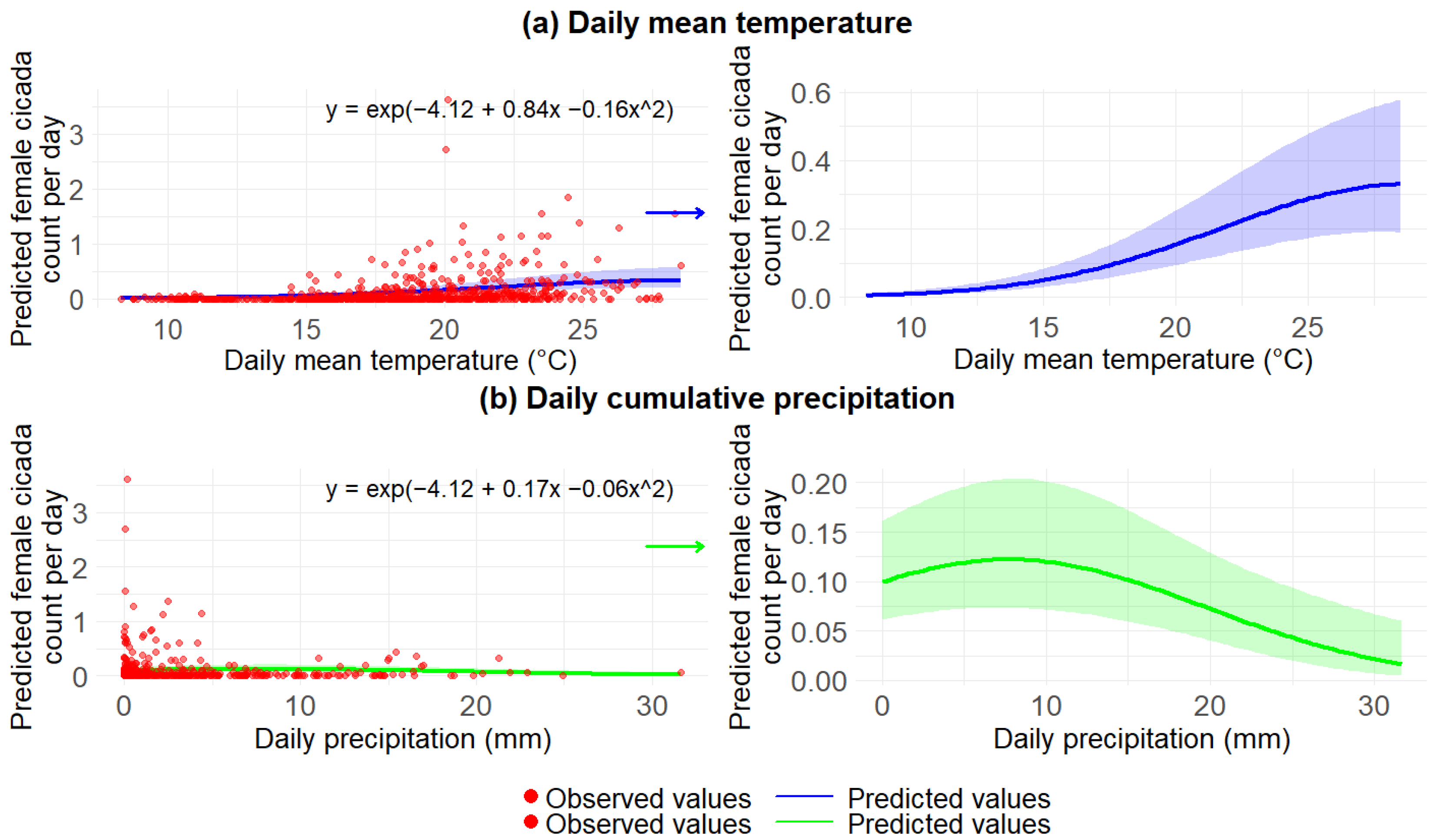
3.2.3. Impact of Daily Weather on Female Cicadas During 2020–2023
3.2.4. Comparison of Long-Term Average and Daily Predictor Variables
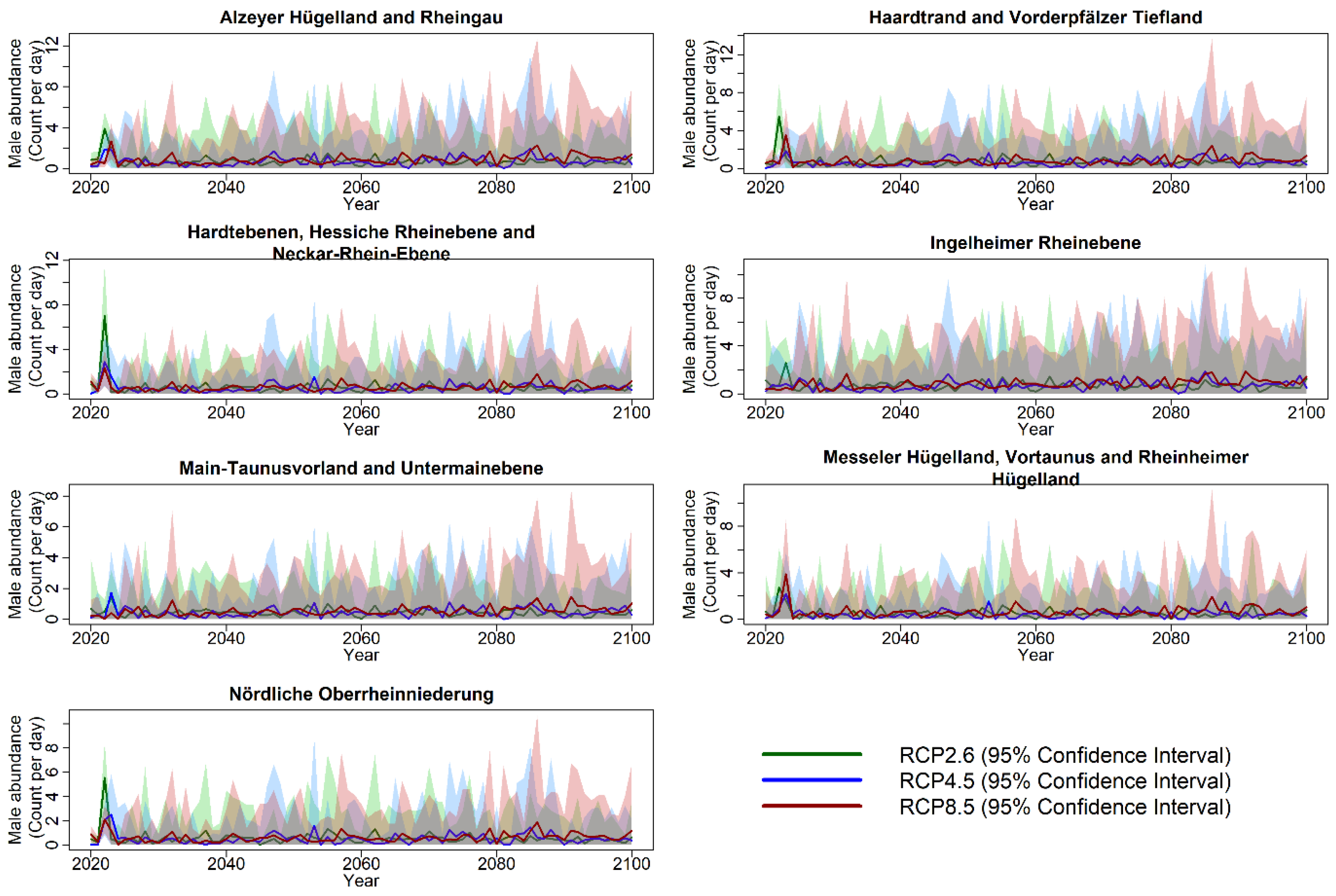
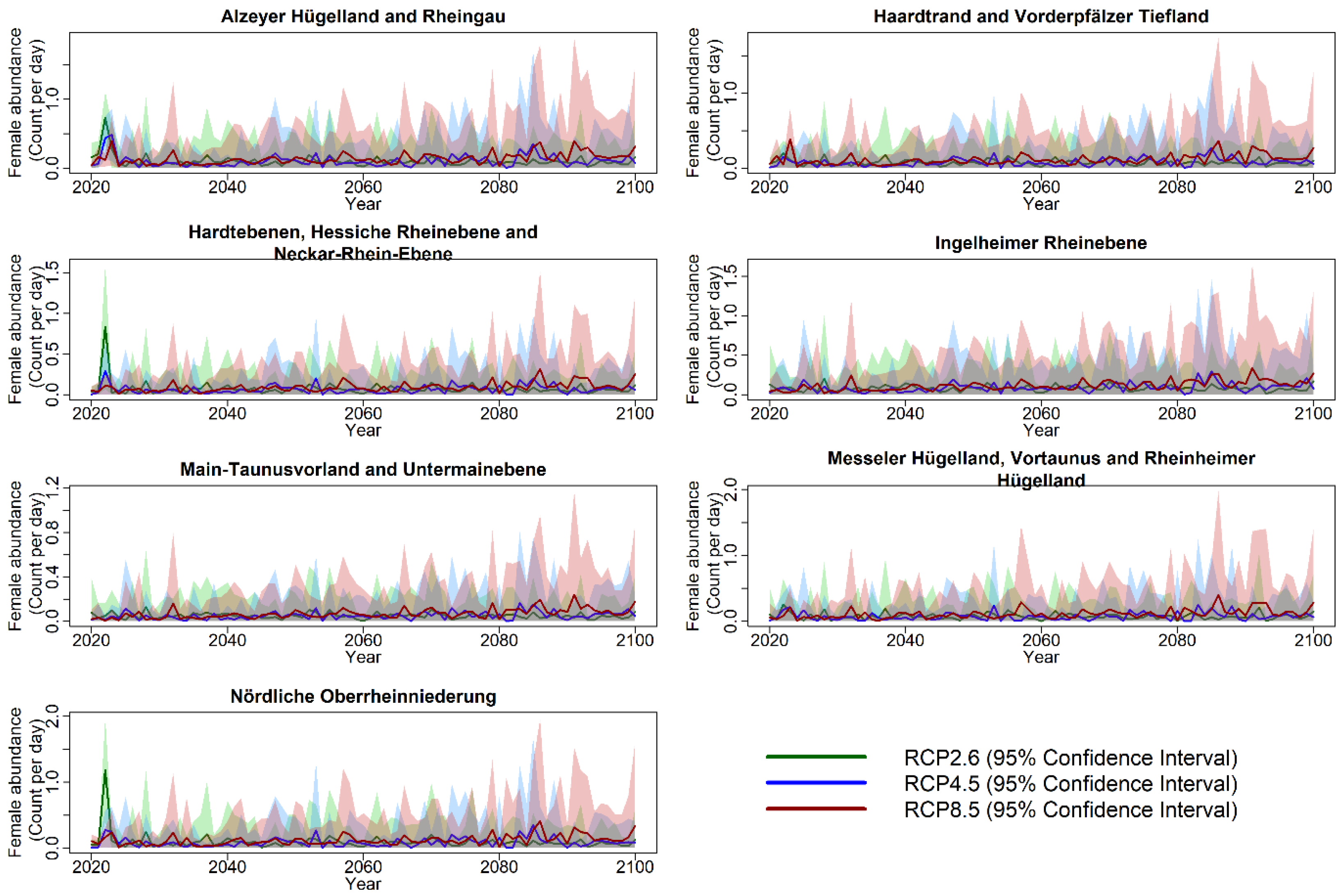
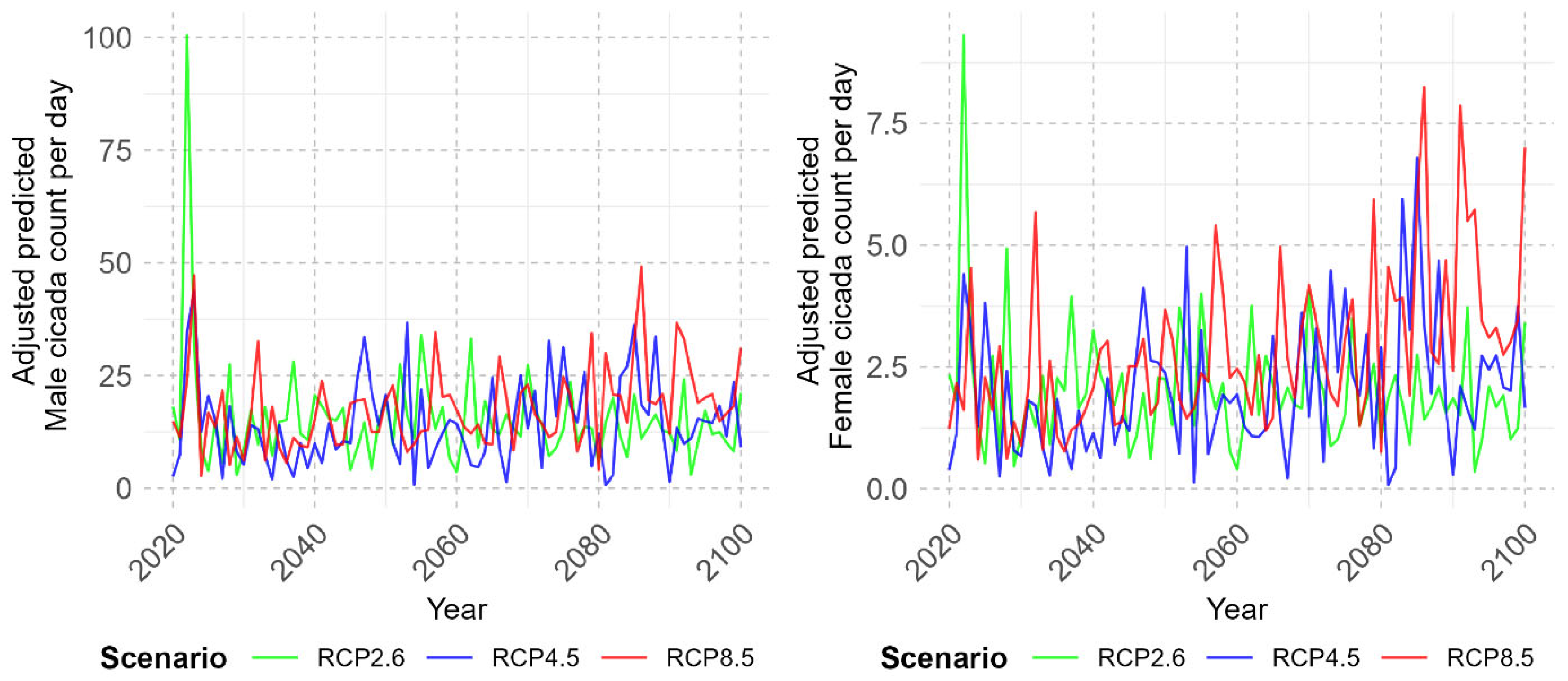
3.3. Projection of Cicada Abundance Using Climate Projections
4. Discussion
4.1. Influence of Climate Regions
4.2. Influence of Temperature and Precipitation
4.3. Limitations of Model 2
4.4. Implications for Crop Diseases
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebert, F.; Bollmann, K.; Schuwirth, N.; Duelli, P.; Weber, D.; Obrist, M.K. Similar Temporal Patterns in Insect Richness, Abundance and Biomass across Major Habitat Types. Insect Conserv. Divers. 2024, 17, 139–154. [Google Scholar] [CrossRef]
- Rumohr, Q.; Baden, C.U.; Bergtold, M.; Marx, M.T.; Oellers, J.; Schade, M.; Toschki, A.; Maus, C. Drivers and Pressures behind Insect Decline in Central and Western Europe Based on Long-Term Monitoring Data. PLoS ONE 2023, 18, e0289565. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, S.C.; Witczak, N.; Lang, C.; Schieler, M.; Dettweiler, A.; Kleinhenz, B.; Schwind, M.; Vilcinskas, A.; Lee, K.-Z. Biology and Rearing of an Emerging Sugar Beet Pest: The Planthopper Pentastiridius leporinus. Insects 2022, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Pfitzer, R.; Varrelmann, M.; Schrameyer, K.; Rostás, M. Life History Traits and a Method for Continuous Mass Rearing of the Planthopper Pentastiridius leporinus, a Vector of the Causal Agent of Syndrome ‘Basses Richesses’ in Sugar Beet. Pest Manag. Sci. 2022, 78, 4700–4708. [Google Scholar] [CrossRef]
- Behrmann, S.C.; Rinklef, A.; Lang, C.; Vilcinskas, A.; Lee, K.-Z. Potato (Solanum tuberosum) as a New Host for Pentastiridius leporinus (Hemiptera: Cixiidae) and Candidatus Arsenophonus Phytopathogenicus. Insects 2023, 14, 281. [Google Scholar] [CrossRef]
- Webster, M.T.; Beaurepaire, A.; Neumann, P.; Stolle, E. Population Genomics for Insect Conservation. Annu. Rev. Anim. Biosci. 2023, 11, 115–140. [Google Scholar] [CrossRef]
- Yazdanian, M.; Kankaanpää, T.; Itämies, J.; Leinonen, R.; Merckx, T.; Pöyry, J.; Sihvonen, P.; Suuronen, A.; Välimäki, P.; Kivelä, S.M. Ecological and Life-history Traits Predict Temporal Trends in Biomass of Boreal Moths. Insect Conserv. Divers. 2023, 16, 600–615. [Google Scholar] [CrossRef]
- Cómbita, J.L.; Giraldo, C.E.; Escobar, F. Environmental Variation Associated with Topography Explains Butterfly Diversity along a Tropical Elevation Gradient. Biotropica 2022, 54, 146–156. [Google Scholar] [CrossRef]
- Moriyama, M.; Numata, H. Ecophysiological Responses to Climate Change in Cicadas. Physiol. Entomol. 2019, 44, 65–76. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Trowbridge, A.M.; Raffa, K.F.; Lindroth, R.L. Consequences of Climate Warming and Altered Precipitation Patterns for Plant-Insect and Multitrophic Interactions. Plant Physiol. 2012, 160, 1719–1727. [Google Scholar] [CrossRef]
- Kremer, P. Die Zuckerrübe im Klimawandel: Agrarökologische Auswirkungen in Rheinland-Pfalz und Hessen; SpringerLink Bücher; Springer Spektrum: Wiesbaden, Germany, 2017; ISBN 978-3-658-18972-3. [Google Scholar]
- World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; LCC MAKS Press: Moscow, Russia, 2024; ISBN 978-5-317-07235-3. [Google Scholar]
- BGR—BÜK1000. Available online: https://www.bgr.bund.de/DE/Themen/Boden/Informationsgrundlagen/Bodenkundliche_Karten_Datenbanken/BUEK1000/buek1000_node.html (accessed on 7 February 2025).
- Naturräumliche Gliederung. Landesamt Für Umwelt Rheinland-Pfalz. Available online: https://lfu.rlp.de/natur/planungsgrundlagen/naturraeumliche-gliederung (accessed on 7 February 2025).
- Razafimaharo, C.; Krähenmann, S.; Höpp, S.; Rauthe, M.; Deutschländer, T. New High-Resolution Gridded Dataset of Daily Mean, Minimum, and Maximum Temperature and Relative Humidity for Central Europe (HYRAS). Theor. Appl. Climatol. 2020, 142, 1531–1553. [Google Scholar] [CrossRef]
- Intergovernmental Panel On Climate Change (IPCC). Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Cambridge University Press: Cambridge, UK, 2023; ISBN 978-1-009-32584-4. [Google Scholar]
- Esri (Environmental Systems Research Institute) ArcGIS Pro 3.2.2. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 8 February 2024).
- Salinas Ruíz, J.; Montesinos López, O.A.; Hernández Ramírez, G.; Crossa Hiriart, J. Generalized Linear Mixed Models with Applications in Agriculture and Biology; Springer Nature: Cham, Switzerland, 2023; ISBN 978-3-031-32799-5. [Google Scholar]
- Udokang Anietie, M.A.; Raji Surajudeen, E.; Bello Latifat Kemi, T. An Empirical Study of Generalized Linear Model for Count Data. J. Appl. Computat Math. 2015, 4, 1000253. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2017, 1.10.7. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 8 February 2024).
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models 2016, 0.4.7. Available online: https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 1 March 2024).
- McCullagh, P.; Nelder, J.A. Generalized Linear Models; Springer: Boston, MA, USA, 1989; ISBN 978-0-412-31760-6. [Google Scholar]
- (PDF) Acoustic Communication in the Subtroglophile Planthopper Trigonocranus Emmeae Fieber, 1876 (Hemiptera: Fulgoromorpha: Cixiidae: Oecleini). Available online: https://www.researchgate.net/publication/259479914_Acoustic_communication_in_the_subtroglophile_planthopper_Trigonocranus_emmeae_Fieber_1876_Hemiptera_Fulgoromorpha_Cixiidae_Oecleini (accessed on 9 June 2025).
- Maixner, M. Transmission of German Grapevine Yellows (Vergilbungskrankheit) by the Planthopper Hyalesthes Obsoletus (Auchenorrhyncha: Cixiidae). VITIS—J. Grapevine Res. 1994, 33, 103. [Google Scholar] [CrossRef]
- Bressan, A.; Sémétey, O.; Nusillard, B.; Clair, D.; Boudon-Padieu, E. Insect Vectors (Hemiptera: Cixiidae) and Pathogens Associated with the Disease Syndrome “Basses Richesses” of Sugar Beet in France. Plant Dis. 2008, 92, 113–119. [Google Scholar] [CrossRef]
- Fernández, G.J.; Carro, M.E. Effects of Nest Predation on a South Temperate House Wren Population. J. Avian Biol. 2021, 52, jav.02632. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in Crop Losses to Insect Pests in a Warming Climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Ziska, L.H.; McConnell, L.L. Climate Change, Carbon Dioxide, and Pest Biology: Monitor, Mitigate, Manage. J. Agric. Food Chem. 2016, 64, 6–12. [Google Scholar] [CrossRef]
- Gu, L.; Pallardy, S.G.; Hosman, K.P.; Sun, Y. Impacts of Precipitation Variability on Plant Species and Community Water Stress in a Temperate Deciduous Forest in the Central US. Agric. For. Meteorol. 2016, 217, 120–136. [Google Scholar] [CrossRef]
- Bressan, A.; Moral García, F.J.; Sémétey, O.; Boudon-Padieu, E. Spatio-Temporal Pattern of Pentastiridius leporinus Migration in an Ephemeral Cropping System. Agric. For. Entomol. 2010, 12, 59–68. [Google Scholar] [CrossRef]
- Pfitzer, R.; Rostás, M.; Häußermann, P.; Häuser, T.; Rinklef, A.; Detring, J.; Schrameyer, K.; Voegele, R.T.; Maier, J.; Varrelmann, M. Effects of Succession Crops and Soil Tillage on Suppressing the Syndrome ‘Basses Richesses’ Vector Pentastiridius leporinus in Sugar Beet. Pest Manag. Sci. 2024, 80, 3379–3388. [Google Scholar] [CrossRef] [PubMed]
- Duduk, B.; Stepanović, J.; Fránová, J.; Zwolińska, A.; Rekanović, E.; Stepanović, M.; Vučković, N.; Duduk, N.; Vico, I. Geographical Variations, Prevalence, and Molecular Dynamics of Fastidious Phloem-Limited Pathogens Infecting Sugar Beet across Central Europe. PLoS ONE 2024, 19, e0306136. [Google Scholar] [CrossRef] [PubMed]
- Mahillon, M.; Brodard, J.; Schoen, R.; Botermans, M.; Dubuis, N.; Groux, R.; Pannell, J.R.; Blouin, A.G.; Schumpp, O. Revisiting a Pollen-Transmitted Ilarvirus Previously Associated with Angular Mosaic of Grapevine. Virus Res. 2024, 344, 199362. [Google Scholar] [CrossRef]
- Blé dur: Vigilance Pucerons et Cicadelles, Notamment sur les Parcelles non Protégées. Available online: https://www.arvalis.fr/infos-techniques/ble-dur-vigilance-pucerons-et-cicadelles-notamment-sur-les-parcelles-non-protegees (accessed on 7 February 2025).
- Eini, O.; Pfitzer, R.; Varrelmann, M. Rapid and Specific Detection of Pentastiridius leporinus by Recombinase Polymerase Amplification Assay. Bull. Entomol. Res. 2024, 114, 309–316. [Google Scholar] [CrossRef]
- Lang, C.; Dettweiler, A.; Benaouda, S.; Kreimer, D.; Löffler, D.; Glaser, E.; Adam, H.; Bojanowicz, S.L. Pentastiridius leporinus as a Plant Disease Vector: The Practical State of Knowledge and Derived Research Objectives. Sugar Ind. Int. 2025, 150, 105–120. [Google Scholar] [CrossRef]
- Duduk, N.; Vico, I.; Kosovac, A.; Stepanović, J.; Ćurčić, Ž.; Vučković, N.; Rekanović, E.; Duduk, B. A Biotroph Sets the Stage for a Necrotroph to Play: ‘Candidatus Phytoplasma Solani’ Infection of Sugar Beet Facilitated Macrophomina phaseolina Root Rot. Front. Microbiol. 2023, 14, 1164035. [Google Scholar] [CrossRef]
- Arsenophonus phytopathogenicus Bressan; et al. 2011. Available online: https://www.gbif.org/species/148925680 (accessed on 7 February 2025).
- Cargnus, E.; Pavan, F.; Mori, N.; Martini, M. Identification and Phenology of Hyalesthes Obsoletus (Hemiptera: Auchenorrhyncha: Cixiidae) Nymphal Instars. Bull. Entomol. Res. 2012, 102, 504–514. [Google Scholar] [CrossRef]
- Rinklef, A.; Behrmann, S.C.; Löffler, D.; Erner, J.; Meyer, M.V.; Lang, C.; Vilcinskas, A.; Lee, K.-Z. Prevalence in Potato of ‘Candidatus Arsenophonus Phytopathogenicus’ and ‘Candidatus Phytoplasma Solani’ and Their Transmission via Adult Pentastiridius leporinus. Insects 2024, 15, 275. [Google Scholar] [CrossRef]
- Therhaag, E.; Schneider, B.; Zikeli, K.; Maixner, M.; Gross, J. Pentastiridius leporinus (Linnaeus, 1761) as a Vector of Phloem-Restricted Pathogens on Potatoes: ‘Candidatus Arsenophonus Phytopathogenicus’ and ‘Candidatus Phytoplasma Solani’. Insects 2024, 15, 189. [Google Scholar] [CrossRef]
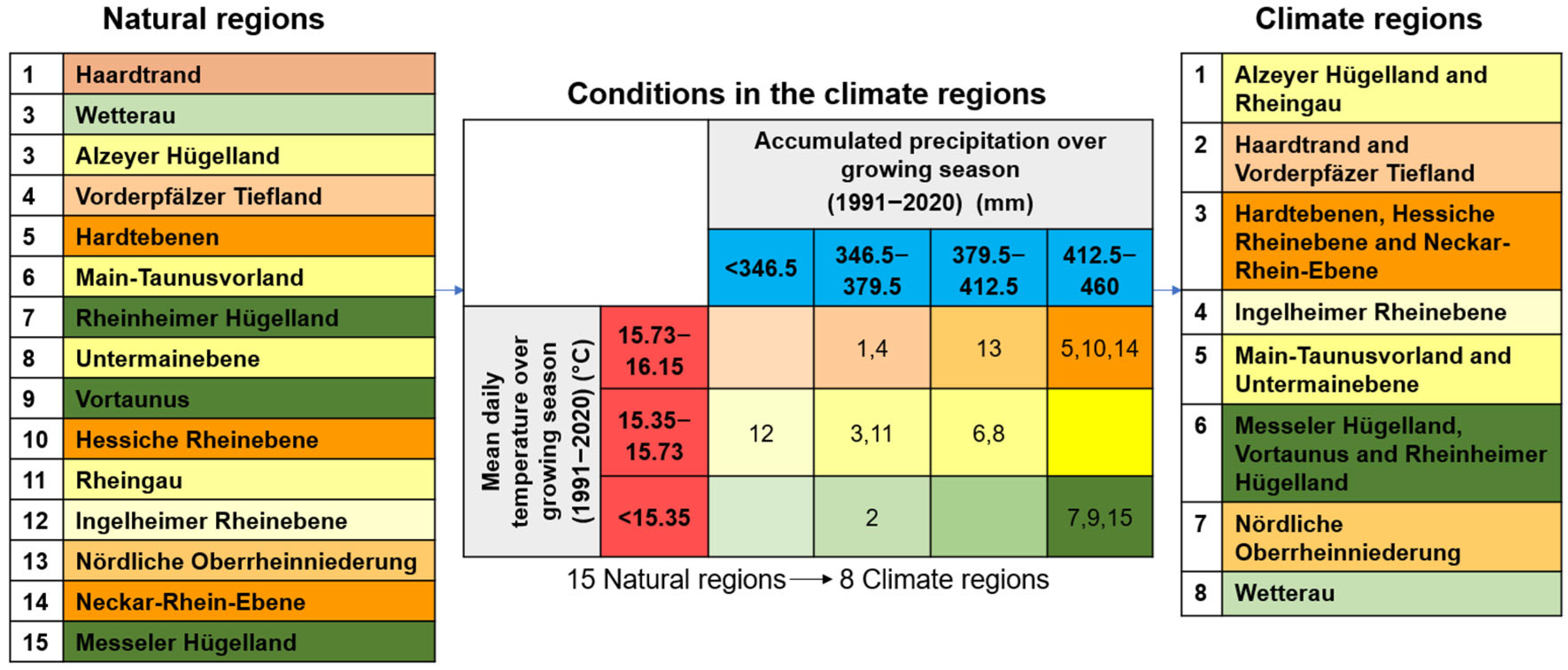
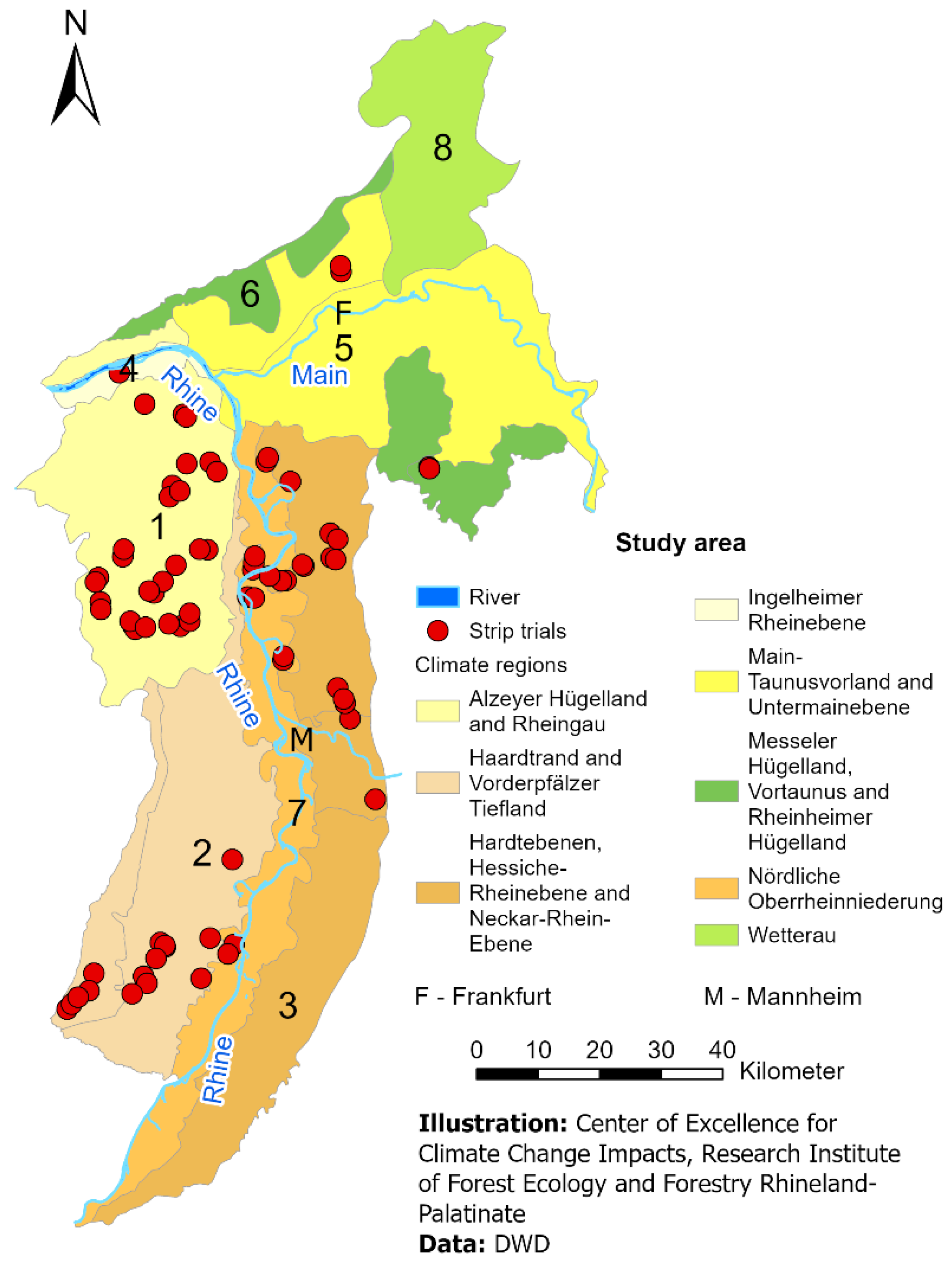
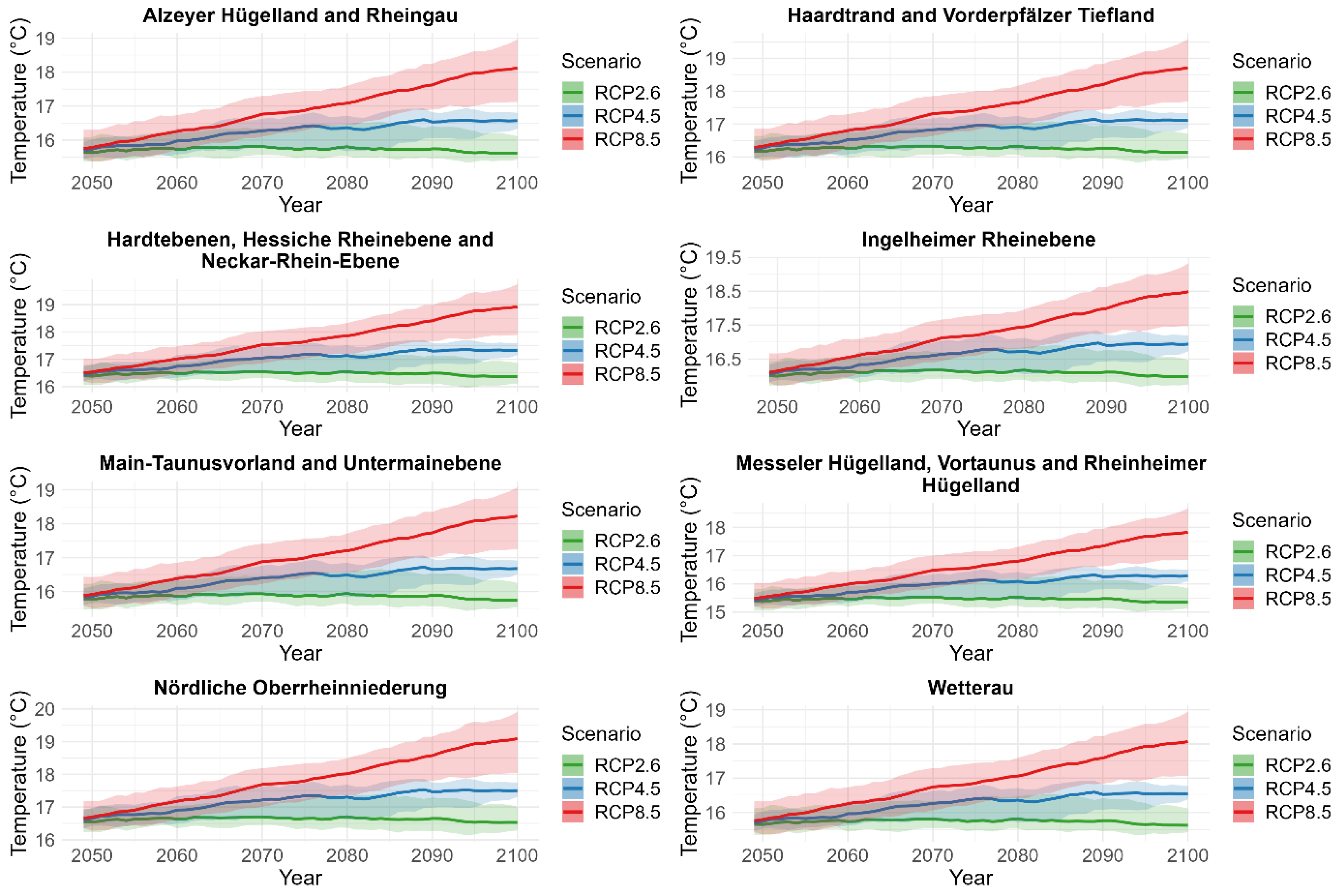
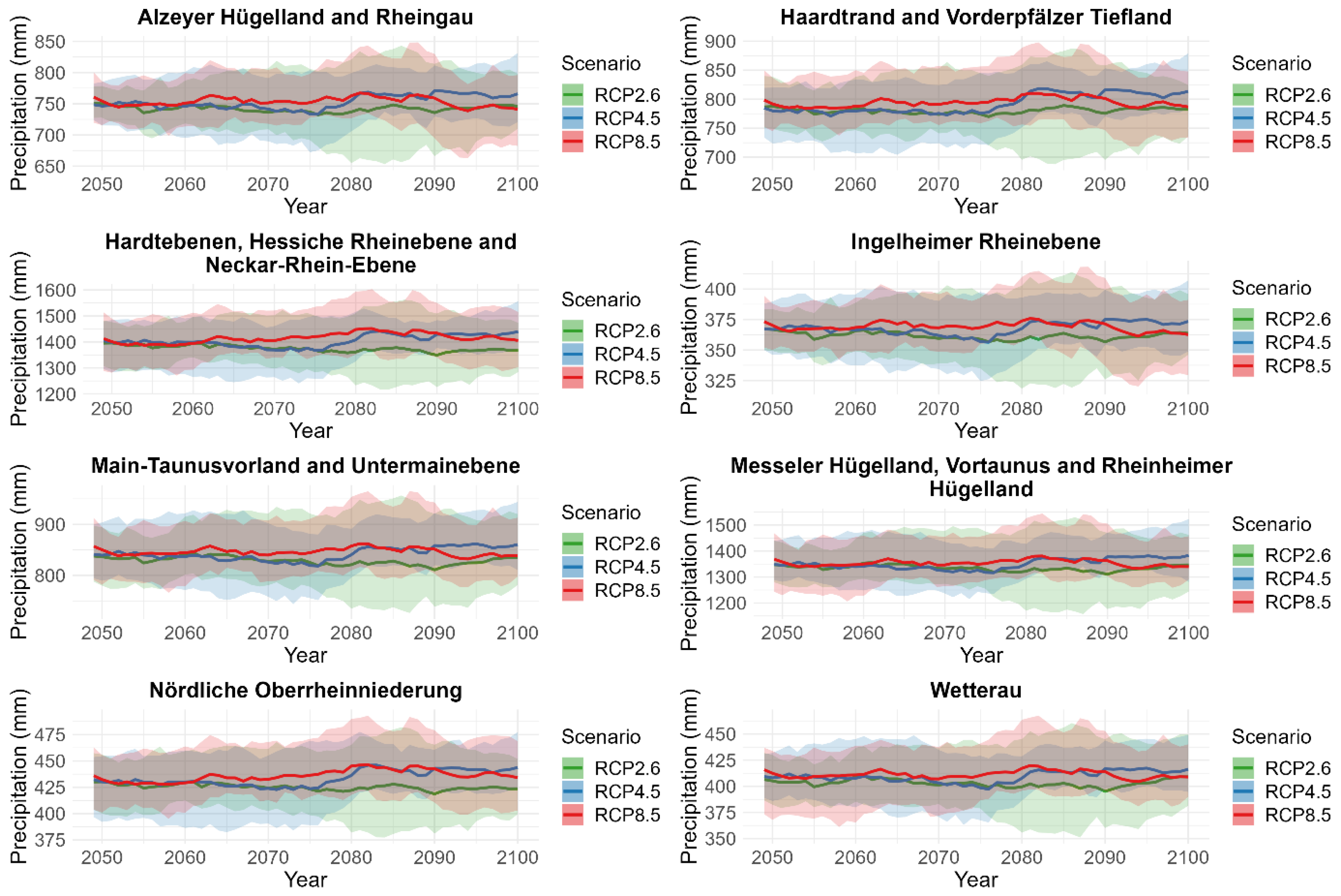

| Variables | Time Span | Data Source |
|---|---|---|
| Mean temperature and cumulative precipitation in the growing season (April–October) are averaged for 30 years across each natural region. | 1991–2020 | Data source: HYRAS. The data is based on measured weather data and gridded to 5 km resolution. |
| Daily mean, minimum, and maximum temperature and daily cumulative precipitation across each natural region. | 2020–2023 | Data source: HYRAS for daily precipitation and ERA5 for daily temperature. |
| Daily mean, minimum, and maximum temperature and daily cumulative precipitation across each natural region. | 2020–2100 | Multi-model ensemble of future climate projections based on three greenhouse gas emissions (RCP2.6, RCP4.5, and RCP8.5). |
| GCM-RCM Combination | RCP2.6 | RCP4.5 | RCP8.5 |
|---|---|---|---|
| ICHEC-EC-EARTH_r12i1p1_CLMcom-CCLM4-8-17 | X | X | X |
| ICHEC-EC-EARTH_r12i1p1_KNMI-RACMO22E | X | X | X |
| ICHEC-EC-EARTH_r12i1p1_SMHI-RCA4 | X | X | X |
| MIROC-MIROC5_r1i1p1_CLMcom-CCLM4-8-17 | X | - | X |
| MPI-M-MPI-ESM-LR_r1i1p1_SMHI-RCA4 | X | X | X |
| MPI-M-MPI-ESM-LR_r1i1p1_UHOH-WRF361H | X | - | X |
| Variance Components | Male Cicadas | Female Cicadas |
|---|---|---|
| Climate region | 0 | 0 |
| Climate region: Year: Month ( | 6.60 | 5.25 |
| Climate region: Location | 0.42 | 0.64 |
| Climate Regions | Male Cicadas (Count per Day) | Female Cicadas (Count per Day) | ||
|---|---|---|---|---|
| Observed Sample Means | Model 1 Backtransformed Means | Observed Sample Means | Model 1 Backtransformed Means | |
| Alzeyer Hügelland and Rheingau | 0.91 | 0.48 | 0.14 | 0.08 |
| Haardtrand and Vorderpfäzer Tiefland | 0.74 | 0.72 | 0.05 | 0.07 |
| Hardtebenen, Hessiche Rheinebene and Neckar-Rhein-Ebene | 0.90 | 1.50 | 0.07 | 0.07 |
| Messeler Hügelland, Vortaunus and Rheinheimer Hügelland | 1.44 | 2.02 | 0.13 | 0.29 |
| Nördliche Oberrheinniederung | 1.16 | 1.11 | 0.13 | 0.15 |
| Ingelheimer Rheinebene | 1.14 | 0.76 | 0.04 | 0.08 |
| Main-Taunusvorland and Untermainebene | 1.39 | 0.39 | 0.06 | 0.02 |
| Parameter | Male Cicadas | Female Cicadas | ||||
|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | Standard Error | p-Value | Estimate | Standard Error | p-Value |
| Intercept | −2.30 | 0.36 | <0.001 *** | −4.45 | 0.48 | <0.001 *** |
| Scaled mean temperature | 0.31 | 0.23 | 0.17 ns | 0.12 | 0.26 | 0.65 ns |
| (Scaled mean temperature)2 | 0.32 | 0.16 | 0.05 . | 0.33 | 0.18 | 0.07 . |
| Scaled precipitation | 0.07 | 0.29 | 0.80 ns | −0.03 | 0.31 | 0.91 ns |
| (Scaled precipitation)2 | −0.17 | 0.24 | 0.47 ns | −0.21 | 0.28 | 0.45 ns |
| Random effects | Variance | Standard Deviation | Variance | Standard Deviation | ||
| Climate region: Year: Month | 6.75 | 2.60 | 5.28 | 2.30 | ||
| Climate region: Location | 0.38 | 0.62 | 0.62 | 0.79 | ||
| Fixed Effects | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | −1.77 | 0.25 | <0.001 *** |
| Scaled mean temperature | 0.58 | 0.04 | <0.001 *** |
| (Scaled mean temperature)2 | −0.29 | 0.02 | <0.001 *** |
| Scaled precipitation | 0.25 | 0.05 | <0.001 *** |
| (Scaled precipitation)2 | −0.08 | 0.01 | <0.001 *** |
| Random effects | Variance | Standard Deviation | |
| Climate region: Year: Month | 5.48 | 2.34 | |
| Climate region: Location | 0.43 | 0.66 | |
| Fixed Effects | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | −4.12 | 0.24 | <0.001 *** |
| Scaled mean temperature | 0.84 | 0.06 | <0.001 *** |
| (Scaled mean temperature)2 | −0.16 | 0.03 | <0.001 *** |
| Scaled precipitation | 0.16 | 0.07 | 0.02 * |
| (Scaled precipitation)2 | −0.06 | 0.02 | 0.003 ** |
| Random effects | Variance | Standard Deviation | |
| Climate region: Year: Month | 3.60 | 1.89 | |
| Climate region: Location | 0.65 | 0.80 | |
| Data Source | Male Cicadas | Female Cicadas | ||
|---|---|---|---|---|
| Long-term averages of temperature and precipitation over 30 years (1991–2020) | 0.02 | 0.83 | 0.01 | 0.78 |
| Daily temperature and cumulative daily precipitation (2020–2023) | 0.07 | 0.83 | 0.12 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakarla, S.K.; Schall, E.; Dettweiler, A.; Stohl, J.; Glaser, E.; Adam, H.; Teubler, F.; Ingwersen, J.; Sauer, T.; Piepho, H.-P.; et al. Dependence of the Abundance of Reed Glass-Winged Cicadas (Pentastiridius leporinus (Linnaeus, 1761)) on Weather and Climate in the Upper Rhine Valley, Southwest Germany. Agriculture 2025, 15, 1323. https://doi.org/10.3390/agriculture15121323
Kakarla SK, Schall E, Dettweiler A, Stohl J, Glaser E, Adam H, Teubler F, Ingwersen J, Sauer T, Piepho H-P, et al. Dependence of the Abundance of Reed Glass-Winged Cicadas (Pentastiridius leporinus (Linnaeus, 1761)) on Weather and Climate in the Upper Rhine Valley, Southwest Germany. Agriculture. 2025; 15(12):1323. https://doi.org/10.3390/agriculture15121323
Chicago/Turabian StyleKakarla, Sai Kiran, Eric Schall, Anna Dettweiler, Jana Stohl, Elisabeth Glaser, Hannah Adam, Franziska Teubler, Joachim Ingwersen, Tilmann Sauer, Hans-Peter Piepho, and et al. 2025. "Dependence of the Abundance of Reed Glass-Winged Cicadas (Pentastiridius leporinus (Linnaeus, 1761)) on Weather and Climate in the Upper Rhine Valley, Southwest Germany" Agriculture 15, no. 12: 1323. https://doi.org/10.3390/agriculture15121323
APA StyleKakarla, S. K., Schall, E., Dettweiler, A., Stohl, J., Glaser, E., Adam, H., Teubler, F., Ingwersen, J., Sauer, T., Piepho, H.-P., Lang, C., & Streck, T. (2025). Dependence of the Abundance of Reed Glass-Winged Cicadas (Pentastiridius leporinus (Linnaeus, 1761)) on Weather and Climate in the Upper Rhine Valley, Southwest Germany. Agriculture, 15(12), 1323. https://doi.org/10.3390/agriculture15121323






