Abstract
Manganese is an important essential micronutrient, and its deficiency causes latent health issues in humans. Agronomic biofortification can promisingly improve the plant nutrient concentration without changing the genetic makeup of plants. This study was designed to assess the best method of Mn application to enhance productivity and grain Mn contents under conventional tillage (CT) and no tillage (NT) systems. Manganese was delivered through seed coating (250-mg kg−1 seed), osmopriming (0.1-M Mn solution), soil application (1 kg ha−1), and foliar application (0.25-M Mn solution). A general control with no seed Mn application was included, whereas hydropriming and water spray were used as positive control treatments for Mn seed priming and Mn foliar spray, respectively. No tillage had a higher total soil porosity (9%), soil organic carbon (16%), soil microbial biomass carbon (4%), nitrogen (2%), and soil nutrients in the CT system. Manganese nutrition through various methods significantly enhanced the yield, grain biofortification, and net benefits for CT and NT systems. Averaged across two years, the maximum improvement in grain productivity was recorded with osmopriming (28%) followed by foliar application (26%). The highest grain Mn concentration (29% over no application) was recorded with Mn foliar applications under both tillage systems. Moreover, the highest economic returns and marginal net benefits were recorded with osmopriming. To improve the wheat production, profitability, and grain Mn concentration, Mn application through priming and foliar application may be opted.
1. Introduction
Hidden hunger is an emerging issue that is adversely affecting the global population and has become a major challenge [1,2]. Globally, there are around 0.8 billion people who are persistently hungry and malnourished [3]. Above two billion people suffer from hidden hunger, particularly in developing countries [4]. The global population is escalating with rapid growth, and food demand is also rising at the same rate. After the green revolution, the major focus of the research was to increase the food quantity, without any focus on the quality of food [5,6,7]. About 50% of the global population is influenced by micronutrient deficiencies, because they are solely dependent upon cereals for their diet [8]. Micronutrients have a chief role in the metabolic and physiological functions of the plant, and their deficiency leads to disturbances in developmental cascades of plants and adversely affects productivity [9]. They are required in minor quantities and, at the above optimum amount, they become toxic [10].
Manganese (Mn) is essentially needed for the better functioning of all living organisms considered as an important micronutrient [11]. At higher concentrations, Mn reduces the availability of iron (Fe), copper (Cu), boron (B), and zinc (Zn) in soil [12]. Manganese is essential for neurotransmitter synthesis, brain functioning, and involved in carbohydrate and lipid metabolism [13,14]. In plants, Mn is necessary for hydrolysis in the oxygen-evolving complex of photosystem II. Additionally, it is required in chloroplast breakdown and chlorophyll biosynthesis [15,16]. The deficiency of Mn impedes root development, reduce tillering, and causes interveinal chlorosis [17,18]. The prevalence of Mn deficiency is higher in alkaline calcareous soils [19], adversely affecting plant growth and productivity. Staple cereals, especially wheat and rice, showed a higher sensitivity of Mn deficiency compared with other crops [19,20]. A survey reported that, globally, 10% of agricultural soils are Mn-deficient [21]. In soil solution, the concentration of micronutrients (Mn, copper (Cu), iron (Fe), and zinc (Zn)) is mainly dependent upon the soil pH, redox potential, and soil organic matter [22]. Reduction in pH or redox potential can enhance the Fe, Cu, Mn, and Zn concentrations [23].
Conventional tillage (CT) significantly alters the soil properties and improves the seed-to-soil contact that ultimately leads to a better crop stand [24]. Nevertheless, intensive soil manipulation adversely affects the soil properties and deteriorates soil health and quality [25]. In this situation, no tillage (NT) is the best option, as it involves no soil disturbance, ensures timely wheat plantation, and increases the productivity and profitability of rice–wheat rotation on a sustained basis [26,27]. Wheat planting in the no tillage system, it helps to conserve energy resources (water and fuel) and improves soil health [28].
Fundamentally, the occurrence of micronutrient deficiency is high in developing countries, because the agriculture systems of these countries do not produce nutrient-rich foods [29]. There are a variety of options to overcome malnutrition problems, including food diversification, postharvest food fortification, pharmaceutical supplementation, and biofortification [30]. Agronomic biofortification is one of the major strategies to enhance the nutrient concentration in grain [31], which is attained by the application of micronutrients through seed treatments (seed coating and priming) and foliar and soil application [32,33]. Biofortification with agronomic interventions is potentially an efficient, economical, easy-to-implement, and more sustainable approach compared to genetic biofortification [34,35].
Although the application of Mn improves the wheat productivity, information about the role of Mn nutrition when applied through various methods on yield, economics, grain Mn accumulation, and use efficiency of wheat under conventional and conservation tillage systems is lacking. Therefore, the present study was carried out with the hypothesis that Mn application through different methods would improve the productivity and wheat grain biofortification of wheat under different tillage systems. The objectives of the present study were (a) to determine the most effective method of Mn application to enhance wheat productivity and grain biofortification under CT and NT and evaluate the economics and Mn efficiencies of Mn application (b) to study the impacts of different tillage permutations on soil health properties and nutrient dynamics.
2. Materials and Methods
2.1. Site, Soil and Climate
The study was executed at the Research Area, University of Agriculture, Faisalabad (latitude 73.89° E, longitude 31.62° N and altitude 183.8 m above sea level), Pakistan during 2017–2018 and 2018–2019. The soil of the experimental field was sandy clay loam in texture at the Lyallpur series, classified as Haplic Yermosols in the Food and Agriculture Organization (FAO) classification system [36] and fine-silty aridisol, hyperthermic Ustalfic, Haplagrid in the US Department of Agriculture (USDA) classification system [37]. Before initiation of the experiment, soil samples were collected for a soil physicochemical analysis [38]. The initial soil analysis indicated that the soil of the study had a pH of 7.1, electrical conductivity (EC) 0.49 dSm−1, total soil organic matter 6.3 g kg−1, total nitrogen (N) 0.358 g kg−1, available phosphorus (P) 0.00702 g kg−1, extractable potassium (K) 0.086 g kg−1, and Mn concentration 3.85 ppm. The climate of Faisalabad is generally hot, subtropical to semi-arid, with maximum temperatures of 43–46 °C during summer and the minimum temperatures of 6–9 °C during the winter. The weather data for both experimental periods are given in Figure 1.
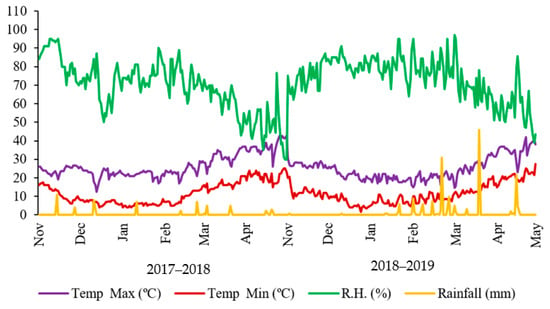
Figure 1.
Meteorological data during the two years of study. Source: Meteorological Cell, University of Agriculture Faisalabad, Pakistan. All the values of sunshine, relative humidity and mean temperature are the monthly averages. R.H.: relative humidity.
2.2. Plant Material
Anaaj-2017 cultivar of wheat was procured from Wheat Research Institute, Faisalabad. Germination potential and moisture contents of the seeds were determined according to [39] and were 94% and 12%, respectively.
2.3. Experimental Details
The experiment was designed as a randomized complete block design under a split-plot arrangement; where tillage systems were kept in the main plots and Mn application treatments were placed in subplots. The tillage system was comprised of conventional tillage and no tillage systems. There were seven Mn treatments viz. no application, Mn seed coating, hydropriming, osmopriming, basal application, foliar application of Mn, and water spray. For performing a coating of the seeds, an adhesive solution was prepared with Arabic gum, and Mn (250 mg kg−1 seed) was added in sticky solution, and the seed was added in the solution for 30 min and allowed to adhere on seeds. For seed priming, seeds were dipped in aerated distilled water (hydropriming) or 0.1-M aerated Mn solution (osmopriming) for 12 h with 1:5 seed weight to a solution: volume ratio. The aquarium pump was used for the provision of artificial aeration to the solution during soaking. From the soaking solution, seeds were removed, washed by using distilled water, and dried for gaining their original weight. Mn soil application was carried out by its broadcasting at the rate of 1 kg ha−1 before seed drilling. For foliar application, 0.25-M Mn solution (Foliar Mn) or water was sprayed using a manual sprayer at the booting stage (BBCH-40) [40]. Hydropriming and water spray were considered as a positive control for osmopriming and Mn foliar application. The source for Mn was MnSO4.7H2O for all treatments. Husbandry practices during both experimental seasons are detailed in Table 1. Based on soil test, fertilizers were applied at 115:85:65 N:P:K kg ha−1, respectively. At the time of sowing, half of the N and complete doses of P and K were applied, while the N remaining dose was applied in two halves with first (25 days after sowing (DAS) and second irrigations (55 DAS).

Table 1.
Crop husbandry details in wheat during 2017 and 2018 and 2018 to 2019.
2.4. Observations, Measurements, and Data Analysis
2.4.1. Soil Properties
For the determination of soil bulk density (BD), total soil porosity (TSP), soil organic carbon (SOC), total N, available P, and extractable K, soil samples were taken at final harvest from two sampling depths (0–10 cm and 10–20 cm). Whereas the soil was sampled at the anthesis stage (BBCH-69) [40] for the estimation of soil microbial biomass carbon (SMBC) and nitrogen (SMBN). Data regarding BD [41] and TSP [42], SOC [43], total N [44], available P [45], and extractable K [46] were estimated. Chloroform extraction method was used for the determination of SMBC and SMBN [47,48].
2.4.2. Yield Parameters
The number of productive tillers were determined from a 1 m−2 unit area in each plot from four random points at final harvest. Twenty spikes were randomly selected from each plot and after threshing grains were separated. From the same spikes, grains were counted to record grains per spike. The crop was manually harvested from each plot, tied into bundles, sundried for a week, and weighed to record the biological yield. The crop, from each plot was threshed with the help of a mini-thresher, grains were separated, and grain yield was recorded. For 1000-grain weight, three subsamples of 1000 grains from each plot were taken and weighed using digital weighing beam. The harvest index was recorded as the ratio of dry grain yield to biological yield and expressed in percentage.
2.4.3. Grain and Straw Mn Concentrations
Mature samples of grain and straw were taken and prepared by wet ashing [49]. Samples were oven-dried, crushed, and weighed. Afterward, these samples were digested in a di-acid mixture (HClO4:HNO3 at 3:10 v/v), and Mn concentration in grains and straw was determined on atomic absorption spectrophotometer (Perkin Elmer, San Jose, CA, USA).
2.4.4. Estimation of Mn Use Efficiency
Manganese use efficiencies, viz., agronomic (AgE), physiological (PE), agro-physiological (AgPE), apparent recovery (ARE), utilization efficiency (UE), and Mn harvest index (MnHI) were calculated by following Fageria [12] and Shivay and Prasad [50].
where GYMn is grain yield of Mn fertilized plots, GYC is the yield of unfertilized plots, Mna is the total amount of Mn applied, YMn is the grain and straw yield of Mn-treated plots, Yc is the grain and straw yield of unfertilized plots, UMn is the Mn uptake in the grain and straw of Mn-fertilized plots, and UC is the uptake of Mn in the grain and straw of untreated plots.
2.4.5. Economic Analysis
For the estimation of net benefits and the benefit:cost ratio (BCR), an economic analysis was performed following [51]. For deriving adjusted grain and straw yield, the actual grain and straw yield was reduced by 10%. Seed, irrigation, fertilizers, plant protection, labor cost, and harvest were included as a fixed cost, whereas tillage operations and Mn nutrition were included as a variable cost. The marginal analysis was performed by the following [52].
2.4.6. Statistical Analysis
Data were statistically analyzed using computer software Statistix 8.1. For mean separation, Tukey’s HSD (honest significant difference) test was applied at the 5% probability level [53]. SigmaPlot 10.0 was used for graphical representation of data.
3. Results
3.1. Soil Properties
Wheat tillage systems (WTs) had a significant impact on the soil BD, TSP, SOC, SMBC, and SMBN at both sampling depths during both years of experimentation (Table 2). Soil BD was recorded higher (3% at 0–10 cm and 4% at 10–20 cm) from A CT system in comparison to NT system for both years (Table 2). Under NT, substantial improvements were observed at 0–10 cm and 10–20 cm sampling depths in TSP (8% and 9%), SMBC (4% and 3%), SMBN (3% and 2%), and SOC (17% and 15%) on an average of two years (Table 2).

Table 2.
Influence of tillage systems on soil health parameters recorded after wheat harvest.
Total N, available P, and extractable K were also affected by tillage systems during 2017–2018 and 2018–2019 (Figure 2), whereas the results were nonsignificant for total N during the first year of study. Total N was 12% higher in the NT system, compared to CT during 2018 to 2019. The highest available phosphorus was recorded (9% and 8%) with a NT system compared to CT during both study years. Likewise, NT showed the highest values (6% and 7%) for extractable K compared with CT during the 2017 to 18 and 2018 to 19 (Figure 2).
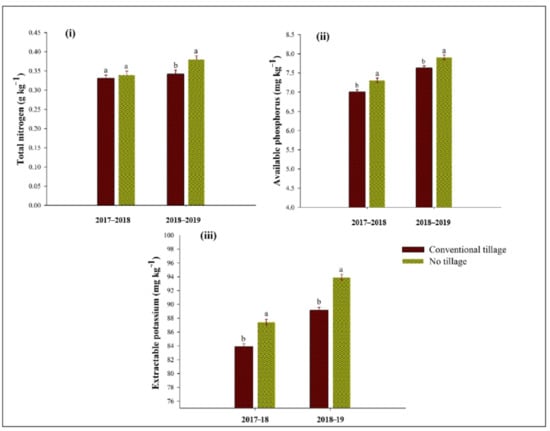
Figure 2.
Influence of conventional tillage and no tillage systems on (i) total nitrogen (g kg−1), (ii) available phosphorous (mg kg−1), and (iii) extractable potassium (mg kg−1) in the soil after wheat harvest. Error bars above indicates the ±S.E. of three replicates. Means sharing the same letter during an experimental year for a parameter do not differ significantly at p ≤ 0.05.
3.2. Yield Parameters
A manganese application considerably influenced the productive tillers during both study years. However, WTs had no considerable impact on the productive tillers. Across different WTs, the highest value for the number of productive tillers i.e., 12% higher during each year, was observed with osmopriming, compared with those in no Mn application treatment. Grains per spike were substantially improved with Mn application, and 23% and 27% higher grains per spike were observed with osmopriming during the first and second years of study, respectively. Results were statistically at par for the first year to foliar-applied Mn in case of grains per spike. The highest 1000-grain weight (20% and 32% over control) was noted with osmopriming during 2017–2018 and 2018–2019, respectively. However, osmopriming was statistically at par with Mn foliar application during both years. The highest biological yield was found by soil-applied Mn in the CT system and NT system for the first and second years, respectively. The grain yield was highest (36% and 26% over control) with osmopriming in the NT system during both study years. Grain yield (26% over control) was found with osmopriming for the second year. The harvest index was highest with a foliar application of Mn for both years (Table 3).

Table 3.
Effect of manganese application on the yield and related traits of wheat planted under two tillage systems.
3.3. Grain and Straw Mn Concentrations
Manganese nutrition significantly improved the grain and straw Mn accumulation. For both years, the highest grain Mn content was recorded by its foliar application under both WTs (Figure 3). The highest straw Mn concentration was noted with osmopriming during both years in the CT and NT systems (Figure 3).
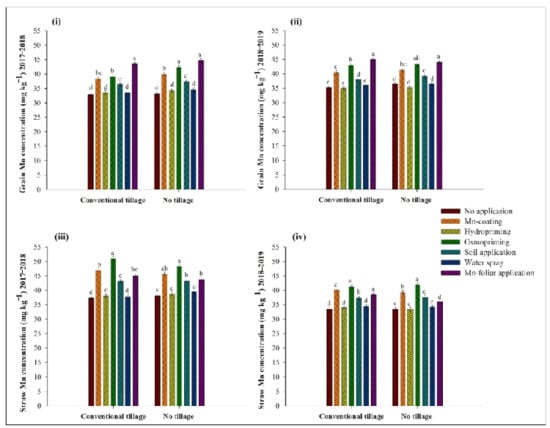
Figure 3.
Influence of Manganese (Mn) application on grain Mn concentrations (mg kg−1) during (i) 2017–2018 and in (ii) 2018–2019, straw Mn concentration (mg kg−1) during (iii) 2017–2018 and in (iv) 2018–2019; Error bars above the means indicate the ± S.E. of three replicates. Means sharing the same letter during an experimental year for a parameter do not differ significantly at p ≤ 0.05.
3.4. Manganese Use Efficiency Indices
The application of Mn considerably influenced the efficiency indices (Table 4). However, the effects of WTs were not significant for efficiency indices. Mn seed coating showed the highest AgE during both years. The PE was highest, with soil-applied Mn during both study years. Likewise, the AgPE was highest, with soil-applied Mn for the first year, whereas the highest AgPE was observed with soil-applied Mn in a CT system during the second experimental year; the results were statistically similar to the soil-applied Mn in the NT system. ARE and UE were highest by seed coating with Mn for both experimental years. MnHI was highest with foliar-applied Mn during both study years, and the results were statistically at par to seed coated with Mn during the second year (Table 4).

Table 4.
Effect of manganese application methods on Mn use efficiencies of wheat under two tillage systems.
3.5. Economic and Marginal Analysis
The economic analysis showed that manganese application improved the net benefits of wheat through either application method grown in both tillage systems. Nevertheless, the highest net benefits and BCR were noted with osmopriming in the CT and NT systems. Among WTs, the NT system had the highest net benefits compared with the CT system (Table 5). Likewise, the highest marginal rate of return was recorded with osmopriming (Table 6).

Table 5.
Economics of Mn application methods in wheat under conventional tillage and no tillage (pooled data for 2017–2018 and 2018–2019).

Table 6.
Marginal analysis for the effects of Mn application on the wheat performance in different tillage systems (pooled data for 2 years).
4. Discussion
The results supported the hypothesis that Mn nutrition in wheat by different application methods increased the productivity, net benefits, grain Mn concentration, and its efficient use in conventional, as well as conservation, tillage systems (Table 3, Table 4, Table 5 and Table 6 and Figure 2); nevertheless, a variation was found for various Mn application methods and WTs. The no tillage system enhanced the soil health attributes in comparison to the CT system, as depicted by a decrease in soil BD and improvement in total soil porosity, soil organic carbon, soil microbial biomass nitrogen, soil microbial biomass carbon, total N, available P, and extractable K for both years of experimentation (Table 2 and Figure 2). Soil BD was higher under the CT system owing to intensive tillage and soil compaction with heavy tillage implements [54,55]. Lower BD under the NT system due to minimizing the soil disturbances, which makes its way towards improving the soil pores’ continuity [56]. It is well-documented that with a decrease in soil BD, TSP increased [57]. Moreover, residues holding on to soil surface results in firm aggregates formation and improves TSP [58,59,60], as less soil disturbance improves the transmission and storage pores of soil [61]. Furthermore, the reduction in soil compaction under the NT system provided a feasible environment for the microbial population in the soil, thus improving the SMBC, SMBN, and SOC [62]. Higher residues retention on the soil cover amended the soil health owing to a greater availability of carbon for decomposition; as reduced soil disturbance in the NT system provide organic C continuously for soil microfauna, thus enhancing the microbial activity and biomass [63,64,65]. No tillage (NT) decreases the decomposition of SOM and reduces the soil C losses and improves the SMBC, SMBN, and SOC [66]. Soil microbial biomass carbon and SOC were improved under the NT system, which may be due to the conservation of mineralizable C from reserved residues that improve the results of biological activities of soil by increasing the concentration of soil enzymes, including phosphatase and urease [67]. Contrarily, the CT exposed stored soil carbon due to an intensive disturbance of soil that may lead to the depletion of SOC, lessening the biological activity, and active microbial biomass [68,69]. In addition, under the CT system, the dispersal of soil particles owing to intensive soil disturbances intensifies carbon-rich macropores and free the SOM particles having higher degradability and poor stability results in SOC loss [70]. Concentrations of total N and available P were improved under the NT system, because N and P are directly associated with the presence of crop residues, as it enhances the storage of N and N in the top layer of the soil [71]. Likewise, no tillage leads to more contents of P stratification near the soil surface [72]. In a CT system, the soil is highly exposed to the aerial environment that leads to N volatilization. Moreover, higher nitrate leaching was observed in a CT system compared with the NT system [73]. In CT, during plowing, soil inversion shifts fertile subsoil to the surface, leading to the possibility of leaching [74].
Both WTs and methods of Mn applications significantly affected the yield and associated traits (Table 3). Better results regarding the productive tillers, grain weight, and grain yield with the NT system were due to better soil properties and nutrient dynamics [75]. Under both tillage systems, Mn nutrition through either method significantly enhanced the grain yield of wheat (Table 3). Manganese has a significant role in photosynthesis and assimilates translocation toward grains during grain development [76]. A deficiency of Mn leads to the poor development of anthers, pollens infertility, reduction in assimilates translocation, poor seed setting, and declined grain yield [77].
Among the application methods, osmopriming and foliar applications effectively improved the yield and yield contributing components. Manganese osmopriming produced the highest number of productive tillers owing to uniform and vigorous stand establishment. Primed seeds have excessive metabolites that are readily available during planting [78]. It plays a significant role to readily start and completes the process of germination, which can lead to uniform crop standing even under adverse conditions [79] and leads to improved seed setting and grain weight [33]. Manganese nutrition improves the number of tillers and seeds set due to better pollen germination and fertilization [80]. After osmopriming, foliar-applied Mn enhanced the wheat yield for both WTs, because it is an efficient method of application owing to various reasons, including lower application rates, uniform application and distribution, and rapid plant response [81]. The lower response of Mn basal application was might due to lower SOM and alkaline calcareous soils that reduced the Mn availability to plants [82]. Grain weight with Mn application on foliage might be due to an improved source-sink relationship that ensures maximum assimilates supply during grain development [83]. Such findings could be helpful for model and non-model plants [84,85].
The manganese foliar application might improve the grain yield; as Mn foliar fertilization at the anthesis stage efficiently translocates the Mn towards reproductive parts, and then accumulates in grains [86,87]. In addition, the foliar application of Mn resulted in absorption in the leaf epidermis, and after remobilization, it was transported into developing grains via xylem [88]. Furthermore, foliar-applied Mn improved the grain Mn contents that may be due to the accumulation of Mn on flag leaf and better translocation toward grains, with the appropriate balance of photosynthates among vegetative, as well as reproductive parts.
Wheat tillage systems, different Mn application methods, and their interactions significantly affect their net benefits and BCR (Table 5). Maximum net income and BCR were achieved by osmopriming in the NT system. The lowest income and BCR were recorded where Mn was not applied in the CT system (Table 5). Nonetheless, the marginal study showed that osmopriming is the better profitable approach of Mn application with the highest marginal rate of return (Table 6).
5. Conclusions
The application of Mn through either method enhanced the productivity, economic returns, and grain biofortification under both tillage permutations. The highest wheat yield and economic returns were achieved with osmopriming followed by foliar-applied Mn, particularly under the no tillage system. The variations in the efficacy of Mn application methods in relation to the grain Mn contents was also found among different tillage systems, because the Mn foliar approach gave the maximum grain of Mn accumulation under both tillage systems. Among the tillage systems, the no till system improved the soil health attributes, as shown by higher TSP, SMBC, SMBN, and SOC due to the improved microbial activity and nutrient concentration compared with a conventional till system. In crux, wheat cultivation under the no tillage system with Mn application as osmopriming and foliar application is helpful in attaining better yield and grain biofortification.
Author Contributions
Conceptualization, methodology, and software, U.Z., M.I., N.A., M.A., F.I., S.H., and M.A.E.-E.; formal analysis, validation, and data curation, U.Z., M.I., N.A., M.A., F.I., M.A.E.-E., and S.H.; investigation, U.Z., S.H., M.I., A.R., C.H., and M.A.E.-E.; resources, S.H. and M.A.E.-E.; visualization, A.R., S.H., M.S.S., C.H., and M.A.E.-E.; writing—original draft preparation, U.Z., M.I., N.A., M.A., F.I., S.H., and M.A.E.-E.; writing—review and editing, U.Z., S.H., M.S.S., A.R., C.H., and M.A.E.-E. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the findings of this study are included in this article.
Acknowledgments
The authors acknowledge Tanta University in Egypt for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hodge, J. Hidden hunger: Approaches to tackling micronutrient deficiencies. In Nourishing Millions: Stories of Change in Nutrition; Gillespie, S., Hodge, J., Yosef, S., Pandya-Lorch, R., Eds.; International Food Policy Research Institute: Washington, DC, USA, 2016; pp. 35–43. [Google Scholar] [CrossRef]
- International Food Policy Research Institute. Global Nutrition Report 2017: Nourishing the SDGs; IFPRI: Washington, DC, USA, 2017. [Google Scholar]
- Food and Agriculture Organization; International Fund for Agricultural Development; United Nations Children’s Fund; World Health Organization. The State of Food Security and Nutrition in the World: Building Resilience for Peace and Food Insecurity; FAO: Rome, Italy, 2017. [Google Scholar]
- Jawaldeh, A.A.; Pena-Rosas, J.P.; McColl, K.; Johnson, Q.; Elmadfa, I.; Nasreddine, L. Wheat Four Fortifcation in the Eastern Mediterranean Region; Licence: CC BY-NC-SA 3.0 IGO; WHO Regional Office for the Eastern Mediterranean: Cairo, Egypt, 2019. [Google Scholar]
- Bouis, H.E.; Eozenou, P.; Rahman, A. Food Prices, Household Income, and Resource Allocation: Socioeconomic Perspectives on Their Effects on Dietary Quality and Nutritional Status. Food Nutr. Bull. 2011, 32, S14–S23. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A New Tool to Reduce Micronutrient Malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef]
- Allen, S.L.; De Brauw, A. Nutrition sensitive value chains: Theory, progress, and open questions. Glob. Food Secur. 2018, 16, 22–28. [Google Scholar] [CrossRef]
- Kenzhebayeva, S.; Abekova, A.; Atabayeva, S.D.; Yernazarova, G.; Omirbekova, N.; Zhang, G.; Turasheva, S.; Asrandina, S.; Sarsu, F.; Wang, Y. Mutant Lines of Spring Wheat with Increased Iron, Zinc, and Micronutrients in Grains and Enhanced Bioavailability for Human Health. BioMed Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Mishra, S.; Chauhan, D.K.; Dubey, N.K. Micronutrients and their diverse role in agricultural crops: Advances and future prospective. Acta Physiol. Plant. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef]
- Millaleo, R.; Diaz, M.R.; Ivanov, A.G.; Mora, M.; Alberdi, M. Manganese as Essential and Toxic Element for Plants: Transport, Accumulation and Resistance Mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC Press: Boca Raton, FL, USA, 2009; pp. 31–77. [Google Scholar] [CrossRef]
- Golub, M.S.; Hogrefe, C.E.; Germann, S.L.; Tran, T.T.; Beard, J.L.; Crinella, F.M.; Lonnerdal, B. Neurobehavioral evaluation of rhesus monkey infants fed cow’s milk formula, soy formula, or soy formula with added manganese. Neurotoxicol. Teratol. 2005, 27, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Finley, E.J.; Chakraborty, S.; Aschner, M. Manganese in Biological Systems. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1297–1303. [Google Scholar] [CrossRef]
- Livorness, J.; Smith, T.D. The role of manganese in photosynthesis. In Bioschemistry; Springer: Berlin/Heidelberg, Germany, 1982; pp. 1–44. [Google Scholar] [CrossRef]
- Grundmeier, A.; Dau, H. Structural models of the manganese complex of photosystem II and mechanistic implications. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 88–105. [Google Scholar] [CrossRef]
- Moosavi, A.A.; Ronaghi, A. Growth and Iron-Manganese Relationships in Dry Bean as Affected by Foliar and Soil Applications of Iron and Manganese in a Calcareous Soil. J. Plant Nutr. 2010, 33, 1353–1365. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese Deficiency in Plants: The Impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Farooq, M.; Rehman, A.; Arshad, M.S.; Shoukat, H.; Nadeem, A.; Nawaz, A.; Wakeel, A.; Nadeem, F. Manganese Nutrition Improves the Productivity and Grain Biofortification of Bread Wheat in Alkaline Calcareous Soil. Exp. Agric. 2017, 54, 744–754. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Nadeem, A.; Rehman, A.; Asad, S.A.; Nawaz, A. Manganese nutrition improves the productivity and grain biofortification of fine grain aromatic rice in conventional and conservation production systems. Paddy Water Environ. 2016, 15, 563–572. [Google Scholar] [CrossRef]
- Graham, R.D. Micronutrient Deficiencies in Crops and Their Global Significance. In Micronutrient Deficiencies in Global Crop Production; Springer: Dodrecht, The Netherlands, 2008; pp. 41–61. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Mackie-Dawson, L.A.; Linehan, D.J. Micronutrient inflow rates and mobilisation into soil solution in the root zone of winter wheat (Triticum aestivum L.). Plant Soil 1990, 122, 143–146. [Google Scholar] [CrossRef]
- Miao, S.; Delaune, R.; Jugsujinda, A. Influence of sediment redox conditions on release/solubility of metals and nutrients in a Louisiana Mississippi River deltaic plain freshwater lake. Sci. Total Environ. 2006, 371, 334–343. [Google Scholar] [CrossRef]
- Dixit, A.K.; Agrawal, R.K.; Das, S.K.; Sahay, C.S.; Choudhary, M.; Rai, A.K.; Palsaniya, D.R. Soil properties, crop productivity and energetics under different tillage practices in fodder sorghum + cowpea—Wheat cropping system. Arch. Agron. Soil Sci. 2019, 65, 492–506. [Google Scholar] [CrossRef]
- Bhowmik, A.; Kukal, S.S.; Saha, D.; Sharma, H.; Kalia, A.; Sharma, S. Potential Indicators of Soil Health Degradation in Different Land Use-Based Ecosystems in the Shiwaliks of Northwestern India. Sustainability 2019, 11, 3908. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Maqsood, M.; Hussain, S.; Anwar-Ul-Haq, M. Iron Nutrition Improves Productivity, Profitability, and Biofortification of Bread Wheat under Conventional and Conservation Tillage Systems. J. Soil Sci. Plant Nutr. 2020, 20, 1298–1310. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Matloob, A.; Ali, N.; Ahmad, M.; Alyemeni, M.N.; Ahmad, P. Zinc-Induced Effects on Productivity, Zinc Use Efficiency, and Grain Biofortification of Bread Wheat under Different Tillage Permutations. Agronomy 2020, 10, 1566. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Nadeem, F.; Siddique, K.H.M.; Lal, R. Rice–wheat cropping systems in South Asia: Issues, options and opportunities. Crop. Pasture Sci. 2019, 70, 395–427. [Google Scholar] [CrossRef]
- Bouis, H.E. Biofortification: An Agricultural Tool to Address Mineral and Vitamin Deficiencies. In Food Fortification in a Globalized World; Venkatesh Manar, M.G., Hurell, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 69–81. [Google Scholar] [CrossRef]
- Lalani, B.; Bechoff, A.; Bennett, B. Which Choice of Delivery Model(s) Works Best to Deliver Fortified Foods? Nutrients 2019, 11, 1594. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- De Valença, A.; Bake, A.; Brouwer, I.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.; Maqsood, M.; Ishfaq, M.; Ali, N. Zinc nutrition to enhance rice productivity, zinc use efficiency, and grain biofortification under different production systems. Crop Sci. 2021, 61, 739–749. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Maqsood, M.; Hussain, S. Biofortification of Rice with Iron and Zinc: Progress and Prospects. In Rice Research for Quality Improvement: Genomics and Genetic Engineering; Springer: Singapore, 2020; pp. 605–627. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2014. [Google Scholar]
- United States Department of Agriculture. Keys to Soil Taxonomy, 12th ed.; Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas: Aleppo, Syria, 2013; pp. 65–119. [Google Scholar]
- International Seed Testing Association. International Rules for Seed Testing; International Seed Testing Association: Zürich, Switzerland, 2015. [Google Scholar]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis, 2nd ed.; Agron. No. 9, Part I; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar] [CrossRef]
- Vomocil, J.A. Porosity. In Methods of Soil Analysis; Blake, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 299–314. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.; Mulvaney, C. Nitrogen-Total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 1119–1123. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate (No. 939); US Department of Agriculture: Washington, DC, USA, 1954.
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; US Department of Agriculture: Washington, DC, USA, 1954; p. 154.
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility. A Handbook of Methods, 2nd ed.; CAB International: Wallingford, UK, 1993. [Google Scholar] [CrossRef]
- Giron, H.C. Comparison between dry ashing and wet digestion in the preparation of plant material for atomic absorption analysis. J. At. Absorpt. Newsl. 1973, 12, 28–29. [Google Scholar]
- Shivay, Y.S.; Prasad, R. Zinc-Coated Urea Improves Productivity and Quality of Basmati Rice (Oryza Sativa L.) under Zinc Stress Condition. J. Plant Nutr. 2012, 35, 928–951. [Google Scholar] [CrossRef]
- The International Maize and Wheat Improvement Center. From Agronomic Data to Farmers Recommendations: An Economics Training Manual; The International Maize and Wheat Improvement Center: El Batan, Mexico, 1998; pp. 31–33. [Google Scholar]
- Byerlee, D. From Agronomic Data to Farmers Recommendations. An Economics Training Manual; CIMMYT: El Batan, Mexico, 1988; pp. 31–33. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; No. 519.5 S8; McGraw Hill College: New York, NY, USA, 1997. [Google Scholar]
- Benjamin, J. Tillage effects on near-surface soil hydraulic properties. Soil Tillage Res. 1993, 26, 277–288. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and cropproductivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A. Weed dynamics and productivity of wheat in conventional and conservation rice-based cropping systems. Soil Tillage Res. 2014, 141, 1–9. [Google Scholar] [CrossRef]
- Nawaz, A.; Farooq, M.; Lal, R.; Rehman, A.; Hussain, T.; Nadeem, A. Influence of Sesbania Brown Manuring and Rice Residue Mulch on Soil Health, Weeds and System Productivity of Conservation Rice–Wheat Systems. Land Degrad. Dev. 2016, 28, 1078–1090. [Google Scholar] [CrossRef]
- Roldan, A. No-tillage, crop residue additions, and legume cover cropping effects on soil quality characteristics under maize in Patzcuaro watershed (Mexico). Soil Tillage Res. 2003, 72, 65–73. [Google Scholar] [CrossRef]
- Hobbs, P.R. Conservation agriculture: What is it and why is it important for future sustainable food production? J. Agric. Sci. 2007, 145, 127–137. [Google Scholar] [CrossRef]
- Jacobs, A.; Rauber, R.; Ludwig, B. Impact of reduced tillage on carbon and nitrogen storage of two Haplic Luvisols after 40 years. Soil Tillage Res. 2009, 102, 158–164. [Google Scholar] [CrossRef]
- Ordoñez-Morales, K.D.; Cadena-Zapata, M.; Zermeño-González, A.; Campos-Magaña, S. Effect of Tillage Systems on Physical Properties of a Clay Loam Soil under Oats. Agriculture 2019, 9, 62. [Google Scholar] [CrossRef]
- Nandan, R.; Singh, V.; Singh, S.S.; Kumar, V.; Hazra, K.K.; Nath, C.P.; Poonia, S.; Malik, R.K.; Bhattacharyya, R.; McDonald, A. Impact of conservation tillage in rice–based cropping systems on soil aggregation, carbon pools and nutrients. Geoderma 2019, 340, 104–114. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-till agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Mandal, K.G.; Mishra, A.K.; Hati, K.M.; Bandyopadhyay, K.K.; Ghosh, P.K.; Mohanty, M. Rice residue management options and effects on soil properties and crop productivity. Food Agric. Environ. 2004, 2, 224–231. [Google Scholar]
- Mohanty, M.; Painuli, D.; Misra, A.; Ghosh, P. Soil quality effects of tillage and residue under rice–wheat cropping on a Vertisol in India. Soil Tillage Res. 2007, 92, 243–250. [Google Scholar] [CrossRef]
- Zikeli, S.; Gruber, S.; Teufel, C.-F.; Hartung, K.; Claupein, W. Effects of Reduced Tillage on Crop Yield, Plant Available Nutrients and Soil Organic Matter in a 12-Year Long-Term Trial under Organic Management. Sustainability 2013, 5, 3876–3894. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Hanson, K.; Harker, K.; Clayton, G.; Blackshaw, R.; O’Donovan, J.; Johnson, E.; Gan, Y.; Irvine, R.; Monreal, M. Soil microbial biomass, functional diversity and enzyme activity in glyphosate-resistant wheat–canola rotations under low-disturbance direct seeding and conventional tillage. Soil Biol. Biochem. 2007, 39, 1418–1427. [Google Scholar] [CrossRef]
- Balota, E.L.; Colozzi-Filho, A.; Andrade, D.S.; Dick, R.P. Microbial biomass in soils under different tillage and crop rotation systems. Biol. Fertil. Soils 2003, 38, 15–20. [Google Scholar] [CrossRef]
- Roscoe, R. Tillage effects on soil organic matter in density fractions of a Cerrado Oxisol. Soil Tillage Res. 2003, 70, 107–119. [Google Scholar] [CrossRef]
- Moussadek, R.; Mrabet, R.; Dahan, R.; Zouahri, A.; El Mourid, M.; Van Ranst, E. Tillage System Affects Soil Organic Carbon Storage and Quality in Central Morocco. Appl. Environ. Soil Sci. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Das, A.; Lyngdoh, D.; Ghosh, P.K.; Lal, R.; Layek, J.; Idapuganti, R.G. Tillage and cropping sequence effect on physico-chemical and biological properties of soil in Eastern Himalayas, India. Soil Tillage Res. 2018, 180, 182–193. [Google Scholar] [CrossRef]
- Stanislawska-Glubiak, E.; Korzeniowska, J. Effect of soil tillage systems on nutrient concentration in winter wheat plants. J. Food Agric. Environ. 2012, 10, 1353–1355. [Google Scholar]
- Meisinger, J.J.; Palmer, R.E.; Timlin, D.J. Effects of tillage practices on drainage and nitrate leaching from winter wheat in the Northern Atlantic Coastal-Plain USA. Soil Tillage Res. 2015, 151, 18–27. [Google Scholar] [CrossRef]
- Busari, M.A.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Bhatt, R.; Khera, K.L.; Arora, S. Effect of tillage and mulching on yield of corn in the submontaneous rainfed region of Punjab, India. Int. J. Agric. Biol. 2004, 6, 126–128. [Google Scholar]
- Longnecker, N.E.; Graham, R.D.; McCarthy, K.W.; Sparrow, D.H.B.; Egan, J.P.; Bassam, N. Screening for manganese efficiency in barley (Hordeum vulgare L.). In Genetic Aspects of Plant Mineral Nutrition; Bassam, N.E., Loughman, B.C., Eds.; Springer: Dodrecht, The Netherlands, 1990; pp. 273–280. [Google Scholar] [CrossRef]
- Marschner, P. (Ed.) Marschner’s Mineral Nutrition of Higher Plants; Academic Press: San Diego, CA, USA, 1995; p. 889. [Google Scholar] [CrossRef]
- Lutts, S.; Benincasa, P.; Wojtyla, Ł.; Kubala, S.S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; IntechOpen: London, UK, 2016; pp. 1–46. [Google Scholar] [CrossRef]
- Haider, M.U.; Hussain, M.; Khan, M.B.; Ijaz, M.; Sattar, A.; Akram, M.S.; Hassan, W. Influence of Seed Priming and Seed Size on Wheat Performance under Different Tillage Systems. Int. J. Agric. Biol. 2016, 18, 858–864. [Google Scholar] [CrossRef]
- Goussias, C.; Boussac, A.; Rutherford, A. Photosystem II and photosynthetic oxidation of water: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Suzana, C.S.; Brunetto, A.; Marangon, D.; Tonello, A.A.E.; Kulczynski, S.M. Influence of fertilization leaf on the quality of soybean seed physiological stored. Enciclopedia Biosf. 2012, 8, 2385–2392. [Google Scholar]
- Barros, H.B.; Souza, M.E.; Dario, A.S.; Santos, M.P.D.A.; Nascimento, V.L. Manganese foliar supplementation impacts rice yield in tropical lowlands. J. Plant Nutr. 2019, 42, 1567–1574. [Google Scholar] [CrossRef]
- Longnecker, N.; Marcar, N.; Graham, R. Increased manganese content of barley seeds can increase grain yield in manganese-deficient conditions. Aust. J. Agric. Res. 1991, 42, 1065–1074. [Google Scholar] [CrossRef]
- El-Esawi, M.; Glascoe, A.; Engle, D.; Ritz, T.; Link, J.; Ahmad, M. Cellular metabolites modulate in vivo signaling of Arabidopsis cryptochrome-1. Plant Signal. Behav. 2015, 10, e1063758. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.; Sammour, R. Karyological and phylogenetic studies in the genus Lactuca L. (Asteraceae). Cytologia 2014, 79, 269–275. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Tian, X.; Wang, S.; Chen, Y. Effect of Nitrogen Fertilizer and Foliar Zinc Application at Different Growth Stages on Zinc Translocation and Utilization Efficiency in Winter Wheat. Cereal Res. Commun. 2014, 42, 81–90. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Ali, N.; Yasin, M.U.; Ali, M.A. Foliar Manganese Supply Enhances Crop Productivity, Net Benefits, and Grain Manganese Accumulation in Direct-Seeded and Puddled Transplanted Rice. J. Plant Growth Regul. 2020, 2020, 1–18. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Ahmadvand, G. Effect of Zinc and Manganese Foliar Application on Yield, Quality and Enrichment on Potato (Solanum tuberosum L.). Asian J. Plant Sci. 2007, 6, 1256–1260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).