Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women
Abstract
1. Introduction
2. Materials and Methods
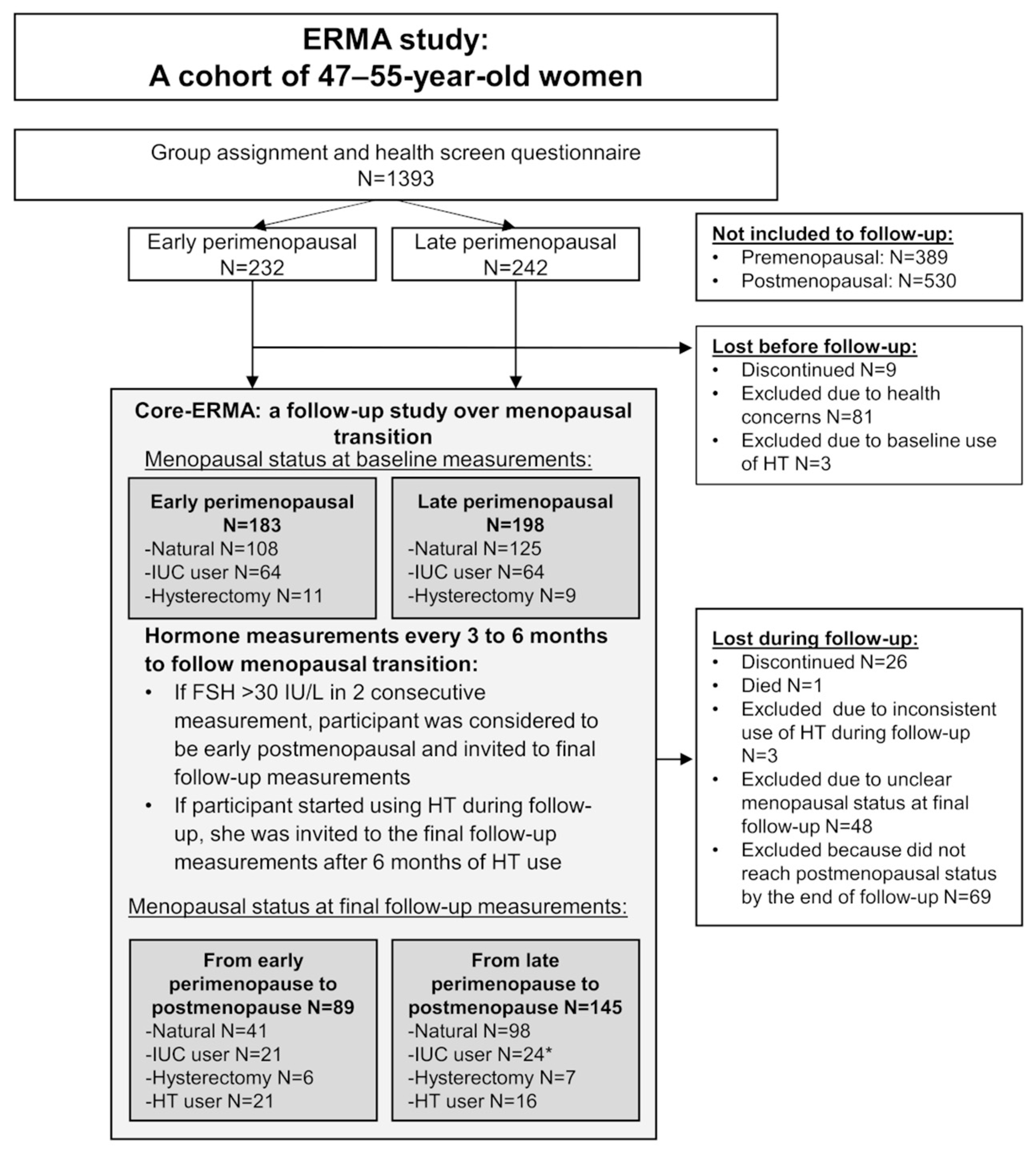
2.1. Study Design and Participants
2.2. Hormone Measurements
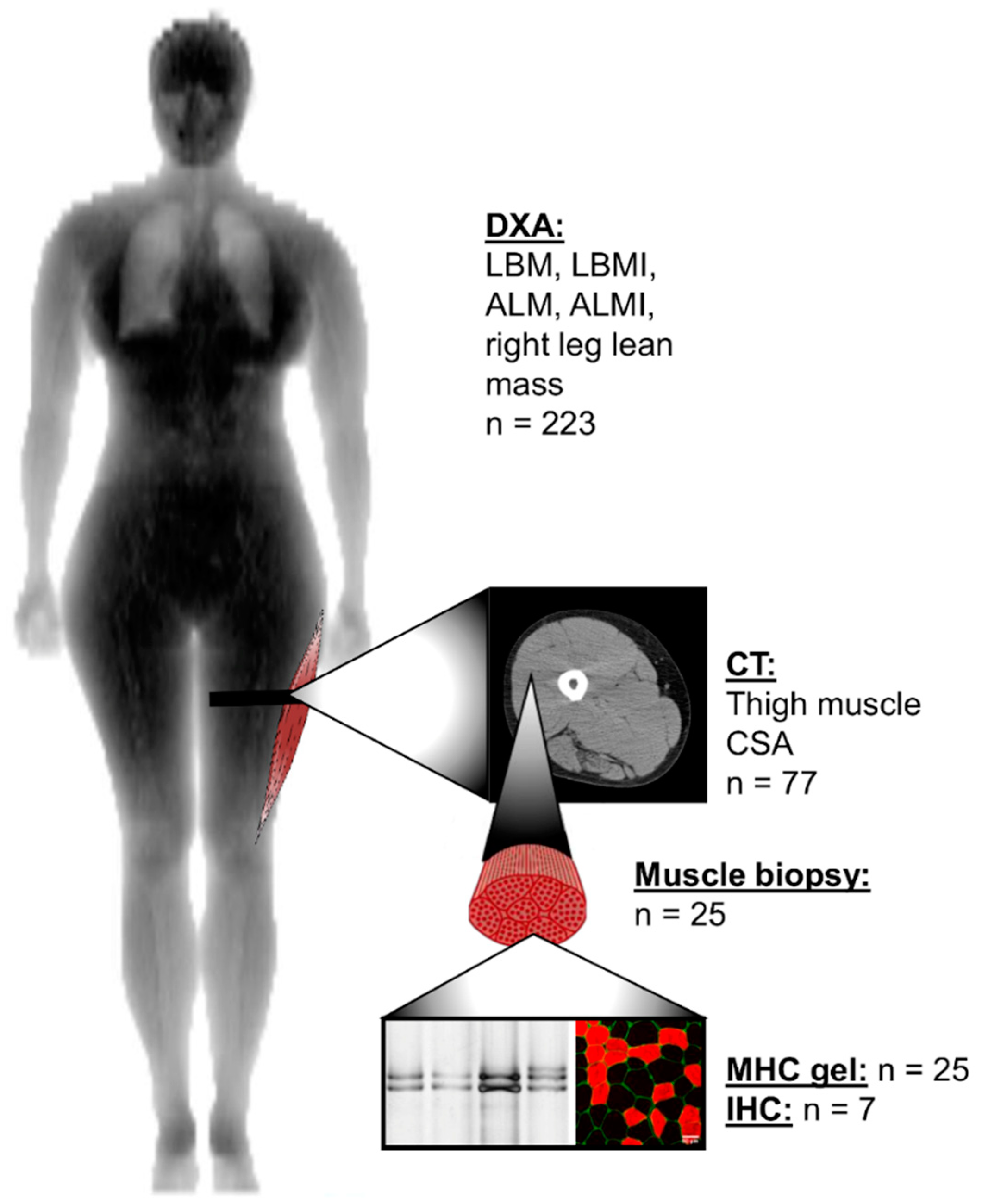
2.3. Lean and Muscle Mass Measurements
2.4. Muscle Biopsies
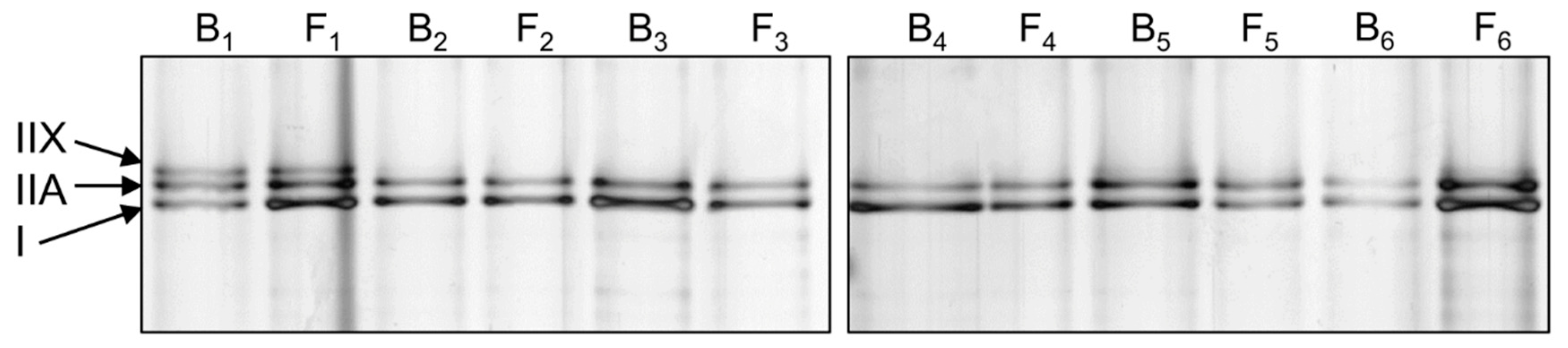
2.5. Myosin Heavy Chain Isoform Separation with SDS-PAGE
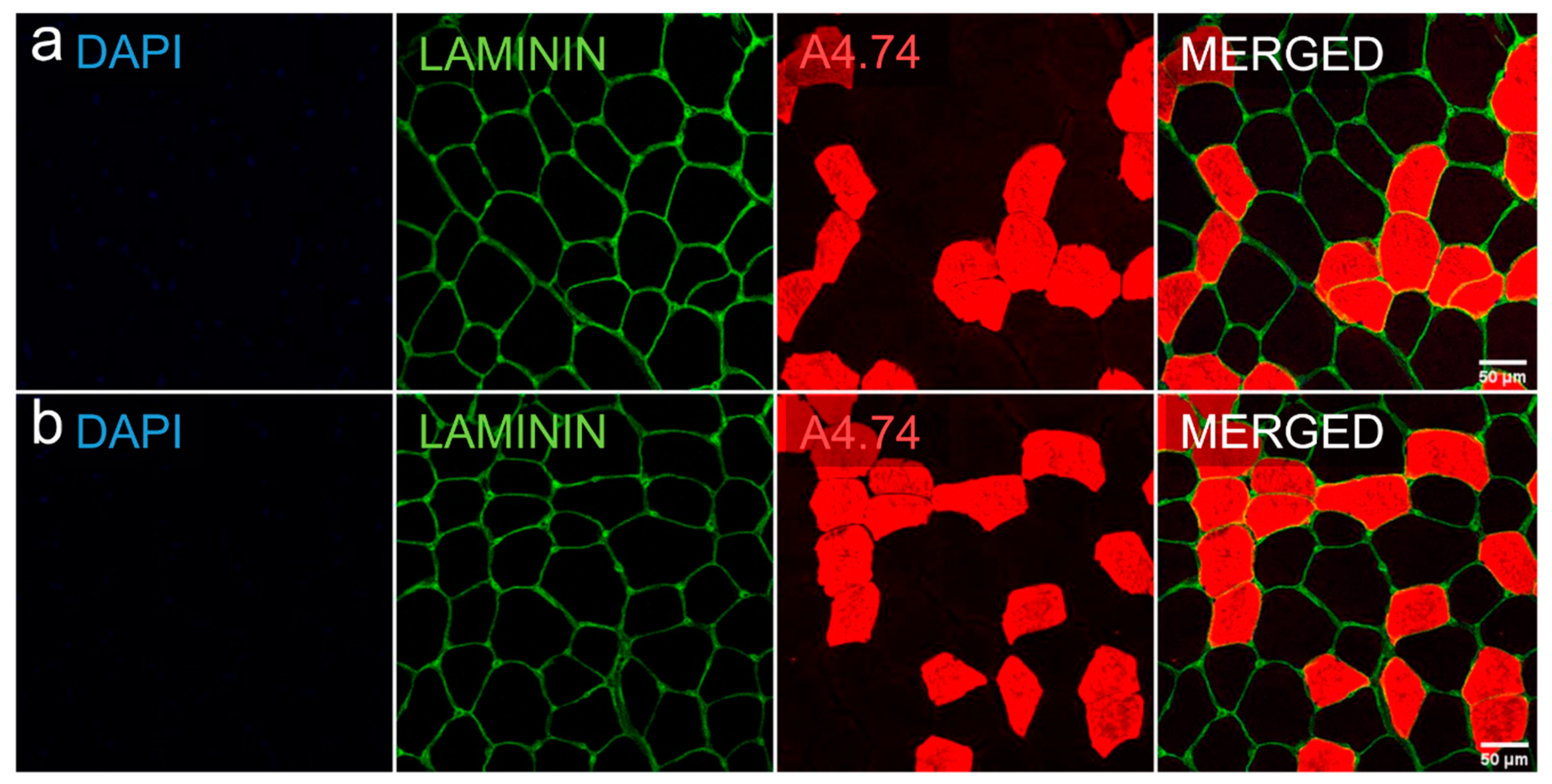
2.6. Myofiber Type Distribution and Size Measurement
2.7. Physical Activity
2.8. Dietary Analysis
2.9. Background Variables
2.10. Statistical Analysis
3. Results
3.1. Characteristics of the Population at Baseline
3.2. Changes in Characteristics and Lean and Muscle Mass Variables during the Follow-Up
3.3. Longitudinal Associations Between Menopausal Status, Covariates and Lean and Muscle Mass Variables
3.4. Changes at The Cellular Level
4. Discussion
4.1. Menopausal Transition Decreases Lean and Muscle Mass
4.2. Associations of Menopausal Transition at the Single Cell Level
4.3. Physical Activity Helps to Maintain Lean and Muscle Mass during Menopause
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2014, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Boil. Med. 2019, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- A Faulkner, J.; Larkin, L.M.; Claflin, D.; Brooks, S.V. Age-Related Changes in the Structure and Function of Skeletal Muscles. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Sherman, B.M.; Korenman, S.G. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J. Clin. Investig. 1975, 55, 699–706. [Google Scholar] [CrossRef]
- Laakkonen, E.K.; Soliymani, R.; Karvinen, S.; Kaprio, J.; Kujala, U.M.; Baumann, M.; Sipilä, S.; Kovanen, V.; Lalowski, M. Estrogenic regulation of skeletal muscle proteome: A study of premenopausal women and postmenopausal MZ cotwins discordant for hormonal therapy. Aging Cell 2017, 16, 1276–1287. [Google Scholar] [CrossRef]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Prim. 2015, 1, 15004. [Google Scholar] [CrossRef]
- Ho, S.C.; Wu, S.; Chan, S.G.; Sham, A. Menopausal transition and changes of body composition: A prospective study in Chinese perimenopausal women. Int. J. Obes. 2010, 34, 1265–1274. [Google Scholar] [CrossRef]
- Lovejoy, J.C.; Champagne, C.M.; De Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef]
- Pöllänen, E.; Sipilä, S.; Alén, M.; Ronkainen, P.H.A.; Ankarberg-Lindgren, C.; Puolakka, J.; Suominen, H.; Hämäläinen, E.; Turpeinen, U.; Konttinen, Y.T.; et al. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell 2011, 10, 650–660. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and bone mass in middle-aged women: Role of menopausal status and physical activity. J. Cachex Sarcopenia Muscle 2020. [Google Scholar] [CrossRef]
- Douchi, T.; Yamamoto, S.; Nakamura, S.; Ijuin, T.; Oki, T.; Maruta, K.; Nagata, Y. The effect of menopause on regional and total body lean mass. Maturitas 1998, 29, 247–252. [Google Scholar] [CrossRef]
- Aloia, J.F.; McGowan, D.M.; Vaswani, A.N.; Ross, P.; Cohn, S.H. Relationship of menopause to skeletal and muscle mass. Am. J. Clin. Nutr. 1991, 53, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.-F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Maury, J.; Molinari, N.; Pomiès, P.; Mercier, J.; Préfaut, C.; Hayot, M. Reference values for vastus lateralis fiber size and type in healthy subjects over 40 years old: A systematic review and metaanalysis. J. Appl. Physiol. 2013, 115, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E.; Booth, F.W. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol. Scand. 2003, 178, 413–424. [Google Scholar] [CrossRef]
- Widrick, J.J.; Maddalozzo, G.F.; Lewis, D.; Valentine, B.A.; Garner, D.P.; Stelzer, J.E.; Shoepe, T.C.; Snow, C.M. Morphological and functional characteristics of skeletal muscle fibers from hormone-replaced and nonreplaced postmenopausal women. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2003, 58, B3–B10. [Google Scholar] [CrossRef]
- Piccone, C.M.; Brazeau, G.A.; McCormick, K.M. Effect of oestrogen on myofibre size and myosin expression in growing rats. Exp. Physiol. 2004, 90, 87–93. [Google Scholar] [CrossRef]
- Dalgaard, L.B.; Dalgas, U.; Andersen, J.L.; Rossen, N.B.; Møller, A.B.; Stødkilde-Jørgensen, H.; Jørgensen, J.O.; Kovanen, V.; Couppé, C.; Langberg, H.; et al. Influence of Oral Contraceptive Use on Adaptations to Resistance Training. Front. Physiol. 2019, 10, 824. [Google Scholar] [CrossRef]
- Kovanen, V.; Aukee, P.; Kokko, K.; Finni, T.; Tarkka, I.M.; Tammelin, T.; Kujala, U.M.; Sipilä, S.; Laakkonen, E.K. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women’s cohort. Menopause 2018, 25, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Applications of Evolutionary Computation; Springer Science and Business Media LLC: Cham, Switzerland, 2015; Volume 9351, pp. 234–241. [Google Scholar]
- Kujala, U.M.; Kaprio, J.; Sarna, S.; Koskenvuo, M. Relationship of Leisure-Time Physical Activity and Mortality. JAMA 1998, 279, 440. [Google Scholar] [CrossRef] [PubMed]
- Laakkonen, E.K.; Kulmala, J.; Aukee, P.; Hakonen, H.; Kujala, U.M.; Lowe, D.A.; Kovanen, V.; Tammelin, T.; Sipilä, S. Female reproductive factors are associated with objectively measured physical activity in middle-aged women. PLoS ONE 2017, 12, e0172054. [Google Scholar] [CrossRef]
- Hyvärinen, M.; Sipilä, S.; Kulmala, J.; Hakonen, H.; Tammelin, T.H.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Validity and Reliability of a Single Question for Leisure-Time Physical Activity Assessment in Middle-Aged Women. J. Aging Phys. Acta 2020, 28, 231–241. [Google Scholar] [CrossRef]
- Sasaki, J.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Brakenridge, C.; Fjeldsoe, B.; Young, D.C.; Winkler, E.A.H.; Dunstan, D.W.; Straker, L.; Healy, G. Evaluating the effectiveness of organisational-level strategies with or without an activity tracker to reduce office workers’ sitting time: A cluster-randomised trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 115. [Google Scholar] [CrossRef]
- Masip, G.; Keski-Rahkonen, A.; Pietiläinen, K.H.; Kujala, U.M.; Rottensteiner, M.; Väisänen, K.; Kaprio, J.; Bogl, L.H. Development of a Food-Based Diet Quality Score from a Short FFQ and Associations with Obesity Measures, Eating Styles and Nutrient Intakes in Finnish Twins. Nutrients 2019, 11, 2561. [Google Scholar] [CrossRef]
- Cabelka, C.A.; Baumann, C.W.; Collins, B.C.; Nash, N.; Le, G.; Lindsay, A.; Spangenburg, E.E.; Lowe, D.A. Effects of ovarian hormones and estrogen receptor α on physical activity and skeletal muscle fatigue in female mice. Exp. Gerontol. 2019, 115, 155–164. [Google Scholar] [CrossRef]
- Smith, G.; Yoshino, J.; Reeds, M.N.; Bradley, D.; Burrows, R.E.; Heisey, H.D.; Moseley, A.C.; Mittendorfer, B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J. Clin. Endocrinol. Metab. 2013, 99, 256–265. [Google Scholar] [CrossRef]
- Sternfeld, B.; Bhat, A.K.; Wang, H.; Sharp, T.; Quesenberry, C.P. Menopause, Physical Activity, and Body Composition/Fat Distribution in Midlife Women. Med. Sci. Sports Exerc. 2005, 37, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O.L.; Hassager, C.; Christiansen, C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 1995, 44, 369–373. [Google Scholar] [CrossRef]
- Ronkainen, P.H.A.; Kovanen, V.; Alén, M.; Pollanen, E.; Palonen, E.M.; Ankarberg-Lindgren, C.; Hämäläinen, E.; Turpeinen, U.; Kujala, U.M.; Puolakka, J.; et al. Postmenopausal hormone replacement therapy modifies skeletal muscle composition and function: A study with monozygotic twin pairs. J. Appl. Physiol. 2009, 107, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sipilä, S.; Taaffe, D.R.; Cheng, S.; Puolakka, J.; Toivanen, J.; Suominen, H. Effects of hormone replacement therapy and high-impact physical exercise on skeletal muscle in post-menopausal women: A randomized placebo-controlled study. Clin. Sci. 2001, 101, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Heikkinen, J.; Kyllönen, E.; Kurttila-Matero, E.; Wilen-Rosenqvist, G.; Lankinen, K.S.; Rita, H.; Väänänen, H. HRT and exercise: Effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2-year prospective trial on two estrogen-progestin regimens in healthy postmenopausal women. Maturitas 1997, 26, 139–149. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Deschenes, M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; Van Kranenburg, J.; Verdijk, L.B.; Van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Oh, S.-L.; Yoon, S.H.; Lim, J.-Y. Age- and sex-related differences in myosin heavy chain isoforms and muscle strength, function, and quality: A cross sectional study. J. Exerc. Nutr. Biochem. 2018, 22, 43–50. [Google Scholar] [CrossRef]
- Bougea, A.; Papadimas, G.; Papadopoulos, C.; Paraskevas, G.P.; Kalfakis, N.; Manta, P.; Kararizou, E. An Age-Related Morphometric Profile of Skeletal Muscle in Healthy Untrained Women. J. Clin. Med. 2016, 5, 97. [Google Scholar] [CrossRef]
- Korhonen, M.T.; Cristea, A.; Alén, M.; Häkkinen, K.; Sipilä, S.; Mero, A.; Viitasalo, J.T.; Larsson, L.; Suominen, H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J. Appl. Physiol. 2006, 101, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.F.; Roth, S.; Ivey, F.M.; Lemmer, J.T.; Tracy, B.L.; Hurlbut, D.E.; Metter, E.J.; Hurley, B.F.; Rogers, M.A. Age and sex affect human muscle fibre adaptations to heavy-resistance strength training. Exp. Physiol. 2006, 91, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lexell, J.; Taylor, C.C. Variability in muscle fibre areas in whole human quadriceps muscle: Effects of increasing age. J. Anat. 1991, 174, 239–249. [Google Scholar] [PubMed]
- Kitajima, Y.; Ono, Y. Estrogens maintain skeletal muscle and satellite cell functions. J. Endocrinol. 2016, 229, 267–275. [Google Scholar] [CrossRef]
- Kadi, F.; Karlsson, C.; Larsson, B.; Eriksson, J.; Larval, M.; Billig, H.; Jonsdottir, I.H. The effects of physical activity and estrogen treatment on rat fast and slow skeletal muscles following ovariectomy. J. Muscle Res. Cell Motil. 2002, 23, 335–339. [Google Scholar] [CrossRef]
- Lai, S.; Collins, B.C.; Colson, B.A.; Kararigas, G.; Lowe, D.A. Estradiol modulates myosin regulatory light chain phosphorylation and contractility in skeletal muscle of female mice. Am. J. Physiol. Metab. 2016, 310, E724–E733. [Google Scholar] [CrossRef]
- Qaisar, R.; Renaud, G.; Hedström, Y.; Pöllänen, E.; Ronkainen, P.; Kaprio, J.; Alén, M.; Sipilä, S.; Artemenko, K.; Bergquist, J.; et al. Hormone replacement therapy improves contractile function and myonuclear organization of single muscle fibres from postmenopausal monozygotic female twin pairs. J. Physiol. 2013, 591, 2333–2344. [Google Scholar] [CrossRef]
- Andersen, J.L. Muscle fibre type adaptation in the elderly human muscle. Scand. J. Med. Sci. Sports 2003, 13, 40–47. [Google Scholar] [CrossRef]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 2007, 5, 129–142. [Google Scholar] [CrossRef]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef]
- Sipilä, S.; Poutamo, J. Muscle performance, sex hormones and training in peri-menopausal and post-menopausal women. Scand. J. Med. Sci. Sports 2003, 13, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Tieland, M.; Verdijk, L.B.; Leenders, M.; Dirks, M.L.; De Groot, L.C.P.G.M.; Van Loon, L.J.C. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J. Am. Med. Dir. Assoc. 2015, 16, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Moreillon, M.; Alonso, S.C.; Broskey, N.T.; Greggio, C.; Besson, C.; Rousson, V.; Amati, F. Hybrid fiber alterations in exercising seniors suggest contribution to fast-to-slow muscle fiber shift. J. Cachex Sarcopenia Muscle 2019, 10, 687–695. [Google Scholar] [CrossRef] [PubMed]
| Early Perimenopausal (n = 89) # | Late Perimenopausal (n = 145) ¤ | P | |
|---|---|---|---|
| Age, y | 51.2 ± 2.0 | 51.8 ± 1.8 | 0.013 a |
| E2, nmol/L | 0.46 ± 0.34 | 0.26 ± 0.18 | <0.001 b |
| FSH, IU/L | 18.3 ± 5.0 | 46.9 ± 20.6 | <0.001 b |
| DHEAS, µmol/L | 2.64 ± 1.42 | 2.91 ± 1.36 | 0.135 b |
| SHBG, nmol/L | 58.5 ± 24.3 | 53.1 ± 22.3 | 0.140 b |
| Education level Primary Secondary Tertiary | 1.1% 59.1% 39.8% | 3.4% 47.6% 49.0% | 0.261 b |
| Smoking Never Quitter Current smoker | 74.2% 23.6% 2.2% | 66.9% 24.1% 9.0% | 0.159 b |
| Alcohol use, units/week | 4.5 ± 4.5 | 3.9 ± 3.2 | 0.528 b |
| Bleeding status Natural IUC Hysterectomy | 56.2% 34.8% 9.0% | 66.9% 29% 4.1% | 0.151 c |
| Physical activity | |||
| MVPA, min/day X | 53.6 ± 22.8 | 52.0 ± 32.9 | 0.195 b |
| MET-hours/day XX | 4.6 ± 4.2 | 4.3 ± 3.8 | 0.743 b |
| Early Perimenopausal (n = 89) # | Late Perimenopausal (n = 145) ¤ | P | |
|---|---|---|---|
| Body mass, kg | 69.2 ± 11.9 | 70.2 ± 10.8 | 0.534 a |
| Body height, cm | 165.2 ± 5.6 | 165.0 ± 5.8 | 0.771 a |
| BMI, kg/m2 Underweight (<18.5) Normal weight (18.5–24.99) Overweight (25.0–29.99) Obese (>30) | 25.4 ± 4.2 0% 57.3% 28.1% 14.6% | 25.8 ± 3.8 0% 47.6% 36.6% 15.9% | 0.287 b |
| DXA-measurements | |||
| LBM, kg | 42.3 ± 4.8 | 41.4 ± 4.1 | 0.141 a |
| LBMI, kg/m2 | 15.4 ± 1.4 | 15.2 ± 1.2 | 0.204 a |
| ALM, kg | 18.2 ± 2.4 | 17.9 ± 2.1 | 0.416 a |
| ALMI, kg/m2 | 6.6 ± 0.7 | 6.6 ± 0.6 | 0.553 a |
| Right leg lean mass, kg | 6.8 ± 0.9 | 6.8 ± 0.8 | 0.494 a |
| Computed tomography | (n = 24) | (n = 53) | |
| Absolute muscle area, cm2 | 166.1 ± 8.1 | 167.3 ± 10.3 | 0.636 a |
| Relative muscle area, % | 69.3 ± 4.2 | 69.8 ± 6.1 * | 0.722 a |
| Baseline (Perimenopausal) n = 234 | Final Follow-Up (Postmenopausal) n = 234 | Difference % | P | |
|---|---|---|---|---|
| Age, y | 51.6 ± 1.9 | 53.0 ± 1.9 | +2.7 | <0.001 a |
| Body mass, kg | 69.8 ± 11.2 | 70.4 ± 11.6 | +0.9 | <0.001 b |
| BMI, kg/m2 | 25.6 ± 4.0 | 25.8 ± 4.1 | +0.8 | <0.001 b |
| E2, nmol/L | 0.34 ± 0.27 | 0.24 ± 0.19 | −30 | <0.001 b |
| FSH, IU/L | 36.0 ± 21.6 | 66.9 ± 28.1 | +86 | <0.001 b |
| DQS, points | 5.7 ± 2.3 | 5.5 ± 2.2 | 0.207 a | |
| Physical activity | ||||
| MVPA, min/day X (n = 173) | 51.8 ± 29.3 | 49.7 ± 23.6 | 0.567 b | |
| MET-hours/day XX (n = 231) | 4.5 ± 3.9 | 4.7 ± 3.6 | 0.057 b | |
| DXA-measurements | ||||
| LBM, kg (n = 223) | 41.7 ± 4.4 | 41.5 ± 4.4 | −0.5 | 0.019 a |
| LBMI, kg/m2 (n = 223) | 15.3 ± 1.3 | 15.2 ± 1.3 | −0.7 | 0.018 a |
| ALM, kg (n = 223) | 18.0 ± 2.2 | 17.8 ± 2.2 | −1.1 | <0.001 a |
| ALMI, kg/m2 (n = 223) | 6.6 ± 0.6 | 6.5 ± 0.6 | −1.1 | <0.001 a |
| Right leg lean mass, kg (n = 223) | 6.8 ± 0.9 | 6.7 ± 0.8 | −1.5 | 0.002 a |
| Computed tomography | ||||
| Absolute muscle area, cm2 (n = 77) | 166.9 ± 9.6 | 165.3 ± 10.1 | −1.0 | <0.001 a |
| Relative muscle area (%) (n = 76) * | 69.6 ± 5.6 | 68.9 ± 6.0 | −1.0 | <0.001 a |
| Model 1 | Adjusted Model | |||
|---|---|---|---|---|
| B | P | B | P | |
| LBM Menopausal status Use of HT Use of progestogen Follow-up time MET-hours/day Age | −0.193 0.000 1.193 0.001 - - | 0.026 0.833 0.057 0.503 - - | −0.203 0.000 1.263 0.001 0.053 0.109 | 0.019 0.836 0.050 0.420 0.036 0.506 |
| LBMI Menopausal status Use of HT Use of progestogen Follow-up time MET-hours/day Age | −0.069 0.000 0.290 0.000 - - | 0.029 0.696 0.110 0.426 - - | −0.073 0.000 0.386 0.000 0.017 0.122 | 0.020 0.753 0.037 0.183 0.054 0.009 |
| ALM Menopausal status Use of HT Use of progestogen Follow-up time MET-hours/day Age | −0.231 0.001 0.555 0.001 - - | <0.001 0.362 0.061 0.304 - - | −0.238 0.001 0.557 0.001 0.038 0.025 | <0.001 0.354 0.069 0.278 0.009 0.766 |
| ALMI Menopausal status Use of HT Use of progestogen Follow-up time MET-hours/day Age | −0.085 0.000 0.143 0.000 - - | <0.001 0.386 0.083 0.200 - - | −0.088 0.000 0.175 0.000 0.014 0.046 | <0.001 0.326 0.039 0.082 0.005 0.055 |
| Right leg lean mass Menopausal status Use of HT Use of progestogen Follow-up time MET-hours/day Age | −0.088 0.000 0.214 0.000 - - | 0.001 0.398 0.054 0.224 - - | −0.091 0.000 0.209 0.000 0.017 0.005 | 0.001 0.395 0.066 0.217 0.011 0.870 |
| Absolute muscle area * Menopausal status Use of HT Follow-up time MET-hours/day Age | −1.597 −0.001 −0.001 - - | <0.001 0.811 0.833 - - | −1.586 −0.001 0.000 0.047 0.236 | 0.001 0.797 0.938 0.801 0.748 |
| Relative muscle area * Menopausal status Use of HT Follow-up time MET-hours/day Age | −0.007 −1.5 × 10−5 −7.5 × 10−6 - - | <0.001 0.488 0.716 - - | −0.007 −6.7 × 10−6 −6.9 × 10−6 0.002 −0.002 | <0.001 0.721 0.747 0.011 0.667 |
| Myosin Isoform | Baseline n = 25 | Final Follow-Up n = 25 | P |
|---|---|---|---|
| Type I (%) | 50.6 ± 11.2 | 52.9 ± 8.5 | 0.619 |
| Type IIA (%) | 40.6 ± 9.6 | 41.4 ± 6.0 | 0.563 |
| Type IIX (%) | 8.8 ± 10.8 | 5.7 ± 9.8 | 0.116 |
| Baseline n = 7 | Final Follow-Up n = 7 | P | |
|---|---|---|---|
| Slow (type I) cells, µm2 | 3526 ± 1334 | 3525 ± 1618 | 0.735 a |
| Percentage | 53.3 ± 10.9 | 51.1 ± 14.4 | 0.398 a |
| Fast (type II) cells, µm2 | 2098 ± 948 | 2399 ± 1218 | 0.128 a |
| Percentage | 46.7 ± 10.9 | 48.9 ± 14.4 | 0.398 a |
| P-value for difference between cell types | <0.001 b | <0.001 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juppi, H.-K.; Sipilä, S.; Cronin, N.J.; Karvinen, S.; Karppinen, J.E.; Tammelin, T.H.; Aukee, P.; Kovanen, V.; Kujala, U.M.; Laakkonen, E.K. Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women. J. Clin. Med. 2020, 9, 1588. https://doi.org/10.3390/jcm9051588
Juppi H-K, Sipilä S, Cronin NJ, Karvinen S, Karppinen JE, Tammelin TH, Aukee P, Kovanen V, Kujala UM, Laakkonen EK. Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women. Journal of Clinical Medicine. 2020; 9(5):1588. https://doi.org/10.3390/jcm9051588
Chicago/Turabian StyleJuppi, Hanna-Kaarina, Sarianna Sipilä, Neil J. Cronin, Sira Karvinen, Jari E. Karppinen, Tuija H. Tammelin, Pauliina Aukee, Vuokko Kovanen, Urho M. Kujala, and Eija K. Laakkonen. 2020. "Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women" Journal of Clinical Medicine 9, no. 5: 1588. https://doi.org/10.3390/jcm9051588
APA StyleJuppi, H.-K., Sipilä, S., Cronin, N. J., Karvinen, S., Karppinen, J. E., Tammelin, T. H., Aukee, P., Kovanen, V., Kujala, U. M., & Laakkonen, E. K. (2020). Role of Menopausal Transition and Physical Activity in Loss of Lean and Muscle Mass: A Follow-Up Study in Middle-Aged Finnish Women. Journal of Clinical Medicine, 9(5), 1588. https://doi.org/10.3390/jcm9051588







