Advances in Understanding the Relationship between Sleep and Attention Deficit-Hyperactivity Disorder (ADHD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
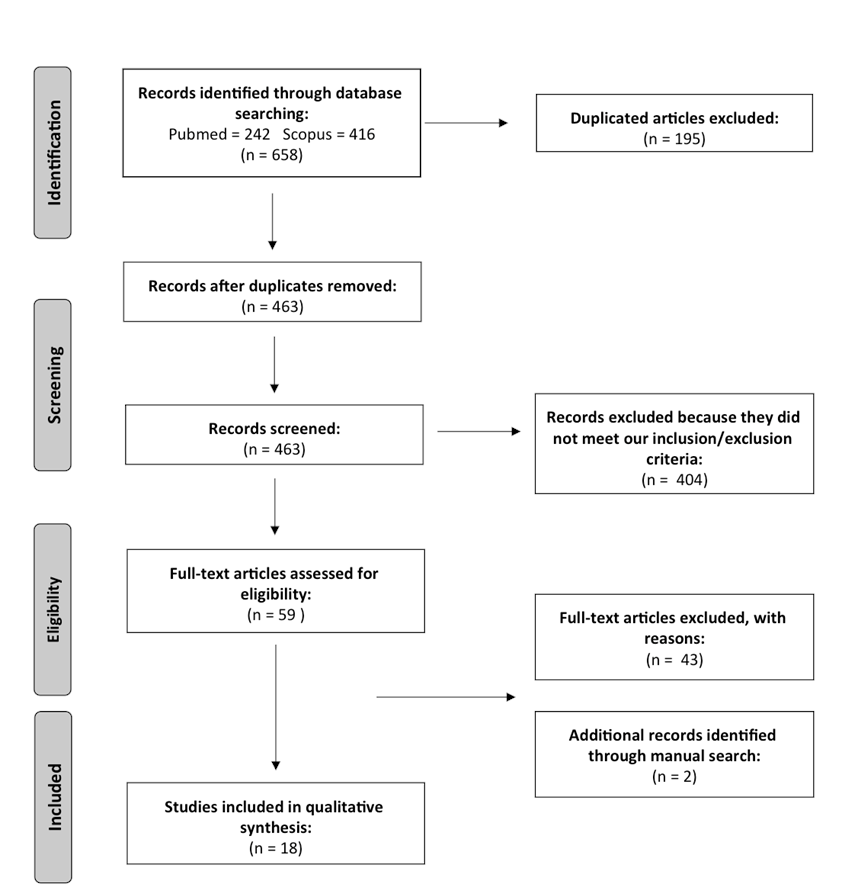
3. Results
- (a)
- (b)
3.1. Macrostructural Pattern
3.2. Microstructural Pattern
3.2.1. NREM Sleep
3.2.2. REM Sleep
4. Discussion
4.1. Macrostructural Pattern
4.2. Microstructural Pattern
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Thomas, R.; Sanders, S.; Doust, J.; Beller, E.; Glasziou, P. Prevalence of Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis. Pediatrics 2015, 135, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Barkley, R.A.; Fischer, M.; Smallish, L.; Fletcher, K. Young Adult Outcome of Hyperactive Children: Adaptive Functioning in Major Life Activities. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 192–202. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Clinical practice guideline: Diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. Pediatrics 2000, 105, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J. Attention-Deficit/Hyperactivity Disorder: A Selective Overview. Boil. Psychiatry 2005, 57, 1215–1220. [Google Scholar] [CrossRef]
- Larson, K.; Russ, S.A.; Kahn, R.S.; Halfon, N. Patterns of comorbidity, functioning, and service use for US children with ADHD, 2007. Pediatrics 2011, 127, 462–470. [Google Scholar] [CrossRef]
- Hvolby, A. Associations of sleep disturbance with ADHD: Implications for treatment. ADHD Atten. Deficit Hyperact. Disord. 2015, 7, 1–18. [Google Scholar] [CrossRef]
- Cortese, S.; Faraone, S.V.; Konofal, E.; Lecendreux, M. Sleep in Children with Attention-Deficit/Hyperactivity Disorder: Meta-Analysis of Subjective and Objective Studies. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 894–908. [Google Scholar] [CrossRef]
- Craig, S.G.; Weiss, M.D.; Hudec, K.L.; Gibbons, C. The Functional Impact of Sleep Disorders in Children With ADHD. J. Atten. Disord. 2017, 29, 1087054716685840. [Google Scholar] [CrossRef]
- Van der Heijden, K.B.; Smits, M.G.; Van Someren, E.J.; Ridderinkhof, K.R.; Gunning, W.B. Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J. Am. Acad. Child Adolesc. Psychiatry 2007, 46, 233–241. [Google Scholar] [CrossRef]
- Corkum, P.; Tannock, R.; Moldofsky, H.; Hogg-Johnson, S.; Humphries, T. Actigraphy and parental ratings of sleep in children with attention-deficit/hyperactivity disorder (ADHD). Sleep 2001, 24, 303–312. [Google Scholar] [CrossRef]
- Owens, J.; Gruber, R.; Brown, T.; Corkum, P.; Cortese, S.; O’Brien, L.; Stein, M.; Weiss, M. Future research directions in sleep and ADHD: Report of a consensus working group. J. Atten. Disord. 2013, 17, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Rao, H.; Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2009, 29, 320–339. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, M.; D’Atri, A.; Scarpelli, S.; Reda, F.; De Gennaro, L. Sleep electroencephalography and brain maturation: Developmental trajectories and the relation with cognitive functioning. Sleep Med. 2019, 4152. [Google Scholar] [CrossRef]
- Andreou, G.; Karapetsas, A.; Agapitou, P.; Gourgoulianis, K. Verbal intelligence and sleep disorders in children with ADHD. Percept. Mot. Ski. 2003, 96, 1283–1288. [Google Scholar] [CrossRef]
- O’Brien, L.M.; Holbrook, C.R.; Mervis, C.B.; Klaus, C.J.; Bruner, J.L.; Raffield, T.J.; Rutherford, J.; Mehl, R.C.; Wang, M.; Tuell, A.; et al. Sleep and neurobehavioral characteristics of 5- to 7-year-old children with parentally reported symptoms of attention-deficit/hyperactivity disorder. Pediatrics 2003, 111, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Kirov, R.; Uebel, H.; Albrecht, B.; Banaschewski, T.; Rothenberger, A. P01-419—Two faces of rem sleep in normal and psychopathological development. Eur. Psychiatry 2011, 26, 422–423. [Google Scholar] [CrossRef]
- Gorgoni, M.; D’Atri, A.; Lauri, G.; Rossini, P.M.; Ferlazzo, F.; De Gennaro, L. Is Sleep Essential for Neural Plasticity in Humans, and How Does It Affect Motor and Cognitive Recovery? Neural Plast. 2013, 2013, 103949. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and synaptic down-selection. Eur. J. Neurosci. 2019. [Google Scholar] [CrossRef]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kirov RKinkelbur, J.; Heipke, S.; Kostanecka-Endress, T.; Westhoff, M.; Cohrs, S.; Ruther, E.; Hajak, G.; Banaschewski, T.; Rothenberger, A. Is there a specific polysomnographic sleep pattern in children with attention deficit/hyperactivity disorder? J. Sleep Res. 2004, 13, 87–93. [Google Scholar] [CrossRef]
- Miano, S.; Donfrancesco, R.; Bruni, O.; Ferri, R.; Galiffa, S.; Pagani, J.; Montemitro, E.; Kheirandish, L.; Gozal, D.; Villa, M.P. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder. Sleep 2006, 29, 797–803. [Google Scholar] [PubMed]
- Gruber, R.; Frenette, S.; Robert, M.; Vannasinh, P.; Carrier, J.; Xi, T. Sleep Disturbances in Prepubertal Children with Attention Deficit Hyperactivity Disorder: A Home Polysomnography Study. Sleep 2009, 32, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Prihodova, I.; Paclt, I.; Kemlink, D.; Skibova, J.; Ptacek, R.; Nevsimalova, S. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactivity disorder: A two-night polysomnographic study with a multiple sleep latency test. Sleep Med. 2010, 11, 922–928. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Molzow, I.; Munz, M.; Wilhelm, I.; Muller, K.; Freytag, D.; Wiesner, C.D.; Baving, L. Sleep restores daytime deficits in procedural memory in children with attention-deficit/hyperactivity disorder. Res. Dev. Disabil. 2011, 32, 2480–2488. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Göder, R.; Fischer, J.; Wilhelm, I.; Seeck-Hirschner, M.; Aldenhoff, J.; Baving, L. Reduced sleep-associated consolidation of declarative memory in attention-deficit/hyperactivity disorder. Sleep Med. 2011, 12, 672–679. [Google Scholar] [CrossRef]
- Gruber, R.; Fontil, L.; Bergmame, L.; Wiebe, S.T.; Amsel, R.; Frenette, S.; Carrier, J. Contributions of circadian tendencies and behavioral problems to sleep onset problems of children with ADHD. BMC Psychiatry 2012, 12, 212. [Google Scholar] [CrossRef]
- Kirov, R.; Uebel, H.; Albrecht, B.; Banaschewski, T.; Yordanova, J.; Rothenberger, A. Attention-deficit/hyperactivity disorder (ADHD) and adaptation night as determinants of sleep patterns in children. Eur. Child Adolesc. Psychiatry 2012, 21, 681–690. [Google Scholar] [CrossRef]
- Prihodova, I.; Paclt, I.; Kemlink, D.; Nevsimalova, S. Sleep microstructure is not altered in children with attention-deficit/hyperactivity disorder (ADHD). Physiol. Res. 2012, 61, 125–133. [Google Scholar]
- Prehn-Kristensen, A.; Munz, M.; Molzow, I.; Wilhelm, I.; Wiesner, C.D.; Baving, L. Sleep Promotes Consolidation of Emotional Memory in Healthy Children but Not in Children with Attention-Deficit Hyperactivity Disorder. PLoS ONE 2013, 8, e65098. [Google Scholar] [CrossRef]
- Wiebe, S.; Carrier, J.; Frenette, S.; Gruber, R. Sleep and sleepiness in children with attention deficit/hyperactivity disorder and controls. J. Sleep Res. 2013, 22, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ringli, M.; Souissi, S.; Kurth, S.; Brandeis, D.; Jenni, O.G.; Huber, R. Topography of sleep slow wave activity in children with attention-deficit/hyperactivity disorder. Cortex 2013, 49, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Oztura, I.; Hiz, S.; Akdogan, O.; Karaarslan, D.; Ozek, H.; Akay, A. Sleep Structure in Children with Attention-Deficit/Hyperactivity Disorder. J. Child Neurol. 2015, 30, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Virring, A.; Lambek, R.; Thomsen, P.H.; Møller, L.R.; Jennum, P.J. Disturbed sleep in attention-deficit hyperactivity disorder (ADHD) is not a question of psychiatric comorbidity or ADHD presentation. J. Sleep Res. 2016, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Saletin, J.M.; Coon, W.G.; Carskadon, M.A. Stage 2 sleep EEG sigma activity and motor learning in childhood ADHD: A pilot study. J. Clin. Child Adolesc. Psychol. 2017, 46, 188–197. [Google Scholar] [CrossRef]
- Cremone, A.; Lugo-Candelas, C.I.; Harvey, E.A.; McDermott, J.M.; Spencer, R.M.C. REM theta activity enhances inhibitory control in typically developing children but not children with ADHD symptoms. Exp. Brain Res. 2017, 235, 1491–1500. [Google Scholar] [CrossRef]
- Wiesner, C.D.; Molzow, I.; Prehn-Kristensen, A.; Baving, L. Sleep-Dependent Consolidation of Rewarded Behavior Is Diminished in Children with Attention Deficit Hyperactivity Disorder and a Comorbid Disorder of Social Behavior. Front. Psychol. 2017, 8, 1520. [Google Scholar] [CrossRef]
- Chin, W.C.; Huang, Y.S.; Chou, Y.H.; Wang, C.H.; Chen, K.T.; Hsu, J.F.; Hsu, S.C. Subjective and objective assessments of sleep problems in children with attention deficit/hyperactivity disorder and the effects of methylphenidate treatment. Biomed. J. 2018, 41, 356–363. [Google Scholar] [CrossRef]
- Krueger, J.M.; Tononi, G. Local use-dependent sleep; synthesis of the new paradigm. Curr. Top. Med. Chem. 2011, 11, 2490–2492. [Google Scholar] [CrossRef]
- Owens, J.; Sangal, R.B.; Sutton, V.K.; Bakken, R.; Allen, A.J.; Kelsey, D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009, 10, 446–456. [Google Scholar] [CrossRef]
- Chervin, R.D.; Archbold, K.H. Hyperactivity and polysomnographic findings in children evaluated for sleep-disordered breathing. Sleep 2001, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Wang, M.; Pope, D.W. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics 2001, 108, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Pergamin, L.; Bar-Haim, Y. Sleep in children with attention-deficit hyperactivity disorder: A meta-analysis of polysomnographic studies. Sleep Med. Rev. 2006, 10, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Wetterling, F.; McCarthy, H.; Tozzi, L.; Skokauskas, N.; O’Doherty, J.P.; Mulligan, A.; Meaney, J.F.; Fagan, A.J.; Gill, M.; Frodl, T. Impaired reward processing in the human prefrontal cortex distinguishes between persistent and remittent attention deficit hyperactivity disorder. Hum. Brain Mapp. 2015, 36, 4648–4663. [Google Scholar] [CrossRef]
- Crabtree, V.M.; Ivanenko, A.; O’Brien, L.M.; Gozal, D. Periodic limb movement disorder of sleep in children. J. Sleep Res. 2003, 12, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Anderer, P.; Klösch, G.; Gruber, G.; Trenker, E.; Pascual-Marqui, R.D.; Zeitlhofer, J.; Barbanoj, M.J.; Rappelsberger, P.; Saletu, B. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience 2001, 103, 581–592. [Google Scholar] [CrossRef]
- Novelli, L.; D’Atri, A.; Marzano, C.; Finotti, E.; Ferrara, M.; Bruni, O.; De Gennaro, L. Mapping changes in cortical activity during sleep in the first 4 years of life. J. Sleep Res. 2016, 25, 381–389. [Google Scholar] [CrossRef]
- Kurth, S.; Ringli, M.; Geiger, A.; LeBourgeois, M.; Jenni, O.G.; Huber, R. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J. Neurosci. 2010, 30, 13211–13219. [Google Scholar] [CrossRef]
- Shaw, P.; Lerch, J.; Greenstein, D.; Sharp, W.; Clasen, L.; Evans, A.; Giedd, J.; Castellanos, F.; Rapoport, J. Longitudinal Mapping of Cortical Thickness and Clinical Outcome in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 2006, 63, 540. [Google Scholar] [CrossRef]
- Massimini, M.; Huber, R.; Ferrarelli, F.; Hill, S.; Tononi, G. The Sleep Slow Oscillation as a Traveling Wave. J. Neurosci. 2004, 24, 6862–6870. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Li, H.; Wu, Z.; Zhao, Q.; Song, Y.; Liu, L.; Qian, Q.; Wang, Y.; Roodenrys, S.; Johnstone, S.J.; et al. Electroencephalogram Theta/Beta Ratio and Spectral Power Correlates of Executive Functions in Children and Adolescents With AD/HD. J. Atten. Disord. 2017, 23, 721–732. [Google Scholar] [CrossRef]
- Hermens DFSoei, E.X.; Clarke, S.D.; Kohn, M.R.; Gordon, E.; Williams, L.M. Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr. Neurol. 2005, 32, 248–256. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Pearsall, J.; Buckner, R.L.; Walker, M.P. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex 2009, 19, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, S.; Gorgoni, M.; Ferrara, M.; De Gennaro, L.; D’Atri, A. EEG oscillations during sleep and dream recall: State- or trait-like individual differences? Front. Psychol. 2015, 6, 605. [Google Scholar] [CrossRef]
- Díaz-Román, A.; Hita-Yáñez, E.; Buela-Casal, G. Sleep Characteristics in Children with Attention Deficit Hyperactivity Disorder: Systematic Review and Meta-Analyses. J. Clin. Sleep Med. 2016, 12, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Reynolds, C.F.; Riemann, D. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Calhoun, S.L.; Bixler, E.O.; Vgontzas, A.N.; Mahr, F.; Hillwig-Garcia, J.; Elamir, B.; Edhere-Ekexie, L.; Parvin, M. ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: Differences in sleep problems. J. Pediatr. Psychol. 2009, 34, 328–337. [Google Scholar] [CrossRef]
- Becker, S.P.; Froehlich, T.E.; Epstein, J.N. Effects of Methylphenidate on Sleep Functioning in Children with Attention-Deficit/Hyperactivity Disorder. J. Dev. Behav. Pediatr. 2016, 37, 395–404. [Google Scholar] [CrossRef]
- Esposito, M.; Carotenuto, M. Intellectual disabilities and power spectra analysis during sleep: A new perspective on borderline intellectual functioning. J. Intell. Disabil. Res. 2014, 58, 421–429. [Google Scholar] [CrossRef]
- Hsieh, L.T.; Ranganath, C. Frontal midline theta oscillations during working memory maintenance and episodic encoding and retrieval. Neuroimage 2014, 85, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Van Vugt, M.K.; Sederberg, P.B.; Kahana, M.J. Comparison of spectral analysis methods for characterizing brain oscillations. J. Neurosci. Methods 2007, 162, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-S.; Sarasso, S.; Ferrarelli, F.; Riedner, B.; Ghilardi, M.F.; Cirelli, C.; Tononi, G. Local Experience-Dependent Changes in the Wake EEG after Prolonged Wakefulness. Sleep 2013, 36, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.B.; Madsen, J.R.; Raghavachari, S.; Kahana, M.J. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. J. Neurophysiol. 2001, 86, 368–380. [Google Scholar] [CrossRef]
- Marzano, C.; Ferrara, M.; Mauro, F.; Moroni, F.; Gorgoni, M.; Tempesta, D.; Cipolli, C.; De Gennaro, L. Recalling and Forgetting Dreams: Theta and Alpha Oscillations during Sleep Predict Subsequent Dream Recall. J. Neurosci. 2011, 31, 6674–6683. [Google Scholar] [CrossRef]
- Scarpelli, S.; Marzano, C.; D’Atri, A.; Gorgoni, M.; Ferrara, M.; De Gennaro, L. State- or trait-like individual differences in dream recall: Preliminary findings from a within-subjects study of multiple nap REM sleep awakenings. Front. Psychol. 2015, 6, 928. [Google Scholar] [CrossRef]
- Marzano, C.; Ferrara, M.; Curcio, G.; De Gennaro, L. The effects of sleep deprivation in humans: Topographical EEG changes in NREM vs. REM sleep. J. Sleep Res. 2010, 19, 260–268. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Munz, M.; Göder, R.; Wilhelm, I.; Korr, K.; Vahl, W.; Wiesner, C.D.; Baving, L. Transcranial Oscillatory Direct Current Stimulation During Sleep Improves Declarative Memory Consolidation in Children with Attention-deficit/hyperactivity Disorder to a Level Comparable to Healthy Controls. Brain Stimul. 2014, 7, 793–799. [Google Scholar] [CrossRef]
- Munz, M.T.; Prehn-Kristensen, A.; Thielking, F.; Mölle, M.; Göder, R.; Baving, L. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/hyperactivity disorder. Front. Cell. Neurosci. 2015, 9, 307. [Google Scholar] [CrossRef]
| Authors. | Sample Size (Sex; Mean Age) | ADHD Subtypes | IQ and Comorbidities | Medications | PSG Recording (Setting; EEG Channels) | Sleep Measures | Main Results |
|---|---|---|---|---|---|---|---|
| Kirov et al. [22] | 17 ADHD (all M; 11.2 ± 2.0) vs. 17 HC (all M; 11.2 ± 2.3) | All combined | Full-scale IQ ≥ 80 ADHD: 12 with dyslexia; 3 with conduct disorder; 1 with panic disorder; 1 with nocturnal enuresis | 11 ADHD stopped medications at least 3 days previous the experimental session | Laboratory recording with adaptation night; 1 EEG channel (C3) | Macrostructure | Children with ADHD had higher TBT, longer SPT, longer REM sleep duration and more sleep cycles than HC. |
| Miano et al. [23] | 20 ADHD (18 M; 9.3 range: 6–13) vs. 20 HC (18 M; 9.3 range: 6–13) | 2 inattentive 18 combined | Full-scale IQ ≥ 70 ADHD: 10 with learning disabilities; 4 with mild neurological signs; 2 with language disorder; 4 with psychiatric comorbidities | None | Laboratory recording with adaptation night; EEG channels not specified (at least 8 electrodes) | Macrostructure CAP parameters | Children with ADHD had lower TST, SPT, TBT and higher rate of SS than HC. Children with ADHD had lower total CAP rates and lower CAP rates during stage 2 than HC. Children with ADHD had lower CAP sequences and a reduced total A1 index in stages 1 and 2. |
| Gruber et al. [24] | 15 ADHD (10 M; 8.45 ± 1.39) vs. 23 HC (13 M; 8.58 ± 1.27) | 1 hyperactive 2 inattentive 12 combined | Full-scale IQ ≥ 80 ADHD: 2 with PLMD HC: 2 with PLMD | ADHD stopped medications at least 7 days previous the experimental session | Home recording; 8 EEG channels (F3, F4, C3, C4, P3, P4, O1, O2) | Macrostructure | Children with ADHD had lower TST, lower percentage of REM sleep than HC. |
| Prihodova et al. [25] | 31 ADHD (26 M; 9.3 ± 1.7) vs. 26 HC (22 M; 9.2 ± 1.5) | 4 inattentive 27 combined | Full-scale IQ ≥ 80 None | None | Laboratory recording with adaptation night; 4 EEG channels (F4–C4, C4–P4, F3–C3, C3–P3, C4–A1, C3–A2) | Macrostructure | No significant differences were found on sleep parameters between groups. |
| Prehn-Kristensen et al. [26] | 16 ADHD (not provided, 10.6 ± 0.88) vs. 16 HC (not provided, 11.00 ± 0.99) | 8 inattentive 8 combined | full-scale IQ ≥ 85 ADHD: 4 with ODD. | 12 ADHD stopped medications 2 days previous the experimental session | Laboratory recording with adaptation night; 2 EEG channels (C3, C4) | Macrostructure | No significant differences were found on sleep parameters between groups. A sleep-associated gain in reaction times of procedural memory task was positively correlated with the amount of stage 4 and REM sleep density in ADHD group. |
| Prehn-Kristensen et al. [27] | 12 ADHD (all M; 12.22 ± 0.52) vs. 2 HC (all M; 12.64 ± 0.24) | Not provided | full-scale IQ ≥ 85 ADHD: 3 with ODD | 5 ADHD stopped medications 2 days previous the experimental session | Laboratory recording with adaptation night; 2 EEG channels (C3, C4) | Macrostructure EEG power analysis at C3 (SWA; delta; theta; alpha; sigma, during REM and NREM sleep) Visual spindle detection in Stage 2 | Children with ADHD had longer REM sleep duration and SOL than HC Children with. ADHD had shorter SWS latency and lower SE than HC. No significant differences on EEG power and spindle density were found between groups. Children with ADHD showed reduced sleep-associated consolidation of declarative memory. HC showed a correlation between sleep-associated recognition enhancement in declarative memory task (IAPS) and <1 Hz power during the first sleep cycle. NREM sleep duration in HC was positively correlated to sleep-related memory consolidation. |
| Gruber et al. [28] | 26 ADHD (17 M; 8.61 ± 1.27) vs. 49 HC (30 M; 8.61 ± 1.27) | 1 hyperactive 8 inattentive 17 combined | full-scale IQ ≥ 80 ADHD: 8 with ODD; 2 with conduct disorder | ADHD stopped medications 2 days previous the experimental session | Home recordings; 8 EEG channels (F3, F4, C3, C4, P3, P4, O1, O2) | Macrostructure | No significant differences were found on sleep parameters between groups. |
| Kirov et al. [29] | 20 ADHD (19 M; 11.24 ± 2.31) vs. 19 HC (17 M; 11.26 ± 2.49) | All combined | full-scale IQ ≥ 80 None | 11 ADHD stopped medications at least 7 days previous the experimental session | Laboratory recordings with adaptation night; 2 EEG channels (C3, C4) | Macrostructure | Children with ADHD had higher TBT, TST, shorter REM sleep latency and longer REM sleep duration than HC. |
| Prihodova et al. [30] | 14 ADHD (12 M; 9.6 ± 1.6) vs. 12 HC (8 M; 9.0 ± 1.6) | 2 inattentive 12 combined | IQ not specified, exclusion of mental retardation None | None | Laboratory recording with adaptation night; 4 bipolar EEG channels (F4-C4, C4-P4, F3-C3, C3-P3, C4-A1, C3-A2) | Macrostructure CAP analysis | No significant differences were found on sleep parameters between groups. |
| Prehn-Kristensen et al. [31] | 16 ADHD (all M; 10.6 ± 0.95) vs. 16 HC (all M; 11.1 ± 0.95) vs. 20 HC adults (all M; 24.7 ± 2.8) | 8 inattentive 8 combined | full-scale IQ ≥ 85 ADHD: 4 with ODD. | 12 ADHD stopped medications 2 days previous the experimental session. | Laboratory recordings with adaptation night; 4 EEG channels (F3, F4, C3, C4) | Macrostructure EEG power analysis at F4. (SWA, delta and sigma during stage 2; theta during REM sleep) | No significant differences were found on sleep parameters between children groups. After merged all healthy subjects (children and adults), a correlation between emotional memory (investigated by IAPS) and slow/delta during SWS was found. ADHD showed negative correlation between performance and <1 Hz power during SWS. The same correlation was found with theta activity during REM sleep. |
| Wiebe et al. [32] | 20 ADHD (13 M; 9.2 ± 1.6) vs. 46 HC (28 M; 8.8 ± 1.1) | 3 hyperactive 13 inattentive 4 combined | mean IQ ADHD =100.4 mean IQ HC = 104.0 None | ADHD stopped medications at least 2 days previous the experimental session. | Home recordings; 8 EEG channels (F3, F4, C3, C4, P3, P4, O1, O2) | Macrostructure | No significant differences were found on sleep parameters between groups. |
| Ringli et al. [33] | 9 ADHD (8 M; 11.9 range: 9.7–13.4) vs. 9 HC (8 M; 11.6 range: 9.6–14.2) | All combined | mean IQ 120±15 None | 2 ADHD were treated at the day of experimental session. The second dose of medications was not given at the day of measurement. | Laboratory recording; High-density EEG (128 channels) | Macrostructure EEG power analysis in all cortical channels. (SWA during NREM sleep) | Children with ADHD had lower duration of stage 1 than HC. Children with ADHD showed higher SWA power over central than HC. |
| Akinci et al. [34] | 28 ADHD (20 M; 10 range: 8–12) vs. 15 HC (9 M; 10 range: 9–13) | 7 inattentive 21 hyperactive or combined | full-scale IQ > 70. None | None | Laboratory recordings with adaptation nigh; 10 EEG channels | Macrostructure CAP analysis | Children with ADHD had higher REM sleep duration than HC. Children with ADHD had lower total CAP rates than HC. Children with ADHD had a reduced total A1 index in stage 2. |
| Virring et al. [35] | 76 ADHD (74% M; 9.6 ± 1.8) vs. 25 HC (68% M; 9.4 ± 1.5). | 5 hyperactive 14 inattentive 57 combined | full-scale IQ > 70 ADHD: 6 with autism, 9 with internalizing comorbidity, 20 with externalizing comorbidity; 7 with tic disorder | None | Home recording; 6 EEG channels (F4, C4, O2, F3, C3, O1) | Macrostructure | Children with ADHD had higher numbers of sleep cycles, lower TST, lower stage 1 and 3 and longer REM sleep duration than HC. When children with comorbidity were excluded from the analyses, ADHD group showed only longer SOL than HC. |
| Saletin et al. [36] | 7 ADHD (5 M; 11.9 ± 0.9) vs. 14 HC (10 M; 11.7 ± 0.9) | Not provided | mean IQ 110.3 ± 14.1. None | ADHD stopped medications 2 days previous the experimental session. | Laboratory recordings with adaptation night; 4 EEG channels (C3, C4, O1, O2) | Macrostructure EEG power analysis at C3, C4 (slow and fast sigma; SWA during Stage 2) | Children with ADHD had lower TBT than HC. Children with ADHD showed reduced sigma power (spindle-related) than HC. Children with ADHD showed lower MST before sleep than HC, but no overnight gain was observed. MST precision was positively associated with slow spindle activity for the children with ADHD. |
| Cremone et al. [37] | 18 ADHD (13 M; 6.70 ± 1.07) vs. 15 HC (11 M; 6.73 ± 0.71) | All hyperactive | IQ not specified, exclusion of mental retardation None | ADHD stopped medications 2 days previous the experimental session | Laboratory recordings; 24 EEG channels | Macrostructure EEG power analysis in all cortical channels. (delta during stage 2 and SWS; theta, during REM and NREM sleep) | No significant differences were found on sleep parameters between groups. HC showed greater accuracy at go/noGo task in the morning vs. baseline after sleep. The performance was significantly associated with REM theta activity at F4. Children with ADHD showed greater theta activity in REM sleep than controls, however they revealed no changes in their performance after sleep. |
| Wiesener et al. [38] | 17 ADHD (All M; 11.3 ± 0.4) vs. 17 HC (all M; 11.1 ± 0.2) | 2 hyperactive 15 combined | full-scale IQ ≥ 85 ADHD: 14 with ODD, 3 with conduct disorder; 6 with learning disabilities. | 13 ADHD stopped medications 2 days previous the experimental session | Laboratory recording with adaptation night; 2 EEG channels (C3, C4) | Macrostructure | No significant differences were found on sleep parameters between groups. Children with ADHD did not show sleep-dependent consolidation of rewarded behavior. Their consolidation of rewarded behavior did not correlate with sleep. Instead, HC consolidated rewarded behavior better during a night of sleep than during a day awake. |
| Chin et al. [39] | 71 ADHD (54 M, 8.83 ± 1.86) vs. 30 HC (15 M, 8.48 ± 2.36) | 35 inattentive 36 hyperactive or combined | full-scale IQ > 70 None | ADHD had no medications in the 6 months previous the experimental session. | Laboratory recordings; 32 EEG channels | Macrostructure | Children with ADHD had lower percentage of SWS and higher apnea-hypopnea index than HC. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpelli, S.; Gorgoni, M.; D’Atri, A.; Reda, F.; De Gennaro, L. Advances in Understanding the Relationship between Sleep and Attention Deficit-Hyperactivity Disorder (ADHD). J. Clin. Med. 2019, 8, 1737. https://doi.org/10.3390/jcm8101737
Scarpelli S, Gorgoni M, D’Atri A, Reda F, De Gennaro L. Advances in Understanding the Relationship between Sleep and Attention Deficit-Hyperactivity Disorder (ADHD). Journal of Clinical Medicine. 2019; 8(10):1737. https://doi.org/10.3390/jcm8101737
Chicago/Turabian StyleScarpelli, Serena, Maurizio Gorgoni, Aurora D’Atri, Flaminia Reda, and Luigi De Gennaro. 2019. "Advances in Understanding the Relationship between Sleep and Attention Deficit-Hyperactivity Disorder (ADHD)" Journal of Clinical Medicine 8, no. 10: 1737. https://doi.org/10.3390/jcm8101737
APA StyleScarpelli, S., Gorgoni, M., D’Atri, A., Reda, F., & De Gennaro, L. (2019). Advances in Understanding the Relationship between Sleep and Attention Deficit-Hyperactivity Disorder (ADHD). Journal of Clinical Medicine, 8(10), 1737. https://doi.org/10.3390/jcm8101737








