Transient Laterality of Cerebral Oxygenation Changes in Response to Head-of-Bed Manipulation in Acute Ischemic Stroke
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Ethics and Study Registration
2.3. Cerebral Hemoglobin Concentration Measurement by Time-Resolved NIRS
2.4. Cerebral Blood Hemoglobin Concentration Measurements Protocol
2.5. Statistical Analysis
3. Results
3.1. Study Participants’ Characteristics
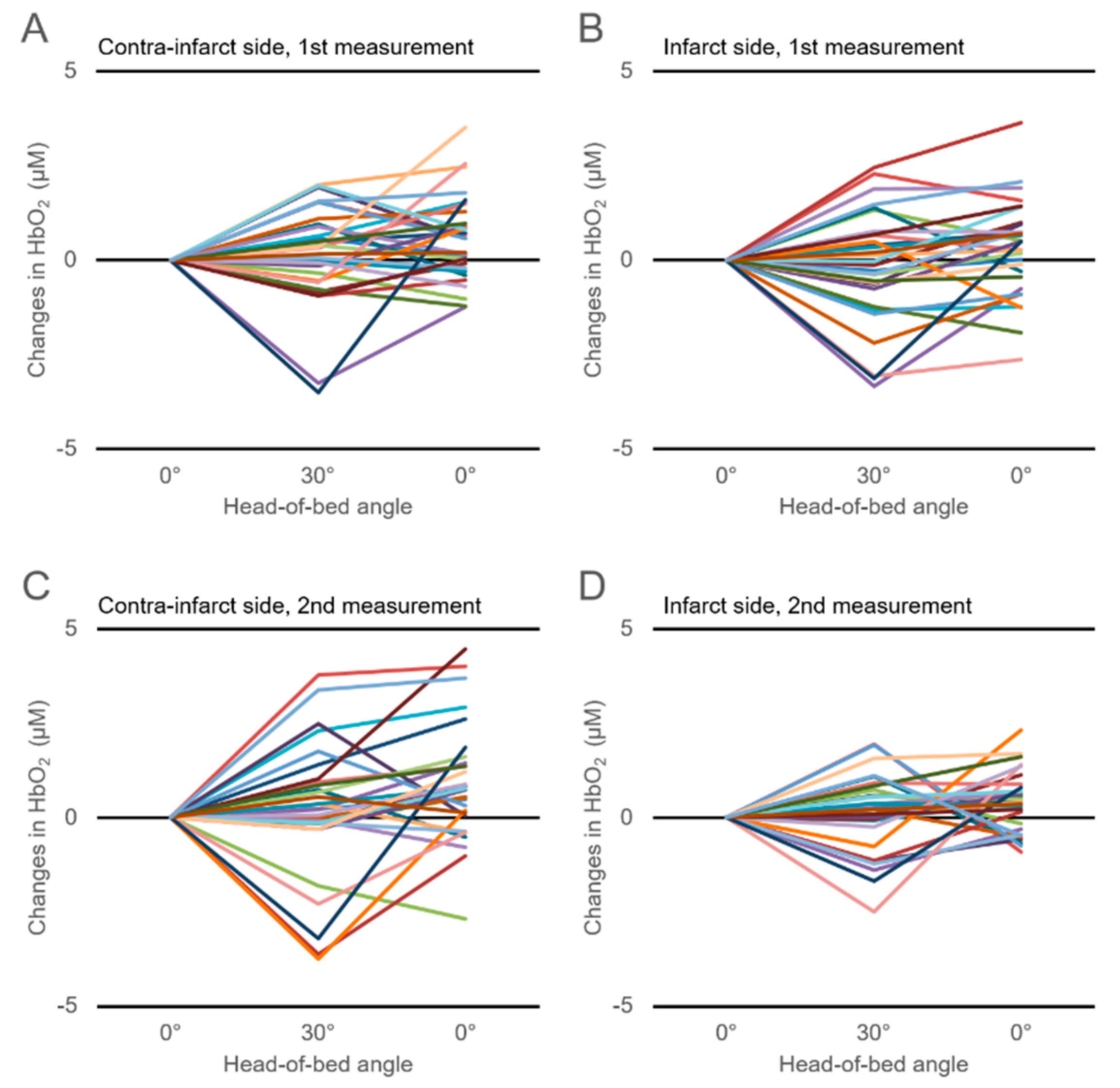
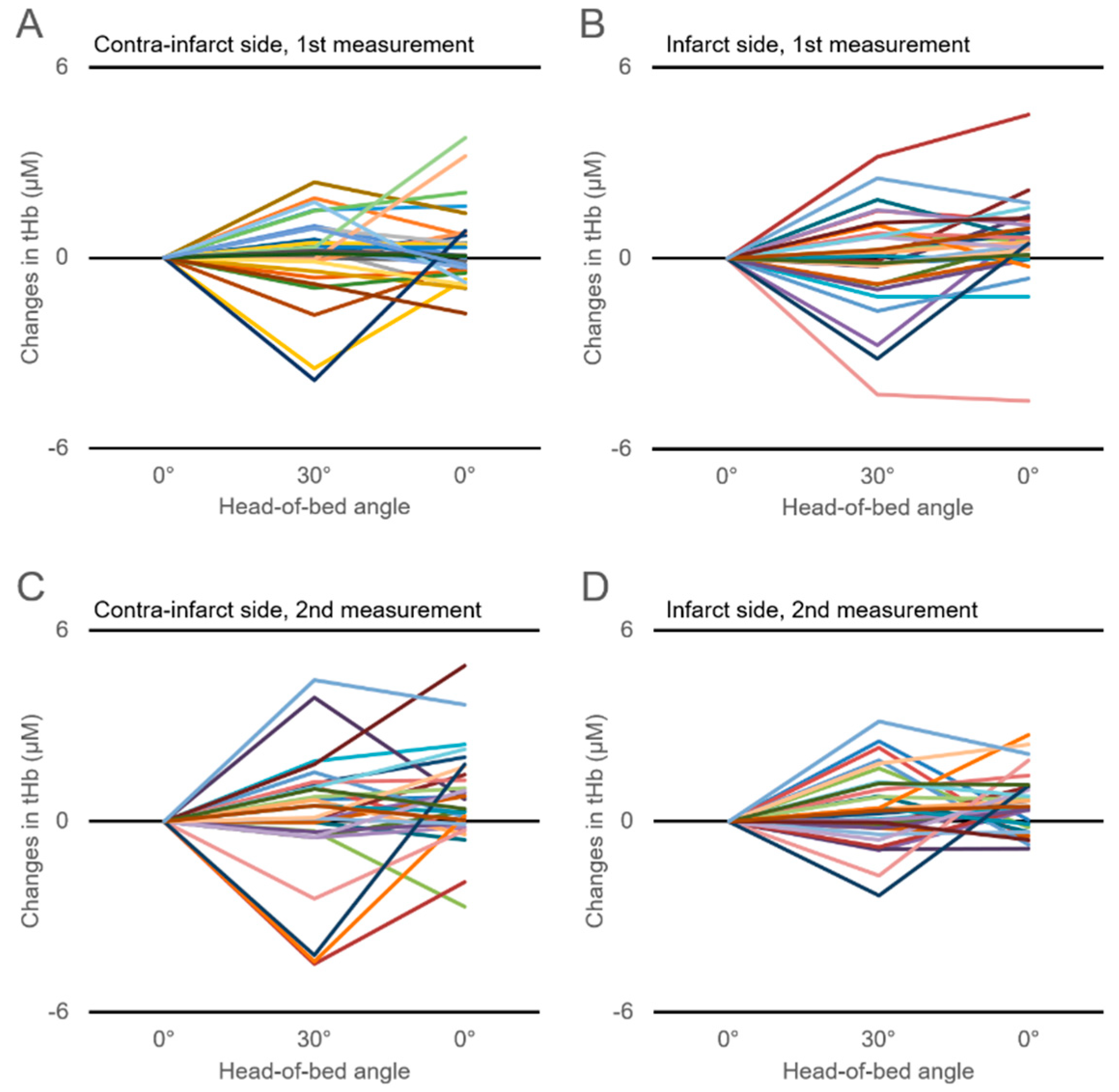
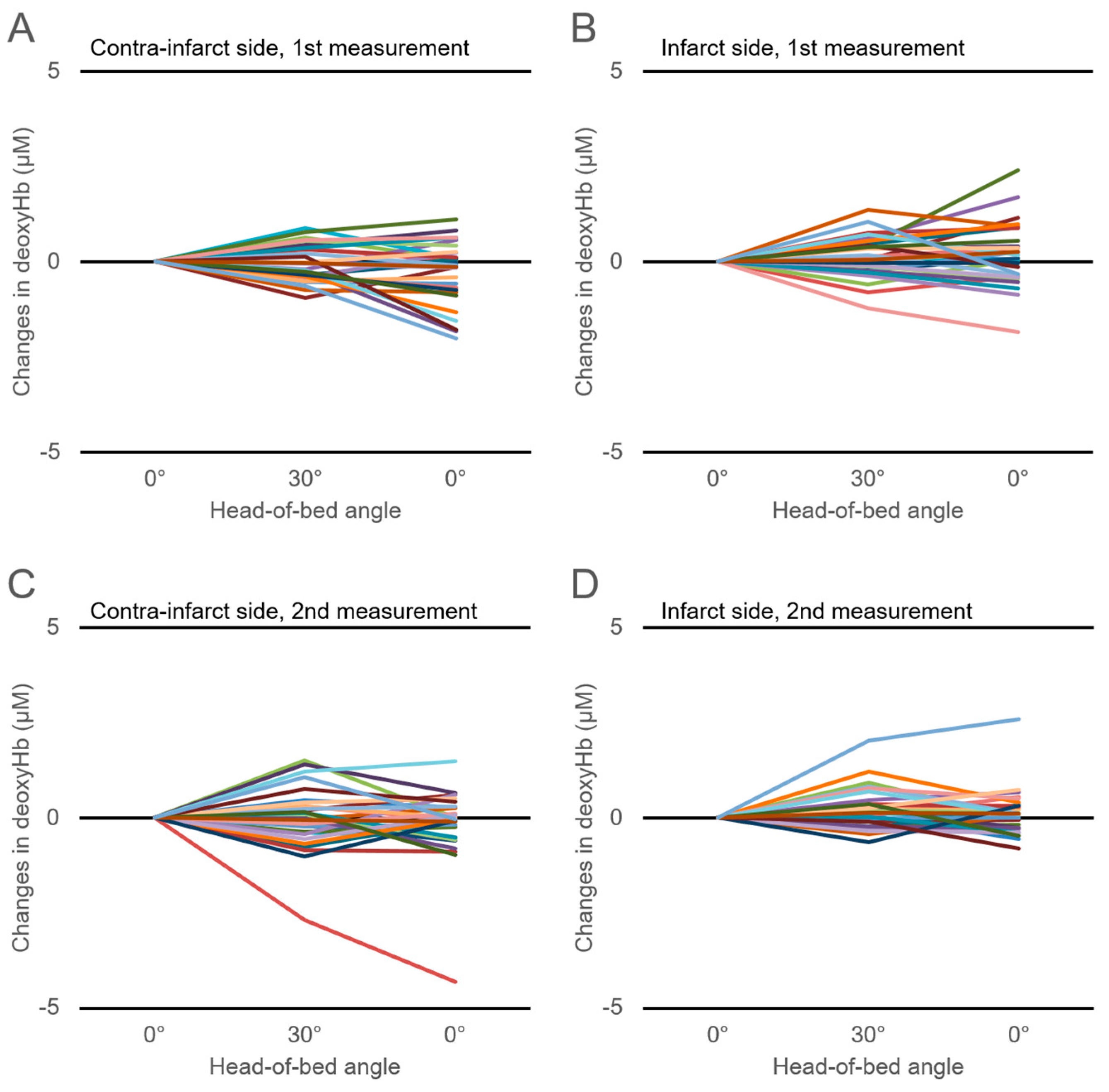
3.2. Changes in Blood Pressure, Heart Rate, tHb, HbO2, and deoxyHb with HOB Elevation
3.3. Changes in Cerebral Hemoglobin Concentrations with HOB Elevation
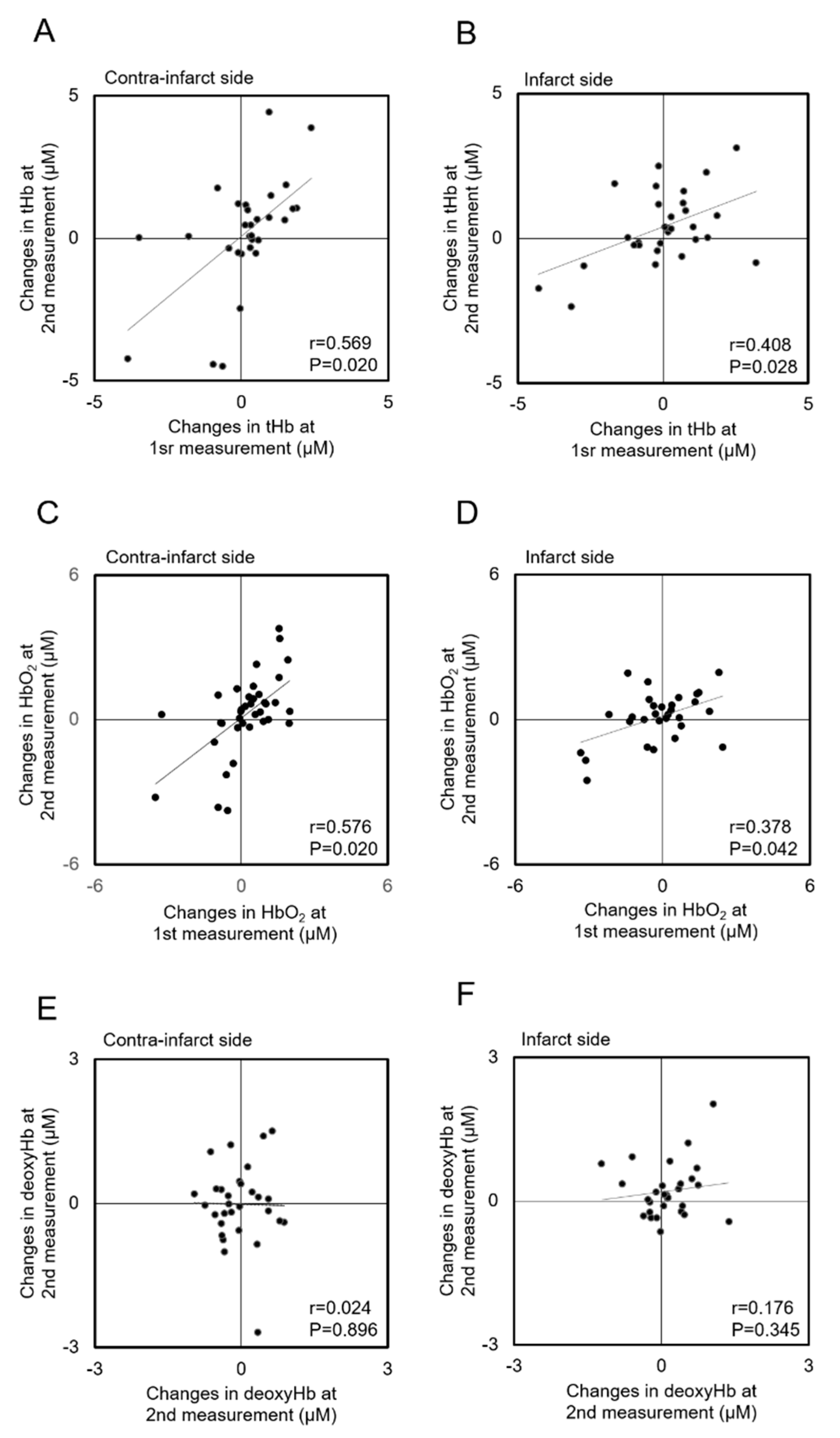
3.4. Correlations of Hemoglobin Concentration Changes with HOB Elevation between Measurements
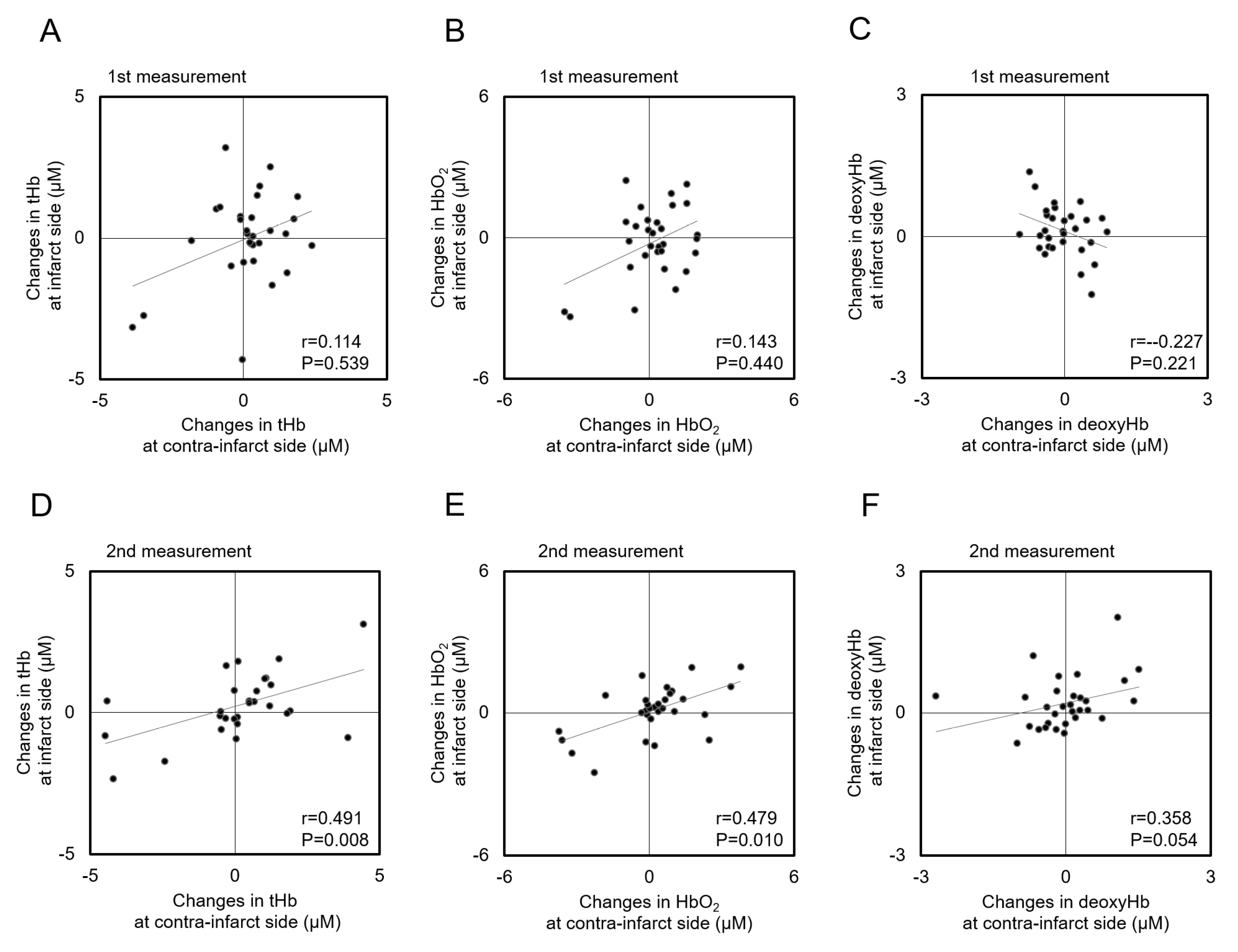
3.5. Correlations of Hemoglobin Concentration Changes with HOB Elevation between Infarct and Contra-Infarct Sides
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for adult stroke rehabilitation and recovery: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (avert): A randomised controlled trial. Lancet 2015, 386, 46–55. [Google Scholar]
- Mehagnoul-Schipper, D.J.; Vloet, L.C.; Colier, W.N.; Hoefnagels, W.H.; Jansen, R.W. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke 2000, 31, 1615–1620. [Google Scholar] [CrossRef]
- Tyson, S.F.; Nightingale, P. The effects of position on oxygen saturation in acute stroke: A systematic review. Clin. Rehabil. 2004, 18, 863–871. [Google Scholar] [CrossRef]
- Hargroves, D.; Tallis, R.; Pomeroy, V.; Bhalla, A. The influence of positioning upon cerebral oxygenation after acute stroke: A pilot study. Age ageing 2008, 37, 581–585. [Google Scholar] [CrossRef][Green Version]
- Olavarria, V.V.; Arima, H.; Anderson, C.S.; Brunser, A.M.; Munoz-Venturelli, P.; Heritier, S.; Lavados, P.M. Head position and cerebral blood flow velocity in acute ischemic stroke: A systematic review and meta-analysis. Cerebrovasc. Dis. 2014, 37, 401–408. [Google Scholar] [CrossRef]
- Anderson, C.S.; Arima, H.; Lavados, P.; Billot, L.; Hackett, M.L.; Olavarria, V.V.; Munoz Venturelli, P.; Brunser, A.; Peng, B.; Cui, L.; et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N. Engl. J. Med. 2017, 376, 2437–2447. [Google Scholar] [CrossRef]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Muller, N.G. Applications of functional near-infrared spectroscopy (fnirs) neuroimaging in exercise(-)cognition science: A systematic, methodology-focused review. J. Clin. Med. 2018. [Google Scholar] [CrossRef]
- Kim, M.N.; Edlow, B.L.; Durduran, T.; Frangos, S.; Mesquita, R.C.; Levine, J.M.; Greenberg, J.H.; Yodh, A.G.; Detre, J.A. Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults. Neurocrit. Care 2014, 20, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Harrer, M.; Waldenberger, F.R.; Weiss, G.; Folkmann, S.; Gorlitzer, M.; Moidl, R.; Grabenwoeger, M. Aortic arch surgery using bilateral antegrade selective cerebral perfusion in combination with near-infrared spectroscopy. Eur. J. Cardiothorac. Surg. 2010, 38, 561–567. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogino, H.; Ueda, Y.; Sugita, T.; Morioka, K.; Sakakibara, Y.; Matsubayashi, K.; Nomoto, T. Monitoring of regional cerebral oxygenation by near-infrared spectroscopy during continuous retrograde cerebral perfusion for aortic arch surgery. Eur. J. Cardiothorac. Surg. 1998, 14, 415–418. [Google Scholar] [CrossRef]
- Weindling, A.M. Peripheral oxygenation and management in the perinatal period. Semin. Fetal Neonatal. Med. 2010, 15, 208–215. [Google Scholar] [CrossRef]
- Aries, M.J.; Elting, J.W.; Stewart, R.; De Keyser, J.; Kremer, B.; Vroomen, P. Cerebral blood flow velocity changes during upright positioning in bed after acute stroke: An observational study. BMJ Open 2013. [Google Scholar] [CrossRef] [PubMed]
- Favilla, C.G.; Mesquita, R.C.; Mullen, M.; Durduran, T.; Lu, X.; Kim, M.N.; Minkoff, D.L.; Kasner, S.E.; Greenberg, J.H.; Yodh, A.G.; et al. Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation. Stroke 2014, 45, 1269–1274. [Google Scholar] [CrossRef]
- Durduran, T.; Zhou, C.; Edlow, B.L.; Yu, G.; Choe, R.; Kim, M.N.; Cucchiara, B.L.; Putt, M.E.; Shah, Q.; Kasner, S.E.; et al. Transcranial optical monitoring of cerebrovascular hemodynamics in acute stroke patients. Opt. express 2009, 17, 3884–3902. [Google Scholar] [CrossRef]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef]
- Sato, C.; Yamaguchi, T.; Seida, M.; Ota, Y.; Yu, I.; Iguchi, Y.; Nemoto, M.; Hoshi, Y. Intraoperative monitoring of depth-dependent hemoglobin concentration changes during carotid endarterectomy by time-resolved spectroscopy. Appl. Opt. 2007, 46, 2785–2792. [Google Scholar] [CrossRef]
- Ohmae, E.; Ouchi, Y.; Oda, M.; Suzuki, T.; Nobesawa, S.; Kanno, T.; Yoshikawa, E.; Futatsubashi, M.; Ueda, Y.; Okada, H.; et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: Correlation with simultaneous positron emission tomography measurements. NeuroImage 2006, 29, 697–705. [Google Scholar] [CrossRef]
- Tutaj, M.; Miller, M.; Krakowska-Stasiak, M.; Piatek, A.; Hebda, J.; Latka, M.; Strojny, J.; Szczudlik, A.; Slowik, A. Dynamic cerebral autoregulation is compromised in ischaemic stroke of undetermined aetiology only in the non-affected hemisphere. Neurol. Neurochir. Pol. 2014, 48, 91–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Petersen, N.H.; Ortega-Gutierrez, S.; Reccius, A.; Masurkar, A.; Huang, A.; Marshall, R.S. Dynamic cerebral autoregulation is transiently impaired for one week after large-vessel acute ischemic stroke. Cerebrovasc. Dis. 2015, 39, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Panerai, R.B.; Jara, J.L.; Saeed, N.P.; Horsfield, M.A.; Robinson, T.G. Dynamic cerebral autoregulation following acute ischaemic stroke: Comparison of transcranial doppler and magnetic resonance imaging techniques. J. Cereb. Blood Flow Metab. 2016, 36, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Guo, Z.N.; Jin, H.; Yan, X.; Liu, J.; Lv, S.; Zhang, P.; Sun, X.; Yang, Y. Preliminary study of dynamic cerebral autoregulation in acute ischemic stroke: Association with clinical factors. Front Neurol. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed]
- Novak, V.; Hu, K.; Desrochers, L.; Novak, P.; Caplan, L.; Lipsitz, L.; Selim, M. Cerebral flow velocities during daily activities depend on blood pressure in patients with chronic ischemic infarctions. Stroke 2010, 41, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Bogert, L.W.; Immink, R.V.; Harms, M.P.; Colier, W.N.; van Lieshout, J.J. Effects of aging on the cerebrovascular orthostatic response. Neurobiol. Aging 2011, 32, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Mehagnoul-Schipper, D.J.; Colier, W.N.; Jansen, R.W. Reproducibility of orthostatic changes in cerebral oxygenation in healthy subjects aged 70 years or older. Clin. Physiol. 2001, 21, 77–84. [Google Scholar] [CrossRef]
- Chieregato, A.; Tanfani, A.; Compagnone, C.; Pascarella, R.; Targa, L.; Fainardi, E. Cerebral blood flow in traumatic contusions is predominantly reduced after an induced acute elevation of cerebral perfusion pressure. Neurosurgery 2007, 60, 115–123. [Google Scholar] [CrossRef]
- Jaeger, M.; Schuhmann, M.U.; Soehle, M.; Nagel, C.; Meixensberger, J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke 2007, 38, 981–986. [Google Scholar] [CrossRef]
- Gatto, R.; Hoffman, W.; Paisansathan, C.; Mantulin, W.; Gratton, E.; Charbel, F.T. Effect of age on brain oxygenation regulation during changes in position. J. Neurosci. Methods 2007, 164, 308–311. [Google Scholar] [CrossRef][Green Version]
- Wojner-Alexander, A.W.; Garami, Z.; Chernyshev, O.Y.; Alexandrov, A.V. Heads down: Flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005, 64, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Wojner, A.W.; El-Mitwalli, A.; Alexandrov, A.V. Effect of head positioning on intracranial blood flow velocities in acute ischemic stroke: A pilot study. Crit. Care Nurs. Q. 2002, 24, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.J.; Snodgrass, S.J.; Quain, D.; Parsons, M.W.; Levi, C.R. Hoboe (head-of-bed optimization of elevation) study: Association of higher angle with reduced cerebral blood flow velocity in acute ischemic stroke. Phys. Ther. 2011, 91, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Bevers, M.B.; Kimberly, W.T. Critical care management of acute ischemic stroke. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 41. [Google Scholar] [CrossRef]
- Boujan, T.; Neuberger, U.; Pfaff, J.; Nagel, S.; Herweh, C.; Bendszus, M.; Mohlenbruch, M.A. Value of contrast-enhanced mra versus time-of-flight mra in acute ischemic stroke mri. AJNR Am. J. Neuroradiol. 2018, 39, 1710–1716. [Google Scholar] [CrossRef]
- Bang, O.Y.; Goyal, M.; Liebeskind, D.S. Collateral circulation in ischemic stroke: Assessment tools and therapeutic strategies. Stroke 2015, 46, 3302–3309. [Google Scholar] [CrossRef]
- Markus, H.S. Cerebral perfusion and stroke. J. Neurol. Neurosurg. Psychiatry 2004, 75, 353–361. [Google Scholar] [CrossRef]
- Dohmen, C.; Bosche, B.; Graf, R.; Reithmeier, T.; Ernestus, R.I.; Brinker, G.; Sobesky, J.; Heiss, W.D. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke 2007, 38, 56–61. [Google Scholar] [CrossRef]
- Aaslid, R.; Lindegaard, K.F.; Sorteberg, W.; Nornes, H. Cerebral autoregulation dynamics in humans. Stroke 1989, 20, 45–52. [Google Scholar] [CrossRef]
- Czosnyka, M.; Smielewski, P.; Kirkpatrick, P.; Menon, D.K.; Pickard, J.D. Monitoring of cerebral autoregulation in head-injured patients. Stroke 1996, 27, 1829–1834. [Google Scholar] [CrossRef]
- Steiner, L.A.; Pfister, D.; Strebel, S.P.; Radolovich, D.; Smielewski, P.; Czosnyka, M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit. Care 2009, 10, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.M.; Lee, J.K.; Kibler, K.K.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Koehler, R.C.; Shaffner, D.H. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007, 38, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Brady, K.; Joshi, B.; Zweifel, C.; Smielewski, P.; Czosnyka, M.; Easley, R.B.; Hogue, C.W., Jr. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010, 41, 1951–1956. [Google Scholar] [CrossRef]
- Moerman, A.; De Hert, S. Recent advances in cerebral oximetry. Assessment of cerebral autoregulation with near-infrared spectroscopy: Myth or reality? F1000Res 2017, 6, 1615. [Google Scholar] [CrossRef]
- Rivera-Lara, L.; Geocadin, R.; Zorrilla-Vaca, A.; Healy, R.; Radzik, B.R.; Palmisano, C.; Mirski, M.; Ziai, W.C.; Hogue, C. Validation of near-infrared spectroscopy for monitoring cerebral autoregulation in comatose patients. Neurocrit. Care 2017, 27, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Rostrup, E.; Law, I.; Pott, F.; Ide, K.; Knudsen, G.M. Cerebral hemodynamics measured with simultaneous pet and near-infrared spectroscopy in humans. Brain Res. 2002, 954, 183–193. [Google Scholar] [CrossRef]
- Claassen, J.A.; Colier, W.N.; Jansen, R.W. Reproducibility of cerebral blood volume measurements by near infrared spectroscopy in 16 healthy elderly subjects. Physiol. Meas. 2006, 27, 255–264. [Google Scholar] [CrossRef]
- Rasmussen, P.; Dawson, E.A.; Nybo, L.; van Lieshout, J.J.; Secher, N.H.; Gjedde, A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J. Cereb. Blood Flow Metab. 2007, 27, 1082–1093. [Google Scholar] [CrossRef]
- Xiong, L.; Tian, G.; Lin, W.; Wang, W.; Wang, L.; Leung, T.; Mok, V.; Liu, J.; Chen, X.; Wong, K.S. Is dynamic cerebral autoregulation bilaterally impaired after unilateral acute ischemic stroke? J. Stroke Cerebrovasc. Dis. 2017, 26, 1081–1087. [Google Scholar] [CrossRef]
- Lam, M.Y.; Haunton, V.J.; Robinson, T.G.; Panerai, R.B. Does gradual change in head positioning affect cerebrovascular physiology? Physiol. Rep. 2018. [Google Scholar] [CrossRef]
- Kety, S.S.; Schmidt, C.F. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J. Clin. Invest. 1948, 27, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Reivich, M. Arterial pco2 and cerebral hemodynamics. Am. J. Physiol. 1964, 206, 25–35. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value |
|---|---|
| Age, year | 72.8 ± 11.3 |
| Female sex, n (%) | 13 (43.3) |
| Height (cm) | 158.0 ± 11.6 |
| Body weight (kg) | 56.0 ± 14.4 |
| Body mass index (kg/m2) | 22.1 ± 3.1 |
| Stroke side (right/left) | 11/19 |
| NIHSS score | 7.6 ± 4.9 |
| TOAST classification, n (%) | |
| Large-artery atherosclerosis | 10 (33.3) |
| Small-vessel occlusion | 10 (33.3) |
| Cardioembolism | 6 (20.0) |
| Stroke of other determined etiology | 4 (13.3) |
| Stroke of undetermined etiology | 0 (0) |
| Vascular territorial segmentation, n (%) | |
| Anterior cerebral artery | 6 (20.0) |
| Middle cerebral artery | 17 (56.7) |
| Posterior cerebral artery | 7 (20.3) |
| Medical history n (%) | |
| Hypertension | 18 (60.0) |
| Diabetes mellitus | 8 (26.7) |
| Dyslipidemia | 22 (73.3) |
| Chronic atrial fibrillation | 4 (13.3) |
| Tobacco use | 12 (40.0) |
| Parameters | Item Measured | HOB 0° | HOB 30° | Difference | p |
|---|---|---|---|---|---|
| First Measurement | |||||
| Contra-infarct Side | sBP (mmHg) | 137.6 ± 20.9 | 137.7 ± 19.8 | 0.1 ± 4.1 | 0.90 |
| dBP(mmHg) | 75.9 ± 11.7 | 76.9 ± 10.6 | 1.0 ± 5.4 | 0.91 | |
| HR (bpm) | 73.5 ± 10.4 | 73.5 ± 9.7 | 0.0 ± 3.5 | 0.95 | |
| Infarct Side | sBP (mmHg) | 137.9 ± 20.6 | 137.3 ± 19.8 | −0.6 ± 3.7 | 0.39 |
| dBP (mmHg) | 75.9 ± 11.2 | 76.4 ± 10.9 | 0.5 ± 4.6 | 0.63 | |
| HR (bpm) | 73.6 ± 11.0 | 73.4 ± 11.4 | −0.2 ± 3.6 | 0.86 | |
| Second Measurement | |||||
| Contra-Infarct Side | sBP (mmHg) | 128.9 ± 16.5 | 129.8 ±15.0 | 0.9 ± 5.1 | 0.53 |
| dBP (mmHg) | 71.0 ± 9.0 | 71.1 ± 8.6 | 0.0 ± 3.9 | 0.51 | |
| HR (bpm) | 75.6 ± 8.1 | 76.5 ± 7.8 | 0.9 ± 3.9 | 0.36 | |
| Infarct Side | sBP (mmHg) | 129.5 ± 15.1 | 128.7 ± 15.6 | −0.8 ±5.3 | 0.10 |
| dBP (mmHg) | 71.0 ± 8.4 | 70.3 ± 8.3 | −0.7 ± 4.6 | 0.24 | |
| HR (bpm) | 74.8 ± 8.4 | 75.1 ± 7.7 | 0.3 ± 3.1 | 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, N.; Odagiri, K.; Hakamata, A.; Inui, N.; Yamauchi, K.; Watanabe, H. Transient Laterality of Cerebral Oxygenation Changes in Response to Head-of-Bed Manipulation in Acute Ischemic Stroke. J. Clin. Med. 2019, 8, 1739. https://doi.org/10.3390/jcm8101739
Katayama N, Odagiri K, Hakamata A, Inui N, Yamauchi K, Watanabe H. Transient Laterality of Cerebral Oxygenation Changes in Response to Head-of-Bed Manipulation in Acute Ischemic Stroke. Journal of Clinical Medicine. 2019; 8(10):1739. https://doi.org/10.3390/jcm8101739
Chicago/Turabian StyleKatayama, Naoki, Keiichi Odagiri, Akio Hakamata, Naoki Inui, Katsuya Yamauchi, and Hiroshi Watanabe. 2019. "Transient Laterality of Cerebral Oxygenation Changes in Response to Head-of-Bed Manipulation in Acute Ischemic Stroke" Journal of Clinical Medicine 8, no. 10: 1739. https://doi.org/10.3390/jcm8101739
APA StyleKatayama, N., Odagiri, K., Hakamata, A., Inui, N., Yamauchi, K., & Watanabe, H. (2019). Transient Laterality of Cerebral Oxygenation Changes in Response to Head-of-Bed Manipulation in Acute Ischemic Stroke. Journal of Clinical Medicine, 8(10), 1739. https://doi.org/10.3390/jcm8101739





