Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal?
Abstract
1. Introduction
2. Materials and methods
2.1. Patients
2.2. Preoperative Assessment
2.3. Blood Sampling and Assays
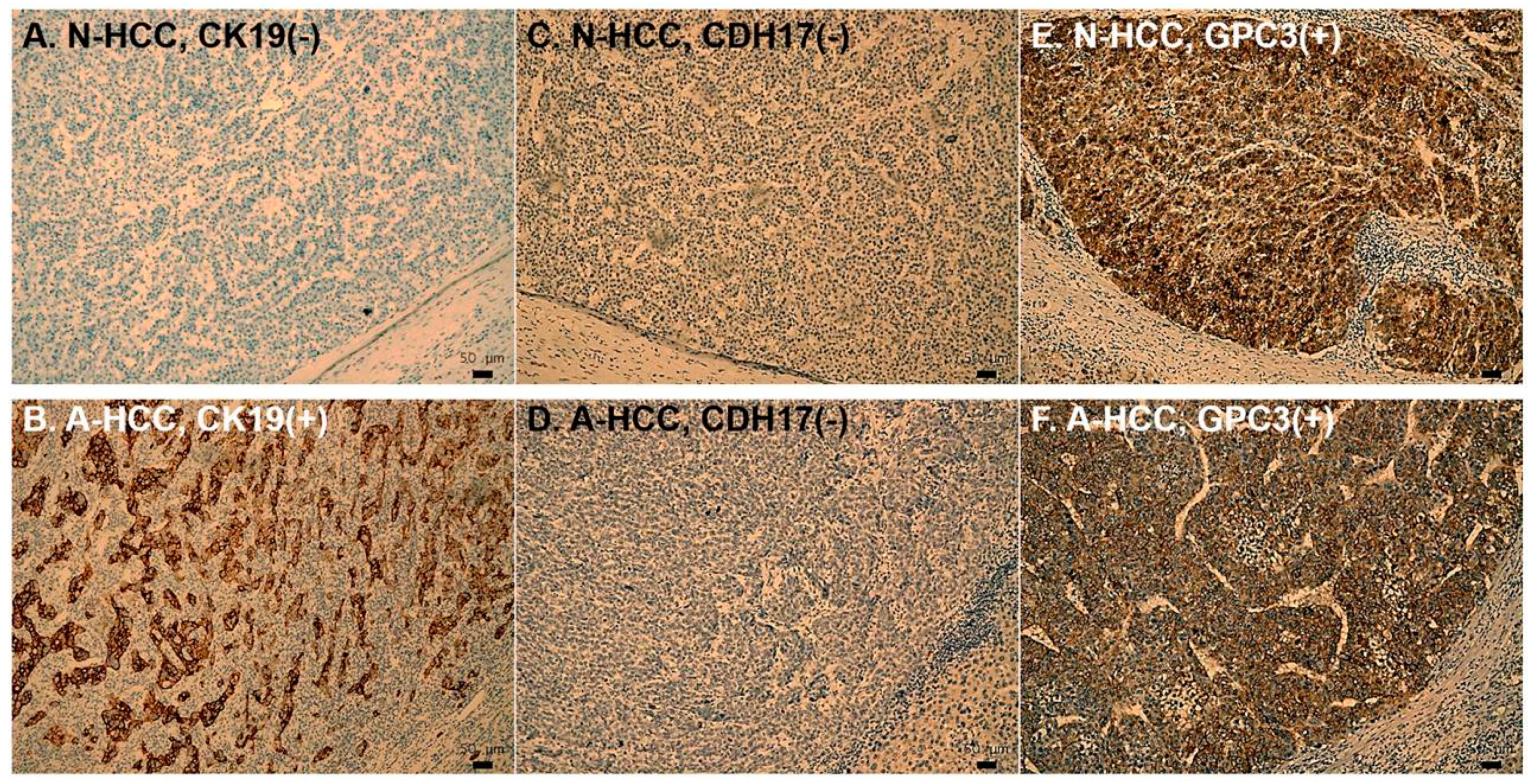
2.4. Immunohistochemistry
2.5. Definition and Statistical Analysis
3. Results
3.1. Clinical-Pathological Characteristics of N-HCC Versus A-HCC
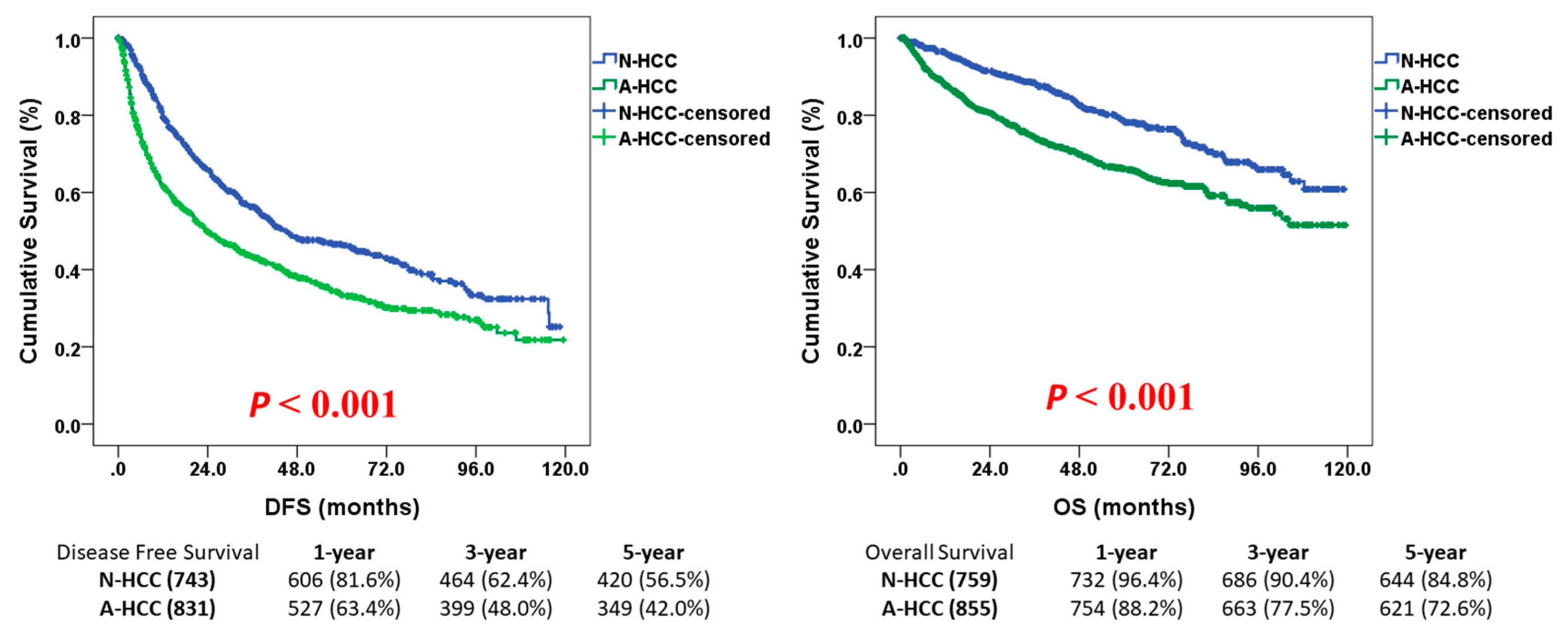
3.2. Survival Outcome of N-HCC After Hepatectomy
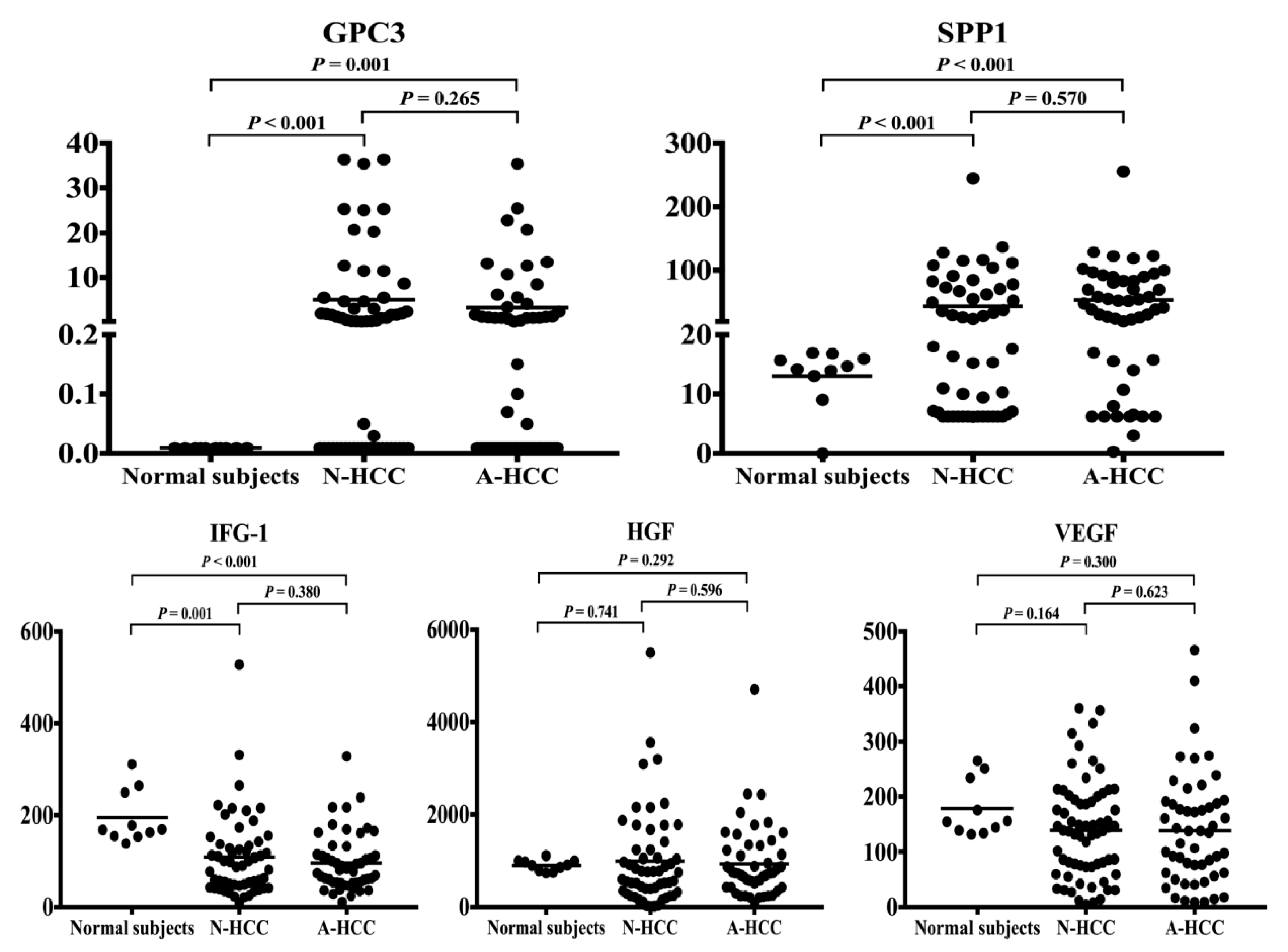
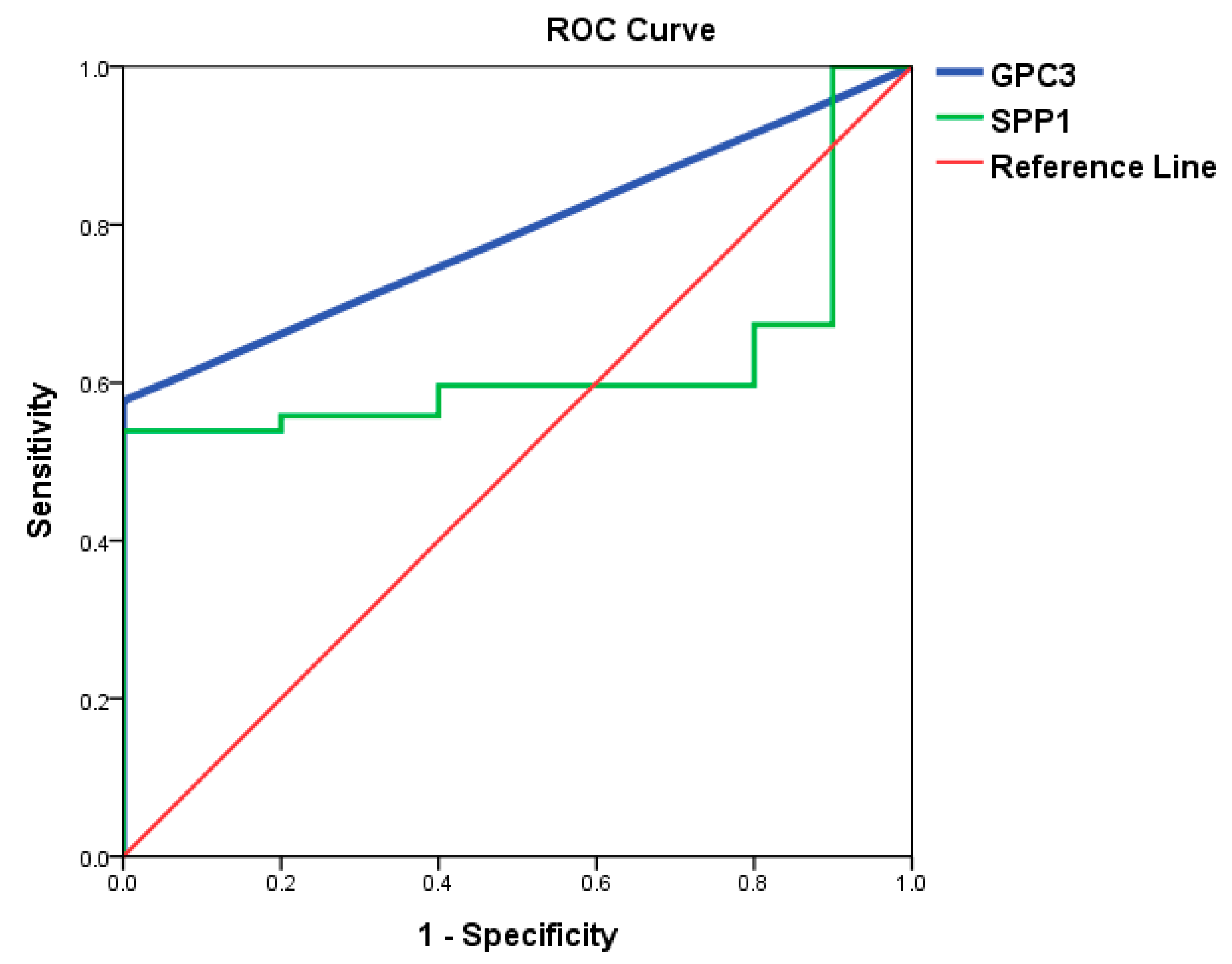
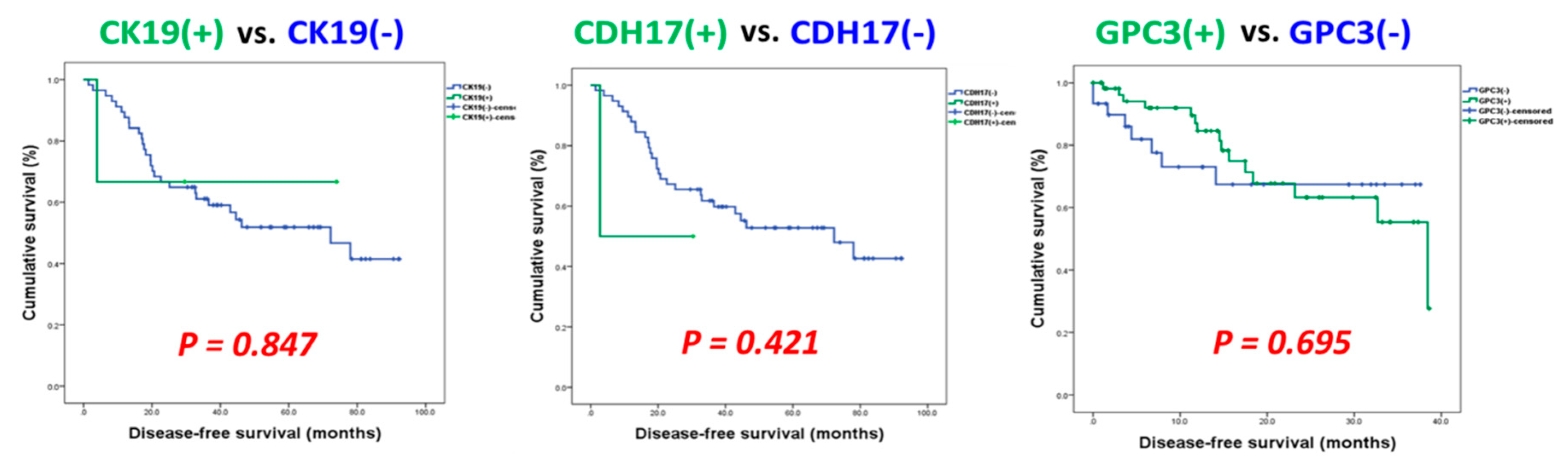
3.3. Identification of Potential Biomarkers for Normal AFP Hepatocellular Carcinoma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA A Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Department of Health ROC. Report of Leading Cancer-Related Death in 2014; Department of Health, Executive Yuan: Taipei, Taiwan, 2015. [Google Scholar]
- Masuzaki, R.; Karp, S.J.; Omata, M. New serum markers of hepatocellular carcinoma. Semin. Oncol. 2012, 39, 434–439. [Google Scholar] [CrossRef]
- Waidely, E.; Al-Yuobi, A.R.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Serum protein biomarkers relevant to hepatocellular carcinoma and their detection. Analyst 2016, 141, 36–44. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Barrat, A.; Askari, F.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Prognosis of hepatocellular carcinoma: Comparison of 7 staging systems in an American cohort. Hepatology 2005, 41, 707–716. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Mavroudis, C.; Jacobs, M.L.; Maruszewski, B.; Tchervenkov, C.I.; Lacour-Gayet, F.G.; Clarke, D.R.; Yeh, T., Jr.; Walters, H.L., 3rd; Kurosawa, H.; et al. What is operative mortality? Defining death in a surgical registry database: A report of the STS Congenital Database Taskforce and the Joint EACTS-STS Congenital Database Committee. Ann. Thorac. Surg. 2006, 81, 1937–1941. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Seppala, M. Studies of carcino-fetal proteins: Physical and chemical properties of human alpha-fetoprotein. Int. J. Cancer 1971, 7, 218–225. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, J.Y.; Jeong, S.W.; Cho, Y.K.; Lee, S.H.; Kim, S.G.; Cha, S.W.; Kim, Y.S.; Cho, Y.D.; Kim, H.S.; et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Medicine 2017, 96, e5811. [Google Scholar] [CrossRef] [PubMed]
- Daniele, B.; Bencivenga, A.; Megna, A.S.; Tinessa, V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology 2004, 127, S108–S112. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Garcia-Tsao, G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int. 2009, 29, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Cancer of the Liver Italian Program (CLIP) Investigators. A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998, 28, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Filmus, J.; Selleck, S.B. Glypicans: Proteoglycans with a surprise. J. Clin. Investig. 2001, 108, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Yoshitake, Y.; Senju, S.; Monji, M.; Komori, H.; Motomura, Y.; Hosaka, S.; Beppu, T.; Ishiko, T.; Kamohara, H.; et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun. 2003, 306, 16–25. [Google Scholar] [CrossRef]
- Capurro, M.I.; Xiang, Y.Y.; Lobe, C.; Filmus, J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005, 65, 6245–6254. [Google Scholar] [CrossRef] [PubMed]
- Midorikawa, Y.; Ishikawa, S.; Iwanari, H.; Imamura, T.; Sakamoto, H.; Miyazono, K.; Kodama, T.; Makuuchi, M.; Aburatani, H. Glypican-3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP-7 signaling. Int. J. Cancer. 2003, 103, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, P.; Zhai, Y.; Qu, C.F.; Zhang, L.J.; Tan, Y.F.; Li, N.; Ding, H.G. Diagnostic value of glypican-3 in serum and liver for primary hepatocellular carcinoma. World J. Gastroenterol. 2010, 16, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Wanless, I.R.; Sherman, M.; Deboer, G.; Shi, W.; Miyoshi, E.; Filmus, J. Glypican-3: A novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003, 125, 89–97. [Google Scholar] [CrossRef]
- Rehem, R.N.; El-Shikh, W.M. Serum IGF-1, IGF-2 and IGFBP-3 as parameters in the assessment of liver dysfunction in patients with hepatic cirrhosis and in the diagnosis of hepatocellular carcinoma. Hepatogastroenterology 2011, 58, 949–954. [Google Scholar] [PubMed]
- Karabulut, S.; Tas, F.; Akyüz, F.; Ormeci, A.C.; Serilmez, M.; Soydinç, H.O.; Vatansever, S.; Yasasever, V. Clinical significance of serum hepatocyte growth factor (HGF) levels in hepatocellular carcinoma. Tumour Biol. 2014, 35, 2327–2333. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Liu, F.; Zhao, X.; Yang, X. Identification of candidate biomarkers for hepatocellular carcinoma through pre-cancerous expression analysis in an HBx transgenic mouse. Cancer Biol. Ther. 2007, 6, 1532–1538. [Google Scholar] [CrossRef][Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Yamagamim, H.; Moriyama, M.; Matsumura, H.; Aoki, H.; Shimizu, T.; Saito, T.; Kaneko, M.; Shioda, A.; Tanaka, N.; Arakawa, Y. Serum concentrations of human hepatocyte growth factor is a useful indicator for predicting the occurrence of hepatocellular carcinomas in C-viral chronic liver diseases. Cancer 2002, 95, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.M.E.S.A.; Atta, H.M.; Gad, M.A.M.; Rashed, L.A.; Said, E.M.; Hassanien, S.E.S.A.; Kaseb, A.O. Clinical significance of vascular endothelial growth factor in hepatitis C related hepatocellular carcinoma in Egyptian patients. J. Hepatocell. Carcinoma 2016, 3, 19–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carr, B.I.; Pancoska, P.; Branch, R.A. Low alpha-fetoprotein hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2010, 25, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.; Man, K.; Fan, S.T.; Yu, W.C.; Lo, C.M.; Wong, J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br. J. Surg. 1997, 84, 1255–1259. [Google Scholar] [CrossRef]
- Lam, C.M.; Fan, S.T.; Lo, C.M.; Wong, J. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br. J. Surg. 1999, 86, 1012–1017. [Google Scholar] [CrossRef]
- Pang, Y.Y. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB 2002, 4, 99–100. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Yu, M.C.; Lee, C.W.; Tsai, H.I.; Sung, C.M.; Chen, C.W.; Huang, S.W.; Lin, C.Y.; Jeng, W.J.; Lee, W.C.; et al. RAM score is an effective predictor for early mortality and recurrence after hepatectomy for hepatocellular carcinoma. BMC Cancer 2017, 17, 742. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Tokushige, K.; Koike, K.; Shima, T.; Kanbara, Y.; Saibara, T.; Uto, H.; Takami, S.; Kawanaka, M.; et al. Clinical and pathological progression of non-alcoholic steatohepatitis to hepatocellular carcinoma. Hepatol. Res. 2012, 42, 767–773. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Lee, C.W.; Chan, K.M.; Tsai, H.I.; Hsieh, Y.C.; Lin, C.Y.; Kuo, Y.C.; Hsu, H.Y.; Yu, M.C. Hepatic resection for hepatocellular carcinoma in the octogenarian: Is it justified? Aging 2019, 11, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lin, S.E.; Tsai, H.I.; Su, P.J.; Hsieh, C.H.; Kuo, Y.C.; Sung, C.M.; Lin, C.Y.; Tsai, C.N.; Yu, M.C. Cadherin 17 is related to recurrence and poor prognosis of cytokeratin 19-positive hepatocellular carcinoma. Oncol. Lett. 2018, 15, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Kuo, W.L.; Yu, M.C.; Chen, T.C.; Tsai, C.N.; Lee, W.C.; Chen, M.F. The expression of cytokeratin 19 in lymph nodes was a poor prognostic factor for hepatocellular carcinoma after hepatic resection. World J. Surg. Oncol. 2013, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.B.; Shi, Y.H.; Zhou, J.; Shi, G.M.; Ke, A.W.; Qiu, S.; Wang, X.Y.; Dai, Z.; Xu, Y.; Fan, J. Liver-intestine cadherin predicts microvascular invasion and poor prognosis of hepatitis B virus-positive hepatocellular carcinoma. Cancer 2009, 115, 4753–4765. [Google Scholar] [CrossRef]
- Fu, S.J.; Qi, C.Y.; Xiao, W.K.; Li, S.Q.; Peng, B.G.; Liang, L.J. Glypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery 2013, 154, 536–544. [Google Scholar] [CrossRef]
- Yu, M.C.; Lee, Y.S.; Lin, S.E.; Wu, H.Y.; Chen, T.C.; Lee, W.C.; Chen, M.F.; Tsai, C.N. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann. Surg. Oncol. 2012, 19 (Suppl. 3), S455–S463. [Google Scholar] [CrossRef]
- Wang, S.K.; Zynger, D.L.; Hes, O.; Yang, X.J. Discovery and diagnostic value of a novel oncofetal protein: Glypican 3. Adv. Anat. Pathol. 2014, 21, 450–460. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, J.; Chen, H.; Sheng, Y.; Zhang, X.; Liu, Z.; Zhang, C. Diagnostic value of immunohistochemical staining of GP73, GPC3, DCP, CD34, CD31, and reticulin staining in hepatocellular carcinoma. J. Histochem. Cytochem. 2013, 61, 639–648. [Google Scholar] [CrossRef]
- Enan, E.T.; El-Hawary, A.K.; El-Tantawy, D.A.; Abu-Hashim, M.M.; Helal, N.M. Diagnostic role of glypican 3 and CD34 for differentiating hepatocellular carcinoma from nonmalignant hepatocellular lesions. Ann. Diagn. Pathol. 2013, 17, 490–493. [Google Scholar] [CrossRef]
- Shafizadeh, N.; Ferrell, L.D.; Kakar, S. Utility and limitations of glypican-3 expression for the diagnosis of hepatocellular carcinoma at both ends of the differentiation spectrum. Mod. Pathol. 2008, 21, 1011–1018. [Google Scholar] [CrossRef]
- Liu, X.F.; Hu, Z.D.; Liu, X.C.; Cao, Y.; Ding, C.M.; Hu, C.J. Diagnostic accuracy of serum glypican-3 for hepatocellular carcinoma: A systematic review and meta-analysis. Clin. Biochem. 2014, 47, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Parpart, S.; Roessler, S.; Dong, F.; Rao, V.; Takai, A.; Ji, J.; Qin, L.X.; Ye, Q.H.; Jia, H.L.; Tang, Z.Y.; et al. Modulation of miR-29 expression by alpha-fetoprotein is linked to the hepatocellular carcinoma epigenome. Hepatology 2014, 60, 872–883. [Google Scholar] [CrossRef] [PubMed]
| Variables c | Total | N-HCC a | A-HCC b | P-Value d | |||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | ||
| Age > 65 year-old | 577 | (35.7%) | 294 | (38.6%) | 283 | (33.1%) | 0.020 |
| Male gender | 1256 | (77.7%) | 619 | (81.3%) | 637 | (74.5%) | 0.001 |
| Comorbidity | 639 | (39.9%) | 339 | (45.0%) | 300 | (35.4%) | <0.001 |
| Diabetes | 348 | (21.7%) | 198 | (26.3%) | 150 | (17.7%) | <0.001 |
| Hypertension | 369 | (34.7%) | 185 | (35.7%) | 184 | (33.7%) | 0.490 |
| ESRD e | 33 | (2.1%) | 23 | (3.1%) | 10 | (1.2%) | 0.008 |
| HBV infection | 878 | (62.7%) | 378 | (56.2%) | 500 | (68.7%) | <0.001 |
| HCV infection | 471 | (36.0%) | 223 | (35.7%) | 248 | (36.3%) | 0.829 |
| Non-B Non-C | 207 | (13.6%) | 134 | (18.6%) | 73 | (9.1%) | <0.001 |
| Cigarette smoking | 339 | (21.0%) | 169 | (22.2%) | 170 | (19.9%) | 0.252 |
| Alcohol consumption | 201 | (12.4%) | 100 | (13.1%) | 101 | (11.8%) | 0.419 |
| Pre-OP symptoms f | 361 | (22.3%) | 164 | (21.6%) | 197 | (23.0%) | 0.473 |
| ICG-15 g (%) | 516 | (34.0%) | 238 | (33.5%) | 278 | (34.5%) | 0.664 |
| Major procedure h | 444 | (28.3%) | 184 | (24.9%) | 260 | (31.3%) | 0.005 |
| Blood loss >800 mL | 188 | (12.3%) | 75 | (10.4%) | 113 | (14.0%) | 0.032 |
| OP duration >270 min | 672 | (43.1%) | 320 | (43.5%) | 352 | (42.7%) | 0.729 |
| Major complication i | 153 | (9.9%) | 66 | (9.0%) | 87 | (10.7%) | 0.271 |
| In-hospital mortality | 26 | (1.6%) | 9 | (1.2%) | 17 | (2.0%) | 0.202 |
| 6-month mortality | 87 | (5.4%) | 21 | (2.8%) | 66 | (7.7%) | <0.001 |
| Mean | SEM j | Mean | SEM i | Mean | SEM i | P-value | |
| ICG-15 (%) g | 9.542 | 0.231 | 9.136 | 0.309 | 9.887 | 0.342 | 0.103 |
| Hemoglobin (g/dL) | 13.492 | 0.048 | 13.461 | 0.070 | 13.525 | 0.068 | 0.515 |
| Albumin (g/dL) | 4.112 | 0.012 | 4.136 | 0.018 | 4.093 | 0.016 | 0.083 |
| Platelet (1000/uL) | 179.268 | 1.826 | 179.199 | 2.569 | 178.779 | 2.617 | 0.909 |
| ALT (U/L) | 55.23 | 1.775 | 53.92 | 3.146 | 56.24 | 1.888 | 0.517 |
| Bilirubin total (mg/dL) | 0.764 | 0.013 | 0.751 | 0.022 | 0.775 | 0.014 | 0.341 |
| Alkaline phosphatase (U/L) | 93.59 | 2.378 | 94.76 | 4.609 | 92.64 | 1.894 | 0.661 |
| α-fetoprotein (ng/mL) k | 14.40 | 222.60 | 4.60 | 4.60 | 227.20 | 1000.95 | <0.001 |
| Variables c | Total | N-HCC a | A-HCC b | P-Value d | ||||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | (%) | |||
| Tumor size (cm) | >5 | 505 | (32.3%) | 201 | (27.3%) | 304 | (36.7%) | <0.001 |
| Encapsulation | Yes | 1282 | (82.2%) | 605 | (82.7%) | 677 | (81.9%) | 0.685 |
| Capsular invasion | Yes | 965 | (62.0%) | 408 | (55.9%) | 557 | (67.4%) | <0.001 |
| Rupture | Yes | 120 | (7.7%) | 47 | (6.4%) | 73 | (8.8%) | 0.074 |
| Vascular invasion | Yes | 527 | (33.8%) | 178 | (24.3%) | 349 | (42.3%) | <0.001 |
| Daughter nodule | Yes | 332 | (21.3%) | 109 | (14.9%) | 223 | (27.0%) | <0.001 |
| Cirrhosis | Yes | 787 | (50.4%) | 338 | (46.2%) | 449 | (54.2%) | 0.002 |
| Necrosis | Yes | 754 | (48.5%) | 331 | (45.4%) | 423 | (51.3%) | 0.021 |
| Edmondson-Steiner grading system | Grade 1/2 | 944 | (61.8%) | 527 | (74.3%) | 417 | (50.9%) | <0.001 |
| Grade 3/4 | 584 | (38.2%) | 182 | (25.7%) | 402 | (49.1%) | ||
| T stage | T1 | 852 | (58.1%) | 456 | (66.4%) | 396 | (50.8%) | <0.001 |
| T2 | 345 | (23.5%) | 152 | (22.1%) | 193 | (24.7%) | ||
| T3a | 133 | (9.1%) | 38 | (5.5%) | 95 | (12.2%) | ||
| T3b | 50 | (3.4%) | 6 | (0.9%) | 44 | (5.6%) | ||
| T4 | 87 | (5.9%) | 35 | (5.1%) | 52 | (6.7%) | ||
| N stage | N1 | 12 | (0.8%) | 4 | (0.6%) | 8 | (1.0%) | 0.343 |
| Variables c | Univariate | Multivariate | ||
|---|---|---|---|---|
| Median DFS a ± SE (months) | P-Value | Hazard Ratio (95% CI) | P-Value | |
| Age (>65 vs. ≤65 (year-old)) | 39.4 ± 4.6 vs. 49.2 ± 8.6 | 0.131 | ||
| Gender (male vs. female) | 41.9 ± 5.8 vs. 70.6 ± 15.7 | 0.115 | ||
| Diabetes mellitus (yes vs. no) | 45.6 ± 12.0 vs. 44.6 ± 6.3 | 0.428 | ||
| Hypertension (yes vs. no) | 41.7 ± 11.5 vs. 41.6 ± 5.0 | 0.523 | ||
| ESRD d (yes vs. no) | 76.0 ± 29.0 vs. 44.6 ± 5.4 | 0.873 | ||
| HBV surface antigen (positive vs. negative) | 47.9 ± 7.4 vs. 41.0 ± 7.1 | 0.310 | ||
| Hepatitis C virus (positive vs. negative) | 32.9 ± 5.2 vs. 46.2 ± 8.0 | 0.182 | ||
| Cigarette smoking (yes vs. no) | 37.6 ± 5.6 vs. 47.0 ± 7.0 | 0.197 | ||
| Alcohol consumption (yes vs. no) | 41.9 ± 11.8 vs. 45.6 ± 6.3 | 0.526 | ||
| Pre-OP symptoms e (yes vs. no) | 31.8 ± 5.4 vs. 60.0 ± 7.5 | 0.004 | 1.278 (0.986–1.657) | 0.064 |
| ICG-15 f (>10 vs. ≤10 (%)) | 32.7 ± 4.8 vs. 57.2 ± 7.8 | 0.002 | 1.520 (1.204–1.919) | <0.001 |
| Procedure type (Major g vs. Minor) | 28.2 ± 7.0 vs. 61.4 ± 8.0 | <0.001 | 1.017 (0.751–1.377) | 0.913 |
| Blood loss (>800 vs. ≤800 (mL)) | 30.7 ± 11.0 vs. 47.0 ± 6.3 | 0.034 | 1.095 (0.750–1.597) | 0.639 |
| OP duration (>270 vs. ≤270 (mins)) | 36.7 ± 4.2 vs. 70.6 ± 9.1 | <0.001 | 1.238 (0.964–1.591) | 0.094 |
| Complication (Major h vs. Minor/none) | 27.7 ± 10.7 vs. 46.2± 6.2 | 0.044 | 1.031 (0.699–1.520) | 0.879 |
| Albumin (≤3.5 vs. >3.5 (g/dL)) | 40.1 ± 5.6 vs. 47.0 ± 6.3 | 0.175 | ||
| Tumor size (>5 vs. ≤5 (cm)) | 26.9 ± 4.6 vs. 67.9 ± 8.7 | <0.001 | 1.823 (1.366–2.433) | <0.001 |
| Capsule (yes vs. no) | 47.0 ± 6.2 vs. 32.9 ± 5.9 | 0.147 | ||
| Capsular invasion (yes vs. no) | 41.9 ± 7.7 vs. 49.2 ± 9.2 | 0.264 | ||
| Rupture (yea vs. no) | 32.0 ± 11.9 vs. 47.9 ± 6.7 | 0.010 | 1.098 (0.709–1.701) | 0.676 |
| Vascular invasion (yes vs. no) | 22.0 ± 4.2 vs. 63.5 ± 8.9 | <0.001 | 1.460 (1.116–1.910) | 0.006 |
| Daughter nodule (yes vs. no) | 22.2 ± 2.6 vs. 61.4 ± 7.5 | <0.001 | 1.565 (1.182–2.072) | 0.002 |
| Cirrhosis (yes vs. no) | 40.1 ± 3.8 vs. 62.3 ± 9.6 | 0.040 | 1.272 (1.008–1.605) | 0.043 |
| Necrosis (yes vs. no) | 40.6 ± 3.8 vs. 63.5 ± 8.8 | 0.061 | ||
| Edmondson–Steiner grading system(grade 3/4 vs. grade 1/2) | 37.4 ± 7.6 vs. 47.0 ± 6.2 | 0.101 | ||
| Variables c | Univariate | Multivariate | ||
|---|---|---|---|---|
| Mean OS a ± SE (Months) | P-Value | Hazard Ratio (95% CI) | P-Value | |
| Age (>65 vs. ≤65 (year-old)) | 90.7 ± 3.2 vs. 96.6 ± 2.1 | 0.084 | ||
| Gender (male vs. female) | 92.7 ± 2.0 vs. 102.8 ± 3.7 | 0.037 | 1.153 (0.838–1.586) | 0.382 |
| Diabetes mellitus (yes vs. no) | 88.0 ± 3.8 vs. 96.4 ± 2.0 | 0.223 | ||
| Hypertension (yes vs. no) | 98.8 ± 3.8 vs. 94.1 ± 2.9 | 0.841 | ||
| ESRD d (yes vs. no) | 70.8 ± 4.2 vs. 95.1 ± 1.8 | 0.984 | ||
| HBV surface antigen (positive vs. negative) | 94.1 ± 2.4 vs. 98.2 ± 2.9 | 0.501 | ||
| Hepatitis C virus (positive vs. negative) | 96.9 ± 3.4 vs. 91.7 ± 2.5 | 0.189 | ||
| Cigarette smoking (yes vs. no) | 88.8 ± 4.3 vs. 96.2 ± 1.9 | 0.032 | 1.075 (0.819–1.411) | 0.604 |
| Alcohol consumption (yes vs. no) | 95.2 ± 4.6 vs. 94.6 ± 1.9 | 0.986 | ||
| Pre-OP symptoms e (yes vs. no) | 91.8 ± 3.8 vs. 95.4 ± 2.0 | 0.129 | ||
| ICG-15 f (>10 vs. ≤10 (%)) | 89.6 ± 3.5 vs. 95.4 ± 2.1 | 0.120 | ||
| Procedure type (Major g vs. Minor) | 80.1 ± 3.6 vs. 98.7 ± 1.9 | <0.001 | 1.023 (0.760–1.376) | 0.881 |
| Blood loss (>800 vs. ≤800 (mL)) | 71.4 ± 6.0 vs. 97.0 ± 1.8 | <0.001 | 1.131 (0.779–1.643) | 0.516 |
| Complication (Major h vs. Minor/none) | 66.3 ± 5.6 vs. 97.2 ± 1.8 | <0.001 | 1.118 (0.762–1.639) | 0.569 |
| Albumin (≤3.5 vs. >3.5 (g/dL)) | 79.6 ± 5.2 vs. 95.3 ± 1.9 | 0.064 | ||
| Tumor size (>5 vs. ≤5 (cm)) | 82.4 ± 3.7 vs. 99.1 ± 1.9 | <0.001 | 1.839 (1.375–2.461) | <0.001 |
| Capsule (yes vs. no) | 94.8 ± 1.9 vs. 93.9 ± 4.4 | 0.709 | ||
| Capsular invasion (yes vs. no) | 92.9 ± 2.4 vs. 96.2 ± 2.7 | 0.287 | ||
| Rupture (yea vs. no) | 85.7 ± 5.9 vs. 94.9 ± 1.8 | 0.585 | ||
| Vascular invasion (yes vs. no) | 78.8 ± 4.2 vs. 99.5 ± 1.9 | <0.001 | 1.549 (1.190–2.015) | 0.001 |
| Daughter nodule (yes vs. no) | 75.9 ± 4.7 vs. 98.0 ± 1.9 | <0.001 | 1.786 (1.359–2.348) | <0.001 |
| Cirrhosis (yes vs. no) | 90.3 ± 2.7 vs. 98.1 ± 2.3 | 0.046 | 1.438 (1.145–1.805) | 0.002 |
| Necrosis (yes vs. no) | 90.9 ± 2.6 vs. 97.9 ± 2.4 | 0.049 | 1.132 (0.889–1.442) | 0.315 |
| Edmondson–Steiner grading system (grade 3/4 vs. grade 1/2) | 90.1 ± 3.7 vs. 96.0 ± 2.0 | 0.068 | ||
| Variables | N-HCC a vs. A-HCC b | N-HCC a vs. Healthy Subject | A-HCC b vs. Healthy Subject | |||
|---|---|---|---|---|---|---|
| Mean ± SEM | P-Value h | Mean ± SEM | P-Value h | Mean ± SEM | P-Value h | |
| GPC3 c (ng/mL) | 5.1± 1.2 vs. 3.4 ± 0.9 | 0.265 | 5.1± 1.2 vs. < 0.01 | <0.001 | 3.4± 0.9 vs. < 0.01 | 0.001 |
| SPP1 d (ng/mL) | 52.2 ± 8.3 vs. 58.2 ± 6.5 | 0.570 | 52.2 ± 8.3 vs. 13.0 ± 1.6 | <0.001 | 58.2 ± 6.5 vs. 13.0 ± 1.6 | <0.001 |
| IGF-1 e (ng/mL) | 104 ± 11 vs. 92 ± 8 | 0.380 | 104 ± 11 vs. 195 ± 18 | 0.001 | 91.7± 7.8 vs. 195 ± 18 | <0.001 |
| HGF f (pg/mL) | 952 ± 123 vs. 1043 ± 120 | 0.596 | 952 ± 123 vs. 909 ± 38 | 0.741 | 1043 ± 120 vs. 909 ± 38 | 0.292 |
| VEGF g (pg/mL) | 137 ± 12 vs. 145 ± 13 | 0.623 | 137 ± 12 vs. 179 ± 16 | 0.164 | 145 ± 12 vs. 179 ± 16 | 0.300 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-W.; Tsai, H.-I.; Lee, W.-C.; Huang, S.-W.; Lin, C.-Y.; Hsieh, Y.-C.; Kuo, T.; Chen, C.-W.; Yu, M.-C. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? J. Clin. Med. 2019, 8, 1736. https://doi.org/10.3390/jcm8101736
Lee C-W, Tsai H-I, Lee W-C, Huang S-W, Lin C-Y, Hsieh Y-C, Kuo T, Chen C-W, Yu M-C. Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? Journal of Clinical Medicine. 2019; 8(10):1736. https://doi.org/10.3390/jcm8101736
Chicago/Turabian StyleLee, Chao-Wei, Hsin-I Tsai, Wei-Chen Lee, Shu-Wei Huang, Cheng-Yu Lin, Yi-Chung Hsieh, Tony Kuo, Chun-Wei Chen, and Ming-Chin Yu. 2019. "Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal?" Journal of Clinical Medicine 8, no. 10: 1736. https://doi.org/10.3390/jcm8101736
APA StyleLee, C.-W., Tsai, H.-I., Lee, W.-C., Huang, S.-W., Lin, C.-Y., Hsieh, Y.-C., Kuo, T., Chen, C.-W., & Yu, M.-C. (2019). Normal Alpha-Fetoprotein Hepatocellular Carcinoma: Are They Really Normal? Journal of Clinical Medicine, 8(10), 1736. https://doi.org/10.3390/jcm8101736






