Involvement of Iliofemoral Arteries in PET/CT Is Associated with Atherosclerotic Risk Factors in Takayasu’s Arteritis
Abstract
1. Introduction
2. Materials and Methods
2.1. FDG-PET/CT Imaging Technique
2.2. Image Interpretation
2.3. Statistical Analysis
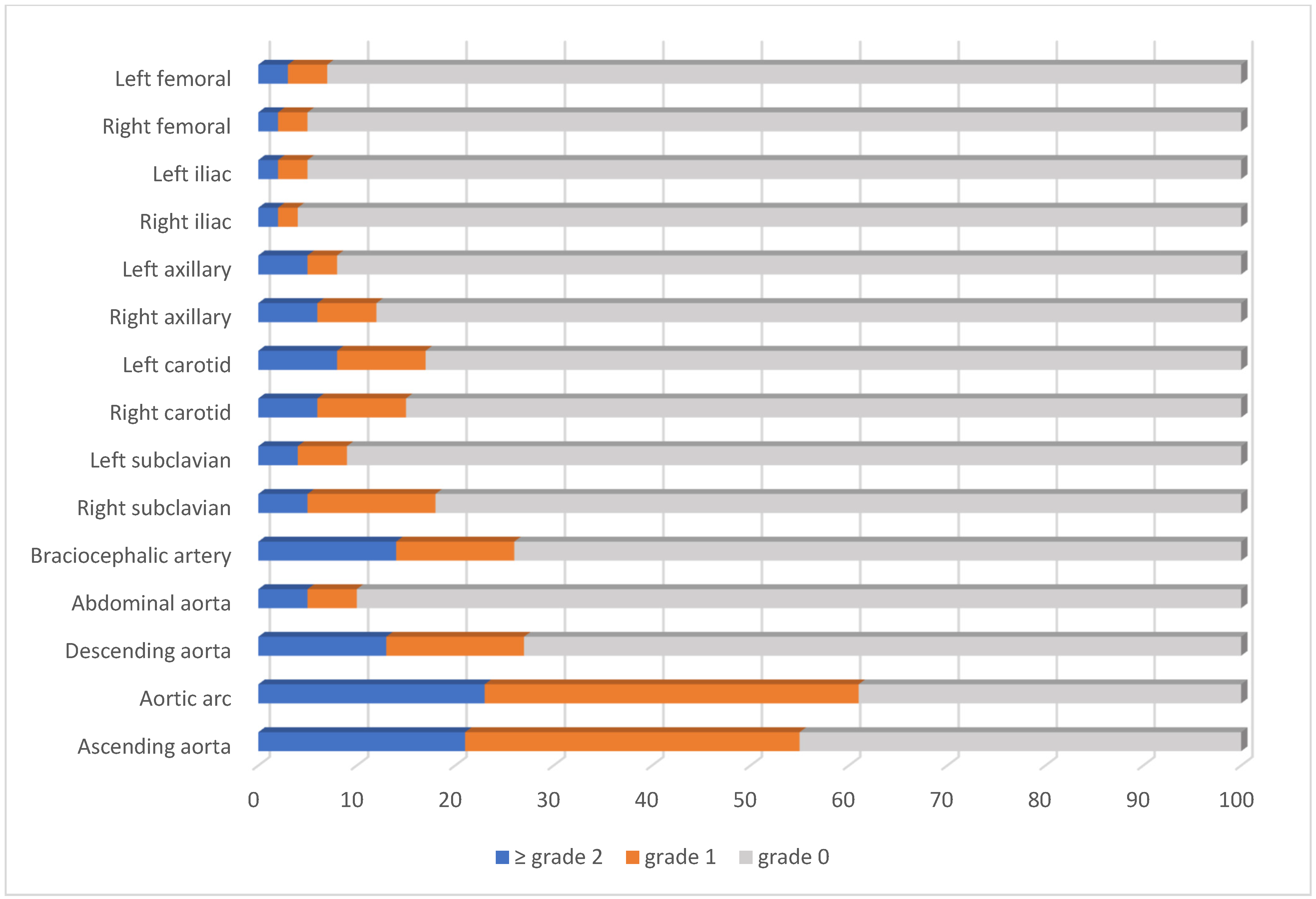
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TAK | Takayasu’s arteritis |
| PETVAS | PET vascular activity score |
| FDG-PET/CT | 18F-fluorodeoxyglucose positron emission tomography/computed tomography |
| ACR | American College of Rheumatology |
| PGA | Physician’s Global Assessment |
| IS | Immunosuppressive |
| GC | Glucocorticoid |
| EULAR | European League Against Rheumatism |
| EANM | The European Association of Nuclear Medicine |
| SNMMI | The Society of Nuclear Medicine and Molecular Imaging |
| PIG | PET Interest Group |
| ASNC | American Society of Nuclear Cardiology |
| VA | Visual analysis |
| OR | Odds ratio |
| CI | Confidence interval |
Appendix A
| Variables | OR (95% CI) | p |
|---|---|---|
| Age, years | 0.97 (0.88–1.07) | 0.58 |
| Gender, male | 2.33 (0.21–25.66) | 0.33 |
| Symptom duration, months | 0.99 (0.98–1.01) | 0.88 |
| BMI, kg/m2 | 1.07 (0.82–1.40) | 0.59 |
| Angiographic type 5 disease | 0.68 (0.09–5.25) | 0.71 |
| Cluster 1 | 1.30 (0.12–13.77) | 0.82 |
| Diabetes | 9.00 (0.62–129.59) | 0.11 |
| Hypertension | 0.47 (0.04–4.89) | 0.53 |
| Hyperlipidemia | 0.64 (0.06–6.67) | 0.71 |
| Smoking | 4.50 (0.43–46.09) | 0.20 |
| Family history for atherosclerosis | 0.37 (0.03–3.85) | 0.41 |
| Cerebrovascular event | 2.66 (0.23–29.90) | 0.42 |
| Coronary artery disease | Not estimable | 0.99 |
| Chronic kidney disease | Not estimable | 0.99 |
| Takayasu arteritis disease activity | 0.41 (0.04–4.22) | 0.45 |
| ESR, mm/h | 1.007 (0.97–1.05) | 0.70 |
| CRP, mg/L | 1.007 (0.97–1.04) | 0.70 |
| Presence of GC treatment | 1.24 (0.16–9.43) | 0.83 |
| Variables | OR (95% CI) | p |
|---|---|---|
| Age, years | 1.10 (0.97–1.24) | 0.11 |
| Gender, male | 7.33 (0.40–133.28) | 0.17 |
| Symptom duration, months | 0.98 (0.96–1.01) | 0.40 |
| BMI, kg/m2 | 1.36 (0.63–2.91) | 0.43 |
| Angiographic type 5 disease | 0.75 (0.04–12.98) | 0.84 |
| Cluster 1 | Not estimable | 0.99 |
| Diabetes | Not estimable | 0.99 |
| Hypertension | Not estimable | 0.99 |
| Hyperlipidemia | 2.70 (0.15–47.39) | 0.49 |
| Family history for atherosclerosis | Not estimable | 0.99 |
| Cerebrovascular event | 7.75 (0.40–149.70) | 0.17 |
| Coronary artery disease | Not estimable | |
| Chronic kidney disease | 16.5 (0.73–372.80) | 0.07 |
| Takayasu arteritis disease activity | Not estimable | |
| ESR, mm/h | 1.06 (0.96–1.18) | 0.21 |
| CRP, mg/L | 1.02 (0.99–1.05) | 0.14 |
| Presence of GC treatment | Not estimable |
| Variables | OR (95% CI) | p |
|---|---|---|
| Age, years | 1.03 (0.96–1.10) | 0.32 |
| Gender, male | 2.12 (0.36–12.49) | 0.40 |
| Symptom duration, months | 1.01 (0.99–1.12) | 0.30 |
| BMI, kg/m2 | 0.94 (0.77–1.16) | 0.60 |
| Angiographic type 5 disease | 0.96 (0.18–5.17) | 0.97 |
| Cluster 1 | 1.89 (0.32–11.03) | 0.47 |
| Diabetes | 4.64 (0.71–30.32) | 0.10 |
| Hypertension | 0.53 (0.09–2.96) | 0.47 |
| Hyperlipidemia | 1.50 (030–7.32) | 0.61 |
| Smoking | 7.61 (0.86–67.26) | 0.06 |
| Family history for atherosclerosis | 0.27 (0.03–2.50) | 0.25 |
| Cerebrovascular event | 1.33 (0.13–12.75) | 0.80 |
| Coronary artery disease | Not estimable | |
| Takayasu arteritis disease activity | 1.66 (0.34–8.07) | 0.52 |
| ESR, mm/h | 1.01 (0.98–1.03) | 0.37 |
| CRP, mg/L | 1.01 (0.99–1.04) | 0.13 |
| Presence of GC treatment | 0.52 (0.09–2.91) | 0.46 |
References
- Kerr, G.S.; Hallahan, C.W.; Giordano, J.; Leavitt, R.Y.; Fauci, A.S.; Rottem, M.; Hoffman, G.S. Takayasu arteritis. Ann. Intern. Med. 1994, 120, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Alibaz-Oner, F.; Koster, M.J.; Unal, A.U.; Yildirim, H.G.; Cikikci, C.; Schmidt, J.; Crowson, C.S.; Makol, A.; Ytterberg, S.R.; Matteson, E.L.; et al. Assessment of the frequency of cardiovascular risk factors in patients with Takayasu’s arteritis. Rheumatology 2017, 56, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Ucar, A.K.; Ozdede, A.; Kayadibi, Y.; Adaletli, I.; Melikoglu, M.; Fresko, I.; Seyahi, E. Increased arterial stiffness and accelerated atherosclerosis in Takayasu arteritis. Semin. Arthritis Rheum. 2023, 60, 152199. [Google Scholar] [CrossRef]
- Du, J.; Ren, Y.; Liu, J.; Li, T.; Zhang, Y.; Yang, S.; Kang, T.; Ning, S.; Chen, L.; Guo, X.; et al. Association of Prolonged Disease Duration and TG/HDL-C Ratio in Accelerating Atherosclerosis in Patients with Takayasu’s Arteritis. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221121297. [Google Scholar] [CrossRef] [PubMed]
- Liddy, S.; Mallia, A.; Collins, C.D.; Killeen, R.P.; Skehan, S.; Dodd, J.D.; Subesinghe, M.; Murphy, D.J. Vascular findings on FDG PET/CT. Br. J. Radiol. 2020, 93, 20200103. [Google Scholar] [CrossRef]
- Allam, M.N.; Baba Ali, N.; Mahmoud, A.K.; Scalia, I.G.; Farina, J.M.; Abbas, M.T.; Pereyra, M.; Kamel, M.A.; Awad, K.A.; Wang, Y.; et al. Multi-Modality Imaging in Vasculitis. Diagnostics 2024, 14, 838. [Google Scholar] [CrossRef]
- Pan, Y.; Jing, J.; Cai, X.; Jin, Z.; Wang, S.; Wang, Y.; Zeng, C.; Meng, X.; Ji, J.; Li, L.; et al. Prevalence and Vascular Distribution of Multiterritorial Atherosclerosis Among Community-Dwelling Adults in Southeast China. JAMA Netw. Open 2022, 5, e2218307. [Google Scholar] [CrossRef]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Slart, R.; Writing Group; Reviewer Group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees; SNMMI Cardiovascular; Members of Council; PET Interest Group; Members of ASNC; et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef]
- Grayson, P.C.; Alehashemi, S.; Bagheri, A.A.; Civelek, A.C.; Cupps, T.R.; Kaplan, M.J.; Malayeri, A.A.; Merkel, P.A.; Novakovich, E.; Bluemke, D.A.; et al. F-Fluorodeoxyglucose-Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018, 70, 439–449. [Google Scholar] [CrossRef]
- Hata, A.; Noda, M.; Moriwaki, R.; Numano, F. Angiographic findings of Takayasu arteritis: New classification. Int. J. Cardiol. 1996, 54 (Suppl. 2), S155–S163. [Google Scholar] [CrossRef]
- Goel, R.; Gribbons, K.B.; Carette, S.; Cuthbertson, D.; Hoffman, G.S.; Joseph, G.; Khalidi, N.A.; Koening, C.L.; Kumar, S.; Langford, C.; et al. Derivation of an angiographically based classification system in Takayasu’s arteritis: An observational study from India and North America. Rheumatology 2020, 59, 1118–1127. [Google Scholar] [CrossRef]
- Alibaz-Oner, F.; Yurdakul, S.; Aytekin, S.; Direskeneli, H. Impaired endothelial function in patients with Takayasu’s arteritis. Acta Cardiol. 2014, 69, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Miyata, T.; Tanemoto, K. Current Clinical Features of New Patients With Takayasu Arteritis Observed From Cross-Country Research in Japan: Age and Sex Specificity. Circulation 2015, 132, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Tomelleri, A.; Campochiaro, C.; Sartorelli, S.; Cavalli, G.; De Luca, G.; Baldissera, E.; Dagna, L. Gender differences in clinical presentation and vascular pattern in patients with Takayasu arteritis. Scand. J. Rheumatol. 2019, 48, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Seyahi, E.; Ugurlu, S.; Cumali, R.; Balci, H.; Seyahi, N.; Yurdakul, S.; Yazici, H. Atherosclerosis in Takayasu arteritis. Ann. Rheum. Dis. 2006, 65, 1202–1207. [Google Scholar] [CrossRef]
- Comarmond, C.; Biard, L.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y.; Chiche, L.; Koskas, F.; Cluzel, P.; et al. Long-Term Outcomes and Prognostic Factors of Complications in Takayasu Arteritis: A Multicenter Study of 318 Patients. Circulation 2017, 136, 1114–1122. [Google Scholar] [CrossRef]
- Gordon, P.; Flanagan, P. Smoking: A risk factor for vascular disease. J. Vasc. Nurs. 2016, 34, 79–86. [Google Scholar] [CrossRef]
- Mirouse, A.; Biard, L.; Comarmond, C.; Lambert, M.; Mekinian, A.; Ferfar, Y.; Kahn, J.E.; Benhamou, Y.; Chiche, L.; Koskas, F.; et al. Overall survival and mortality risk factors in Takayasu’s arteritis: A multicenter study of 318 patients. J. Autoimmun. 2019, 96, 35–39. [Google Scholar] [CrossRef]
- Venugopal, S.K.; Devaraj, S.; Jialal, I. Effect of C-reactive protein on vascular cells: Evidence for a proinflammatory, proatherogenic role. Curr. Opin. Nephrol. Hypertens. 2005, 14, 33–37. [Google Scholar] [CrossRef]
- Matusik, P.S.; Matusik, P.T.; Stein, P.K. Cardiovascular reflex tests in patients with systemic lupus erythematosus: Clinical performance and utility. Lupus 2018, 27, 1759–1768. [Google Scholar] [CrossRef]
| Patients with Iliofemoral Involvement (n = 9) | Patients Without Iliofemoral Involvement (n = 68) | p | |
|---|---|---|---|
| Age, years, mean ± SD | 52.5 ± 17.4 | 41.3 ± 12.1 | 0.098 |
| Gender, male, n (%) | 4 (44) | 4 (6) | 0.015 * |
| Symptom duration, months | 64 (48–456) | 92 (1–340) | 0.98 |
| BMI, kg/m2, mean ± SD | 23.3 ± 5.6 | 25.4 ± 4.1 | 0.39 |
| Angiographic type 5 disease, n (%) | 4 (44) | 35 (51) | 0.87 |
| Cluster 1, n (%) | 2 (22) | 13 (19) | 0.85 |
| Cluster 2, n (%) | 3 (33) | 31 (46) | 0.44 |
| Diabetes, n (%) | 1 (11) | 6 (9) | 0.82 |
| Hypertension, n (%) | 4 (44) | 31 (46) | 0.94 |
| Hyperlipidemia, n (%) | 5 (56) | 25 (37) | 0.27 |
| Smoking, n (%) | 7 (77) | 27 (40) | 0.045 * |
| Obesity, BMI ≥ 30, n (%) | 0 (0) | 8 (12) | 0.27 |
| Family history for atherosclerosis, n (%) | 2 (22) | 26 (38) | 0.32 |
| Cerebrovascular event, n (%) | 1 (11) | 8 (12) | 0.26 |
| Coronary artery disease, n (%) | 1 (11) | 7 (10) | 0.96 |
| Chronic kidney disease, n (%) | 2 (22) | 3 (4) | 0.046 |
| Images with Iliofemoral Involvement (n = 9) | Images Without Iliofemoral Involvement (n = 91) | p | |
|---|---|---|---|
| Treatment | |||
| GC present, n (%) | 2 (22) | 44 (48) | 0.11 |
| GC dose *, mg/d | 0 (0–7.5) | 2.5 (0–80) | 0.15 |
| Methotrexate, n (%) | 1 (11) | 11 (12) | 0.87 |
| Azathioprine, n (%) | 2 (22) | 28 (31) | 0.17 |
| Leflunomide, n (%) | 3 (33) | 15 (17) | 0.25 |
| Mycophenolate mofetil, n (%) | 0 (0) | 3 (3) | 0.56 |
| TNF inhibitors, n (%) | 1 (11) | 10 (11) | 0.95 |
| Tocilizumab, n (%) | 0 (0) | 1 (1) | 0.74 |
| TAK disease activity ** | |||
| Active disease, n (%) | 6 (67) | 38 (42) | 0.15 |
| Inactive disease, n (%) | 3 (33) | 53 (58) | |
| Laboratory | |||
| ESR, mm/h, mean ± SD | 32 (7–120) | 35 (4–113) | 0.71 |
| CRP, mg/l, median (min-max) | 38.5 (3.1–77.4) | 10.7 (1.5–126) | 0.026 † |
| Imaging | |||
| PETVAS, median (min-max) | 7.5 (0–26) | 2 (0–17) | 0.05 |
| Active PET *** | 6 (67) | 25 (27) | 0.015 † |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | O.R. (95% CI) | p | O.R. (95% CI) | p |
| Age, years | 1.07 (1.01–1.14) | 0.031 * | 1.07 (1.002–1.15) | 0.044 * |
| Gender, male | 5.80 (1.24–27.05) | 0.025 * | 6.68 (1.10–40.64) | 0.039 * |
| Symptom duration, months | 1.00 (0.99–1.01) | 0.64 | - | |
| BMI, kg/m2 | 0.89 (0.73–1.07) | 0.22 | - | |
| Angiographic type 5 disease | 0.88 (0.20–3.84) | 0.87 | - | |
| Cluster 1 | 1.16 (0.21–6.27) | 0.86 | - | |
| Cluster 2 | 0.56 (0.13–2.45) | 0.45 | - | |
| Diabetes mellitus | 2.95 (0.49–17.52) | 0.82 | - | |
| Hypertension | 0.95 (0.23–3.86) | 0.95 | - | |
| Hyperlipidemia | 2.15 (0.52–8.75) | 0.29 | - | |
| Smoking | 4.79 (0.92–24.92) | 0.062 | - | |
| Family history for atherosclerosis | 0.43 (0.08–2.33) | 0.33 | - | |
| Cerebrovascular event | 0.91 (0.10–8.23) | 0.93 | - | |
| Coronary artery disease | 1.05 (0.11–9.71) | 0.96 | - | |
| Chronic kidney disease | 6.00 (0.85–42.26) | 0.072 | - | |
| Takayasu arteritis disease activity | 2.39 (0.55–10.33) | 0.25 | - | |
| ESR, mm/h | 1.01 (0.99–1.04) | 0.35 | - | |
| CRP, mg/L | 1.02 (1.00–1.04) | 0.055 | - | |
| Presence of GC treatment | 0.37 (0.07–1.95) | 0.25 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaymaz-Tahra, S.; Ozguven, S.; Avcu, A.; Filizoglu, N.; Unal, A.U.; Ones, T.; Erdil, T.Y.; Alibaz-Oner, F.; Direskeneli, H. Involvement of Iliofemoral Arteries in PET/CT Is Associated with Atherosclerotic Risk Factors in Takayasu’s Arteritis. J. Clin. Med. 2025, 14, 8607. https://doi.org/10.3390/jcm14238607
Kaymaz-Tahra S, Ozguven S, Avcu A, Filizoglu N, Unal AU, Ones T, Erdil TY, Alibaz-Oner F, Direskeneli H. Involvement of Iliofemoral Arteries in PET/CT Is Associated with Atherosclerotic Risk Factors in Takayasu’s Arteritis. Journal of Clinical Medicine. 2025; 14(23):8607. https://doi.org/10.3390/jcm14238607
Chicago/Turabian StyleKaymaz-Tahra, Sema, Salih Ozguven, Aysegul Avcu, Nuh Filizoglu, Ali Ugur Unal, Tunc Ones, Tanju Yusuf Erdil, Fatma Alibaz-Oner, and Haner Direskeneli. 2025. "Involvement of Iliofemoral Arteries in PET/CT Is Associated with Atherosclerotic Risk Factors in Takayasu’s Arteritis" Journal of Clinical Medicine 14, no. 23: 8607. https://doi.org/10.3390/jcm14238607
APA StyleKaymaz-Tahra, S., Ozguven, S., Avcu, A., Filizoglu, N., Unal, A. U., Ones, T., Erdil, T. Y., Alibaz-Oner, F., & Direskeneli, H. (2025). Involvement of Iliofemoral Arteries in PET/CT Is Associated with Atherosclerotic Risk Factors in Takayasu’s Arteritis. Journal of Clinical Medicine, 14(23), 8607. https://doi.org/10.3390/jcm14238607





