Behavioral Changes in Caenorhabditis elegans After Exposure to Radial Extracorporeal Shock Waves
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematodes
2.2. Liquid Cultures
2.3. Assays
2.4. Application of rESWs
2.5. Transfer and Video Capture
2.6. Locomotion Data Collection, Processing and Analysis
2.7. Statistical Analysis
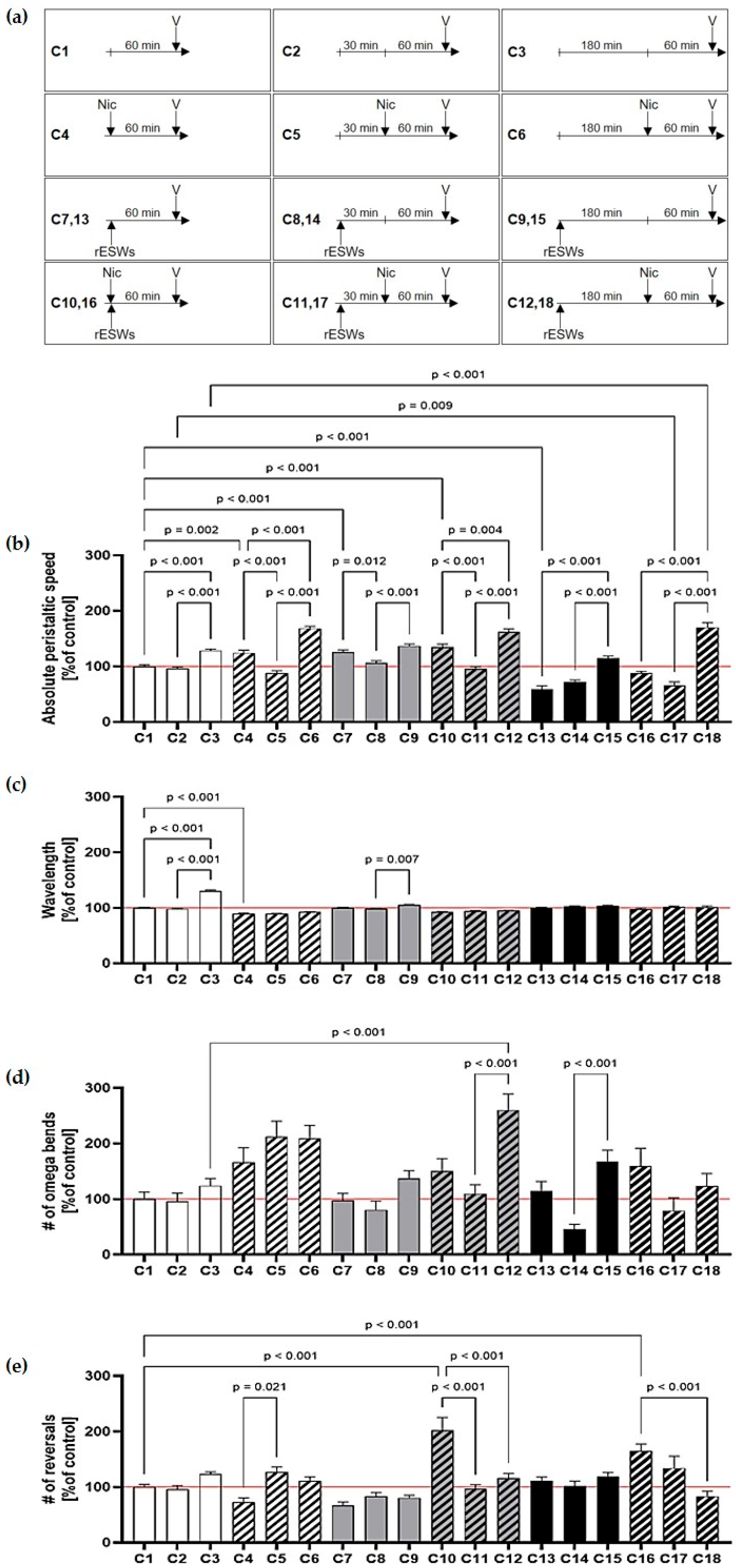
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | acetylcholine |
| AChR | acetylcholine receptor |
| CCh | carbachol |
| C. elegans | Caenorhabditis elegans |
| CNS | central nervous system |
| CP | cerebral palsy |
| E. coli | Escherichia coli |
| mAChR | muscarinic acetylcholine receptor |
| nAChR | nicotinic acetylcholine receptor |
| Nic | nicotine |
| NGM | nematode growth medium |
| NMJ | neuromuscular junction |
| PNS | peripheral nervous system |
| rESW | radial extracorporeal shock wave |
| rESWT | radial extracorporeal shock wave therapy |
| SEM | standard error of the mean |
References
- Patel, D.R.; Bovid, K.M.; Rausch, R.; Ergun-Longmire, B.; Goetting, M.; Merrick, J. Cerebral palsy in children: A clinical practice review. Curr. Probl. Pediatr. Adolesc. Health Care 2024, 54, 101673. [Google Scholar] [CrossRef]
- Vidal, X.; Martí-Fàbregas, J.; Canet, O.; Roqué, M.; Morral, A.; Tur, M.; Schmitz, C.; Sitjà-Rabert, M. Efficacy of radial extracorporeal shock wave therapy compared with botulinum toxin type a injection in treatment of lower extremity spasticity in subjects with cerebral palsy: A randomized, controlled, cross-over study. J. Rehabil. Med. 2020, 52, jrm00076. [Google Scholar] [CrossRef] [PubMed]
- Vidal, X.; Morral, A.; Costa, L.; Tur, M. Radial extracorporeal shock wave therapy (rESWT) in the treatment of spasticity in cerebral palsy: A randomized, placebo-controlled clinical trial. Neuro. Rehabil. 2011, 29, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, L.; Shan, L.; Dong, H.; Feng, J.; Kiessling, M.C.; Angstman, N.B.; Schmitz, C.; Jia, F. A prospective case-control study of radial extracorporeal shock wave therapy for spastic plantar flexor muscles in very young children with cerebral palsy. Medicine 2016, 95, e3649. [Google Scholar] [CrossRef] [PubMed]
- Császár, N.B.; Angstman, N.B.; Milz, S.; Sprecher, C.M.; Kobel, P.; Farhat, M.; Furia, J.P.; Schmitz, C. Radial shock wave devices generate cavitation. PLoS ONE 2015, 10, e0140541. [Google Scholar] [CrossRef]
- Guo, X.; Li, L.; Yan, Z.; Li, Y.; Peng, Z.; Yang, Y.; Zhang, Y.; Schmitz, C.; Feng, Z. Efficacy and safety of treating chronic nonspecific low back pain with radial extracorporeal shock wave therapy (rESWT), reswt combined with celecoxib and eperisone (C + E) or C + E alone: A prospective, randomized trial. J. Orthop. Surg. Res. 2021, 16, 705. [Google Scholar] [CrossRef]
- Kenmoku, T.; Iwakura, N.; Ochiai, N.; Saisu, T.; Ohtori, S.; Takahashi, K.; Nakazawa, T.; Fukuda, M.; Takaso, M. Influence of different energy patterns on efficacy of radial shock wave therapy. J. Orthop. Sci. 2021, 26, 698–703. [Google Scholar] [CrossRef]
- Kenmoku, T.; Nemoto, N.; Iwakura, N.; Ochiai, N.; Uchida, K.; Saisu, T.; Ohtori, S.; Nakagawa, K.; Sasho, T.; Takaso, M. Extracorporeal shock wave treatment can selectively destroy end plates in neuromuscular junctions. Muscle Nerve 2018, 57, 466–472. [Google Scholar] [CrossRef]
- Kenmoku, T.; Ochiai, N.; Ohtori, S.; Saisu, T.; Sasho, T.; Nakagawa, K.; Iwakura, N.; Miyagi, M.; Ishikawa, T.; Tatsuoka, H.; et al. Degeneration and recovery of the neuromuscular junction after application of extracorporeal shock wave therapy. J. Orthop. Res. 2012, 30, 1660–1665. [Google Scholar] [CrossRef]
- Changeux, J.P. The nicotinic acetylcholine receptor: The founding father of the pentameric ligand-gated ion channel superfamily. J. Biol. Chem. 2012, 287, 40207–40215. [Google Scholar] [CrossRef]
- Papke, R.L. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem. Pharmacol. 2014, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.A.; Tietje, K.M.; Subers, E.M.; Scherer, N.M.; Habecker, B.A.; Nathanson, N.M. Regulation of muscarinic acetylcholine receptor function in cardiac cells and in cells expressing cloned receptor genes. Trends Pharmacol. Sci. 1989. Suppl. 43–46. [Google Scholar]
- Leiber, D.; Marc, S.; Harbon, S. Pharmacological evidence for distinct muscarinic receptor subtypes coupled to the inhibition of adenylate cyclase and to the increased generation of inositol phosphates in the guinea pig myometrium. J. Pharmacol. Exp. Ther. 1990, 252, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.I.; Kitt, C.A.; Simonds, W.F.; Price, D.L.; Brann, M.R. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 1991, 11, 3218–3226. [Google Scholar] [CrossRef]
- Hogg, R.C.; Raggenbass, M.; Bertrand, D. Nicotinic acetylcholine receptors: From structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003, 147, 1–46. [Google Scholar] [CrossRef]
- Häggblad, J.; Eriksson, H.; Hedlund, B.; Heilbronn, E. Forskolin blocks carbachol-mediated ion-permeability of chick myotube nicotinic receptors and inhibits binding of 3h-phencyclidine to torpedo microsac nicotinic receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1987, 336, 381–386. [Google Scholar] [CrossRef]
- Jones, C.K.; Byun, N.; Bubser, M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 2012, 37, 16–42. [Google Scholar] [CrossRef]
- Yanuar, R.; Semba, S.; Nezu, A.; Tanimura, A. Muscarinic acetylcholine receptor-mediated phosphorylation of extracellular signal-regulated kinase in HSY salivary ductal cells involves distinct signaling pathways. J. Oral Biosci. 2024, 66, 447–455. [Google Scholar] [CrossRef]
- Towers, P.R.; Edwards, B.; Richmond, J.E.; Sattelle, D.B. The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J. Neurochem. 2005, 93, 1–9. [Google Scholar] [CrossRef]
- Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002, 3, 102–114. [Google Scholar] [CrossRef]
- Mongan, N.P.; Baylis, H.A.; Adcock, C.; Smith, G.R.; Sansom, M.S.; Sattelle, D.B. An extensive and diverse gene family of nicotinic acetylcholine receptor alpha subunits in Caenorhabditis elegans. Recept. Channels 1998, 6, 213–228. [Google Scholar] [PubMed]
- Lewis, J.A.; Wu, C.H.; Berg, H.; Levine, J.H. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics 1980, 95, 905–928. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.T.; Squire, M.D.; Barnes, T.M.; Tornoe, C.; Matsuda, K.; Ahnn, J.; Fire, A.; Sulston, J.E.; Barnard, E.A.; Sattelle, D.B.; et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J. Neurosci. 1997, 17, 5843–5857. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.E.; Jorgensen, E.M. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 1999, 2, 791–797. [Google Scholar] [CrossRef]
- Ballivet, M.; Alliod, C.; Bertrand, S.; Bertrand, D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996, 258, 261–269. [Google Scholar] [CrossRef]
- Francis, M.M.; Evans, S.P.; Jensen, M.; Madsen, D.M.; Mancuso, J.; Norman, K.R.; Maricq, A.V. The ROR receptor tyrosine kinase cam-1 is required for acr-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron 2005, 46, 581–594. [Google Scholar] [CrossRef]
- Touroutine, D.; Fox, R.M.; Von Stetina, S.E.; Burdina, A.; Miller, D.M., 3rd; Richmond, J.E. Acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005, 280, 27013–27021. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 1–340. [Google Scholar] [CrossRef]
- Emmons, S.W.; Yemini, E.; Zimmer, M. Methods for analyzing neuronal structure and activity in Caenorhabditis elegans. Genetics 2021, 218, iyab072. [Google Scholar] [CrossRef]
- Schafer, W.R. Deciphering the neural and molecular mechanisms of C. elegans behavior. Curr. Biol. 2005, 15, R723–R729. [Google Scholar] [CrossRef]
- Feng, Z.; Li, W.; Ward, A.; Piggott, B.J.; Larkspur, E.R.; Sternberg, P.W.; Xu, X.Z. A C. elegans model of nicotine-dependent behavior: Regulation by TRP-family channels. Cell 2006, 127, 621–633. [Google Scholar] [CrossRef]
- Husson, S.J.; Costa, W.S.; Schmitt, C.; Gottschalk, A. Keeping track of worm trackers. WormBook 2013, 1–17. [Google Scholar] [CrossRef]
- Roussel, N.; Sprenger, J.; Tappan, S.J.; Glaser, J.R. Robust tracking and quantification of C. elegans body shape and locomotion through coiling, entanglement, and omega bends. Worm 2014, 3, e982437. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Chang, I.; Kim, S. Development of equation of motion deciphering locomotion including omega turns of Caenorhabditis elegans. eLife 2024, 12, RP92562. [Google Scholar] [CrossRef] [PubMed]
- Avery, L.; Horvitz, H.R. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990, 253, 263–270. [Google Scholar] [CrossRef]
- Raizen, D.M.; Avery, L. Electrical activity and behavior in the pharynx of Caenorhabditis elegans. Neuron 1994, 12, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.R.; Dobbins, D.L.; Kobet, R.A.; Farwell, M.A.; Zhang, B.; Lee, M.H.; Pan, X. Drug-dependent behaviors and nicotinic acetylcholine receptor expressions in Caenorhabditis elegans following chronic nicotine exposure. Neurotoxicology 2015, 47, 27–36. [Google Scholar] [CrossRef]
- Taki, F.A.; Pan, X.; Zhang, B. Nicotine exposure caused significant transgenerational heritable behavioral changes in Caenorhabditis elegans. EXCLI J. 2013, 12, 793–806. [Google Scholar]
- Angstman, N.B.; Kiessling, M.C.; Frank, H.G.; Schmitz, C. High interindividual variability in dose-dependent reduction in speed of movement after exposing C. elegans to shock waves. Front. Behav. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef]
- Angstman, N.B.; Frank, H.G.; Schmitz, C. Hypothermia ameliorates blast-related lifespan reduction of C. elegans. Sci. Rep. 2018, 8, 10549. [Google Scholar] [CrossRef]
- Consortium, C.E.D.M. Large-scale screening for targeted knockouts in the Caenorhabditis elegans genome. G3 2012, 2, 1415–1425. [Google Scholar] [CrossRef]
- Sulston, J.E.; Brenner, S. The DNA of Caenorhabditis elegans. Genetics 1974, 77, 95–104. [Google Scholar] [CrossRef]
- Portman, D.S. Profiling, C. elegans gene expression with DNA microarrays. WormBook 2006, 1–11. [Google Scholar] [CrossRef]
- Schafer, W.R. Genetic analysis of nicotinic signaling in worms and flies. J. Neurobiol. 2002, 53, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Sobkowiak, R.; Kaczmarek, P.; Kowalski, M.; Kabaciński, R.; Lesicki, A. Behavior of Caenorhabditis elegans in a nicotine gradient modulated by food. Drug Chem. Toxicol. 2019, 42, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Angstman, N.B.; Frank, H.G.; Schmitz, C. Advanced behavioral analyses show that the presence of food causes subtle changes in C. elegans movement. Front. Behav. Neurosci. 2016, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef]
- Gray, J.M.; Hill, J.J.; Bargmann, C.I. A circuit for navigation in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 3184–3191. [Google Scholar] [CrossRef]
- Matta, S.G.; Balfour, D.J.; Benowitz, N.L.; Boyd, R.T.; Buccafusco, J.J.; Caggiula, A.R.; Craig, C.R.; Collins, A.C.; Damaj, M.I.; Donny, E.C.; et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 2007, 190, 269–319. [Google Scholar] [CrossRef]
- Deng, L.; Denham, J.E.; Arya, C.; Yuval, O.; Cohen, N.; Haspel, G. Inhibition underlies fast undulatory locomotion in Caenorhabditis elegans. eNeuro 2021, 8, ENEURO.0241-20.2020. [Google Scholar] [CrossRef]
- Maricq, A.V.; Peckol, E.; Driscoll, M.; Bargmann, C.I. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 1995, 378, 78–81. [Google Scholar] [CrossRef]


| Assay | Strain | Chemical | Number of Worms | Concentration [mM] | Number of rESWs | Recovery Period [min] |
|---|---|---|---|---|---|---|
| A1 (control) | N2 | nicotine | 1309–1311 | 0 | 0 | - |
| A2 | N2 | nicotine | 658 | 2 | 0 | - |
| B1 (control) | N2 | nicotine | 265 | 0 | 100 | - |
| B2 | N2 | nicotine | 245 | 2 | 100 | - |
| C1 (control) | N2 | nicotine | 343–351 | 0 | 0 | 0 |
| C2 | N2 | nicotine | 174–206 | 0 | 0 | 30 |
| C3 | N2 | nicotine | 307–348 | 0 | 0 | 180 |
| C4 | N2 | nicotine | 97 | 2 | 0 | 0 |
| C5 | N2 | nicotine | 108 | 2 | 0 | 30 |
| C6 | N2 | nicotine | 44 | 2 | 0 | 180 |
| C7 | N2 | nicotine | 175–182 | 0 | 100 | 0 |
| C8 | N2 | nicotine | 216–219 | 0 | 100 | 30 |
| C9 | N2 | nicotine | 250–253 | 0 | 100 | 180 |
| C10 | N2 | nicotine | 107 | 2 | 100 | 0 |
| C11 | N2 | nicotine | 125 | 2 | 100 | 30 |
| C12 | N2 | nicotine | 108 | 2 | 100 | 180 |
| C13 | N2 | nicotine | 273–275 | 0 | 500 | 0 |
| C14 | N2 | nicotine | 174–178 | 0 | 500 | 30 |
| C15 | N2 | nicotine | 208–215 | 0 | 500 | 180 |
| C16 | N2 | nicotine | 90 | 2 | 500 | 0 |
| C17 | N2 | nicotine | 52 | 2 | 500 | 30 |
| C18 | N2 | nicotine | 77 | 2 | 500 | 180 |
| D1 (control) | N2 | carbachol | 245–257 | 0 | 0 | - |
| D2 | N2 | carbachol | 168 | 0 | 100 | - |
| D3 | N2 | carbachol | 139–145 | 1 | 0 | - |
| D4 | N2 | carbachol | 249 | 1 | 100 | - |
| D5 | N2 | carbachol | 406–413 | 0 | 500 | - |
| D6 | N2 | carbachol | 398–402 | 1 | 500 | - |
| E1 (control) | RB918 | carbachol | 131 | 0 | 0 | - |
| E2 | RB918 | carbachol | 90 | 0 | 100 | - |
| E3 | RB918 | carbachol | 179 | 1 | 0 | - |
| E4 | RB918 | carbachol | 65 | 1 | 100 | - |
| E5 | RB918 | carbachol | 99 | 10 | 0 | - |
| E6 | RB918 | carbachol | 136 | 10 | 100 | - |
| Behavioral Parameter | Definition |
|---|---|
| Absolute peristaltic speed | Total peristaltic track length (i.e., the sum of forward and reverse movement distances) divided by time, expressed in micrometers per second (µm/s). |
| Wavelength | Twice the distance between the negative and positive inflection points of the body waveform, measured in micrometers (µm). |
| Number of omega bends | An omega bend is recorded when the bending angle drops below 90° and continues until the angle exceeds 90° again. |
| Number of reversals | A reversal is recorded when the worm transitions into backward movement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochstrasser, T.; Kaub, L.; Maier, L.; Angstman, N.B.; Kenmoku, T.; Nussbaum-Krammer, C.; Schmitz, C. Behavioral Changes in Caenorhabditis elegans After Exposure to Radial Extracorporeal Shock Waves. J. Clin. Med. 2025, 14, 7206. https://doi.org/10.3390/jcm14207206
Hochstrasser T, Kaub L, Maier L, Angstman NB, Kenmoku T, Nussbaum-Krammer C, Schmitz C. Behavioral Changes in Caenorhabditis elegans After Exposure to Radial Extracorporeal Shock Waves. Journal of Clinical Medicine. 2025; 14(20):7206. https://doi.org/10.3390/jcm14207206
Chicago/Turabian StyleHochstrasser, Tanja, Leon Kaub, Leonard Maier, Nicholas B. Angstman, Tomonori Kenmoku, Carmen Nussbaum-Krammer, and Christoph Schmitz. 2025. "Behavioral Changes in Caenorhabditis elegans After Exposure to Radial Extracorporeal Shock Waves" Journal of Clinical Medicine 14, no. 20: 7206. https://doi.org/10.3390/jcm14207206
APA StyleHochstrasser, T., Kaub, L., Maier, L., Angstman, N. B., Kenmoku, T., Nussbaum-Krammer, C., & Schmitz, C. (2025). Behavioral Changes in Caenorhabditis elegans After Exposure to Radial Extracorporeal Shock Waves. Journal of Clinical Medicine, 14(20), 7206. https://doi.org/10.3390/jcm14207206







