Effectiveness of Electrical Stimulation on Upper Limb Function in Children and Young People with Hemiplegic Cerebral Palsy: A Systematic Review
Abstract
1. Introduction
Research Aim and Research Questions
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- -
- Participants aged 2 to 21 years old, both males and females. The lower limit reflects the age when CP-related motor impairments are most consistently recognised in clinical practice and diagnosed [31,32,33]. The upper limit extends into adolescence and early adulthood, when neuroplasticity and motor skill development are still responsive to rehabilitation. Additionally, the upper limit is consistent with current evidence and clinical practice in both research and clinical settings [34,35,36].
- -
- Diagnosis of HCP.
- -
- English studies published of any design (except case studies with fewer than 3 participants).
- -
- Studies utilising one of the following ES modalities: FES, TENS, NMES, TSCS, or tDCS.
- -
- Studies utilising ES plus physical therapy, occupational therapy, or ES alone.
- -
- -
- Upper limb and hand function outcomes (including fine and gross motor skills, range of motion, muscle strength, functional grip and release, isolated finger movements, protective responses, weight-bearing capacity, object manipulation, movement fluidity, placement accuracy, and performance of daily activities).
2.2.2. Exclusion Criteria
- -
- Studies including mixed CP or other neurological disorder subtypes without subgroup analysis for the HCP population.
- -
- Participants younger than 2 years or older than 21 years.
- -
- No confirmed diagnosis of HCP.
- -
- Non-English publications.
- -
- Grey literature (e.g., dissertations, conference abstracts, preprint reports not peer-reviewed).
- -
- Case studies with fewer than three participants.
- -
- Studies not utilising any of the following ES modalities: FES, TENS, NMES, TSCS, or tDCS.
- -
- Studies using ES combined with interventions other than physical therapy, occupational therapy, or ES alone.
- -
- Studies without upper limb or hand function outcomes.
2.3. Study Selection
2.4. Study Synthesis
2.5. Study Appraisal
3. Results
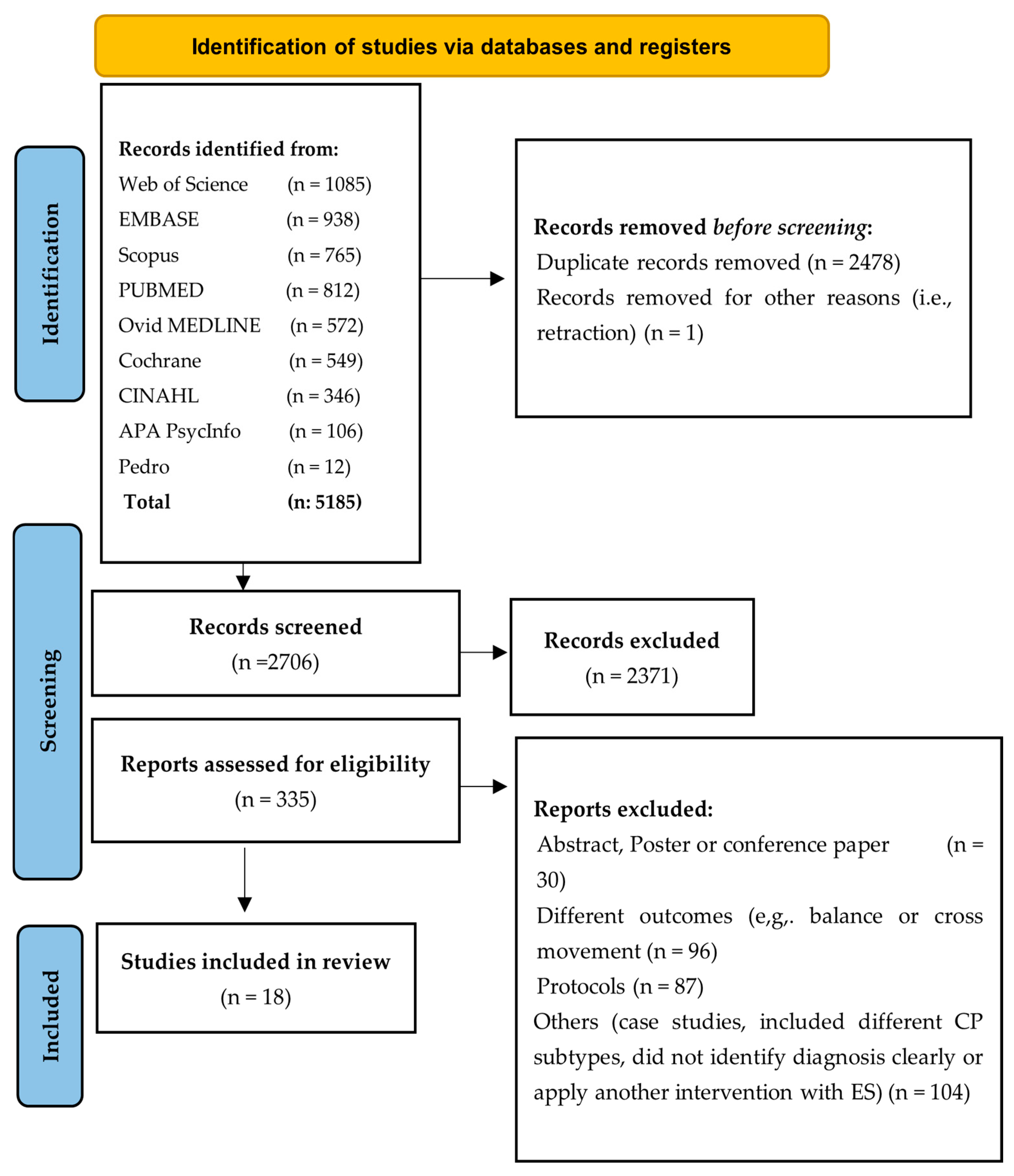
3.1. Search Results
3.2. Study Characteristics
3.3. Electrical Stimulation
3.3.1. tDCS Interventions
3.3.2. FES Interventions
3.3.3. TENS Interventions
3.3.4. NMES Interventions
3.3.5. TSCS Interventions
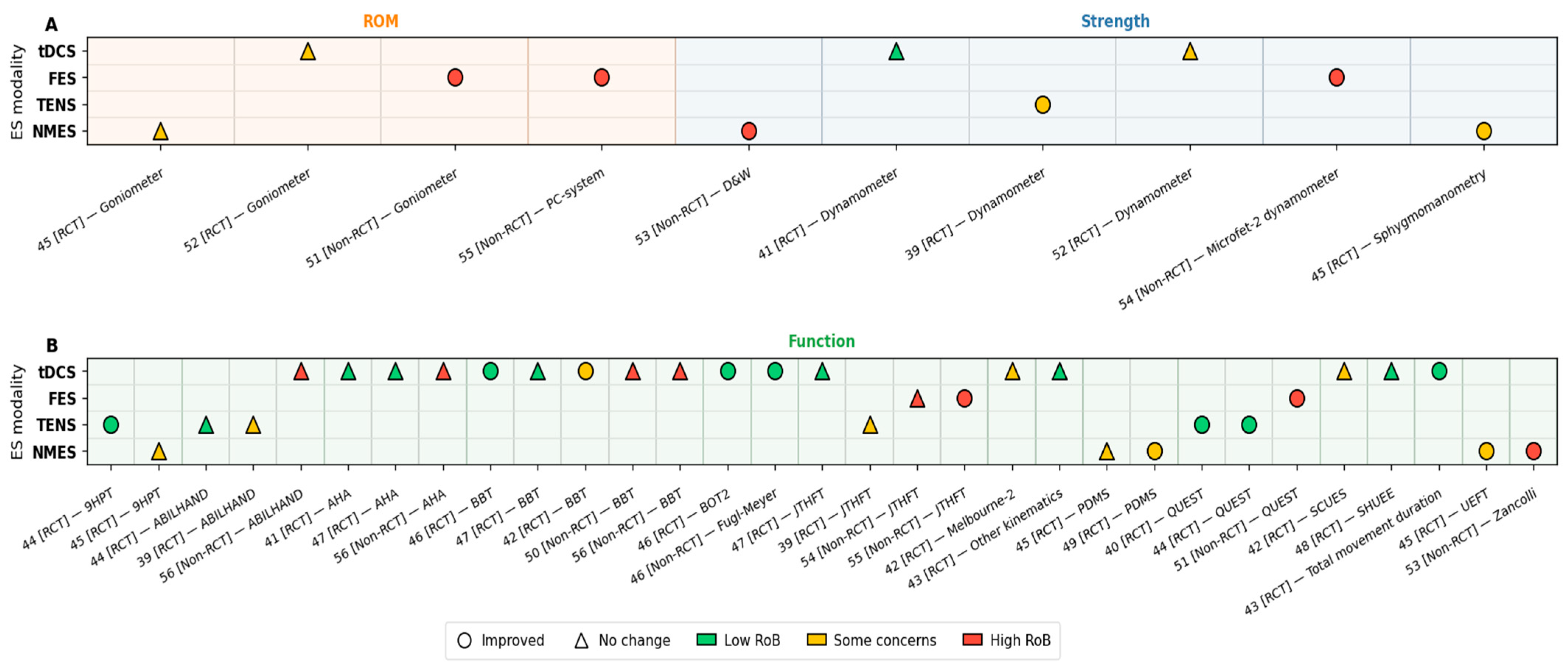
3.4. Outcome Measures Summary
3.4.1. Upper Extremity Function and Hand Skills
3.4.2. Muscle Strength
3.4.3. Other Rehabilitation Outcomes (Kinematics and Range of Motion)
3.5. Methodological Quality
4. Discussion
4.1. Transcranial Direct Current Stimulation
4.2. Functional Electrical Stimulation
4.3. Transcutaneous Electrical Nerve Stimulation
4.4. Neuromuscular Electrical Stimulation
4.5. Transcutaneous Spinal Cord Stimulation Interventions
4.6. Practical and Future Research Recommendations
- -
- tDCS is not currently recommended for routine clinical use in CYP with HCP. If applied, it should be limited to research or closely monitored settings, paired with motor practice, and include both immediate and follow-up assessments. Future studies should explore dose–response effects (e.g., 0.7–1.5 mA and session number).
- -
- FES may support hand function improvement in CYP with CP, particularly when applied daily using parameters around 30–50 Hz, 300 µs pulse width, for at least 30 min per session over 3–6 weeks. However, all supporting studies had a high risk of bias, and outcomes varied with application frequency. Well-designed, controlled trials are needed to determine optimal dosing and confirm efficacy.
- -
- TENS may be considered as an adjunct to upper limb training in CYP with HCP, using standardised parameters (100 Hz, 200–250 µs, 60 min, three times weekly for 8 weeks), especially for unilateral function. Outcome measures may include QUEST, 9HPT, and dynamometry. Given limited effects on bimanual outcomes (e.g., ABILHAND-Kids), future studies should combine TENS with bimanual tasks with follow-up periods.
- -
- NMES may improve upper limb function in CYP with CP, particularly when combined with interventions such as hand orthoses or constraint-based therapy. Future RCTs should compare NMES alone versus NMES combined with orthoses to evaluate the added benefit.
- -
- TSCS: Feasibility and pilot studies are needed to evaluate the use of transcutaneous spinal cord stimulation (TSCS) for upper limb goals in CYP with HCP, focusing on safety, acceptability, and preliminary efficacy given the current evidence gap.
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHA | Assisting hand assessment |
| BBT | Box-and-blocks test |
| ES | Electrical stimulation |
| CP | Cerebral palsy |
| CYP | Children and young people |
| CIMT | Constraint-induced movement therapy |
| FES | Functional electrical stimulation |
| HCP | Hemiplegic cerebral palsy |
| JTHFT | Jebsen–Taylor hand function test |
| Melbourne-2 | Melbourne assessment of unilateral upper limb function-2 |
| NMES | Neuromuscular electrical stimulation |
| PDMS | Peabody developmental motor scales |
| QUEST | Quality of Upper Extremity Skills Test |
| RCTs | Randomised controlled trials |
| SCUES | Selective Control of the Upper Extremity Scale |
| TENS | Transcutaneous electrical nerve stimulation |
| TSCS | Transcutaneous spinal cord stimulation |
| tDCS | Transcranial direct current stimulation |
| 9HPT | Nine-hole peg test |
References
- Richards, C.L.; Malouin, F. Chapter 18-Cerebral palsy: Definition, assessment and rehabilitation. Handb. Clin. Neurol. 2013, 111, 183–195. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Krägeloh-Mann, I.; Cans, C. Cerebral palsy update. Brain Dev. 2009, 31, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Sankar, C.; Mundkur, N. Cerebral palsy-definition, classification, etiology and early diagnosis. Indian J. Pediatr. 2005, 72, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Beaman, J.; Kalisperis, F.R.; Miller-Skomorucha, K. The Infant and Child with Cerebral Palsy. In Pediartric Physical Therapy, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2015; pp. 191–193. [Google Scholar]
- Arner, M.; Eliasson, A.C.; Nicklasson, S.; Sommerstein, K.; Hägglund, G. Hand Function in Cerebral Palsy. Report of 367 Children in a Population-Based Longitudinal Health Care Program. J. Hand Surg. 2008, 33, 1337–1347. [Google Scholar] [CrossRef]
- Martin, L.; Baker, R.; Harvey, A. A Systematic Review of Common Physiotherapy Interventions in School-Aged Children with Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2010, 30, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Badaru, U.; Olubunmi Ogwumike, O.; Fatai Adeniyi, A. Effect of task-oriented exercise training program on the functional performance of children with cerebral palsy. Physiother. Q. 2021, 29, 40–48. [Google Scholar] [CrossRef]
- Sakzewski, L.; Ziviani, J.; Boyd, R.N. Efficacy of Upper Limb Therapies for Unilateral Cerebral Palsy: A Meta-analysis. Pediatrics 2014, 133, e175–e204. [Google Scholar] [CrossRef]
- Das, S.P.; Ganesh, G.S. Evidence-based approach to physical therapy in cerebral palsy. Indian J. Orthop. 2019, 53, 20–34. [Google Scholar] [CrossRef]
- Steultjens, E.M.; Dekker, J.; Bouter, L.M.; van de Nes, J.C.; Lambregts, B.L.; van den Ende, C.H. Occupational therapy for children with cerebral palsy: A systematic review. Clin. Rehabil. 2004, 18, 1–14. [Google Scholar] [CrossRef]
- Doucet, B.M.; Lam, A.; Griffin, L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J. Biol. Med. 2012, 85, 201–215. [Google Scholar]
- Moe, J.H.; Post, H.W. Functional electrical stimulation for ambulation in hemiplegia. J. Lancet 1962, 82, 285–288. [Google Scholar] [PubMed]
- Peckham, P.H.; Knutson, J.S. Functional Electrical Stimulation for Neuromuscular Applications. Annu. Rev. Biomed. Eng. 2005, 7, 327–360. [Google Scholar] [CrossRef]
- Meesen, R.L.; Cuypers, K.; Rothwell, J.C.; Swinnen, S.P.; Levin, O. The effect of long-term TENS on persistent neuroplastic changes in the human cerebral cortex. Hum. Brain Mapp. 2011, 32, 872–882. [Google Scholar] [CrossRef]
- Kesikburun, S. Non-invasive brain stimulation in rehabilitation. Turk. J. Phys. Med. Rehabil. 2022, 68, 1–8. [Google Scholar] [CrossRef]
- Gad, P.; Hastings, S.; Zhong, H.; Seth, G.; Kandhari, S.; Edgerton, V.R. Transcutaneous Spinal Neuromodulation Reorganizes Neural Networks in Patients with Cerebral Palsy. Neurotherapeutics 2021, 18, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Hastings, S.; Zhong, H.; Feinstein, R.; Zelczer, G.; Mitrovich, C.; Gad, P.; Edgerton, V.R. A pilot study combining noninvasive spinal neuromodulation and activity-based neurorehabilitation therapy in children with cerebral palsy. Nat. Commun. 2022, 13, 5660. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application and Evidence. Rev. Pain 2007, 1, 7–11. [Google Scholar] [CrossRef]
- Dewald, J.A.; Given, J.D.; Rymer, W.Z. Long-lasting reductions of spasticity induced by skin electrical stimulation. IEEE Trans. Rehabil. Eng. 1996, 4, 231–242. [Google Scholar] [CrossRef]
- Singleton, C.; Jones, H.; Maycock, L. Functional electrical stimulation (FES) for children and young people with cerebral palsy. Paediatr. Child Health 2019, 29, 498–502. [Google Scholar] [CrossRef]
- Arsovski, D.; Chichevska-Jovanova, N.; Jovanovska, T. Advancing Forward: The Role of Functional Electrical Stimulation in Enhancing Lower Limb Function in Children with Cerebral Palsy. Futur. Med. 2025, 4, 61–78. [Google Scholar] [CrossRef]
- Roche, N.; Geiger, M.; Bussel, B. Mechanisms underlying transcranial direct current stimulation in rehabilitation. Ann. Phys. Rehabil. Med. 2015, 58, 214–219. [Google Scholar] [CrossRef]
- Hassanzahraee, M.; Nitsche, M.A.; Zoghi, M.; Jaberzadeh, S. Determination of anodal tDCS intensity threshold for reversal of corticospinal excitability: An investigation for induction of counter-regulatory mechanisms. Sci. Rep. 2020, 10, 16108. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function After Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tahmasbi, F.; Sanaie, S.; Salehi-Pourmehr, H.; Ghaderi, S.; Rahimi-Mamaghani, A. The role of transcutaneous electrical nerve stimulation (TENS) in rehabilitation of cerebral palsy: A systematic review. Dev. Neurorehabil. 2025, 28, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Zhang, Y.; Luo, J.; Chen, T.; Zhang, J.; Peng, T., Sr.; Han, M.; Le, W.; Peng, T., Jr.; Xu, K. Safety and effectiveness of non-invasive brain stimulation on mobility and balance function in children with cerebral palsy: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2025, 22, 111. [Google Scholar] [CrossRef]
- Ou, C.H.; Shiue, C.C.; Kuan, Y.C.; Liou, T.H.; Chen, H.C.; Kuo, T.J. Neuromuscular Electrical Stimulation of Upper Limbs in Patients with Cerebral Palsy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Am. J. Phys. Med. Rehabil. 2023, 102, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Louwers, A.; MEESTER-DELVER, A.; Folmer, K.; Nollet, F.; Beelen, A. Immediate effect of a wrist and thumb brace on bimanual activities in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 321–326. [Google Scholar] [CrossRef]
- Gilliaux, M.; Renders, A.; Dispa, D.; Holvoet, D.; Sapin, J.; Dehez, B.; Detrembleur, C.; Lejeune, T.M.; Stoquart, G. Upper Limb Robot-Assisted Therapy in Cerebral Palsy: A Single-Blind Randomized Controlled Trial. Neurorehabilit. Neural Repair 2014, 29, 183–192. [Google Scholar] [CrossRef]
- Granild-Jensen, J.B.; Rackauskaite, G.; Flachs, E.M.; Uldall, P. Predictors for early diagnosis of cerebral palsy from national registry data. Dev. Med. Child Neurol. 2015, 57, 931–935. [Google Scholar] [CrossRef]
- Hubermann, L.; Boychuck, Z.; Shevell, M.; Majnemer, A. Age at Referral of Children for Initial Diagnosis of Cerebral Palsy and Rehabilitation: Current Practices. J. Child Neurol. 2016, 31, 364–369. [Google Scholar] [CrossRef]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Martinie, O.; Mercier, C.; Gordon, A.M.; Robert, M.T. Upper Limb Motor Planning in Individuals with Cerebral Palsy Aged between 3 and 21 Years Old: A Systematic Review. Brain Sci. 2021, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.L.; Menk, J.S.; Rudser, K.D.; Feyma, T.; Gillick, B.T. Less-Affected Hand Function in Children with Hemiparetic Unilateral Cerebral Palsy: A Comparison Study With Typically Developing Peers. Neurorehabilit. Neural Repair 2017, 31, 965–976. [Google Scholar] [CrossRef]
- Olivier, I.; Hay, L.; Bard, C.; Fleury, M. Age-related differences in the reaching and grasping coordination in children: Unimanual and bimanual tasks. Exp. Brain Res. 2007, 179, 17–27. [Google Scholar] [CrossRef]
- Risk-of-Bias Tools. Robvis (Visualisation Tool). 2025. Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool (accessed on 17 September 2025).
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Alhusaini, A.A.; Fallatah, S.; Melam, G.R.; Buragadda, S. Efficacy of transcutaneous electrical nerve stimulation combined with therapeutic exercise on hand function in children with hemiplegic cerebral palsy. Somat. Mot. Res. 2019, 36, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, S.; Kumar, D.; Rajasenthil, K. The Effects and Safety of Transcutaneous Electrical Nerve Stimulation to Improve Upper Limb Function in Hemiplegic Cerebral Palsy–A Preliminary Report. Indian J. Physiother. Occup. Ther. 2017, 11, 124–129. [Google Scholar] [CrossRef]
- Gillick, B.; Rich, T.; Nemanich, S.; Chen, C.-Y.; Menk, J.; Mueller, B.; Chen, M.; Ward, M.; Meekins, G.; Feyma, T.; et al. Transcranial direct current stimulation and constraint-induced therapy in cerebral palsy: A randomized, blinded, sham-controlled clinical trial. Eur. J. Paediatr. Neurol. 2018, 22, 358–368. [Google Scholar] [CrossRef]
- He, W.; Huang, Y.; He, L.; Liu, L.; Zeng, P.; Qiu, H.; Wang, X.; Zhou, H.; Chen, Z.; Xu, Y.; et al. Safety and effects of transcranial direct current stimulation on hand function in preschool children with hemiplegic cerebral palsy: A pilot study. Front. Behav. Neurosci. 2022, 16, 925122. [Google Scholar] [CrossRef]
- Moura, R.C.F.; Santos, C.; Collange Grecco, L.; Albertini, G.; Cimolin, V.; Galli, M.; Oliveira, C. Effects of a single session of transcranial direct current stimulation on upper limb movements in children with cerebral palsy: A randomized, sham-controlled study. Dev. Neurorehabilit. 2017, 20, 368–375. [Google Scholar] [CrossRef]
- Satheeskumar, D.; Dhaneshkumar, K.; Rajasenthil, K. A Comparative study to identify the effects of transcutaneous electrical nerve stimulation combined with sensorimotor task oriented training to improve the hand function in hemiplegic cerebral palsy children. J. Clin. Diagn. Res. 2018, 12, YC17–YC21. [Google Scholar] [CrossRef]
- Xu, K.; Wang, L.; Mai, J.; He, L. Efficacy of constraint-induced movement therapy and electrical stimulation on hand function of children with hemiplegic cerebral palsy: A controlled clinical trial. Disabil. Rehabil. 2012, 34, 337–346. [Google Scholar] [CrossRef]
- Ebrahimabadi, Z.; Ghaderian, B.; Kachoosangy, R.A. Transcranial Direct Current Stimulation Combined with Occupational Therapy Improves Upper Limb Function in Children with Unilateral Cerebral Palsy: A Randomized Controlled Trial. Iran. J. Pediatr. 2025, 35, e148515. [Google Scholar] [CrossRef]
- Hilderley, A.J.; Dunbar, M.; Andersen, J.; Fehlings, D.; Metzler, M.; Carlson, H.L.; Zewdie, E.; Hodge, J.; O’Grady, K.; Carsolio, L.; et al. Neuromodulation for Children with Hemiparesis and Perinatal Stroke: A Randomized Clinical Trial. JAMA Neurol. 2025, 82, 267–275. [Google Scholar] [CrossRef]
- Merino-Andrés, J.; Palomo-Carrión, R.; Gómez-Soriano, J.; Fernández-Pérez, J.J.; Serrano-Muñoz, D.; Muñoz-Marrón, E.; López-Muñoz, P. Transcranial direct current stimulation combined with an intensive training program for upper limb rehabilitation in children with unilateral cerebral palsy. A randomized controlled pilot study. Res. Dev. Disabil. 2025, 161, 105001. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, E.E.; Ibrahim, M.B.; Bakr, B.E.M.; Elsayeh, A.A.; Abdullatif, H.M.; Kamar, S.M.; Barakat, N.E. Effect of reciprocal electrical stimulation versus kinesio taping on fine motor skills in hemiparetic cerebral palsy children. Revista Iberoamericana de Psicología del Ejercicio y el Deporte. Rev. Iberoam. Psicol. Ejerc. Deporte 2024, 19, 276–280. [Google Scholar]
- Christopher, P.; Sutter, E.N.; Gavioli, M.; Lench, D.H.; Nytes, G.; Mak, V.; Simpson, E.A.; Ikonomidou, C.; Villegas, M.A.; Saiote, C.; et al. Safety, tolerability and feasibility of remotely-instructed home-based transcranial direct current stimulation in children with cerebral palsy. Brain Stimul. 2023, 16, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Demesi-Drljan, C.; Mikov, A.; Krasnik, R.; Karaba-Jakovljevic, D.; Filipovic, K.; Tomasevic-Todorovic, S. Functional electric stimulation of children with cerebral palsy: A pilot study. HealthMED 2011, 5, 522–527. [Google Scholar]
- Farzamfar, P.; Heirani, A.; Amiri, E.; Sedighi, M.; Machado, D.G.D. The Effect of Transcranial Direct Current Stimulation on M1 with and without Mirror Visual Feedback on Range of Motion and Hand Grip Strength of the Affected Upper Limb in Children with Spastic Hemiplegic Cerebral Palsy. Iran. J. Child Neurol. 2024, 18, 93–106. [Google Scholar] [CrossRef]
- Mäenpää, H.; Jaakkola, R.; Sanström, M.; Airi, T.; Von Wendt, L. Electrostimulation at sensory level improves function of the upper extremities in children with cerebral palsy: A pilot study. Dev. Med. Child Neurol. 2004, 46, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Vaz, D.V.; Mancini, M.C.; da Fonseca, S.T.; Arantes, N.F.; da Silva Pinto, T.P.; de Aljo, P.A. Effects of strength training aided by electrical stimulation on wrist muscle characteristics and hand function of children with hemiplegic cerebral palsy. Phys. Occup. Ther. Pediatr. 2008, 28, 308–324. [Google Scholar] [CrossRef]
- Wright; Granat, H. Therapeutic effects of functional electrical stimulation of the upper limb of eight children with cerebral palsy. Dev. Med. Child Neurol. 2000, 42, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.L.; Nemanich, S.; Chen, M.; Friel, K.; Feyma, T.; Krach, L.; Nawshin, T.; Meekins, G.; Gillick, B.T. Transcranial direct current stimulation (tDCS) paired with occupation-centered bimanual training in children with unilateral cerebral palsy: A preliminary study. Neural Plast. 2018, 2018, 9610812. [Google Scholar] [CrossRef]
- Tedesco Triccas, L.; Burridge, J.H.; Hughes, A.M.; Pickering, R.M.; Desikan, M.; Rothwell, J.C.; Verheyden, G. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: A review and meta-analysis. Clin. Neurophysiol. 2016, 127, 946–955. [Google Scholar] [CrossRef]
- Patel, R.; Ashcroft, J.; Patel, A.; Ashrafian, H.; Woods, A.J.; Singh, H.; Darzi, A.; Leff, D.R. The Impact of Transcranial Direct Current Stimulation on Upper-Limb Motor Performance in Healthy Adults: A Systematic Review and Meta-Analysis. Front. Neurosci. 2019, 13, 1213. [Google Scholar] [CrossRef]
- Arnould, C.; Penta, M.; Renders, A.; Thonnard, J.-L. ABILHAND-Kids. Neurology 2004, 63, 1045–1052. [Google Scholar] [CrossRef]
- Yildizgoren, M.T.; Nakipoglu Yuzer, G.F.; Ekiz, T.; Ozgirgin, N. Effects of neuromuscular electrical stimulation on the wrist and finger flexor spasticity and hand functions in cerebral palsy. Pediatr. Neurol. 2014, 51, 360–364. [Google Scholar] [CrossRef]
- Ozer, K.; Chesher, S.P.; Scheker, L.R. Neuromuscular electrical stimulation and dynamic bracing for the management of upper-extremity spasticity in children with cerebral palsy. Dev. Med. Child Neurol. 2006, 48, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.J.; Rosenbaum, P.L.; Cadman, D.T.; Gowland, C.; Hardy, S.; Jarvis, S. The Gross motor function measure: A means to evaluate the effects of physical therapy. Dev. Med. Child Neurol. 1989, 31, 341–352. [Google Scholar] [CrossRef]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lucas, K.; Keller, A.; Martin, R.; Behrman, A.; Vissarionov, S.; Gerasimenko, Y.P. Transcutaneous Spinal Stimulation from Adults to Children: A Review. Top. Spinal Cord Inj. Rehabil. 2022, 29, 16–32. [Google Scholar] [CrossRef] [PubMed]


| Study | Study Design | Participants’ Details | Country | |||||
|---|---|---|---|---|---|---|---|---|
| Age Range | Number of Participants | Participants’ Distribution | Manual Ability Classification System L: Level | |||||
| Years | N= | Experimental | Control | Experimental | Control | |||
| [42] | RCT | 3–6 | 30 | 15 | 15 | L: I = 14 L: II = 1 | L: I = 14 L: II = 1 | China |
| [41] | RCT | 7–21 | 20 | 10 | 10 | L: I = 1 L: II = 8 L: III =1 | L: I = 1 L: II = 8 L: IV = 1 | USA |
| [43] | RCT | 6–12 | 20 | 10 | 10 | L: I: = 3 L: II: = 7 | L: I: = 1 L: II: = 9 | Brazil |
| [56] | Single-group, multiple-baselines, open-label design | 8–19 | 8 | 8 | - | L: I = 1 L: II = 5 L: III = 2 | - | United States |
| [54] | Pre-test/post-test design | 7–11 | 9 | 9 | - | L: I = 1 L: II = 8 | - | Brazil |
| [51] | Pre-test/post-test design | 4–8 | 13 | 13 | - | L: I = 7 L: II = 3 L: III = 3 | - | Serbia |
| [55] | Pre-test/post-test design | Mean age 10, no range | 8 | 8 | - | - | - | United Kingdom |
| [40] | RCT | 4–12 | 30 | 15 | 15 | L: II–III | India | |
| [39] | RCT | 6–12 | 29 | 15 | 14 | L: I–III = 29 | Saudi Arabia | |
| [44] | RCT | 4–12 | 60 | 30 | 30 | L: I = 4 L: II = 18 L: III = 8 | L: I = 9 L: II = 14 L: III = 7 | India |
| [45] | RCT | 2–14 | 68 | 23 | 45 | - | - | China |
| [53] | Pre-test/post-test design | 2–12 | 12 | 12 | - | - | - | Finland |
| [46] | RCT | 5–10 | 50 | 25 | 25 | - | Iran | |
| [48] | RCT | 4–8 | 18 | 9 | 9 | L: I–III | Spain | |
| [47] | RCT | 6–18 | 83 | 41 | 42 | L: I–IV | Canada | |
| [49] | RCT | 4–5 | 60 | 30 | 30 | - | Egypt | |
| [52] | RCT | 6–12 | 10 | 10 | - | L: I–II | Iran | |
| [50] | Pre-test/post-test design | 10–19 | 10 | 10 | - | - | USA | |
| Study | ES Modality | Freq (Hz) | PW (µs) | Current (mA) | Dose/Exposure Time |
|---|---|---|---|---|---|
| [42] | tDCS | - | - | 1.5 | 20 min × 1 session |
| [41] | tDCS | - | - | 0.7 | 20 min × 10 sessions |
| [43] | tDCS | - | - | 1 | 20 min × 1 session |
| [56] | tDCS | - | - | 1.5 | 20 min × 10 sessions |
| [46] | tDCS | - | - | 1.5 | 20 min × 20 sessions |
| [48] | tDCS | - | - | 1 | 20 min × 13 sessions |
| [47] | tDCS | - | - | 1 | 20 min × 10 sessions |
| [52] | tDCS | - | - | 1 | 20 min × 1 session (each, 1 wk apart × 4 conditions) |
| [50] | tDCS | - | - | 1.5 | 20 min × 3 sessions |
| [54] | FES | 30 | 300 | until visible contractions | 5 s precontraction; 3 sessions/wk × 8 wk (contractions) |
| [51] | FES | 50 | 300 | 10–40 | 15–30 min; 5 sessions/wk × 3 wk |
| [55] | FES | 30 | 300 | 10–40 | 30 min daily × 6 wk |
| [40] | TENS | 100 | 200 | until sensation threshold | 60 min; 3 sessions/wk × 8 wk |
| [39] | TENS | 100 | 250 | 50 | 30 min; 3 sessions/wk × 8 wk |
| [44] | TENS | 100 | 200 | until initial current sensation | 60 min; 3 sessions/wk × 8 wk |
| [45] | NMES | 50 | 300 | ≤100 | 20 min; 5 sessions/wk × 2 wk |
| [53] | NMES | 40 | 300 | 2–10 | 20–40 min; 12 sessions (over 5 wk) |
| [49] | NMES | 30 | 300 | to tolerate | 20 min; 3 sessions/wk × 12 wk |
| Study | ES Modality | Outcomes and Key Finding Between-Group End/Interaction or Intervention Group (⇧ = Significant, → = no Change) | Experimental Arm | Control/Comparison | Stim. Parameters Freq (Hz) PW (µs) Current (mA) | Dose/Exposure Time (min) × Sessions (per wk × wks) | Follow-Up |
|---|---|---|---|---|---|---|---|
| tDCS | |||||||
| [42] | tDCS | BBT (affected hand) ⇧ (immediate and ≥24 h; Melbourne-2 →; SCUES → | tDCS | Sham tDCS | n/a · n/a · 1.5 | 20 × 1 | 90 min |
| [41] | tDCS | Dynamometer, AHA → at post and 6 mo | tDCS + CIMT | Sham tDCS + CIMT | n/a · n/a · 0.7 | 20 × 10 | 6 mo |
| [43] | tDCS | Reduction in total movement duration ⇧; other kinematics → | tDCS + CIMT | Sham tDCS + CIMT. | n/a · n/a · 1 | 20 × 1 | — |
| [56] | tDCS | 3/8 AHA SDD →; 2/8 BBT SEM →; 3/8 Abilhand LMD → | tDCS+ training | — | n/a · n/a · 1.5 | 20 × 10 | — |
| [46] | tDCS | Fugl-Meyer assessment ⇧, BBT ⇧, Bruininks–Oseretsky ⇧ | tDCS + OT | Sham tDCS + OT | n/a · n/a · 1.5 | 20 × 20 | — |
| [48] | tDCS | SHUEE (spontaneous use, grasp-release) → | tDCS + CIMT/BT | Sham tDCS + CIMT/BT | n/a · n/a · 1 | 20 × 13 | 3 mo |
| [47] | tDCS | AHA →, BBT →, JTHFT → | tDCS + CIMT+ Training | Sham tDCS + CIMT + Training | n/a · n/a · 1 | 20 × 10 | 6 mo |
| [52] | tDCS | Dynamometer tDCS-offline ⇧; sham-tDCS-offline ⇧; sham-tDCS-online ⇧; wrist ROM (ROM-W) tDCS-offline ⇧; sham-tDCS-offline ⇧; sham-tDCS-online ⇧; elbow ROM (ROM-E) tDCS-offline ⇧; sham-tDCS-offline ⇧; sham-tDCS-online ⇧ | tDCS † + MVF | — | n/a · n/a · 1 | 20 × 1 (each, 1 wk apart) (4 sessions/conditions) | — |
| [50] | tDCS | BBT → | tDCS (1× mock = 3 × active + 1 × sham) | — | n/a · n/a · 1.5 | 20 × 3 | — |
| FES | |||||||
| [54] | FES | Microfet-2 dynamometer wrist (extensor strength: 30° ⇧, neutral ⇧, flexed 30° →; flexor strength: extended 30° ⇧, other →; JTHFT → | FES + training | — | 30 · 300 · until visible contractions | 5 s precontraction 3/wk × 8 wk Contractions/Session not stated | — |
| [51] | FES | QUEST ⇧; goniometer (wrist) ⇧ (post and 3 mo; n:7/13 missed follow-up) | FES + NDT | — | 50 · 300 · 10–40 | 15–30. 5/wk × 3 wk | 1 mo and 3 mo |
| [55] | FES | JTHFT (draughts ⇧, cards ⇧, objects ⇧) PC system (active wrist extension: ⇧ (not in severe contracture; n:2)), extension moment → | FES | — | 30 · 300 · 10–40 | 30 daily × 6 wk | 6 wk |
| TENS | |||||||
| [40] | TENS | QUEST (grasp and dissociated move) ⇧ | TENS + training + CIMT | Sham TENS + TOT +CIMT | 100 · 200 · until sensation threshold | 60, 3/wk × 8 wk | — |
| [39] | TENS | Dynamometer ⇧; JTHFT, ABILHAND → | TENS + training | training only | 100 · 250 · 50 | 30, 3/wk × 8 wk | — |
| [44] | TENS | 9HPT ⇧; QUEST (grasp) ⇧; ABILHAND → | TENS + SM-TOT + CIMT | Sham TENS + SM-TOT + CIMT | 100 · 200 · until the subject felt the initial current sensation | 60, 3/wk × 8 wk | — |
| NME | |||||||
| [45] | NMES | Sphygmomanometry, UEFT ⇧ (3 and 6 mo); goniometer, 9HPT, PDMS (grasping) → | NMES + CIMT/OT | CIMT/OT | 50 · 300 · ≤ 100 | 20 × 5/wk × 2 wk | 3 and 6 mo |
| [53] | NMES | Daniels and Worthingham (arm flexed, all children ⇧; <4 years ⇧; ≥4 years: ⇧; arm extended, all children; <4 years ⇧; ≥4 years →) Zancolli classification (tone/hand posture) ⇧. | NMES + NDT | — | 40 ·300 · 2–10 | 20–40 × 12 (5 wk) | 3 mo |
| [49] | NMES | PDMS ⇧ | PT + NMES | PT + KT | 30 · 300 · to tolerate | 20 × 3/wk × 12 wk | - |
| [42] | [43] | [41] | [56] | [54] | [51] | [40] | [39] | [44] | [45] | [53] | [55] | [46] | [48] | [47] | [52] | [50] | [49] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Low | Low | Low | High | High | High | Low | Low | Low | Low | High | High | Low | Low | Low | Low | High | Some |
| Random sequence generation | Low | Low | Low | N/A | N/A | N/A | Low | Low | Low | Low | N/A | N/A | Low | Low | Low | Low | N/A | Low |
| Allocation concealment | Low | Low | Low | N/A | N/A | N/A | Low | Low | Low | Low | N/A | N/A | Low | Low | Low | Some | N/A | Some |
| Baseline differences | Some | Low | Low | Some | Some | Some | Low | Low | Low | Low | Some | Some | Low | Low | Low | Low | Some | Low |
| Blinding of participants | Low | Low | Low | High | High | Some | Low | Some | Low | Some | High | Some | Low | Low | Low | Low | High | Some |
| Blinding of personnel | Some | Low | Low | High | Some | High | Low | Some | Low | Low | High | Some | Some | Some | Low | Some | High | Some |
| Deviations from intended intervention | Low | Low | Low | Low | Low | Low | Low | Low | Low | Some | Low | Some | Low | Low | Low | Low | Low | Low |
| Effect of assignment intervention analysis | Low | Low | Low | Some | Low | Some | Low | Low | Low | Low | Some | Some | Low | Low | Low | Low | Low | Some |
| Concurrent intervention/unintended exposure | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Some | Some | Low | Low | Low | Low | Low | Low |
| Incomplete outcome data | Low | Low | Low | Low | Low | Some | Low | Low | Low | Low | Some | Some | Low | Low | Low | Low | Some | Some |
| Method of measuring the outcome | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Blinding of outcome assessor | Low | Low | Low | Some | High | Some | Some | Some | Low | Low | High | Some | Low | Low | Low | Low | Some | Low |
| Selective reporting | Low | Low | Low | Low | Some | Low | Low | Low | Low | Low | Some | Some | Low | Low | Low | Low | Low | Low |
| Overall | Some | Low | Low | High | High | High | Low | Some | Low | Some | High | High | Low | Low | Low | Some | High | Some |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahhas, O.; Astill, S.L.; Chakrabarty, S.; Burdon, J.; Capozio, A. Effectiveness of Electrical Stimulation on Upper Limb Function in Children and Young People with Hemiplegic Cerebral Palsy: A Systematic Review. J. Clin. Med. 2025, 14, 6718. https://doi.org/10.3390/jcm14196718
Nahhas O, Astill SL, Chakrabarty S, Burdon J, Capozio A. Effectiveness of Electrical Stimulation on Upper Limb Function in Children and Young People with Hemiplegic Cerebral Palsy: A Systematic Review. Journal of Clinical Medicine. 2025; 14(19):6718. https://doi.org/10.3390/jcm14196718
Chicago/Turabian StyleNahhas, Omar, Sarah L. Astill, Samit Chakrabarty, Joanna Burdon, and Antonio Capozio. 2025. "Effectiveness of Electrical Stimulation on Upper Limb Function in Children and Young People with Hemiplegic Cerebral Palsy: A Systematic Review" Journal of Clinical Medicine 14, no. 19: 6718. https://doi.org/10.3390/jcm14196718
APA StyleNahhas, O., Astill, S. L., Chakrabarty, S., Burdon, J., & Capozio, A. (2025). Effectiveness of Electrical Stimulation on Upper Limb Function in Children and Young People with Hemiplegic Cerebral Palsy: A Systematic Review. Journal of Clinical Medicine, 14(19), 6718. https://doi.org/10.3390/jcm14196718








