Abstract
Background: Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy. Complete tumor resection (R0) is critical for prognosis and may require multivisceral resection in locally advanced cases. However, data on outcomes after multivisceral resection for ACC remain limited. This study evaluates the perioperative and oncologic outcomes of patients undergoing multivisceral resection for suspected ACC. Methods: We retrospectively analyzed 21 patients who underwent multivisceral resection with curative intent for suspected ACC. Three were later diagnosed with other tumor entities (sarcoma, non-small cell lung carcinoma metastasis and ganglioneuroma). The remaining 18 patients with histologically confirmed ACC were compared with 19 patients who underwent isolated adrenalectomy during the same study period. Results: Patients undergoing multivisceral resection were significantly younger (p = 0.003), had larger (p < 0.001) and more advanced tumors according to ENSAT classification (p < 0.001). All but one had open surgery; laparoscopic or hybrid approaches were more common in the isolated adrenalectomy group. Multivisceral resections were associated with longer operative times (p = 0.002), all required an ICU admission (p < 0.001), and had longer hospital stays (p = 0.001). Lymphnode metastases were observed only in the multivisceral group (p = 0.002). No significant differences were found in complication rates (p = 0.081), resection status (p = 0.091), progression-free survival (p = 0.095), or overall survival (p = 0.71). Conclusions: Multivisceral resection is a safe and feasible approach in specialized centers and may achieve comparable oncologic outcomes to isolated adrenalectomy, even in patients with more advanced disease. It should be considered when R0 resection is required and technically achievable.
1. Introduction
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy originating from the adrenal cortex, with an incidence of approximately 1–2 cases per million people per year [1]. Complete surgical resection with negative margins (R0 resection) remains the only potentially curative treatment [1]. However, due to the frequently advanced local extent of disease at the time of diagnosis [2], achieving an R0 resection often requires multivisceral resection [3], involving adjacent organs such as the kidney, liver, pancreas, spleen, or diaphragm. Although technically demanding, this approach offers the best chance for durable oncologic outcomes in selected patients [1].
Suspicion of ACC typically arises in the context of large adrenal tumors (>4 cm) [4], rapid tumour progression, radiologic features of necrosis [5,6], signs of local invasion into surrounding organs or vessels, suspected lymph node metastasis [6] or native computed tomography (CT) density > 20 Hounsfield Units (HU) [3]. A thorough patient and family history, along with a comprehensive hormonal workup to assess for cortisol and androgen secretion, is essential [3,4,7]. Known or suspected familial syndromes associated with elevated risk for ACC, such as Li–Fraumeni [8] or Lynch syndrome [9], as well as rapidly progressive Cushing’s syndrome [1], should raise strong suspicion for adrenal malignancy. It is also critical to exclude pheochromocytoma through biochemical testing of metanephrines and normetanephrines [3,4,7]. Around 50% of ACCs are hormonally inactive [10], and in these cases, differentiating ACC from other adrenal or retroperitoneal pathologies, such as metastases or sarcomas, can be particularly challenging [11]. Adrenal biopsy is not recommended, as it may negatively impact outcomes by compromising the likelihood of achieving a subsequent R0 resection [3]. Consequently, when ACC is suspected, surgical intervention may be the only valid management option.
In this study, we evaluate the indications, outcomes, and surgical considerations associated with multivisceral resection in patients with suspected ACC. Furthermore, we compare clinical and tumour characteristics, as well as perioperative and postoperative outcomes, between patients undergoing multivisceral resection and those receiving isolated adrenalectomy for histologically confirmed ACC.
2. Materials and Methods
2.1. Study Design
This was a retrospective, single-center study conducted at the Tertiary Center for Endocrine Surgery at the Charité—Universitätsmedizin Berlin, Germany.
2.2. Patient Demographic and Clinical Data
Patients who underwent multivisceral resection for suspected adrenocortical carcinoma (ACC) with curative intent, as well as all patients who underwent isolated adrenalectomy for ACC between January 2008 and April 2024 at the Tertiary Centre for Endocrine Surgery, Charité—Universitätsmedizin Berlin, were retrieved from a retrospectively collected database. A total of 24 patients who underwent multivisceral resection for suspected ACC were identified. One patient with bilateral ACC resection and two patients with distant metastases who underwent adrenalectomy for symptom control of hypercortisolism without curative intent were excluded. Thus, 21 patients who underwent multivisceral resection were included in the final analysis. In addition, 19 patients who underwent isolated adrenalectomy for ACC within the same study period were identified. One patient with ACC who underwent cholecystectomy for a non-oncologic reason (cholecystolithiasis) was assigned to the isolated adrenalectomy group.
For all patients, data were collected on patient-specific factors (age, sex, body mass index (BMI) and physical status according to the American Society of Anaesthesiologists (ASA) classification), tumour-specific factors (tumour entity, size and location, and hormone production, presence of vascular and/or adjacent organ infiltration, lymphnode metastases), and intraoperative and postoperative outcomes (surgical technique, duration of surgery, resection status, pathological confirmation of organ infiltration, need for blood transfusion, requirement for postoperative intensive care unit (ICU) monitoring, ICU length of stay, and total postoperative hospital stay). Postoperative complications were recorded and classified according to the Clavien-Dindo system [12], with minor complications defined as grades I to IIIa, and major complications as grade IIIb 3b or higher. For patients who underwent multivisceral resection for suspected ACC, the indications and extent of the multivisceral resection were documented. Additionally, for all ACC patients, data were collected on Ki67 index, ENSAT stage (according to the European Network for the Study of Adrenal Tumours (ENSAT) system) [13], and the S-GRAS score [14], which includes ENSAT stage, Ki67 index, resection status, age, and tumour-related symptoms. This study was conducted in compliance with the principles of the Declaration of Helsinki, following approval from the institutional review board (EA1/394/20, 21 January 2021, and Gliedkörgerschaft der Freien Universität Berlin und der Humboldt-Universität zu Berlin Charitéplatz, EA1/097/21, 23 April 2021).
2.3. Statistical Analysis
Normally distributed continuous variables are displayed as means (standard deviation, SD), non-normally distributed continuous variables as medians [range] and categorical variables as frequencies. Normality was assessed using Shapiro–Wilk test. The Mann–Whitney U test was used to compare groups for non-normally distributed metric variables, and the two-sided t-test was applied for normally distributed metric variables. The Fischer’s exact test was used for group comparison of categorical variables. Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method. OS was defined as the time from adrenalectomy to death from any cause, while PFS was defined as interval between adrenalectomy and the first postoperative progression in patients with adrenocortical carcinoma. Patients who had neither died nor shown disease progression, or who were lost to follow-up, were censored at the time of their last recorded visit. Individuals without any follow-up data were excluded from both OS and PFS analyses. Survival curves were compared using the log-rank tests. The significance level was set at p < 0.05. All statistical analyses were conducted using R (version 024.12.0 + 467; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
We included 21 patients who underwent multivisceral resection for suspected adrenocortical carcinoma (ACC) with curative intent. Patient and tumor characteristics are summarized in Table 1. The cohort had a mean age of 42 years (SD 15 years) and consisted of 14 women (66.7%) and 7 men (33.3%). The mean tumor size was 142 mm (SD 54 mm). Hormonal activity was present in 15 patients (71.4%). Lymph node metastases were detected in 6 patients (28.6%). Vascular infiltration was observed macroscopically in 11 (52.4%) and microscopically in 5 patients (23.8%). The results of the Shapiro–Wilk normality test for this patient group are presented in Table A1.

Table 1.
Multivisceral resection for suspected adrenocortical carcinoma with curative intention—patient and tumour characteristics (n = 21).
The intra- and postoperative outcomes are shown in Table 2. Open surgery was performed in 18 patients (85.7%) and a hybrid approach in 3 patients (14.3%). The median duration of surgery was 236 min [range 102–825 min]. R0 resection was achieved in 16 patients (76.2%). Blood transfusion was required in 6 cases (28.6%). Most patients (20 of 21; 95.2%) required an ICU stay, with a median duration of 1 day [range 1–38]. The median length of postoperative hospital stay was 12 days [range 6–78]. Postoperative complications occurred in 9 patients (42.9%), including 6 minor (28.6%) and 3 major complications (14.3%). The most commonly resected organs were the kidney (n = 11; 52.4%) and the liver (n = 6; 28.6%). Indications for multivisceral resection included adhesions to adjacent organs (n = 4; 19.1%), suspected infiltration of surrounding organs or vessels (n = 7; 33.3%), liver metastases (n = 4; 19.1%), the need for improved surgical exposure (n = 1; 4.8%), and the presence of tumor thrombus (n = 1; 4.8%). Of the four patients (19.1%) who underwent multivisceral resection due to suspected organ infiltration, liver involvement was histologically confirmed in two (9.5%) and diaphragmatic infiltration in one (4.8%). No pathological evidence of infiltration was found in the resected kidneys, pancreas, or spleen. In 18 patients (85.7%), the suspected diagnosis of ACC was confirmed histologically. The remaining three patients (14.3%) were diagnosed with other tumor entities: one sarcoma, one adrenal metastasis of non-small cell lung carcinoma, and one ganglioneuroma. Examples of preoperative CT scans of patients with suspected ACC and organ infiltration are presented in Figure 1.

Table 2.
Multivisceral resection for suspected adrenocortical carcinoma with curative intention—intra- and postoperative outcomes (n = 21).
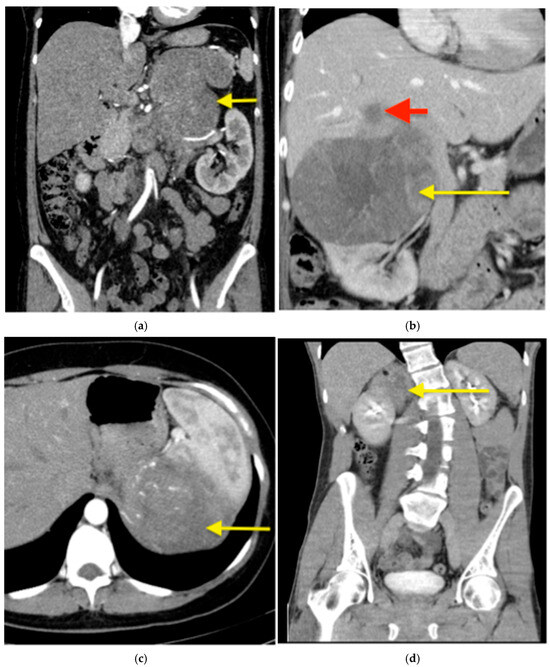
Figure 1.
Suspected ACC in computed tomography (CT)—examples. (a) A CT with contrast of a 42-year-old female patient with a left sided ACC (arrow) producing cortisol, testosterone and dehydroepiandrosterone, measuring 170 mm in the largest diameter with a suspected infiltration of renal hilum. The adrenal tumour was resected en bloc with the left kidney. (b) A CT with contrast of a 49-year-old female patient with a right-sided, cortisol producing ACC (yellow arrow), measuring 150 mm in largest diameter with a liver metastasis in segment VII (red arrow), which was resected en bloc with the adrenal tumour. (c) A CT with contrast of a 39-year-old female patient with a hormonally inactive left-sided ACC (arrow), measuring 215 mm in the largest diameter. Preoperatively suspected adhesions to the spleen in the preoperative cross-sectional imaging were intraoperatively loosened. This resulted in a bleeding that required a blood transfusion and ultimately a splenectomy. (d) A native CT of a 31-year-old patient with a hormonally inactive tumour (arrow). Infiltration of the kidney by the tumour was suspected both preoperatively on cross-sectional imaging and intraoperatively. A partial nephrectomy was performed en bloc with the adrenal tumour. Histopathological examination confirmed a ganglioneuroma—a benign tumour without kidney infiltration.
For patients with ACC, patient and tumour characteristics, as well as intraoperative and postoperative outcomes, were compared between the multivisceral resection (n = 18, 48.6%) and isolated adrenalectomy (n = 19, 51.4%) groups (Table 3 and Table 4, respectively). The results of the Shapiro–Wilk normality test conducted prior to comparative analyses are presented in Table A2. Patients who underwent multivisceral resection were younger and had larger and more advanced tumors. Lymph node metastases were found only in patients who underwent multivisceral resection. Periadrenal lymph node metastases were detected in five (27.8%) patients, and a paracaval lymph node metastasis was observed in one (5.6%) patient.

Table 3.
Comparison of patient and tumour characteristics in patients undergoing multivisceral resection and isolated adrenalectomy for ACC with curative intention (n = 37).

Table 4.
Comparison of intra- and postoperative outcomes in patients undergoing multivisceral resection and isolated adrenalectomy for ACC with curative intention (n = 37).
Tumour hormonal activity did not differ between the groups. The most common hormone excess was combined cortisol and androgen secretion, observed in 5 (27.7%) patients in the multivisceral resection group and 6 (33.3%) patients in the isolated adrenalectomy group. Isolated androgen excess was present in 6 (33.3%) patients in the multivisceral resection group and 1 (5.5%) patient in the isolated adrenalectomy group. Isolated cortisol excess was found in 3 (16.7%) patients in the multivisceral resection group and 3 (16.7%) patients in the isolated adrenalectomy group. Aldosterone secretion was detected in 2 (10.5%) patients in the isolated adrenalectomy group, while no cases were reported in the multivisceral resection group. An overview of hormonal oversecretion by adrenal tumors is presented in Table A3.
All but one patient who underwent multivisceral resection had open surgery (94.4%); one case (5.6%) required conversion from a laparoscopic to an open approach. No procedures in this group were completed laparoscopically. Multivisceral resections had longer operative times compared to isolated adrenalectomies. All patients in this group required postoperative ICU care and had longer hospital stays. There was no statistically significant increase in complication rates in the multivisceral resection group compared to isolated adrenalectomy group.
Follow-up data were collected in June 2025 and were not available for two ACC patients who underwent multivisceral resection and one patient who underwent isolated adrenalectomy; therefore, these patients were excluded from the survival analysis. Among the ACC patients included in the survival analysis, the median follow-up time was 46.5 months (range: 4–154 months), and the median progression-free survival was 20.5 months (range: 3–154 months). Survival analysis revealed no statistically significant difference in overall survival (OS) between surgical techniques (log-rank p = 0.71). The progression-free (PFS) survival was 9.5 months [3–154 months] for patients undergoing multivisceral resections and 31 months [4–92 months] for those undergoing isolated adrenalectomies. However, the difference in PFS between surgical techniques was not statistically significant (log-rank p > 0.05), as shown in Figure 2.
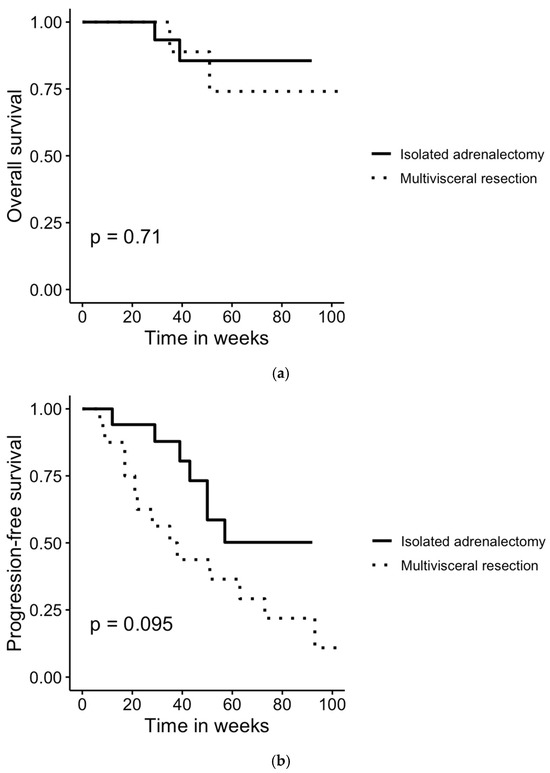
Figure 2.
Comparison of (a) overall and (b) progression-free survival in patients undergoing multivisceral resection and isolated adrenalectomy for histologically confirmed ACC. Survival rates were compared using log-rank test. No statistically significant differences were found between the groups.
In the multivisceral resection group with available follow-up, two patients (12.5%) did not receive any adjuvant therapy. Six patients (37.5%) received mitotane monotherapy, and seven (43.8%) received combined therapy with mitotane and etoposide, doxorubicin, and paclitaxel (EDP). One patient (6.3%) additionally received radiotherapy. None of the patients received a neoadjuvant therapy.
Among those who experienced disease recurrence in this group, two patients (12.5%) had a local recurrence, eight (50.0%) developed new distant metastases, and one had both. One patient (6.3%) had lymph node metastasis, and another (6.3%) had both new distant metastases and progression of previously known metastases. Among the nine patients with distant metastases, six (66.7%) had pulmonary metastases, two (22.2%) had liver metastases, and one (11.1%) had concurrent pulmonary, hepatic, and lymph node metastases.
In the isolated adrenalectomy group with available follow-up, four patients (22.2%) did not receive any adjuvant therapy. Eleven patients (61.1%) received mitotane monotherapy, one (5.6%) received mitotane and EDP, and one (5.6%) received mitotane and radiotherapy.
Among patients in this group who experienced disease recurrence (n = 7), one (14.3%) had a local recurrence, five (71.4%) developed new distant metastases, and one (14.3%) had both a new distant metastasis and lymph node metastasis. Among the six patients with distant metastases, four (66.7%) had pulmonary metastases, one (16.7%) had bone metastasis, and one (16.7%) had liver metastasis.
4. Discussion
In this retrospective cohort study, we analyzed outcomes in 21 patients who underwent multivisceral resection for suspected adrenocortical carcinoma (ACC). We compared those with histologically confirmed ACC (n = 18) to 19 patients who underwent isolated adrenalectomy for ACC. We found that patients in the multivisceral resection group had significantly larger and more advanced tumors, with a higher ENSAT stage and greater S-GRAS scores. Despite more aggressive disease features, R0 resection was achieved in over 70% of multivisceral cases. Importantly, lymph node metastases were identified exclusively in this group, highlighting the need of lymphadenectomy in advanced cases. Although multivisceral resections were associated with longer operative times and consistently required intensive postoperative care, complication rates and overall survival did not differ significantly between the groups. Histologic confirmation of true organ invasion was limited, underscoring the difficulty of preoperative assessment. These findings support the feasibility and potential oncologic benefit of multivisceral resection in selected patients with advanced ACC.
ACC presents unique diagnostic challenges, particularly in differentiating it preoperatively from benign or metastatic lesions. In our series, 3 of 21 patients who underwent multivisceral resection were ultimately diagnosed with non-ACC histologies (sarcoma, adrenal metastasis, ganglioneuroma), despite radiological suspicion. Yalon et al. [6] introduced a score based on identification of the following features: size, attenuation, thin and thick rim enhancement patterns, heterogeneity, calcification, necrosis, fat infiltration, and lymph node prominence for the differentiation of ACC from lipid-poor adrenal adenoma with 100% sensitivity and 80% specificity [6]. Furthermore, Mihai et al. [3] suggested that the boarder for suspicions of ACC should be elevated to 20 HU to improve specificity of this diagnostic tool [3]. While cross-sectional imaging cannot definitively confirm malignancy [1], and tumor biopsy is discouraged due to the risk of tumor dissemination and impaired resectability [3], advances in liquid biopsy, including circulating tumor cells, tumor DNA and specific microRNAs (miRNAs; e.g., miR-483-5p, miR-210), offer promising approaches for non-invasive diagnosis and monitoring of ACC [15,16,17,18]. These tools may improve preoperative diagnostic accuracy and help avoid unnecessary extensive surgeries for benign lesions, though prospective validation is needed.
Multivisceral resection was pursued in our cohort primarily due to suspected infiltration of adjacent organs or vessels, adhesions, and tumor thrombus. However, histopathological confirmation of organ invasion was limited—found only in the liver (2 cases) and diaphragm (1 case)—highlighting the challenge of preoperatively recognizing true invasion. Notably, no pathological evidence of kidney infiltration was found in any case, despite nephrectomy being performed in over half of the patients (52.4%). This observation supports the recommendation by Mihai et al. and that kidney preservation should be prioritized whenever oncologically safe, as the adrenal tumor rarely invades the kidney directly [3]. Furthermore, data by Propiglia et al. [19] reinforce this conclusion, showing that nephrectomy does not improve oncologic outcomes in patients with stage II ACC, and thus should be avoided in the absence of overt renal involvement [19]. In our cohort, R0 resection was still achieved in 72.2% of multivisceral resection cases, demonstrating that complete tumor removal is feasible in anatomically challenging cases. Compared to isolated adrenalectomy, patients undergoing multivisceral resection had significantly larger tumors, higher S-GRAS and ENSAT stage scores, and more frequent vascular invasion. These procedures required open or converted approaches, longer operative times, and extended ICU care. Despite these challenges, complication rates and overall survival did not significantly differ between multivisceral and isolated adrenalectomy groups, even though the former had significantly more advanced disease. Our findings are consistent with previous studies. Procopio et al. [20] demonstrated that stage III ACC patients who underwent extended en bloc R0 resections had overall and disease-free survival comparable to those with stage I/II disease, and without increased postoperative morbidity [20]. Furthermore, it had previously been shown, that stage IV patients with metastatic disease had a longer overall survival, if metastases were resected. Shariq et al. [21] reported that multivisceral resection was associated with longer median overall survival (median 33 months) compared to isolated adrenalectomy (median 22 months), with both outperforming systemic therapies alone [21]. Baur et al. [22] focused solely on ACC patients with liver metastasis and could show that while disease-free survival was short (median 9.1 months), the overall survival was significantly better in patients with resected vs. not resected metastases (median 76.1 vs. 10.1 months) [22]. These data, along with our findings, reinforce that multivisceral resection, when performed in high-volume centers, can offer meaningful oncologic benefit in selected patients, particularly when complete resection is achievable.
Lymphnode metastases were revealed in one-third of patients in the multivisceral group. It is recommended to include locoregional lymph node dissection as part of surgery for suspected ACC, especially when preoperative cross-sectional imaging suggests nodal involvement [3,23]. The absence of nodal metastases in the isolated adrenalectomy group highlights the association between advanced disease and lymphatic spread. While the therapeutic benefit of lymphadenectomy remains debated, studies such as those by Reibetanz et al. [24] and Deschner et al. [25] emphasize its prognostic value. In our series, nodal dissection contributed to accurate staging and informed postoperative therapy decisions.
None of the patients in our cohort received neoadjuvant therapy prior to surgery. Neoadjuvant EDP-M therapy has shown potential to downstage tumors, facilitate R0 resection, and select biologically favorable tumors for surgery [1,26,27]. Given the limited progression-free survival observed in the multivisceral group (median 9.5 months), the potential role of preoperative systemic therapy in improving outcomes deserves exploration in future studies.
All multivisceral resections in this study were performed via open approaches, except for one case that required conversion from a minimally invasive approach. Although a laparoscopic approach for ENSAT stage I–III adrenocortical carcinoma (ACC) offers comparable oncologic outcomes to open surgery when an R0 resection is achieved [28], current guidelines discourage laparoscopy in cases of suspected local invasion [7]. While both laparoscopic [28] and retroperitoneoscopic [29] approaches are feasible for adrenalectomy in malignant tumors, robotic adrenalectomy may offer additional advantages in ACC surgery, particularly by reducing conversion rates [30]. Given the technical complexity of these procedures and the need for intensive postoperative care, current recommendations support performing ACC surgeries in specialized, high-volume centers [31,32,33]. In our series, high R0 resection rates and acceptable morbidity underscore the value of surgical expertise in optimizing outcomes.
This study has several limitations. First, the retrospective design raises the possibility of selection bias. Second, the study was conducted at a single tertiary referral center, which may affect the generalizability of the findings to other settings with varying surgical expertise and case volumes. Finally, the relatively small sample size, particularly within subgroup comparisons, limits statistical power and precludes multivariable analysis to adjust for potential confounding factors. Despite these limitations, the study provides valuable insights into the role and outcomes of multivisceral resection in the management of suspected ACC and highlights key areas for future multicentric prospective investigation.
5. Conclusions
Our study demonstrates that multivisceral resection for suspected or advanced adrenocortical carcinoma is feasible and can achieve high rates of complete (R0) resection, even in cases with aggressive disease features. Importantly, although baseline disease severity was consistently higher in patients requiring multivisceral resection, outcomes in terms of morbidity and overall survival were comparable to those of isolated adrenalectomy. This indicates that, in the hands of a skilled and highly experienced surgical team, multivisceral resection can offer prognostic results equivalent to single-adrenalectomy, thereby reinforcing surgery as the cornerstone of a multimodal treatment approach. Moreover, our findings underscore the importance of lymphadenectomy for accurate staging and highlight the ongoing need for improved preoperative diagnostic tools to optimize treatment of patients with other adrenal or retroperitoneal tumour entities. Future prospective, multicenter studies are needed to refine patient selection and clarify the role of neoadjuvant therapies in the management of this challenging malignancy.
Author Contributions
Conceptualization, A.D., F.B. and M.T.M.; methodology, A.D. and M.T.M.; formal analysis, A.D., F.B. and P.I.G.; investigation, A.D., F.B. and P.I.G.; data curation, A.D., D.S. and P.I.G.; writing—original draft preparation, A.D. and P.I.G.; writing—review and editing, A.D., F.B., P.I.G., D.S., K.M., W.S., R.O., J.P. and M.T.M.; supervision, A.D., F.B. and M.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in compliance with the principles of the Declaration of Helsinki, following approval from the institutional review board (EA1/394/20, 21 January 2021 and Gliedkörgerschaft der Freien Universität Berlin und der Humboldt-Univers’tät zu Berlin Charitéplatz, EA1/097/21, 23 April 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective and non-interventional character of the study.
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to reasons of sensitivity.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | Adrenocortical carcinoma |
| ENSAT | European Network for the Study of Adrenal Tumors |
| ICU | Intensive Care Unit |
| CT | Computed tomography |
| HU | Hounsfield Units |
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| VCI | Vena cava inferior |
| S-GRAS | tumor stage, grade, resection status, age, symptoms |
| EDP | etoposide, doxorubicin, paclitaxel |
| PFS | Progression-free survival |
| OS | Overall survival |
| DNA | Deoxyribonucleic acid |
| miRNA | microRNA |
| EDP-M | Etoposide, doxorubicin, paclitaxel—mitotane |
Appendix A

Table A1.
Shapiro–Wilk test results assessing data distribution in patients with suspected adrenocortical carcinoma.
Table A1.
Shapiro–Wilk test results assessing data distribution in patients with suspected adrenocortical carcinoma.
| Multivisceral Resection for Suspected ACC (n = 21) | ||
|---|---|---|
| Variable | p-Value | Distribution |
| Age (years) | 0.806 | Normal |
| BMI (kg/m2) (n.a. = 1) | <0.001 | Non-normal |
| Tumour size (mm) | 0.453 | Normal |
| Duration of surgery (minutes) | <0.001 | Non-normal |
| Length of postoperative ICU stay | <0.001 | Non-normal |
| Length of postoperative hospital stay | <0.001 | Non-normal |
ACC, adrenocortical carcinoma; BMI, body mass index; ICU, intensive care unit; n.a., not available.

Table A2.
Shapiro–Wilk test results assessing data distribution in patients with confirmed adrenocortical carcinoma.
Table A2.
Shapiro–Wilk test results assessing data distribution in patients with confirmed adrenocortical carcinoma.
| Multivisceral Resection (n = 18) | Isolated Adrenalectomy (n = 19) | |||
|---|---|---|---|---|
| Variable | p-Value | Distribution | p-Value | Distribution |
| Age (years) | 0.919 | Normal | 0.02732 | Non-normal |
| BMI (kg/m2) (n.a. = 1) | 0.629 | Normal | 0.590 | Normal |
| Tumour size (mm) | 0.579 | Normal | 0.314 | Normal |
| Ki67 | <0.001 | Non-normal | 0.003 | Non-normal |
| Duration of surgery (min) | 0.002 | Non-normal | 0.017 | Non-normal |
| Length of postoperative ICU stay | <0.001 | Non-normal | 0.002 | Non-normal |
| Length of postoperative hospital stay | <0.001 | Non-normal | <0.001 | Non-normal |
BMI, body mass index; ICU, intensive care unit; n.a., not available.

Table A3.
Overview of hormonal oversecretion by adrenal tumors.
Table A3.
Overview of hormonal oversecretion by adrenal tumors.
| Hormonal Secretion | Multivisceral Resection (n = 18) | Isolated Adrenalectomy (n = 19; n.a. = 1) |
|---|---|---|
| None | 4 (22.2%) | 6 (33.3%) |
| Aldosterone | 0 (0%) | 2 (11.1%) |
| Cortisole | 3 (16.7%) | 3 (16.7%) |
| Androgen | ||
| 17-OH-Progesterone | 2 (11.1%) | 1 (5.5%) |
| DHEAS | 2 (11.1%) | 0 (0%) |
| Androstendione | 1 (5.5%) | 0 (0%) |
| 17-OH-Progesterone, DHEAS and | 1 (5.5%) | 0 (0%) |
| Oestradiole | ||
| Cortisole and Androgen | ||
| Cortisol and 17-OH-Progesterone | 0 (0%) | 1 (5.5%) |
| Cortisole and DHEAS | 1 (5.5%) | 0 (0%) |
| Cortisole and Androstendione | 1 (5.5%) | 0 (0%) |
| Cortisole, 17-OH-Progesterone and DHEAS | 0 (0%) | 2 (11.1%) |
| Cortisole, 17-OH-Progesterone and Androstendione | 3 (16.7%) | 0 (0%) |
| Cortisole, 17-OH-Progesterone, DHEAS and Androstendione | 0 (0%) | 3 (16.7%) |
DHEAS, dehydroepiandrosterone sulfate; n.a., not available.
References
- Fassnacht, M.; Puglisi, S.; Kimpel, O.; Terzolo, M. Adrenocortical carcinoma: A practical guide for clinicians. Lancet Diabetes Endocrinol. 2025, 13, 438–452. [Google Scholar] [CrossRef]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef]
- Mihai, R.; De Crea, C.; Guerin, C.; Torresan, F.; Agcaoglu, O.; Simescu, R.; Walz, M.K. Surgery for advanced adrenal malignant disease: Recommendations based on European Society of Endocrine Surgeons consensus meeting. Br. J. Surg. 2024, 111, znad266. [Google Scholar] [CrossRef]
- Bancos, I.; Chortis, V.; Jenkinson, C.; Tsagarakis, S.; Macech, M.; Riester, A.; Deutschbein, T.; Kienitz, T.; Prete, A.; Gilligan, L.C.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Walz, M.K.; Metz, K.A.; Theurer, S.; Myland, C.; Alesina, P.F.; Schmid, K.W. Differentiating benign from malignant adrenocortical tumors by a single morphological parameter—A clinicopathological study on 837 adrenocortical neoplasias. Indian J. Surg. Oncol. 2020, 11, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Yalon, T.; Yalon, M.; Assaf, D.; Lenartowicz, K.; Foster, T.; Lyden, M.; Dy, B.; Bancos, I.; McKenzie, T. Differentiating between adrenocortical carcinoma and lipid-poor cortical adenoma: A novel cross-sectional imaging-based score. Surgery 2023, 173, 35–42. [Google Scholar] [CrossRef]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef] [PubMed]
- Jouinot, A.; Bertherat, J. Diseases Predisposing to Adrenocortical Malignancy (Li-Fraumeni Syndrome, Beckwith-Wiedemann Syndrome, and Carney Complex). Exp. Suppl. 2019, 111, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Raymond, V.M.; Everett, J.N.; Furtado, L.V.; Gustafson, S.L.; Jungbluth, C.R.; Gruber, S.B.; Hammer, G.D.; Stoffel, E.M.; Greenson, J.K.; Giordano, T.J.; et al. Adrenocortical carcinoma is a lynch syndrome-associated cancer. J. Clin. Oncol. 2013, 31, 3012–3018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nastos, C.; Papaconstantinou, D.; Paspala, A.; Pararas, N.; Vryonidou, A.; Pikouli, A.; Chronopoulou, E.; Lechou, A.; Peppa, M.; Pikoulis, E. The impact of adrenocortical carcinoma hormone secreting status as a predictor of poor survival: A systematic review and meta-analysis. Langenbecks Arch. Surg. 2024, 409, 316. [Google Scholar] [CrossRef] [PubMed]
- Dobrindt, E.M.; Saeger, W.; Bläker, H.; Mogl, M.T.; Bahra, M.; Pratschke, J.; Rayes, N. The challenge to differentiate between sarcoma or adrenal carcinoma—An observational study. Rare Tumors 2021, 13, 20363613211057746. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.; Hahner, S.; Allolio, B.; et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: Proposal for a revised TNM classification. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Altieri, B.; Berhane, S.; Cosentini, D.; Calabrese, A.; Haissaguerre, M.; Kastelan, D.; Fragoso, M.C.B.V.; Bertherat, J.; Al Ghuzlan, A.; et al. S-GRAS score for prognostic classification of adrenocortical carcinoma: An international, multicenter ENSAT study. Eur. J. Endocrinol. 2021, 186, 25–36. [Google Scholar] [CrossRef]
- Perge, P.; Nyirő, G.; Vékony, B.; Igaz, P. Liquid biopsy for the assessment of adrenal cancer heterogeneity: Where do we stand? Endocrine 2022, 77, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Pinzani, P.; Scatena, C.; Salvianti, F.; Corsini, E.; Canu, L.; Poli, G.; Paglierani, M.; Piccini, V.; Pazzagli, M.; Nesi, G.; et al. Detection of circulating tumor cells in patients with adrenocortical carcinoma: A monocentric preliminary study. J. Clin. Endocrinol. Metab. 2013, 98, 3731–3738. [Google Scholar] [CrossRef]
- Garinet, S.; Nectoux, J.; Neou, M.; Pasmant, E.; Jouinot, A.; Sibony, M.; Orhant, L.; da Fonseca, J.P.; Perlemoine, K.; Bricaire, L.; et al. Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr. Relat. Cancer 2018, 25, L13–L17. [Google Scholar] [CrossRef]
- Mytareli, C.; Kalotychou, V.; Lafioniatis, A.; Kaltsas, G.; Zografos, G.N.; Markou, A.; Papanastasiou, L.; Fountas, A.; Vasilliadi, D.A.; Kassi, E.; et al. Evaluation of microRNAs as liquid biopsy markers in adrenocortical tumors. Front. Endocrinol. 2025, 16, 1511520. [Google Scholar] [CrossRef]
- Porpiglia, F.; Fiori, C.; Daffara, F.C.; Zaggia, B.; Ardito, A.; Scarpa, R.M.; Papotti, M.; Berruti, A.; Scagliotti, G.V.; Terzolo, M. Does nephrectomy during radical adrenalectomy for stage II adrenocortical cancer affect patient outcome? J. Endocrinol. Investig. 2016, 39, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Procopio, P.F.; Pennestrì, F.; Laurino, A.; Rossi, E.D.; Schinzari, G.; Pontecorvi, A.; De Crea, C.; Raffaelli, M. Impact of en bloc extended R0 resections on oncological outcome of locally advanced adrenocortical carcinoma. Updates Surg. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Shariq, O.A.; Kensing, B.; Landry, J.P.; Tzeng, C.-W.D.; Habra, M.A.; Campbell, M.T.; Fisher, S.B.; Perrier, N.D.; Lee, J.E.; Graham, P.H. Surgical management of adrenocortical carcinoma: Is there a role for multivisceral resection? In Proceedings of the AAES 2025 Annual Meeting, Birmingham, AL, USA, 26–28 April 2025; Available online: https://aaes.memberclicks.net/assets/2025_Meeting/AAES_2025_Program_Book-Final.pdf (accessed on 27 August 2025).
- on behalf of the German Adrenocortical Carcinoma Study Group; Baur, J.; Büntemeyer, T.O.; Megerle, F.; Deutschbein, T.; Spitzweg, C.; Quinkler, M.; Nawroth, P.; Kroiss, M.; Germer, C.-T.; et al. Outcome after resection of adrenocortical carcinoma liver metastases: A retrospective study. BMC Cancer 2017, 17, 522. [Google Scholar] [CrossRef]
- de Ponthaud, C.; Bekada, S.; Buffet, C.; Roy, M.; Bachelot, A.; Ayed, A.; Menegaux, F.; Gaujoux, S. Which lymphadenectomy for adrenocortical carcinoma? Surgery 2024, 176, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Reibetanz, J.; Jurowich, C.; Erdogan, I.; Nies, C.; Rayes, N.; Dralle, H.; Behrend, M.; Allolio, B.; Fassnacht, M. Impact of lymphadenectomy on the oncologic outcome of patients with adrenocortical carcinoma. Ann. Surg. 2012, 255, 363–369. [Google Scholar] [CrossRef]
- Deschner, B.W.; Stiles, Z.E.; DeLozier, O.M.; Drake, J.A.; Tsao, M.; Glazer, E.S.; Do, J.L.D.; Yakoub, D.; Dickson, P.V. Critical analysis of lymph node examination in patients undergoing curative-intent resection for adrenocortical carcinoma. J. Surg. Oncol. 2020, 122, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, B.K.; Habra, M.A.; Phan, A.; Milton, D.R.; Wood, C.; Vauthey, N.; Evans, D.B.; Katz, M.H.; Ng, C.S.; Perrier, N.D.; et al. Borderline resectable adrenal cortical carcinoma: A potential role for preoperative chemotherapy. World J. Surg. 2014, 38, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Laganà, M.; Grisanti, S.; Cosentini, D.; Ferrari, V.D.; Lazzari, B.; Ambrosini, R.; Sardini, C.; Volta, A.D.; Palumbo, C.; Poliani, P.L.; et al. Efficacy of the EDP-M scheme plus adjunctive surgery in the management of patients with advanced adrenocortical carcinoma: The Brescia experience. Cancers 2020, 12, 941. [Google Scholar] [CrossRef]
- Mpaili, E.; Moris, D.; Tsilimigras, D.I.; Oikonomou, D.; Pawlik, T.M.; Schizas, D.; Papalampros, A.; Felekouras, E.; Dimitroulis, D. Laparoscopic versus open adrenalectomy for localized/locally advanced primary adrenocortical carcinoma (ENSAT I–III) in adults: Is margin-free resection the key surgical factor that dictates outcome? A review of the literature. J. Laparoendosc. Adv. Surg. Tech. A 2018, 28, 408–414. [Google Scholar] [CrossRef]
- Alesina, P.F.; Knyazeva, P.; Walz, M.K. Adrenocortical carcinoma: The posterior minimally invasive approach. In Primary Adrenal Malignancies; Springer Nature: Cham, Switzerland, 2024; pp. 91–97. [Google Scholar]
- Hue, J.J.; Ahorukomeye, P.; Bingmer, K.; Drapalik, L.; Ammori, J.B.; Wilhelm, S.M.; Rothermel, L.D.; Towe, C.W. A comparison of robotic and laparoscopic minimally invasive adrenalectomy for adrenal malignancies. Surg. Endosc. 2022, 36, 5374–5381. [Google Scholar] [CrossRef]
- Gaujoux, S.; Mihai, R.; Joint Working Group of ESES and ENSAT. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br. J. Surg. 2017, 104, 358–376. [Google Scholar] [CrossRef]
- Terzolo, M.; Fassnacht, M. Endocrine tumours: Our experience with the management of patients with non-metastatic adrenocortical carcinoma. Eur. J. Endocrinol. 2022, 187, R27–R40. [Google Scholar] [CrossRef]
- Fassnacht, M.; Assié, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardière, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).