Effectiveness of Non-Immersive Virtual Reality on Gross Motor Function, Balance, and Functional Independence in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Search Process and Databases
2.4. Data Collection and Selection Procedure
2.5. Methodological Quality Assessment
2.6. Data Synthesis
2.7. Risk of Bias in Individual Studies
2.8. Summary Measures for Meta-Analysis
2.9. Factor Analysis of Single-Training
2.10. Assessment of the Certainty of the Evidence
3. Results
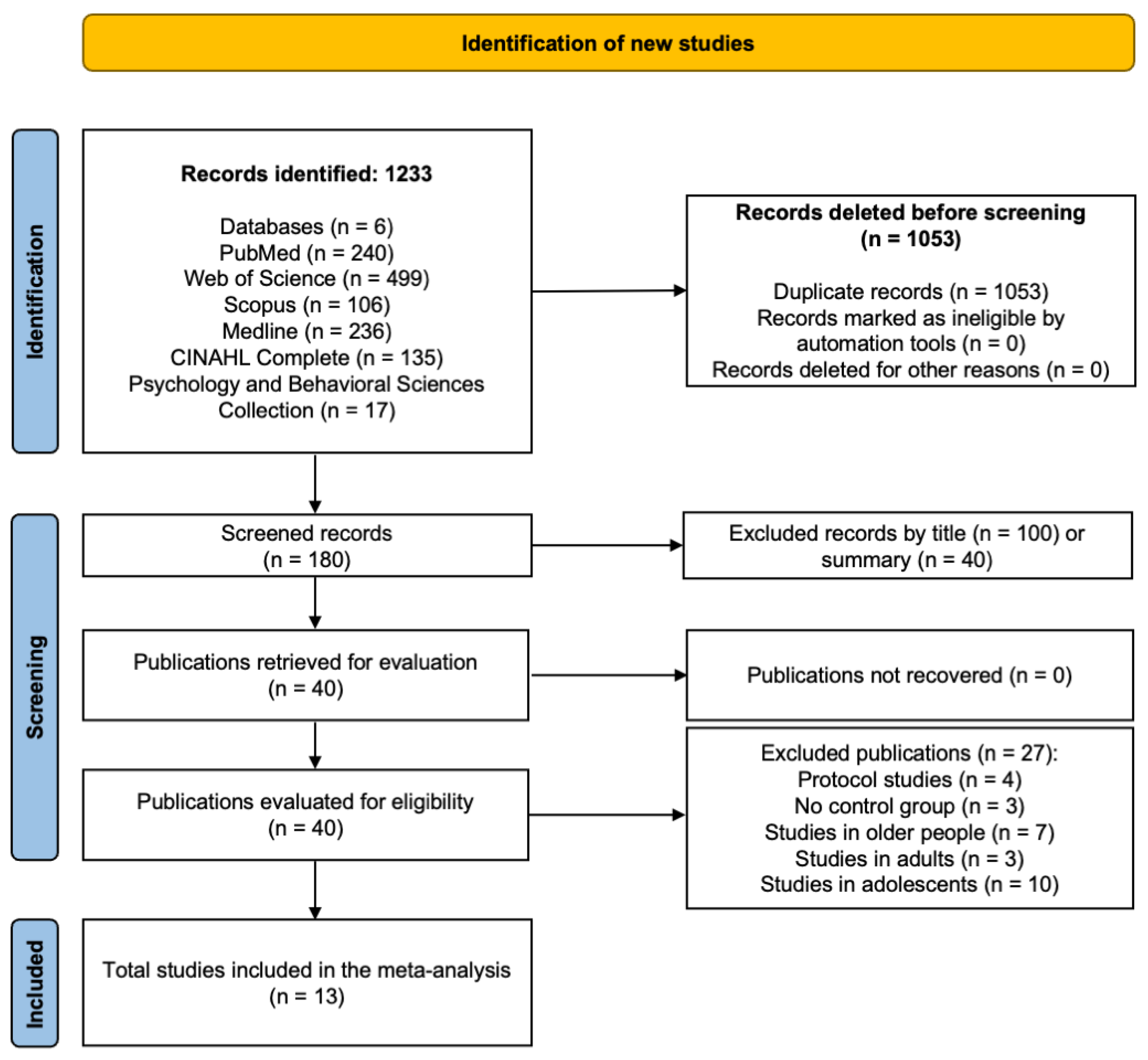
3.1. Study Selection
3.2. Methodological Quality
3.3. Characteristics of the Studies
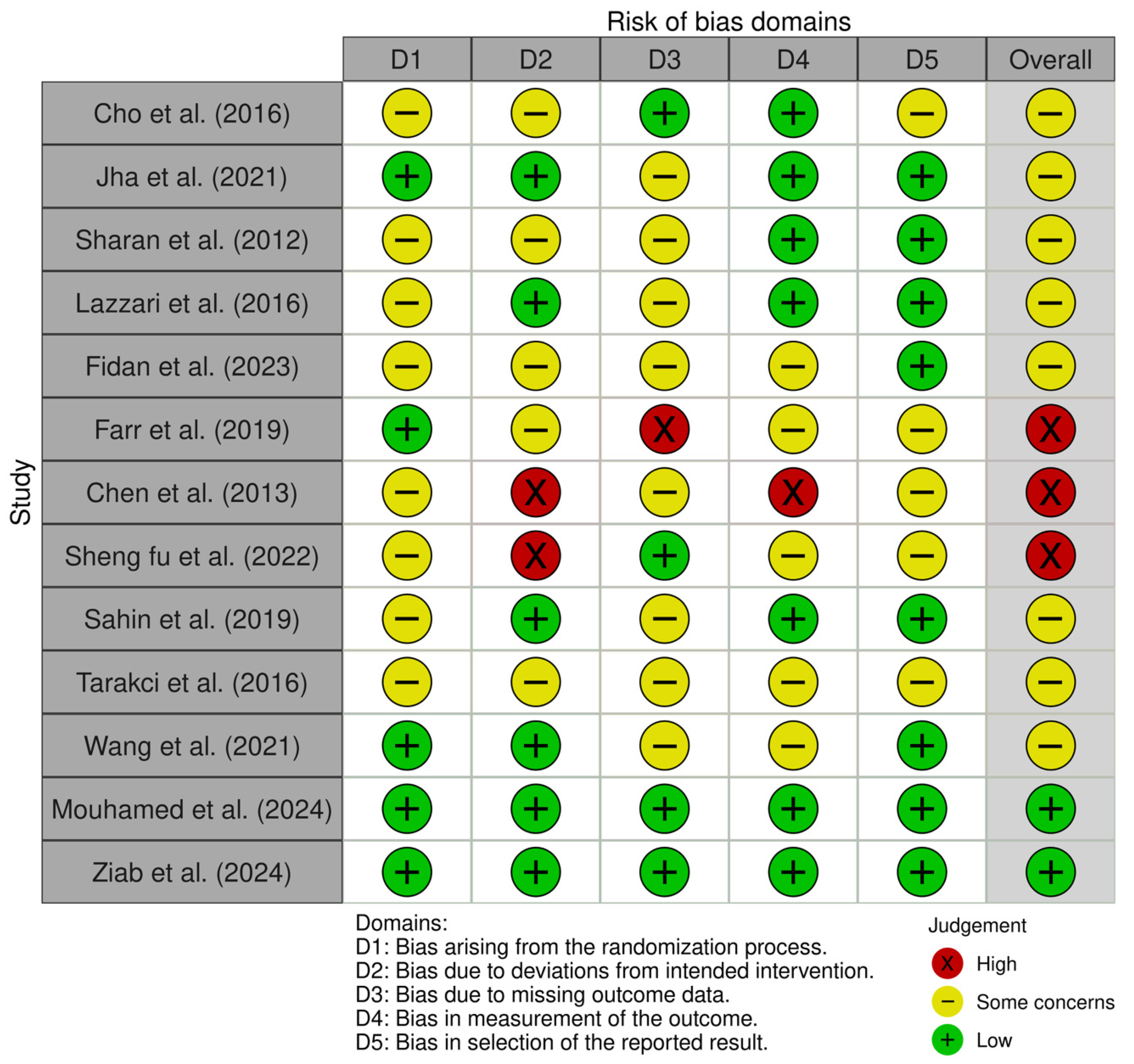
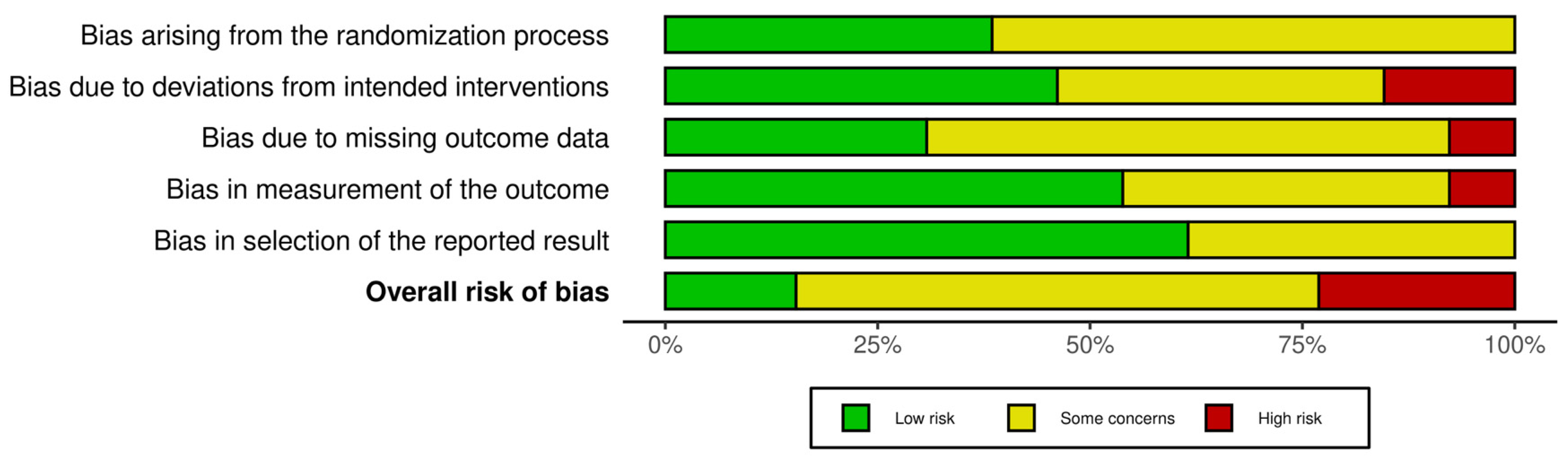
3.4. Risk of Bias
3.5. Sample Characteristics
3.6. Dosing and Conducted Interventions
3.7. Meta-Analysis Results
3.8. Moderator Analysis
3.9. Certainty of Evidence
3.10. Adverse Events and Adherence
4. Discussion
4.1. Gross Motor Function
4.2. Balance
4.3. Functional Independence
4.4. Subgroup Analysis by Session Duration (Minutes)
4.5. Subgroup Analysis by Training Duration
4.6. Subgroup Analysis by Total Sessions
4.7. Limitations and Strengths
4.8. Practical Applications
4.9. Epidemiological Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A Report: The Definition and Classification of Cerebral Palsy April 2006. Dev. Med. Child Neurol. Suppl. 2007, 109, 8–14. [Google Scholar]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An Update on the Prevalence of Cerebral Palsy: A Systematic Review and Meta-Analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Maher, C.A.; Williams, M.T.; Olds, T.; Lane, A.E. An Internet-Based Physical Activity Intervention for Adolescents with Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child Neurol. 2010, 52, 448–455. [Google Scholar] [CrossRef]
- Verschuren, O.; Darrah, J.; Novak, I.; Ketelaar, M.; Wiart, L. Health-Enhancing Physical Activity in Children with Cerebral Palsy: More of the Same Is Not Enough. Phys. Ther. 2014, 94, 297–305. [Google Scholar] [CrossRef]
- Bloemen, M.; Van Wely, L.; Mollema, J.; Dallmeijer, A.; de Groot, J. Evidence for Increasing Physical Activity in Children with Physical Disabilities: A Systematic Review. Dev. Med. Child Neurol. 2017, 59, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Smits, D.W.; Gorter, J.W.; Ketelaar, M.; Van Schie, P.E.; Dallmeijer, A.J.; Lindeman, E.; Jongmans, M.J. Relationship between Gross Motor Capacity and Daily-Life Mobility in Children with Cerebral Palsy. Dev. Med. Child Neurol. 2010, 52, e60–e66. [Google Scholar] [CrossRef]
- Verschuren, O.; Ketelaar, M.; Takken, T.; Helders, P.J.; Gorter, J.W. Exercise Programs for Children with Cerebral Palsy: A Systematic Review of the Literature. Am. J. Phys. Med. Rehabil. 2008, 87, 404–417. [Google Scholar] [CrossRef]
- Sharan, D.; Ajeesh, P.S.; Rameshkumar, R.; Mathankumar, M.; Paulina, R.J.; Manjula, M. Virtual Reality Based Therapy for Post Operative Rehabilitation of Children with Cerebral Palsy. Work 2012, 41, 3612–3615. [Google Scholar] [CrossRef] [PubMed]
- Meriggi, P.; Mandalà, M.; Randazzo, M.; Brazzoli, E.; Castagna, A.; Di Giusto, V.; Cavallini, A.; Marzegan, A.; Lencioni, T.; Olivieri, I. Non-Immersive Virtual Reality Based Treatment for Children with Unilateral Cerebral Palsy: Preliminary Results. J. Pediatr. Rehabil. Med. 2024, 17, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.E.; Borbely, M.; Filler, J.; Huhn, K.; Guarrera-Bowlby, P. Use of a Low-Cost, Commercially Available Gaming Console (Wii) for Rehabilitation of an Adolescent with Cerebral Palsy. Phys. Ther. 2008, 88, 1196–1207. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Weiss, P.L.; Rand, D.; Katz, N.; Kizony, R. Video Capture Virtual Reality as a Flexible and Effective Rehabilitation Tool. J. Neuroeng. Rehabil. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.; Roopchand-Martin, S.; Gregg, A. Potential of the Nintendo Wii™ as a Rehabilitation Tool for Children with Cerebral Palsy in a Developing Country: A Pilot Study. Physiotherapy 2012, 98, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Sandlund, M.; Waterworth, E.L.; Häger, C. Using Motion Interactive Games to Promote Physical Activity and Enhance Motor Performance in Children with Cerebral Palsy. Dev. Neurorehabil. 2011, 14, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.; Glegg, S.M.; Sveistrup, H.; Colquhoun, H.; Miller, P.A.; Finestone, H.; DePaul, V.; Harris, J.E.; Velikonja, D. A Knowledge Translation Intervention to Enhance Clinical Application of a Virtual Reality System in Stroke Rehabilitation. BMC Health Serv. Res. 2016, 16, 557. [Google Scholar] [CrossRef]
- Fidan, Ö.; Genç, A. Effect of Virtual Reality Training on Balance and Functionality in Children with Cerebral Palsy: A Randomized Controlled Trial. Türk Fizyoter. Rehabil. Derg. 2023, 34, 64–72. [Google Scholar] [CrossRef]
- Tarakci, D.; Ersoz Huseyinsinoglu, B.; Tarakci, E.; Razak Ozdincler, A. Effects of Nintendo Wii-Fit® Video Games on Balance in Children with Mild Cerebral Palsy. Pediatr. Int. 2016, 58, 1042–1050. [Google Scholar] [CrossRef]
- Mouhamed, H.A.; Abo-Zaid, N.A.; Khalifa, H.A.; Ali, M.E.; Elserty, N.S.; Behiry, M.A.; Heneidy, W.E. Efficacy of Virtual Reality on Balance Impairment in Ataxic Cerebral Palsy Children: Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 949–955. [Google Scholar] [CrossRef]
- Goyal, C.; Vardhan, V.; Naqvi, W. Virtual Reality-Based Intervention for Enhancing Upper Extremity Function in Children with Hemiplegic Cerebral Palsy: A Literature Review. Cureus 2022, 14, e21693. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Convention on the Rights of the Child: Children’s Version; UNICEF: New York, NY, USA, 2019. [Google Scholar]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a New Tool for the Assessment of Study Quality and Reporting in Exercise Training Studies: TESTEX. Int. J. Evid. Based Healthc. 2015, 13, 9–18. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Davey, J.; Turner, R.M.; Clarke, M.J.; Higgins, J.P. Characteristics of Meta-Analyses and Their Component Studies in the Cochrane Database of Systematic Reviews: A Cross-Sectional, Descriptive Analysis. BMC Med. Res. Methodol. 2011, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Størvold, G.V.; Jahnsen, R.B.; Evensen, K.A.I.; Bratberg, G.H. Is More Frequent Physical Therapy Associated with Increased Gross Motor Improvement in Children with Cerebral Palsy? A National Prospective Cohort Study. Disabil. Rehabil. 2020, 42, 1430–1438. [Google Scholar] [CrossRef]
- Anttila, H.; Autti-Rämö, I.; Suoranta, J.; Mäkelä, M.; Malmivaara, A. Effectiveness of Physical Therapy Interventions for Children with Cerebral Palsy: A Systematic Review. BMC Pediatr. 2008, 8, 14. [Google Scholar] [CrossRef]
- Meyns, P.; Blanckaert, I.; Bras, C.; Jacobs, N.; Harlaar, J.; van de Pol, L.; Plasschaert, F.; Van Waelvelde, H.; Buizer, A.I. Non-immersive virtual reality (VR) Improves Balance in Children with Spastic Cerebral Palsy with Low Balance Performance: Results from a Multicenter Controlled Trial. Disabil. Rehabil. 2022, 44, 5990–5999. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley: Chichester, UK, 2019; pp. 205–228. [Google Scholar]
- Xie, C.X.; Machado, G.C. Clinimetrics: Grading of Recommendations, Assessment, Development and Evaluation (GRADE). J. Physiother. 2021, 67, 66. [Google Scholar] [CrossRef]
- Jha, K.K.; Karunanithi, G.B.; Sahana, A.; Karthikbabu, S. Randomised Trial of Virtual Reality Gaming and Physiotherapy on Balance, Gross Motor Performance and Daily Functions among Children with Bilateral Spastic Cerebral Palsy. Somatosens. Mot. Res. 2021, 38, 117–126. [Google Scholar] [CrossRef]
- Cho, C.; Hwang, W.; Hwang, S.; Chung, Y. Treadmill Training with Virtual Reality Improves Gait, Balance, and Muscle Strength in Children with Cerebral Palsy. Tohoku J. Exp. Med. 2016, 238, 213–218. [Google Scholar] [CrossRef]
- Lazzari, R.D.; Politti, F.; Belina, S.F.; Collange Grecco, L.A.; Santos, C.A.; Dumont, A.J.L.; Lopes, J.B.P.; Cimolin, V.; Galli, M.; Santos Oliveira, C. Effect of Transcranial Direct Current Stimulation Combined with Virtual Reality Training on Balance in Children with Cerebral Palsy: A Randomized, Controlled, Double-Blind, Clinical Trial. J. Mot. Behav. 2017, 49, 329–336. [Google Scholar] [CrossRef]
- Farr, W.J.; Green, D.; Bremner, S.; Male, I.; Gage, H.; Bailey, S.; Speller, S.; Colville, V.; Jackson, M.; Memon, A.; et al. Feasibility of a Randomised Controlled Trial to Evaluate Home-Based Virtual Reality Therapy in Children with Cerebral Palsy. Disabil. Rehabil. 2021, 43, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Chen, C.Y.; Liaw, M.Y.; Chung, C.Y.; Wang, C.J.; Hong, W.H. Efficacy of Home-Based Virtual Cycling Training on Bone Mineral Density in Ambulatory Children with Cerebral Palsy. Osteoporos. Int. 2013, 24, 1399–1406. [Google Scholar] [CrossRef]
- Fu, W.S.; Song, Y.C.; Wu, B.A.; Qu, C.H.; Zhao, J.F. Virtual Reality Combined with Robot-Assisted Gait Training to Improve Walking Ability of Children with Cerebral Palsy: A Randomized Controlled Trial. Technol. Health Care 2022, 30, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Köse, B.; Aran, O.T.; Bahadır Ağce, Z.; Kayıhan, H. The Effects of Virtual Reality on Gross motor functions and Daily Life Activities in Unilateral Spastic Cerebral Palsy: A Single-Blind Randomized Controlled Trial. Games Health J. 2020, 9, 45–52. [Google Scholar] [CrossRef]
- Wang, T.N.; Chen, Y.L.; Shieh, J.Y.; Chen, H.L. Commercial Non-immersive virtual reality (VR) in Home-Based Pediatric Constraint-Induced Therapy: A Randomized Trial. Occup. Ther. J. Res. 2021, 41, 90–100. [Google Scholar] [CrossRef]
- Ziab, H.; Saleh, S.; Talebian, S.; Olyaei, G.; Mazbouh, R.; Sarraj, A.R.; Hadian, M.R. Effectiveness of Virtual Reality Training Compared to Balance-Specific Training and Conventional Training on Balance and Gross Gross motor functions of Children with Cerebral Palsy: A Double Blinded Randomized Controlled Trial. J. Pediatr. Rehabil. Med. 2024, 17, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Chen, R.; Zhang, J. The Effects of Virtual Reality Training on Balance, Gross Gross motor function, and Daily Living Ability in Children with Cerebral Palsy: Systematic Review and Meta-Analysis. JMIR Serious Games 2022, 10, e38972. [Google Scholar] [CrossRef]
- Komariah, M.; Amirah, S.; Abdurrahman, M.F.; Handimulya, M.F.S.; Platini, H.; Maulana, S.; Nugrahani, A.D.; Mulyana, A.M.; Qadous, S.G.; Mediani, H.S.; et al. Effectivity of Virtual Reality to Improve Balance, Gross motor function, Activities of Daily Living, and Upper Limb Function in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Ther. Clin. Risk Manag. 2024, 20, 95–109. [Google Scholar] [CrossRef]
- Chen, Y.; Fanchiang, H.D.; Howard, A. Effectiveness of Virtual Reality in Children with Cerebral Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys. Ther. 2018, 98, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Yohn, S.E.; López-Cruz, L.; San Miguel, N.; Correa, M. Activational and Effort-Related Aspects of Motivation: Neural Mechanisms and Implications for Psychopathology. Brain 2016, 139, 1325–1347. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.E.; Huber, M.E.; Sternad, D. Learning and Transfer of Complex Motor Skills in Virtual Reality: A Perspective Review. J. Neuroeng. Rehabil. 2019, 16, 121. [Google Scholar] [CrossRef]
- Slater, C.; Liu, Y.; Weiss, E.; Yu, K.; Wang, Q. The Neuromodulatory Role of the Noradrenergic and Cholinergic Systems and Their Interplay in Cognitive Functions: A Focused Review. Brain Sci. 2022, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Schonfeld, D.; Kwak, Y.; Bohnen, N.I.; Seidler, R. Dopamine Overdose Hypothesis: Evidence and Clinical Implications. Mov. Disord. 2013, 28, 1920–1929. [Google Scholar] [CrossRef]
- Cerasa, A.; Fasano, A.; Morgante, F.; Koch, G.; Quattrone, A. Maladaptive Plasticity in Levodopa-Induced Dyskinesias and Tardive Dyskinesias: Old and New Insights on the Effects of Dopamine Receptor Pharmacology. Front. Neurol. 2014, 5, 49. [Google Scholar] [CrossRef]
- Goble, D.J.; Cone, B.L.; Fling, B.W. Using the Wii Fit as a Tool for Balance Assessment and Neurorehabilitation: The First Half Decade of “Wii-Search”. J. Neuroeng. Rehabil. 2014, 11, 12. [Google Scholar] [CrossRef]
- Mao, Y.; Chen, P.; Li, L.; Huang, D. Virtual Reality Training Improves Balance Function. Neural Regen. Res. 2014, 9, 1628–1634. [Google Scholar] [CrossRef]
- Park, E.Y.; Nam, S.J. Time Burden of Caring and Depression among Parents of Individuals with Cerebral Palsy. Disabil. Rehabil. 2019, 41, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Song, C.S. Relationships between Physical and Cognitive Functioning and Activities of Daily Living in Children with Cerebral Palsy. J. Phys. Ther. Sci. 2013, 25, 619–622. [Google Scholar] [CrossRef]
- Di Lieto, M.C.; Matteucci, E.; Martinelli, A.; Beani, E.; Menici, V.; Martini, G.; Barzacchi, V.; Dubbini, N.; Sgandurra, G. Impact of Social Participation, Motor, and Cognitive Functioning on Quality of Life in Children with Cerebral Palsy. Res. Dev. Disabil. 2025, 161, 105004. [Google Scholar] [CrossRef]
- Tobaiqi, M.A.; Albadawi, E.A.; Fadlalmola, H.A.; Albadrani, M.S. Application of Virtual Reality-Assisted Non-immersive virtual reality (VR) on the Rehabilitation of Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7091. [Google Scholar] [CrossRef]
- Kilcioglu, S.; Schiltz, B.; Araneda, R.; Bleyenheuft, Y. Short- to Long-Term Effects of Virtual Reality on Motor Skill Learning in Children with Cerebral Palsy: Systematic Review and Meta-Analysis. JMIR Serious Games 2023, 11, e42067. [Google Scholar] [CrossRef]
- Sutcliffe, T.L.; Gaetz, W.C.; Logan, W.J.; Cheyne, D.O.; Fehlings, D.L. Cortical Reorganization after Modified Constraint-Induced Movement Therapy in Pediatric Hemiplegic Cerebral Palsy. J. Child Neurol. 2007, 22, 1281–1287. [Google Scholar] [CrossRef]
- Chan-Víquez, D.; Fernández-Huertas, H.; Montserrat-Gonzalez, C.; Khan, A.; Fehlings, D.; Munce, S.; Wright, F.V.; Biddiss, E. Feasibility of a Home-Based Videogaming Intervention with a Family-Centered Approach for Children with Cerebral Palsy: A Randomized Multiple Baseline Single-Case Experimental Design. J. Neuroeng. Rehabil. 2024, 21, 151. [Google Scholar] [CrossRef]
- Arnoni, J.L.B.; Lima, C.R.G.; Verdério, B.N.; Kleiner, A.F.R.; de Campos, A.C.; Rocha, N. Active Videogame Training Combined with Conventional Therapy Alters Body Oscillation in Children with Cerebral Palsy: A Randomized Controlled Trial. Games Health J. 2022, 11, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Rosie, J.A.; Ruhen, S.; Hing, W.A.; Lewis, G.N. Virtual Rehabilitation in a School Setting: Is It Feasible for Children with Cerebral Palsy? Disabil. Rehabil. Assist. Technol. 2015, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Participants under 12 years of age, diagnosed with cerebral palsy, regardless of sex [22]. | Single clinical case reports or studies including adults (>18 years). |
| Intervention | Interventions using non-immersive virtual reality or active non-immersive video games (e.g., Wii Sports, Kinect, Switch Sports), with a minimum duration of 2 weeks. | Immersive or semi-immersive VR; interventions lacking procedural detail. |
| Comparator | Active controls (e.g., conventional physiotherapy, task-specific, or neurodevelopmental training) or inactive controls (usual care or daily activities). | Studies without control groups. |
| Outcome | At least one pre- and post-intervention assessment of gross motor function (GMFM-D/E), balance (PBS), or functional independence (WeeFIM). | Studies not reporting baseline or follow-up outcomes. |
| Study Design | Randomized controlled trials with pre- and post-assessments. | Nonrandomized studies, protocols, or reviews. |
| Study | Eligibility Criteria Specified | Randomly Allocated Participants | Allocation Concealed | Groups Similar at Baseline | Assessors Blinded | Outcome Measures Assessed > 85% of Participants * | Intention to Treat Analysis | Reporting of Between-Group Statistical Comparisons | Point Measures and Measures of Variability Reported ** | Activity Monitoring in the Control Group | Relative Exercise Intensity Reviewed | Exercise Volume and Energy Expended | Overall TESTEX # |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [9] | Yes | Yes | No | Yes | No | Yes (3) | No | Yes | Yes (2) | No | No | No | 8/15 |
| [17] | Yes | Yes | Yes | Yes | No | Yes (1) | No | Yes | Yes (2) | No | No | No | 8/15 |
| [18] | Yes | Yes | No | Yes | No | Yes (1) | No | Yes | Yes (2) | No | No | No | 9/15 |
| [19] | Yes | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | No | Yes | No | 10/15 |
| [34] | Yes | Yes | Yes | Yes | Yes | Yes (2) | Yes | Yes | Yes (2) | No | Yes | No | 12/15 |
| [35] | Yes | Yes | No | Yes | Yes | Yes (1) | No | Yes | Yes (2) | No | Yes | No | 9/15 |
| [36] | Yes | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | No | No | No | 9/15 |
| [37] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes (2) | Yes | No | No | 9/15 |
| [38] | Yes | Yes | Yes | Yes | No | Yes (1) | No | Yes | Yes (2) | No | Yes | No | 9/15 |
| [39] | Yes | Yes | Yes | Yes | No | Yes (1) | No | Yes | Yes (2) | No | Yes | No | 9/15 |
| [40] | Yes | Yes | No | Yes | Yes | Yes (1) | No | Yes | Yes (2) | No | No | No | 9/15 |
| [41] | Yes | Yes | Yes | Yes | Yes | Yes (1) | No | Yes | Yes (2) | No | Yes | No | 10/15 |
| [42] | Yes | Yes | Yes | Yes | Yes | Yes (2) | No | Yes | Yes (2) | No | No | No | 11/15 |
| Author | Country | CP Type | Groups (n) | Mean Age (y) | Console/Device | Settings | Training Volume | Outcomes Evaluated | Main Results | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | Frequency (Sessions/Weeks) | Session Duration (Min) | |||||||||
| [9] | IN | NR | VRT: 8; CG: 8 | VRT: 8.8 ± 3.2; CG: 10.3 ± 4.4 | Nintendo Wii Fit/Wii Fit Plus | NR | 3 | 3 | 30 | PBS, MACS | ↑ PBS, MACS ↔ |
| [17] | TR | Spastic | VRT: 27; CG: 25 | VRT: 9.2 ± 2.0; CG: 9.4 ± 2.2 | Xbox Kinect | Clinic | 8 | 2 | 45 | PBS, GMFM-88 | ↑ PBS, GMFM-88 ↔ |
| [18] | TR | Dysplegic, hemiplegic, dyskinetic | VRT: 15; CG: 15 | VRT: 10.2.6; CG: 10.5 ± 2.7 | Nintendo Wii/Xbox Kinect | Clinic | 12 | 2 | 50 | WeeFIM | ↑ WeeFIM |
| [19] | EG | Ataxic | VRT: 32; CG: 32 | VRT: 10.7 ± 1.2; CG: 11.2 ± 1.4 | Nintendo Wii/Xbox Kinect | Clinic | 12 | 3 | 60 | PBS | ↑ PBS |
| [34] | IN | Bilateral spastic | VRT: 19; CG: 19 | VRT: 8.9 ± 1.9; CG: 8.7 ± 1.6 | Customized Kinect-based VR system | Clinic | 6 | 4 | 60 | GMFM-88, PBS, WeeFIM | GMFM-88 ↔, ↑ PBS, WeeFIM ↔ |
| [35] | SK | Spastic | VRT: 9; CG: 9 | VRT: 10.2 ± 3.4; CG: 9.4 ± 3.8 | Treadmill with VR | Clinic | 8 | 3 | 30 | GMFM-E, PBS | ↑ GMFM-E, ↑ PBS |
| [36] | BR | NR | VRT: 10; CG: 10 | VRT: 7.6 ± 2.2; CG: 7.4 ± 2.0 | Not specified | Clinic | 2 | 5 | 20 | PBS, TUG | ↑ PBS, ↑ TUG |
| [37] | UK | NR | VRT: 8; CG: 12 | VRT: 9.7 ± 2.1; CG: 9.5 ± 2.3 | Nintendo Wii/Wii Fit | Home | 12 | 3 | 30 | GMFM-66, TUG | GMFM-66 ↔, TUG ↔ |
| [38] | TW | Spastic | VRT: 13; CG: 14 | VRT: 8.7 ± 2.1; CG: 8.6 ± 2.2 | Electro Sync Cycle VR system | Home | 12 | 3 | 40 | GMFM-66 | ↑ GMFM-66 |
| [39] | CN | NR | VRT1: 15; VRT2: 15; VRT3: 15; CG: 15 | VRT1: 5.0 ± 1.6; VRT2: 5.3 ± 2.9; VRT3: 5.6 ± 1.5; CG: 4.5 ± 1.7 | Nintendo Wii | Clinic | 12 | 4 | 50 | GMFM-D, GMFM-E | ↑ GMFM-D, ↑ GMFM-E |
| [40] | TR | Unilateral spastic | VRT: 30; CG: 30 | VRT: 10.5 ± 3.6; CG: 10.0 ± 3.2 | Nintendo Wii/Xbox Kinect | Clinic | 8 | 2 | 45 | NR | NR |
| [41] | TW | Unilateral | VRT: 9; CG: 9 | VRT: 8.5 ± 2.0; CG: 8.5 ± 2.1 | Custom VR device | Home | 8 | 2 | 135 | WeeFIM | ↑ WeeFIM |
| [42] | LB | Spastic hemiplegia, diplegia, monoplegia | VRT: 14; CG: 15 | VRT: 8.2 ± 2.0; CG: 7.4 ± 3.0 | Custom VR systems | Clinic | 6 | 3 | 60 | PBS, GMFM-D, GMFM-E | PBS ↔, GMFM-D ↔, GMFM-E ↔ |
| Gross Motor Function, Balance, and Functional Independence | n of Studies | n of Experimental Groups | n of Control Groups | Total Participants | ES (95%CI) | p | I2 (%) | Egger’s Test (p) | RW (%) |
|---|---|---|---|---|---|---|---|---|---|
| Gross motor function | |||||||||
| GMFM-D | 5 | 5 | 5 | 137 | 2.04 (0.73 to 3.35) | 0.02 | 89.86 | <0.001 | 0.14 to 0.22 |
| GMFM-E | 5 | 5 | 5 | 137 | 2.04 (0.97 to 3.08) | <0.001 | 84.70 | <0.001 | 0.34 to 0.52 |
| Balance | |||||||||
| PBS | 7 | 7 | 7 | 237 | 1.34 (0.17 to 2.52) | 0.02 | 93.42 | <0.001 | 0.13 to 0.17 |
| Functional Independence | |||||||||
| WeeFIM | 4 | 4 | 4 | 146 | 0.99 (0.65 to 1.33) | <0.001 | 32.15 | 0.21 | 3.95 to 6.22 |
| Certainty of Evidence | Nº of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nº of Studies | Study Design | Risk Assessment | Inconsistency | Indirect Evidence | Vagueness | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | ||
| Gross motor function | ||||||||||||
| 4 | RCT | It is not serious | It is not serious | It is not serious | It is not serious | None | 69/137 (50.4%) | 68/137 (49.6%) | Not estimable | ++++ High | IMPORTANT | |
| Balance | ||||||||||||
| 7 | RCT | Serious a | It is not serious | It is not serious | It is not serious | None | 119/237 (50.2%) | 118/237 (49.8%) | Not estimable | +++ Moderate | IMPORTANT | |
| Functional Independence | ||||||||||||
| 4 | RCT | Serious a | It is not serious | It is not serious | It is not serious | None | 73/146 (50.0%) | 73/146 (50.0%) | Not estimable | +++ Moderate | IMPORTANT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Carcamo, J.; Hernandez-Martinez, J.; Vásquez-Carrasco, E.; Fernandez-Cardenas, D.; Branco, B.H.M.; Sandoval, C.; Carmine-Peña, E.; Peña, F.; Aristegui-Mondaca, J.; Valdés-Badilla, P. Effectiveness of Non-Immersive Virtual Reality on Gross Motor Function, Balance, and Functional Independence in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. J. Clin. Med. 2025, 14, 7582. https://doi.org/10.3390/jcm14217582
Perez-Carcamo J, Hernandez-Martinez J, Vásquez-Carrasco E, Fernandez-Cardenas D, Branco BHM, Sandoval C, Carmine-Peña E, Peña F, Aristegui-Mondaca J, Valdés-Badilla P. Effectiveness of Non-Immersive Virtual Reality on Gross Motor Function, Balance, and Functional Independence in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine. 2025; 14(21):7582. https://doi.org/10.3390/jcm14217582
Chicago/Turabian StylePerez-Carcamo, Joaquín, Jordan Hernandez-Martinez, Edgar Vásquez-Carrasco, Diego Fernandez-Cardenas, Braulio Henrique Magnani Branco, Cristian Sandoval, Eduardo Carmine-Peña, Francisca Peña, Juan Aristegui-Mondaca, and Pablo Valdés-Badilla. 2025. "Effectiveness of Non-Immersive Virtual Reality on Gross Motor Function, Balance, and Functional Independence in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis" Journal of Clinical Medicine 14, no. 21: 7582. https://doi.org/10.3390/jcm14217582
APA StylePerez-Carcamo, J., Hernandez-Martinez, J., Vásquez-Carrasco, E., Fernandez-Cardenas, D., Branco, B. H. M., Sandoval, C., Carmine-Peña, E., Peña, F., Aristegui-Mondaca, J., & Valdés-Badilla, P. (2025). Effectiveness of Non-Immersive Virtual Reality on Gross Motor Function, Balance, and Functional Independence in Children with Cerebral Palsy: A Systematic Review with Meta-Analysis. Journal of Clinical Medicine, 14(21), 7582. https://doi.org/10.3390/jcm14217582










