Value of Automatically Derived Full Thrombus Characteristics: An Explorative Study of Their Associations with Outcomes in Ischemic Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
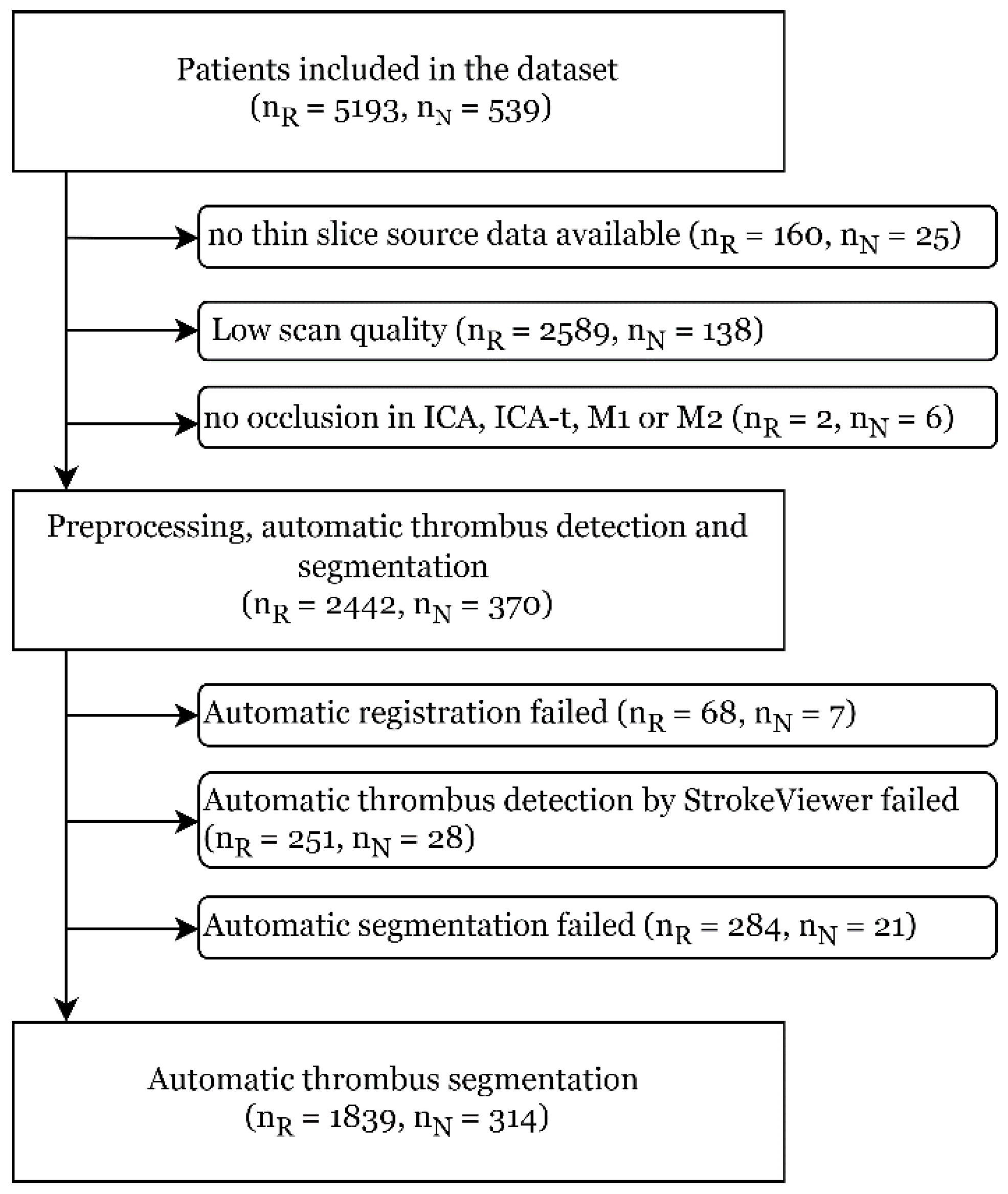
2.1. Patient Selection
2.2. Automatic Thrombus Segmentation
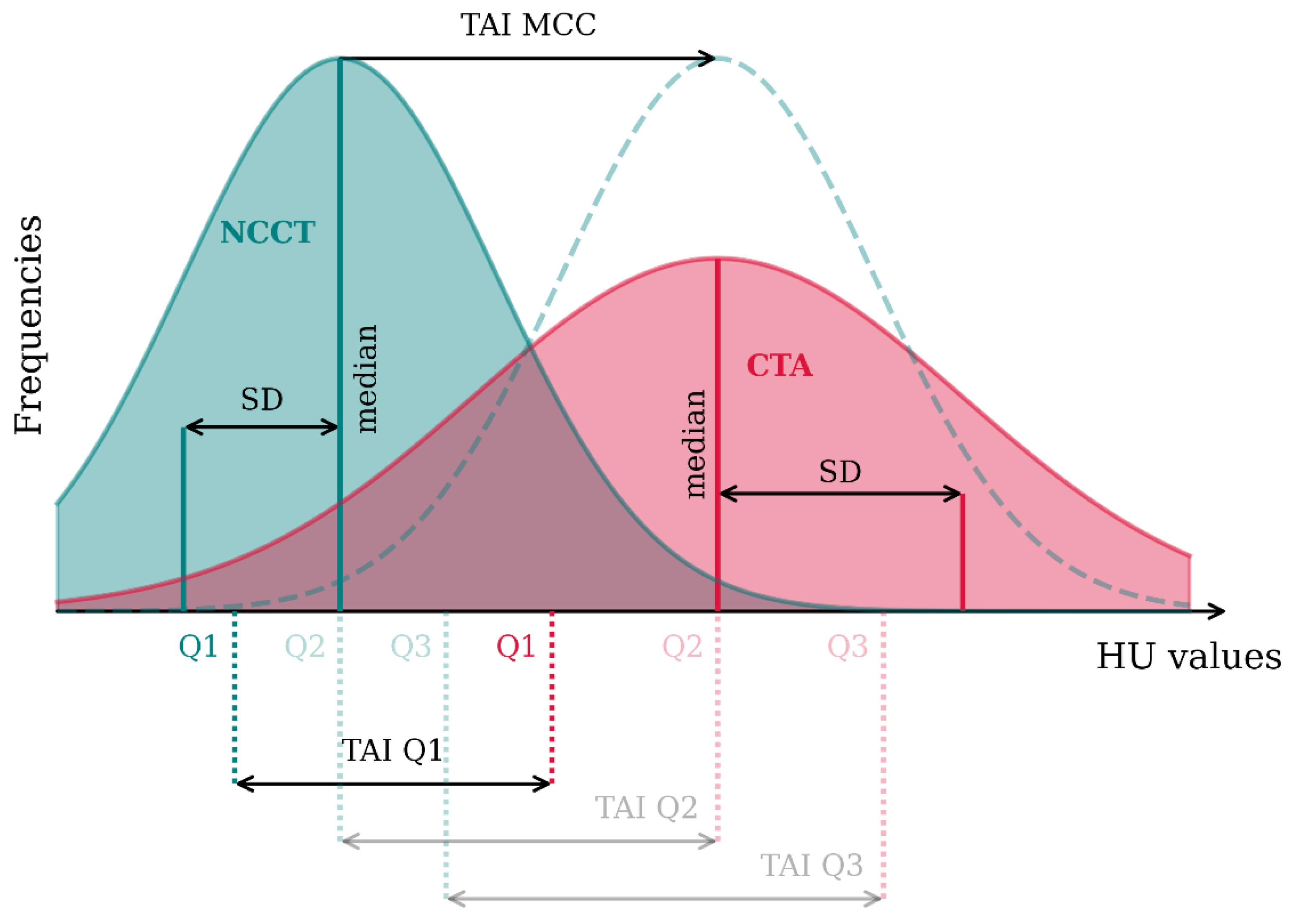
2.3. Thrombus Imaging Characteristics
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Associations with the Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| VOLUME | DENSITY | PERVIOUSNESS | HETEROGENEITY | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (per 0.1 mL) | NCCT Median (per 10 HU) | TAIQ1 (per 10 HU) | TAIQ2 (per 10 HU) | TAIQ3 (per 10 HU) | TAIMCC (per 10 HU) | CTA Median (per 10 HU) | CTA SD (per 10 HU) | NCCT SD (per 10 HU) | ||
| Functional Outcome | Registry | 0.98 (0.93–1.03) | 0.94 (0.83–1.05) | 1.03 (0.97–1.10) | 1.02 (0.96–1.07) | 1.01 (0.97–1.04) | 1.03 (0.97–1.09) | 1.00 (0.95–1.06) | 0.95 (0.89–1.02) | 0.92 (0.83–1.03) |
| NO IV | 1.02 (0.90–1.17) | 0.84 (0.58–1.22) | 1.18 (0.97–1.43) | 1.16 (0.98–1.38) | 1.09 (0.96–1.25) | 1.13 (0.97–1.32) | 1.12 (0.95–1.32) | 1.16 (0.94–1.43) | 0.83 (0.56–1.23) | |
| FPS | Registry | 0.97 (0.90–1.04) | 0.97 (0.83–1.12) | 1.05 (0.95–1.17) | 1.03 (0.95–1.10) | 1.02 (0.97–1.07) | 1.04 (0.96–1.13) | 1.02 (0.95–1.10) | 1.01 (0.91–1.12) | 0.99 (0.85–1.14) |
| NO IV | 0.95 (0.80–1.12) | 0.69 (0.41–1.15) | 1.02 (0.81–1.27) | 1.05 (0.86–1.28) | 1.09 (0.93–1.29) | 1.03 (0.84–1.26) | 0.99 (0.81–1.20) | 1.06 (0.83–1.35) | 0.58 (0.32–1.02) | |
| eTICI2b+ | Registry | 1.01 (0.95–1.07) | 0.91 (0.80–1.04) | 0.94 (0.87–1.01) | 0.96 (0.90–1.02) | 0.98 (0.93–1.02) | 0.97 (0.90–1.04) | 0.94 † (0.88–1.00) | 0.96 (0.89–1.04) | 0.87 † (0.78–0.99) |
| NO IV | 0.97 (0.79–1.19) | 1.07 (0.63–1.82) | 0.94 (0.68–1.29) | 0.93 (0.72–1.21) | 0.96 (0.79–1.15) | 1.00 (0.80–1.26) | 0.95 (0.75–1.22) | 1.04 (0.76–1.43) | 0.83 (0.50–1.36) | |
Appendix B
| VOLUME | DENSITY | PERVIOUSNESS | HETEROGENEITY | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (per 0.1 mL) | NCCT Median (per 10 HU) | TAIQ1 (per 10 HU) | TAIQ2 (per 10 HU) | TAIQ3 (per 10 HU) | TAIMCC (per 10 HU) | CTA Median (per 10 HU) | CTA SD (per 10 HU) | NCCT SD (per 10 HU) | ||
| Functional Outcome | Registry | 0.90 † (0.86–0.95) | 0.96 (0.86–1.07) | 1.14 † (1.07–1.22) | 1.10 † (1.04–1.16) | 1.06 † (1.02–1.10) | 1.11 † (1.05–1.18) | 1.09 † (1.04–1.15) | 0.93 † (0.86–0.99) | 0.85 † (0.75–0.96) |
| NO IV | 0.91 (0.81–1.02) | 1.15 (0.82–1.62) | 1.20 † (1.01–1.44) | 1.22 † (1.04–1.42) | 1.16 † (1.02–1.30) | 1.17 † (1.01–1.35) | 1.23 † (1.06–1.44) | 1.14 (0.94–1.40) | 0.76 (0.53–1.09) | |
| FPS | Registry | 0.93 † (0.87–0.99) | 0.96 (0.83–1.12) | 1.11 † (1.00–1.22) | 1.06 (0.98–1.15) | 1.02 (0.97–1.08) | 1.09 † (1.00–1.19) | 1.05 (0.97–1.14) | 1.03 (0.94–1.13) | 0.96 (0.84–1.11) |
| NO IV | 0.91 (0.79–1.04) | 0.68 (0.44–1.04) | 1.02 (0.82–1.27) | 1.09 (0.90–1.32) | 1.13 (0.97–1.32) | 1.09 (0.90–1.31) | 1.01 (0.84–1.21) | 1.03 (0.81–1.31) | 0.54 † (0.30–0.97) | |
| eTICI2b+ | Registry | 1.01 (0.95–1.06) | 0.93 (0.82–1.06) | 0.96 (0.89–1.03) | 0.96 (0.91–1.03) | 0.98 (0.94–1.03) | 0.98 (0.92–1.05) | 0.95 (0.89–1.01) | 0.96 (0.88–1.04) | 0.87 † (0.77–0.98) |
| NO IV | 1.05 (0.88–1.24) | 1.15 (0.69–1.90) | 0.93 (0.72–1.21) | 0.95 (0.76–1.19) | 0.97 (0.82–1.16) | 0.99 (0.79–1.23) | 0.98 (0.79–1.22) | 1.01 (0.76–1.36) | 0.76 (0.47–1.24) | |
Appendix C
| VOLUME | DENSITY | PERVIOUSNESS | HETEROGENEITY | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Volume | NCCT Median | TAIQ1 | TAIQ2 | TAIQ3 | TAIMCC | CTA Median | CTA SD | NCCT SD | |
| ICC | |||||||||
References
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 h with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Dutra, B.G.; Tolhuisen, M.L.; Alves, H.C.B.R.; Treurniet, K.M.; Kappelhof, M.; Yoo, A.J.; Jansen, I.G.H.; Dippel, D.W.J.; Van Zwam, W.H.; Van Oostenbrugge, R.J.; et al. Thrombus Imaging Characteristics and Outcomes in Acute Ischemic Stroke Patients Undergoing Endovascular Treatment. Stroke 2019, 50, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.M.; Marquering, H.A.; Den Blanken, M.D.; Berkhemer, O.A.; Boers, A.M.M.; Yoo, A.J.; Beenen, L.F.; Treurniet, K.M.; Wismans, C.; Van Noort, K.; et al. Thrombus Permeability Is Associated with Improved Functional Outcome and Recanalization in Patients with Ischemic Stroke. Stroke 2016, 47, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Baek, J.H.; Park, H.; Song, D.; Kim, K.; Hwang, I.G.; Kim, Y.D.; Kim, S.H.; Lee, H.S.; Ahn, S.H.; et al. Thrombus volume as a predictor of nonrecanalization after intravenous thrombolysis in acute stroke. Stroke 2018, 49, 2108–2115. [Google Scholar] [CrossRef]
- Riedel, C.H.; Zimmermann, P.; Jensen-Kondering, U.; Stingele, R.; Deuschl, G.; Jansen, O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011, 42, 1775–1777. [Google Scholar] [CrossRef]
- van Voorst, H.; Bruggeman, A.A.E.; Andriessen, J.; Hoving, J.W.; Konduri, P.R.; Yang, W.; Kappelhof, M.; Arrarte Terreros, N.; Roos, Y.B.W.E.M.; van Zwam, W.H.; et al. Prognostic Value of Thrombus Volume and Interaction With First-Line Endovascular Treatment Device Choice. Stroke 2023, 54, 1056–1065. [Google Scholar] [CrossRef]
- Bala, F.; Cimflova, P.; Singh, N.; Zhang, J.; Kappelhof, M.; Kim, B.J.; Najm, M.; Golan, R.; Elebute, I.; Benali, F.; et al. Impact of vessel tortuosity and radiological thrombus characteristics on the choice of first-line thrombectomy strategy: Results from the ESCAPE-NA1 trial. Eur. Stroke J. 2023, 8, 675–683. [Google Scholar] [CrossRef]
- Warner, J.J.; Harrington, R.A.; Sacco, R.L.; Elkind, M.S.V. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke. Stroke 2019, 50, 3331–3332. [Google Scholar] [CrossRef]
- Hofmeister, J.; Bernava, G.; Rosi, A.; Vargas, M.I.; Carrera, E.; Montet, X.; Burgermeister, S.; Poletti, P.A.; Platon, A.; Lovblad, K.O.; et al. Clot-Based Radiomics Predict a Mechanical Thrombectomy Strategy for Successful Recanalization in Acute Ischemic Stroke. Stroke 2020, 51, 2488–2494. [Google Scholar] [CrossRef]
- Santos, E.M.M.; Yoo, A.J.; Beenen, L.F.; Berkhemer, O.A.; den Blanken, M.D.; Wismans, C.; Niessen, W.J.; Majoie, C.B.; Marquering, H.A. Observer variability of absolute and relative thrombus density measurements in patients with acute ischemic stroke. Neuroradiology 2016, 58, 133–139. [Google Scholar] [CrossRef]
- Zhu, K.; Bala, F.; Zhang, J.; Benali, F.; Cimflova, P.; Kim, B.J.; McDonough, R.; Singh, N.; Hill, M.D.; Goyal, M.; et al. Automated Segmentation of Intracranial Thrombus on NCCT and CTA in Patients with Acute Ischemic Stroke Using a Coarse-to-Fine Deep Learning Model. Am. J. Neuroradiol. 2023, 44, 641–648. [Google Scholar] [CrossRef]
- Dumitriu LaGrange, D.; Hofmeister, J.; Rosi, A.; Vargas, M.I.; Wanke, I.; Machi, P.; Lövblad, K.-O. Predictive value of clot imaging in acute ischemic stroke: A systematic review of artificial intelligence and conventional studies. Neurosci. Inform. 2023, 3, 100114. [Google Scholar] [CrossRef]
- Mojtahedi, M.; Kappelhof, M.; Ponomareva, E.; Tolhuisen, M.; Jansen, I.; Bruggeman, A.A.E.; Dutra, B.G.; Yo, L.; Lecouffe, N.; Hoving, J.W.; et al. Fully Automated Thrombus Segmentation on CT Images of Patients with Acute Ischemic Stroke. Diagnostics 2022, 12, 698. [Google Scholar] [CrossRef]
- Gliklich, R.E.; Leavy, M.B.; Dreyer, N.A. (Eds.) Chapter 3, Registry Design. In Registries for Evaluating Patient Outcomes: A User’s Guide, 4th ed.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562568/ (accessed on 21 February 2024).
- Jansen, I.G.H.; Mulder, M.J.H.L.; Goldhoorn, R.J.B. Endovascular treatment for acute ischaemic stroke in routine clinical practice: Prospective, observational cohort study (MR CLEAN Registry). BMJ 2018, 360, K949. [Google Scholar] [CrossRef] [PubMed]
- LeCouffe, N.E.; Kappelhof, M.; Treurniet, K.M.; Rinkel, L.A.; Bruggeman, A.E.; Berkhemer, O.A.; Wolff, L.; van Voorst, H.; Tolhuisen, M.L.; Dippel, D.W.J.; et al. A Randomized Trial of Intravenous Alteplase before Endovascular Treatment for Stroke. N. Engl. J. Med. 2021, 385, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu LaGrange, D.; Reymond, P.; Brina, O.; Zboray, R.; Neels, A.; Wanke, I.; Lövblad, K.O. Spatial heterogeneity of occlusive thrombus in acute ischemic stroke: A systematic review. J. Neuroradiol. 2023, 50, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Kuang, H.; Nair, J.; Assis, Z.; Najm, M.; McDougall, C.; McDougall, B.; Chung, K.; Wilson, A.T.; Goyal, M.; et al. Radiomics-Based Intracranial Thrombus Features on CT and CTA Predict Recanalization with Intravenous Alteplase in Patients with Acute Ischemic Stroke. Am. J. Neuroradiol. 2019, 40, 39–44. [Google Scholar] [CrossRef]
- Baek, J.H.; Yoo, J.; Song, D.; Kim, Y.D.; Nam, H.S.; Kim, B.M.; Kim, D.J.; Lee, H.S.; Heo, J.H. Predictive value of thrombus volume for recanalization in stent retriever thrombectomy. Sci. Rep. 2017, 7, 15938. [Google Scholar] [CrossRef]
- Borst, J.; Berkhemer, O.A.; Santos, E.M.M.; Yoo, A.J.; Den Blanken, M.; Roos, Y.B.W.E.M.; Van Bavel, E.; Van Zwam, W.H.; Van Oostenbrugge, R.J.; Lingsma, H.F.; et al. Value of thrombus CT Characteristics in Patients with Acute Ischemic Stroke. Am. J. Neuroradiol. 2017, 38, 1758–1764. [Google Scholar] [CrossRef]
- Jagani, M.; Kallmes, D.F.; Brinjikji, W. Correlation between clot density and recanalization success or stroke etiology in acute ischemic stroke patients. Interv. Neuroradiol. 2017, 23, 274–278. [Google Scholar] [CrossRef]
- Liu, Y.; Brinjikji, W.; Abbasi, M.; Dai, D.; Arturo Larco, J.L.; Irfan Madhani, S.; Shahid, A.H.; Madalina Mereuta, O.; Nogueira, R.G.; Kvamme, P.; et al. Quantification of clot spatial heterogeneity and its impact on thrombectomy. J. Neurointerv. Surg. 2021; ahead of print. [Google Scholar] [CrossRef]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef]
- Santos, E.M.M.; Arrarte Terreros, N.; Kappelhof, M.; Borst, J.; Boers, A.M.M.; Lingsma, H.F.; Berkhemer, O.A.; Dippel, D.W.J.; Majoie, C.B.; Marquering, H.A.; et al. Associations of thrombus perviousness derived from entire thrombus segmentation with functional outcome in patients with acute ischemic stroke. J. Biomech. 2021, 128, 110700. [Google Scholar] [CrossRef]
- Kappelhof, M.; Tolhuisen, M.L.; Treurniet, K.M.; Dutra, B.G.; Alves, H.; Zhang, G.; Brown, S.; Muir, K.W.; Dávalos, A.; Roos, Y.B.W.E.M.; et al. Endovascular Treatment Effect Diminishes With Increasing Thrombus Perviousness. Stroke 2021, 52, 3633–3641. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.-S.; Nicholson, P.; Hilditch, C.A.; Chun, A.; Tsang, O.; Pereira, M.; Krings, T.; Fang, Y.; Brinjikji, W. Thrombus perviousness is not associated with first-pass revascularization using stent retrievers. Interv. Neuroradiol. 2018, 25, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Boodt, N.; Compagne, K.C.J.; Dutra, B.G.; Samuels, N.; Tolhuisen, M.L.; Alves, H.C.B.R.; Kappelhof, M.; Lycklama à Nijeholt, G.J.; Marquering, H.A.; Majoie, C.B.L.M.; et al. Stroke Etiology and Thrombus Computed Tomography Characteristics in Patients with Acute Ischemic Stroke: A MR CLEAN Registry Substudy. Stroke 2020, 51, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.C.; Treurniet, K.M.; Dutra, B.G.; Jansen, I.G.H.; Boers, A.M.M.; Santos, E.M.M.; Berkhemer, O.A.; Dippel, D.W.J.; Van Der Lugt, A.; Van Zwam, W.H.; et al. Associations between collateral status and thrombus characteristics and their impact in anterior circulation stroke. Stroke 2018, 49, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
| MR CLEAN Registry (n = 1839) | MR CLEAN-NO IV (n = 314) | p-Value | |
|---|---|---|---|
| Age, median (year, IQR) | 72 (63–81) | 70 (63–78) | 0.03 † |
| Sex (men), n (%) | 1007 (54.8%) | 191 (60.8%) | 0.05 |
| Baseline NIHSS, median (IQR) | 16 (11–20); (n = 1822) | 17 (11–20) | 0.09 |
| Onset-to-arterial puncture time, median (IQR) | 190 (138–269); (n = 1789) | 92 (68–141) | <0.0001 † |
| Atherosclerosis (yes), n (%) | 216 (11.7%) | 49 (16.2%); (n = 303) | 0.04 † |
| Diabetes (yes), n (%) | 286 (15.7%); (n = 1825) | 46 (14.6%) | 0.71 |
| Hypertension, n (%) | 912 (50.6%); (n = 1801) | 152 (48.6%) | 0.54 |
| Time between NCCT and CTA (minute), median (IQR) | 13.4 (7.7–34.7); (n = 1609) | 9.3 (6.8–13.4); (n = 197) | <0.0001 † |
| Pre-stroke mRS | (n = 1786) | (n = 313) | 0.003 † |
| 0 | 1166 (65.3%) | 215 (68.7%) | |
| 1 | 261 (14.6%) | 59 (18.8%) | |
| ≥2 | 359 (20.1%) | 39 (12.5%) | |
| ASPECTS | (n = 1829) | 0.21 | |
| 0–4 | 79 (4.3%) | 11 (3.5%) | |
| 5–7 | 360 (19.7%) | 50 (15.9%) | |
| 8–10 | 1390 (76%) | 253 (80.6%) | |
| Collateral score | (n = 1802) | (n = 311) | 0.004 † |
| Score 0 (absent collaterals) | 96 (5.3%) | 21 (6.8%) | |
| Score 1 (filling = 50% of occluded area) | 712 (39.5%) | 90 (28.9%) | |
| Score 2 (>50% but less than <100%) | 701 (38.9%) | 137 (44.1%) | |
| 3 (100% of the occluded area) | 293 (16.3%) | 63 (20.3%) | |
| Thrombus location | (n = 1825) | 0.002 † | |
| ICA | 72 (3.9%) | 1 (3%) | |
| ICA-t | 380 (20.8%) | 78 (24.8%) | |
| M1 | 1115 (61.1%) | 200 (63.7%) | |
| M2 | 258 (14.1%) | 35 (11.1%) | |
| Antiplatelet use (yes), n (%) | 549 (30.2%); (n = 1819) | 104 (33.1%) | 0.33 |
| IVT (yes), n (%) | 1251 (68.4%); (n = 1830) | 166 (52.9%) | <0.0001 † |
| First-pass success (yes), n(%) | 467 (34%); (n = 1373) | 143 (51.8%); (n = 276) | <0.0001 † |
| eTICI 2b/2c/3 | 1156 (65.8%); (n = 1757) | 227 (79.1%); (n = 287) | <0.0001 † |
| mRS 90 days | (n = 1673) | <0.0001 † | |
| 0 | 129 (7.7%) | 11 (3.5%) | |
| 1 | 294 (17.6%) | 36 (11.5%) | |
| 2 | 296 (17.7%) | 110 (35.0%) | |
| 3 | 219 (13.1%) | 35 (11.1%) | |
| 4 | 188 (11.2%) | 29 (9.2%) | |
| 5 | 90 (5.4%) | 31 (9.9%) | |
| 6 | 457(27.3%) | 62 (19.7%) |
| MR CLEAN Registry (n = 1839) | MR CLEAN-NO IV (n = 314) | p-Value | ||
|---|---|---|---|---|
| Volume (mm3), median (IQR) | 128 (49–261) | 132 (53–271) | 0.46 | |
| Density (HU) | NCCT median median (IQR) | 47.6 (44.8–50.8) | 49.2 (45.8–53.1) | <0.0001 † |
| Perviousness (HU) | TAIQ1 median (IQR) | 0.51 (−5.0–6.5) | 3.5 (−2.8–8.1) | <0.0001 † |
| TAIQ2 median (IQR) | 5.8 (−0.3–13.0) | 9.6 (2.9–16.0) | <0.0001 † | |
| TAIQ3 median (IQR) | 11.9 (3.9–22.0) | 15.7 (8.6–25.0) | <0.0001 † | |
| TAIMCC median (IQR) | 4.3 (−1.6–10.9) | 7.3 (0.0–12.7) | <0.0001 † | |
| CTA median median (IQR) | 54.0 (47.0–61.7) | 58.0 (53.0–65.7) | <0.0001 † | |
| Heterogeneity (HU) | CTA SD median (IQR) | 21.2 (15.7–18.1) | 24.0 (18.3–31.3) | <0.0001 † |
| NCCT SD (HU) | 8.6 (6.7–10.6) | 9.1 (7.1–11.1) | 0.01 † | |
| VOLUME | DENSITY | PERVIOUSNESS | HETEROGENEITY | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume (per 0.1 mL) | NCCT median (per 10 HU) | TAIQ1 (per 10 HU) | TAIQ2 (per 10 HU) | TAIQ3 (per 10 HU) | TAIMCC (per 10 HU) | CTA median (per 10 HU) | CTA SD (per 10 HU) | NCCT SD (per 10 HU) | ||
| Functional Outcome | Registry | 0.91 † (0.87–0.95) | 0.98 (0.87–1.09) | 1.14 † (1.07–1.21) | 1.10 † (1.04–1.15) | 1.05 † (1.02–1.09) | 1.11 † (1.05–1.17) | 1.09 † (1.03–1.15) | 0.93 † (0.87–0.99) | 0.87 † (0.77–0.98) |
| NO IV | 0.91 (0.81–1.02) | 1.15 (0.81–1.62) | 1.20 † (1.01–1.44) | 1.22 † (1.04–1.42) | 1.16 † (1.02–1.30) | 1.17 † (0.01–1.35) | 1.23 † (1.06–1.44) | 1.14 (0.94–1.40) | 0.76 (0.53–1.09) | |
| FPS | Registry | 0.94 (0.88–1.00) | 0.93 (0.80–1.08) | 1.07 (0.97–1.18) | 1.04 (0.96–1.12) | 1.03 (0.98–1.08) | 1.05 (0.97–1.14) | 1.02 (0.96–1.10) | 1.01 (0.92–1.12) | 0.97 (0.84–1.13) |
| NO IV | 0.92 (0.79–1.06) | 0.70 (0.44–1.12) | 1.01 (0.82–1.25) | 1.07 (0.89–1.29) | 1.11 (0.95–1.29) | 1.05 (0.87–1.27) | 1.00 (0.84–1.20) | 1.06 (0.84–1.34) | 0.55 † (0.31–0.98) | |
| eTICI2b+ | Registry | 1.01 (0.95–1.06) | 0.93 (0.82–1.05) | 0.96 (0.89–1.03) | 0.97 (0.91–1.03) | 0.98 (0.94–1.03) | 0.98 (0.92–1.05) | 0.95 (0.90–1.01) | 0.96 (0.89–1.04) | 0.88 † (0.78–0.99) |
| NO IV | 0.99 (0.84–1.17) | 1.20 (0.72–2.00) | 0.94 (0.70–1.26) | 0.94 (0.75–1.17) | 0.98 (0.83–1.16) | 1.00 (0.81–1.23) | 0.97 (0.77–1.22) | 1.03 (0.76–1.38) | 0.75 (0.47–1.21) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojtahedi, M.; Bruggeman, A.E.; van Voorst, H.; Ponomareva, E.; Kappelhof, M.; van der Lugt, A.; Hoving, J.W.; Dutra, B.G.; Dippel, D.; Cavalcante, F.; et al. Value of Automatically Derived Full Thrombus Characteristics: An Explorative Study of Their Associations with Outcomes in Ischemic Stroke Patients. J. Clin. Med. 2024, 13, 1388. https://doi.org/10.3390/jcm13051388
Mojtahedi M, Bruggeman AE, van Voorst H, Ponomareva E, Kappelhof M, van der Lugt A, Hoving JW, Dutra BG, Dippel D, Cavalcante F, et al. Value of Automatically Derived Full Thrombus Characteristics: An Explorative Study of Their Associations with Outcomes in Ischemic Stroke Patients. Journal of Clinical Medicine. 2024; 13(5):1388. https://doi.org/10.3390/jcm13051388
Chicago/Turabian StyleMojtahedi, Mahsa, Agnetha E. Bruggeman, Henk van Voorst, Elena Ponomareva, Manon Kappelhof, Aad van der Lugt, Jan W. Hoving, Bruna G. Dutra, Diederik Dippel, Fabiano Cavalcante, and et al. 2024. "Value of Automatically Derived Full Thrombus Characteristics: An Explorative Study of Their Associations with Outcomes in Ischemic Stroke Patients" Journal of Clinical Medicine 13, no. 5: 1388. https://doi.org/10.3390/jcm13051388
APA StyleMojtahedi, M., Bruggeman, A. E., van Voorst, H., Ponomareva, E., Kappelhof, M., van der Lugt, A., Hoving, J. W., Dutra, B. G., Dippel, D., Cavalcante, F., Yo, L., Coutinho, J., Brouwer, J., Treurniet, K., Tolhuisen, M. L., LeCouffe, N., Arrarte Terreros, N., Konduri, P. R., van Zwam, W., ... Marquering, H. A. (2024). Value of Automatically Derived Full Thrombus Characteristics: An Explorative Study of Their Associations with Outcomes in Ischemic Stroke Patients. Journal of Clinical Medicine, 13(5), 1388. https://doi.org/10.3390/jcm13051388








