Current Trends in Gait Rehabilitation for Stroke Survivors: A Scoping Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Stage 1: Identifying Research Questions
2.2. Stage 2: Identifying Relevant Studies
- S1: “Stroke”
- S2: “Rehabilitation”
- S3: “Gait”
2.3. Stage 3: Study Selection
2.4. Stage 4: Data Charting
2.5. Stage 5: Collating, Summarizing, and Reporting the Results
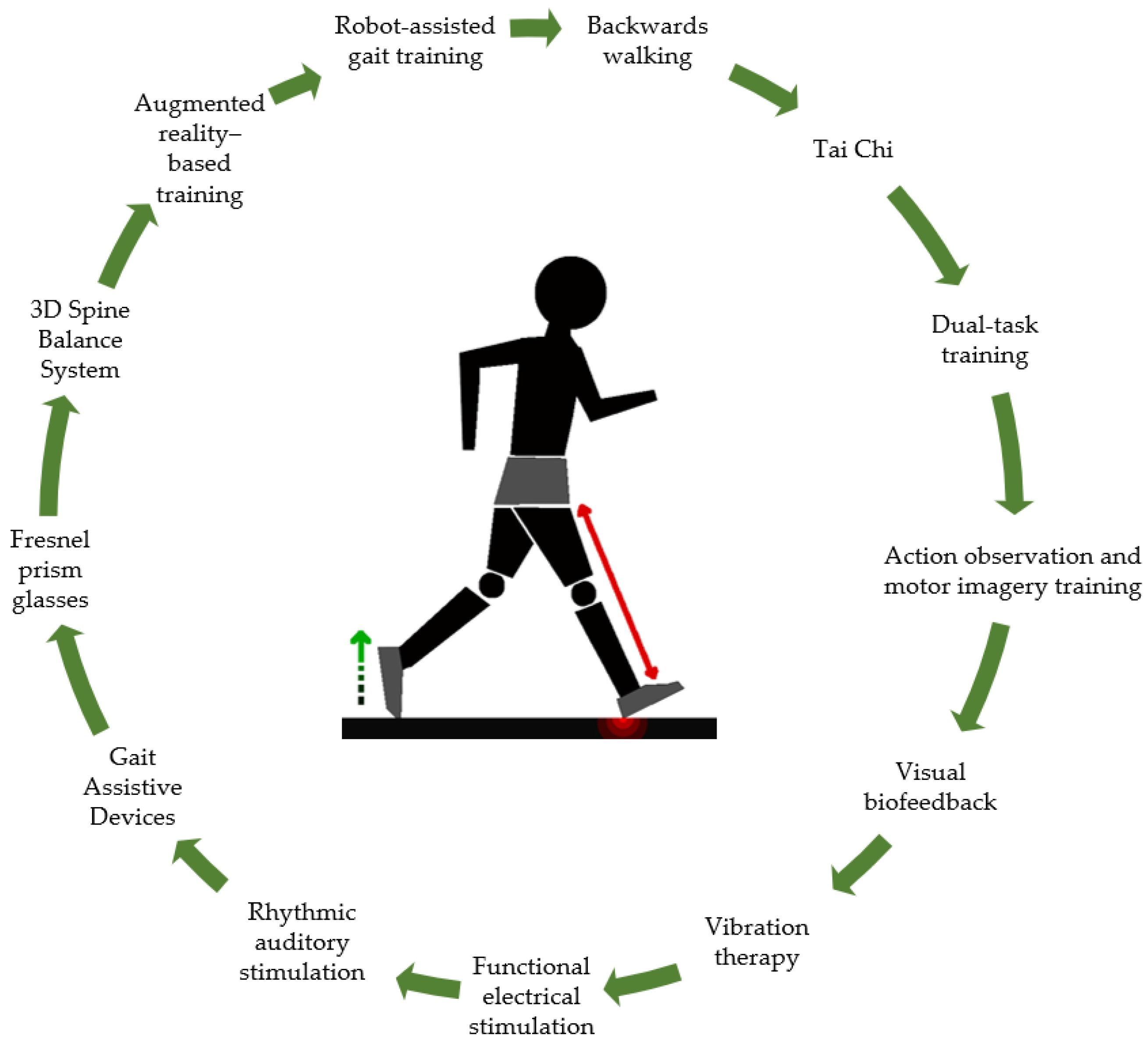
3. Results
3.1. Backwards Walking
3.2. Tai Chi
3.3. Dual-Task Training
3.4. Action Observation Training and Motor Imagery Training
3.5. Visual Biofeedback
3.6. Vibration Therapy
3.7. Functional Electrical Stimulation
3.8. Rhythmic Auditory Stimulation
3.9. Gait Assistive Devices
3.10. Fresnel Prism Glasses
3.11. 3D Spine Balance System
3.12. Augmented Reality-Based Training
3.13. Robot-Assisted Gait Training
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Gittins, M.; Lugo-Palacios, D.; Vail, A.; Bowen, A.; Paley, L.; Bray, B.; Tyson, S. Stroke impairment categories: A new way to classify the effects of stroke based on stroke-related impairments. Clin. Rehabil. 2021, 35, 446–458. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Wei, Z.J.; Zhang, Y.C.; Li, X.; Gong, L.; Zhou, J.W.; Wang, Y.; Zhang, Y.Y.; Wang, R.P. Functional Disability After Ischemic Stroke: A Community-Based Cross-Sectional Study in Shanghai, China. Front. Neurol. 2021, 12, 649088. [Google Scholar] [CrossRef]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.; Tallis, R.C. Balance disability after stroke. Phys. Ther. 2006, 86, 30–38. [Google Scholar] [CrossRef]
- Kim, Y.W. Update on Stroke Rehabilitation in Motor Impairment. Brain NeuroRehabil. 2022, 15, e12. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.; Iwarsson, S.; Jönsson, A.C.; Lindgren, A.; Månsson Lexell, E. Living and ageing with stroke: An exploration of conditions influencing participation in social and leisure activities over 15 years. Brain Inj. 2018, 32, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Bower, K.; Thilarajah, S.; Pua, Y.H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. Neuroeng. Rehabil. 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Goljar, N.; Globokar, D.; Puzić, N.; Kopitar, N.; Vrabič, M.; Ivanovski, M.; Vidmar, G. Effectiveness of a fall-risk reduction programme for inpatient rehabilitation after stroke. Disabil. Rehabil. 2016, 38, 1811–1819. [Google Scholar] [CrossRef]
- Goto, Y.; Otaka, Y.; Suzuki, K.; Inoue, S.; Kondo, K.; Shimizu, E. Incidence and circumstances of falls among community-dwelling ambulatory stroke survivors: A prospective study. Geriatr. Gerontol. Int. 2019, 19, 240–244. [Google Scholar] [CrossRef]
- Schinkel-Ivy, A.; Inness, E.L.; Mansfield, A. Relationships between fear of falling, balance confidence, and control of balance, gait, and reactive stepping in individuals with sub-acute stroke. Gait Posture 2016, 43, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ramnemark, A.; Nilsson, M.; Borssén, B.; Gustafson, Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 2000, 31, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Ursin, M.H.; Bergland, A.; Fure, B.; Tørstad, A.; Tveit, A.; Ihle-Hansen, H. Balance and Mobility as Predictors of Post-Stroke Cognitive Impairment. Dement. Geriatr. Cogn. Disord. Extra 2015, 5, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.; Burger, M.; Inglis-Jassiem, G. Post stroke health-related quality of life, stroke severity and function: A longitudinal cohort study. Afr. J. Disabil. 2022, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Poomalai, G.; Prabhakar, S.; Sirala Jagadesh, N. Functional Ability and Health Problems of Stroke Survivors: An Explorative Study. Cureus 2023, 15, e33375. [Google Scholar] [CrossRef] [PubMed]
- Olawale, O.A.; Usman, J.S.; Oke, K.I.; Osundiya, O.C. Evaluation of Predictive Factors Influencing Community Reintegration in Adult Patients with Stroke. J. Neurosci. Rural Pract. 2018, 9, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Dionne, T.P. Interventions to Improve Movement and Functional Outcomes in Adult Stroke Rehabilitation: Review and Evidence Summary. J. Particip. Med. 2018, 10, e3. [Google Scholar] [CrossRef]
- Vincent-Onabajo, G.; Shaphant, N.D. Relationship between functional independence and psychosocial quality of life of stroke survivors undergoing outpatient rehabilitation in Maiduguri, Nigeria. J. Patient-Rep. Outcomes 2019, 3, 18. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Lane, D.A.; Lenarczyk, R.; Boriani, G.; Doehner, W.; Benjamin, L.A.; Fisher, M.; Lowe, D.; Sacco, R.L.; Schnabel, R.; et al. Integrated care for optimizing the management of stroke and associated heart disease: A position paper of the European Society of Cardiology Council on Stroke. Eur. Heart J. 2022, 43, 2442–2460. [Google Scholar] [CrossRef]
- Kernan, W.N.; Viera, A.J.; Billinger, S.A.; Bravata, D.M.; Stark, S.L.; Kasner, S.E.; Kuritzky, L.; Towfighi, A.; American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; et al. Primary Care of Adult Patients After Stroke: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2021, 52, e558–e571. [Google Scholar] [CrossRef]
- Kalavina, R.; Chisati, E.; Mlenzana, N.; Wazakili, M. The challenges and experiences of stroke patients and their spouses in Blantyre, Malawi. Malawi Med. J. J. Med. Assoc. Malawi 2019, 31, 112–117. [Google Scholar] [CrossRef]
- Lu, Q.; Mårtensson, J.; Zhao, Y.; Johansson, L. Needs of family members caring for stroke survivors in china: A deductive qualitative content analysis study by using the caregiver task inventory-25. BMC Geriatr. 2022, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Tavares, E.; Coelho, J.; Rogado, P.; Correia, R.; Castro, C.; Fernandes, J.B. Barriers to Gait Training among Stroke Survivors: An Integrative Review. J. Funct. Morphol. Kinesiol. 2022, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V.; Hayfield, N.; Terry, G. Handbook of Research Methods in Health Social Sciences; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bang, D.-H.; Shin, W.-S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: A randomized controlled pilot trial. NeuroRehabilitation 2016, 38, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Kim, Y.; Hwang, S.; Chung, Y. Intensive gait training with rhythmic auditory stimulation in individuals with chronic hemiparetic stroke: A pilot randomized controlled study. NeuroRehabilitation 2014, 35, 681–688. [Google Scholar] [CrossRef]
- Choi, W.; Han, D.; Kim, J.; Lee, S. Whole-Body Vibration Combined with Treadmill Training Improves Walking Performance in Post-Stroke Patients: A Randomized Controlled Trial. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 4918–4925. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Moon, Y.; Choi, J.-D. Effects of Cognitive Task Training on Dynamic Balance and Gait of Patients with Stroke: A Preliminary Randomized Controlled Study. Med. Sci. Monit. Basic Res. 2020, 26, e925264. [Google Scholar] [CrossRef]
- Hwang, D.-Y.; Lee, H.-J.; Lee, G.-C.; Lee, S.-M. Treadmill training with tilt sensor functional electrical stimulation for improving balance, gait, and muscle architecture of tibialis anterior of survivors with chronic stroke: A randomized controlled trial. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2015, 23, 443–452. [Google Scholar] [CrossRef]
- Kang, Y.S.; Oh, G.B.; Cho, K.H. Walking Training with a Weight Support Feedback Cane Improves Lower Limb Muscle Activity and Gait Ability in Patients with Chronic Stroke: A Randomized Controlled Trial. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e931565. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, B.-H. Action observation training for functional activities after stroke: A pilot randomized controlled trial. NeuroRehabilitation 2013, 33, 565–574. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, K.B.; Bae, Y.-H.; Fong, S.S.M.; Lee, S.M. Effects of progressive backward body weight suppoted treadmill training on gait ability in chronic stroke patients: A randomized controlled trial. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2017, 25, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Cynn, H.-S.; Yi, C.-H.; Yoon, T.-L.; Lee, J.-H. Wearable tubing assistive walking device immediately enhances gait parameters in subjects with stroke: A randomized controlled study. NeuroRehabilitation 2017, 40, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-J.; Kim, T.-H. Effect of three-dimensional spine stabilization exercise on trunk muscle strength and gait ability in chronic stroke patients: A randomized controlled trial. NeuroRehabilitation 2017, 41, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.-Y.; Sung, Y.-H. Effects of Fresnel prism glasses on balance and gait in stroke patients with hemiplegia: A randomized controlled trial pilot study. Technol. Health Care Off. J. Eur. Soc. Eng. Med. 2020, 28, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Druzbicki, M.; Guzik, A.; Przysada, G.; Kwolek, A.; Brzozowska-Magon, A. Efficacy of Gait Training Using a Treadmill with and without Visual Biofeedback in Patients After Stroke: A Randomized Study. J. Rehabil. Med. 2015, 47, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Dujović, S.D.; Malešević, J.; Maleševićc, N.; Vidaković, A.S.; Bijelić, G.; Keller, T.; Konstantinović, L. Novel multi-pad functional electrical stimulation in stroke patients: A single-blind randomized study. NeuroRehabilitation 2017, 41, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Arsh, A.; Hammad, S.M.; Haq, I.U.; Darain, H. Comparison of dual task specific training and conventional physical therapy in ambulation of hemiplegic stroke patients: A randomized controlled trial. J. Pak. Med. Assoc. 2020, 70, 7–10. [Google Scholar] [CrossRef]
- Kelley, C.P.; Childress, J.; Boake, C.; Noser, E.A. Over-ground and robotic-assisted locomotor training in adults with chronic stroke: A blinded randomized clinical trial. Disabil. Rehabil. Assist. Technol. 2013, 8, 161–168. [Google Scholar] [CrossRef]
- Munari, D.; Serina, A.; Disarò, J.; Modenese, A.; Filippetti, M.; Gandolfi, M.; Smania, N.; Picelli, A. Combined effects of backward treadmill training and botulinum toxin type A therapy on gait and balance in patients with chronic stroke: A pilot, single-blind, randomized controlled trial. NeuroRehabilitation 2020, 46, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Mustafaoglu, R.; Erhan, B.; Yeldan, I.; Gunduz, B.; Tarakci, E. Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trial. Acta Neurol. Belg. 2020, 120, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.S.M.; Rehab, N.I.; Aly, S.M.A. Effect of aquatic versus land motor dual task training on balance and gait of patients with chronic stroke: A randomized controlled trial. NeuroRehabilitation 2019, 44, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, C.; Roerdink, M.; Meskers, C.G.M.; Beek, P.J.; Janssen, T.W.J. Walking-adaptability therapy after stroke: Results of a randomized controlled trial. Trials 2021, 22, 923. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-R.; Mi, P.-L.; Huang, S.-F.; Chiu, S.-L.; Liu, Y.-C.; Wang, R.-Y. Effects of neuromuscular electrical stimulation on gait performance in chronic stroke with inadequate ankle control—A randomized controlled trial. PLoS ONE 2018, 13, e0208609. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-M.; Jin, X.-M.; Lu, Y.; Gao, Y.; Xu, H.-C.; Xue, X.; Fang, L.; Hu, J. Effects of Body Weight Support-Tai Chi Footwork Training on Balance Control and Walking Function in Stroke Survivors with Hemiplegia: A Pilot Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. ECAM 2020, 2020, 9218078. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Finklestein, S.P.; Cramer, S.C. New Directions in Treatments Targeting Stroke Recovery. Stroke 2018, 49, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Domingos, J.; Dean, J.; Cruickshank, T.M.; Śmiłowska, K.; Fernandes, J.B.; Godinho, C. A Novel Boot Camp Program to Help Guide Personalized Exercise in People with Parkinson Disease. J. Pers. Med. 2021, 11, 938. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Teixeira, F.; Godinho, C. Personalized Care and Treatment Compliance in Chronic Conditions. J. Pers. Med. 2022, 12, 737. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Domingos, J.; Família, C.; Veríssimo, J.; Castanheira, P.; Menezes, C.; Vicente, C.; Santos, C.; Marvão, E.; Coelho, J.; et al. Adapted Portuguese folk dance intervention for subacute rehabilitation post-stroke: Study protocol. Front. Public Health 2023, 11, 1200093. [Google Scholar] [CrossRef]
- Fernandes, J.B.; Fernandes, S.B.; Almeida, A.S.; Vareta, D.A.; Miller, C.A. Older Adults’ Perceived Barriers to Participation in a Falls Prevention Strategy. J. Pers. Med. 2021, 11, 450. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [PubMed]
- Bryck, R.L.; Fisher, P.A. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am. Psychol. 2012, 67, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Dhami, P.; Moreno, S.; DeSouza, J.F. New framework for rehabilitation—Fusion of cognitive and physical rehabilitation: The hope for dancing. Front. Psychol. 2015, 5, 1478. [Google Scholar] [CrossRef] [PubMed]
- Milewska-Jędrzejczak, M.; Głąbiński, A. The Influence of Conventional and Innovative Rehabilitation Methods on Brain Plasticity Induction in Patients with Multiple Sclerosis. J. Clin. Med. 2023, 12, 1880. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef]
- Poli, P.; Morone, G.; Rosati, G.; Masiero, S. Robotic technologies and rehabilitation: New tools for stroke patients’ therapy. BioMed Res. Int. 2013, 2013, 153872. [Google Scholar] [CrossRef]
- Aprile, I.; Conte, C.; Cruciani, A.; Pecchioli, C.; Castelli, L.; Insalaco, S.; Germanotta, M.; Iacovelli, C. Efficacy of Robot-Assisted Gait Training Combined with Robotic Balance Training in Subacute Stroke Patients: A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 5162. [Google Scholar] [CrossRef]
- Chen, B.; Ma, H.; Qin, L.Y.; Gao, F.; Chan, K.M.; Law, S.W.; Qin, L.; Liao, W.H. Recent developments and challenges of lower extremity exoskeletons. J. Orthop. Transl. 2015, 5, 26–37. [Google Scholar] [CrossRef]
- Morone, G.; Bragoni, M.; Iosa, M.; De Angelis, D.; Venturiero, V.; Coiro, P.; Pratesi, L.; Paolucci, S. Who may benefit from robotic-assisted gait training? A randomized clinical trial in patients with subacute stroke. Neurorehabil. Neural Repair 2011, 25, 636–644. [Google Scholar] [CrossRef]
- Duret, C.; Grosmaire, A.G.; Krebs, H.I. Robot-Assisted Therapy in Upper Extremity Hemiparesis: Overview of an Evidence-Based Approach. Front. Neurol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Saraiva, J.; Rosa, G.; Fernandes, S.; Fernandes, J.B. Current Trends in Balance Rehabilitation for Stroke Survivors: A Scoping Review of Experimental Studies. Int. J. Environ. Res. Public Health 2023, 20, 6829. [Google Scholar] [CrossRef]
- Christoforou, E.G.; Avgousti, S.; Ramdani, N.; Novales, C.; Panayides, A.S. The Upcoming Role for Nursing and Assistive Robotics: Opportunities and Challenges Ahead. Front. Digit. Health 2020, 2, 585656. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tyson, S.; Weightman, A. Professionals’ Views and Experiences of Using Rehabilitation Robotics with Stroke Survivors: A Mixed Methods Survey. Front. Med. Technol. 2021, 3, 780090. [Google Scholar] [CrossRef] [PubMed]
- Verrienti, G.; Raccagni, C.; Lombardozzi, G.; De Bartolo, D.; Iosa, M. Motivation as a Measurable Outcome in Stroke Rehabilitation: A Systematic Review of the Literature. Int. J. Environ. Res. Public Health 2023, 20, 4187. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.B.; Ferreira, N.; Domingos, J.; Ferreira, R.; Amador, C.; Pardal, N.; Castro, C.; Simões, A.; Fernandes, S.; Bernardes, C.; et al. Health Professionals’ Motivational Strategies to Enhance Adherence in the Rehabilitation of People with Lower Limb Fractures: Scoping Review. Int. J. Environ. Res. Public Health 2023, 20, 7050. [Google Scholar] [CrossRef] [PubMed]
- Shahid, J.; Kashif, A.; Shahid, M.K. A Comprehensive Review of Physical Therapy Interventions for Stroke Rehabilitation: Impairment-Based Approaches and Functional Goals. Brain Sci. 2023, 13, 717. [Google Scholar] [CrossRef]
- Amin, F.; Waris, A.; Iqbal, J.; Gilani, S.; Rehman, M.; Mushtaq, S.; Khan, N.; Khan, M.; Jameel, M.; Tamam, N. Maximizing stroke recovery with advanced technologies: A comprehensive assessment of robot-assisted, EMG-Controlled robotics, virtual reality, and mirror therapy interventions. Results Eng. 2024, 21, 101725. [Google Scholar] [CrossRef]
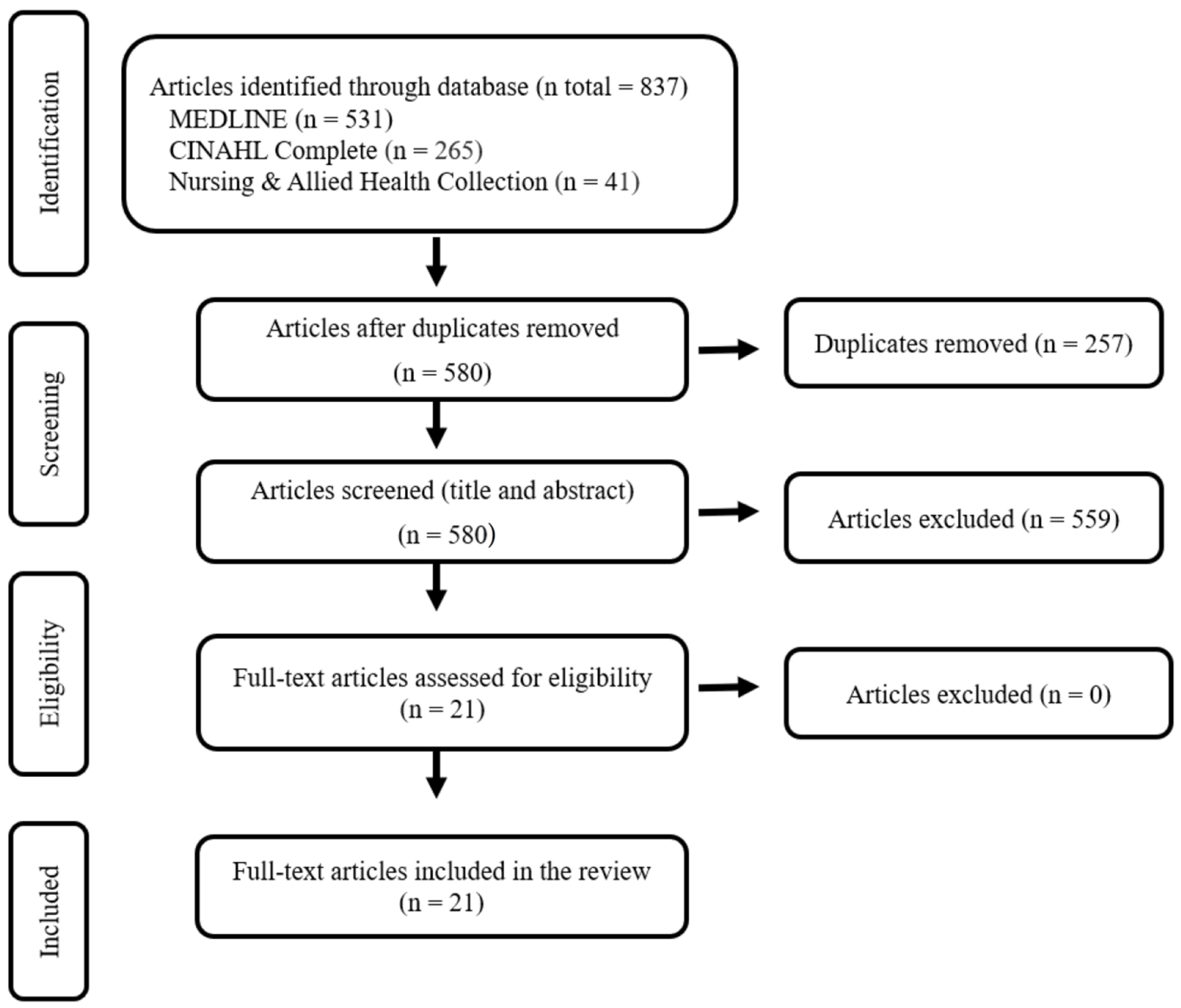
| Parameter | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Stroke survivors; Adults ≥ 18 years old. | Other health conditions besides stroke; Participants < 18 years old. |
| Concept | Studies that explore interventions for promoting gait. | Studies that do not explore interventions for promoting gait. |
| Context | Studies conducted in rehabilitation settings. | Studies conducted in non-rehabilitation settings. |
| Study design | Randomized controlled trials focusing on interventions that promote gait in stroke survivors. | Other type of studies. |
| Author/Year/Country | Study Design/Study Aim | Intervention |
|---|---|---|
| Bang and Shin [27] (2016) South Korea | Randomized controlled trial To compare the effects of robot-assisted gait training versus treadmill on spatiotemporal gait parameters, balance, and activities-specific balance confidence with stroke patients. | Robot-assisted gait training |
| Cha et al. [28] (2014) South Korea | Randomized controlled trial To investigate the effect of intensive gait training with rhythmic auditory stimulation on postural control and gait performance in individuals with chronic hemiparetic stroke. | Rhythmic auditory stimulation |
| Choi et al. [29] (2017) South Korea | Randomized controlled trial To investigate the effect of whole-body vibration combined with treadmill training on walking performance in patients with chronic stroke. | Vibration therapy |
| Druzbicki et al. [38] (2015) Poland | Randomized controlled trial To evaluate the effects of gait training using a treadmill with and without visual biofeedback in patients late after stroke and to compare both training methods. | Visual biofeedback |
| Dujović et al. [39] (2017) Serbia | Single-blind randomized trial To evaluate the efficacy of an additional novel FES system to conventional therapy in facilitating motor recovery in the lower extremities and improving walking ability after stroke. | Functional electrical stimulation |
| Ha and Sung [37] (2020) North Korea | Randomized controlled trial To investigate the effect of Fresnel prism glasses on balance and gait in stroke patients with hemiplegia. | Fresnel prism glasses |
| Hong et al. [30] (2020) South Korea | Randomized controlled trial To determine whether cognitive task training improves walking and balancing abilities for stroke survivors. | Dual-task training |
| Hwang et al. [31] (2015) South Korea | Randomized controlled trial To investigate the effects of treadmill training with tilt sensor FES on the balance, gait, and muscle architecture of the tibialis anterior in stroke survivors. | Functional electrical stimulation |
| Iqbal et al. [40] (2020) Pakistan | Randomized controlled trial To compare the effectiveness of dual task-specific training and conventional physical therapy in the ambulation of patients with chronic stroke. | Dual-task training |
| Kang et al. [32] (2021) South Korea | Randomized controlled trial To investigate the effect of walking training with a weight support feedback cane on chronic stroke patients’ lower limb muscle activity and gait ability. | Gait Assistive Devices |
| Kelley et al. [41] (2013) United States of America | Blinded randomized controlled trial To compare the effectiveness of robotic-assisted body weight-supported treadmill training using the Lokomat® for over-ground gait training in adults with chronic stroke. | Robot-assisted gait training |
| Kim and Lee [33] (2013) South Korea | Randomized controlled trial To compare the effects of action observation and motor imagery training on recovery from chronic stroke. | Action observation training and motor imagery training |
| Kim et al. [34] (2017) South Korea | Randomized controlled trial To examine the effect of progressive backward body weight-supported treadmill training on gait in chronic stroke patients with hemiplegic gait. | Backwards walking |
| Lee et al. [35] (2017) South Korea | Randomized controlled trial To investigate the effects of a wearable tubing assistive walking device on gait parameters (gait speed, cadence, step length, and stride length on affected and less affected sides) in patients with stroke. | Gait Assistive Devices |
| Moon and Kim [36] (2017) South Korea | Randomized controlled trial To investigate the effects of the newly developed Spine Balance three-dimensional (3D) system on chronic stroke patients’ trunk strength and gait abilities. | 3D Spine Balance System |
| Munari et al. [42] (2020) Italy | Randomized controlled trial To compare the effects of backward treadmill training versus standard forward treadmill training on motor impairment in patients with chronic stroke receiving botulinum toxin type A Therapy. | Backwards walking |
| Mustafaoglu et al. [43] (2020) Turkey | Randomized controlled trial To investigate the effects of robot-assisted gait training on mobility, activities of daily living, and quality of life in stroke Rehabilitation. | Robot-assisted gait training |
| Saleh et al. [44] (2019) Egypt | Randomized controlled trial To compare the effect of aquatic versus land motor dual-task training on chronic stroke patients’ balance and gait. | Dual-task training |
| Timmermans et al. [45] (2021) The Netherlands | Randomized controlled trial To compare the efficacy of two walking-adaptability interventions: a novel treadmill-based C-Mill therapy and the standard overground FALLS program. | Augmented reality-based rehabilitation |
| Yang [46] (2018) Taiwan | Randomized controlled trial To evaluate the effects of applying NMES over ankle dorsiflexion or plantar flexors on ankle control during walking and gait performance in chronic stroke patients. | Functional electrical stimulation |
| Yu et al. [47] (2020) China | Randomized controlled trial To examine the effects of body weight support Tai Chi training on balance control and walking function in stroke survivors with hemiplegia. | Tai Chi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teodoro, J.; Fernandes, S.; Castro, C.; Fernandes, J.B. Current Trends in Gait Rehabilitation for Stroke Survivors: A Scoping Review of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 1358. https://doi.org/10.3390/jcm13051358
Teodoro J, Fernandes S, Castro C, Fernandes JB. Current Trends in Gait Rehabilitation for Stroke Survivors: A Scoping Review of Randomized Controlled Trials. Journal of Clinical Medicine. 2024; 13(5):1358. https://doi.org/10.3390/jcm13051358
Chicago/Turabian StyleTeodoro, Joana, Sónia Fernandes, Cidália Castro, and Júlio Belo Fernandes. 2024. "Current Trends in Gait Rehabilitation for Stroke Survivors: A Scoping Review of Randomized Controlled Trials" Journal of Clinical Medicine 13, no. 5: 1358. https://doi.org/10.3390/jcm13051358
APA StyleTeodoro, J., Fernandes, S., Castro, C., & Fernandes, J. B. (2024). Current Trends in Gait Rehabilitation for Stroke Survivors: A Scoping Review of Randomized Controlled Trials. Journal of Clinical Medicine, 13(5), 1358. https://doi.org/10.3390/jcm13051358







