Effects of SGLT2 Inhibitors with and without Metformin in High-Risk, Treatment-Naïve Patients with Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Covariate Measurements
2.4. Outcome Definitions
2.5. Statistical Analysis
3. Results
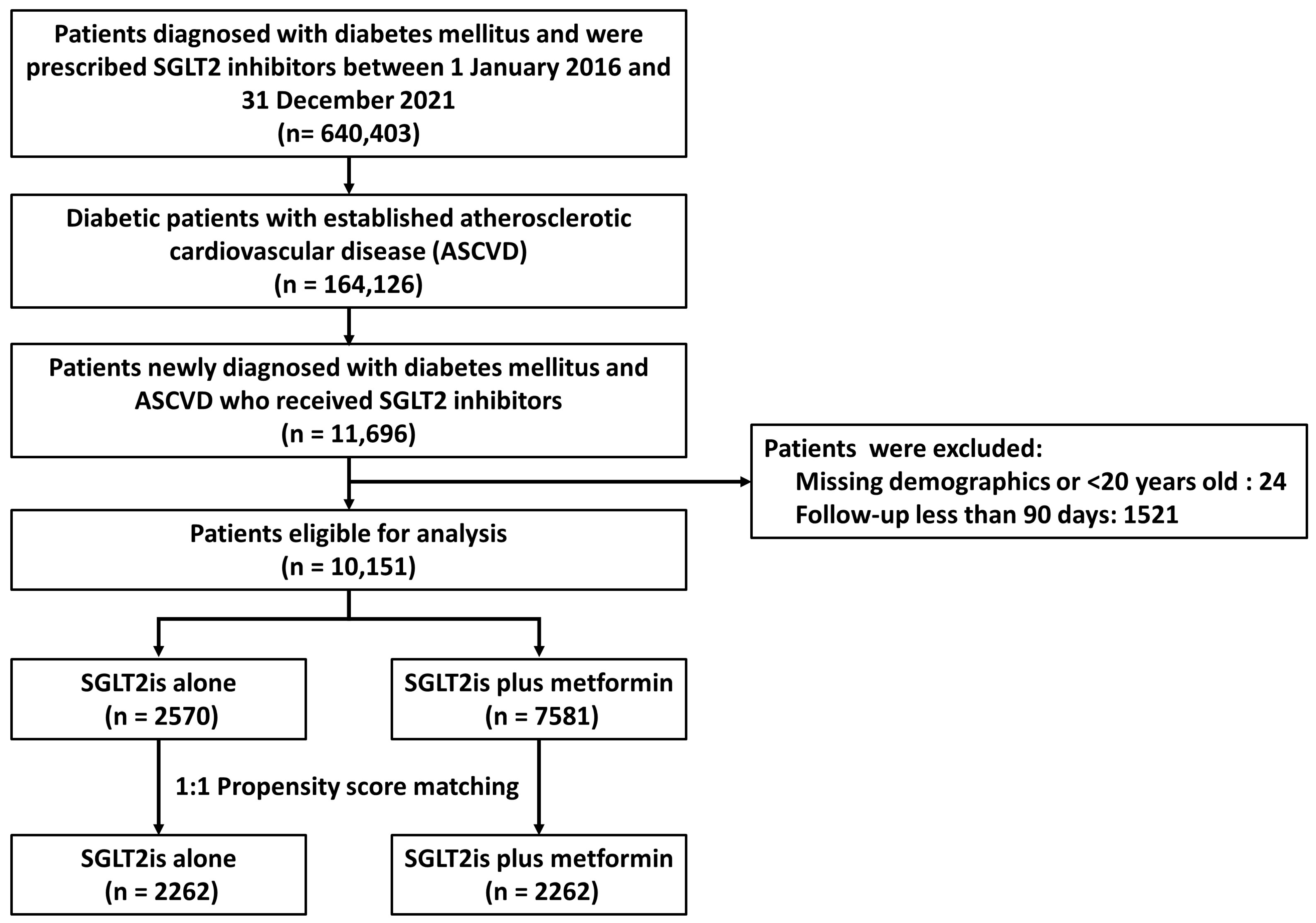
3.1. Patient Inclusion
3.2. Baseline Characteristics
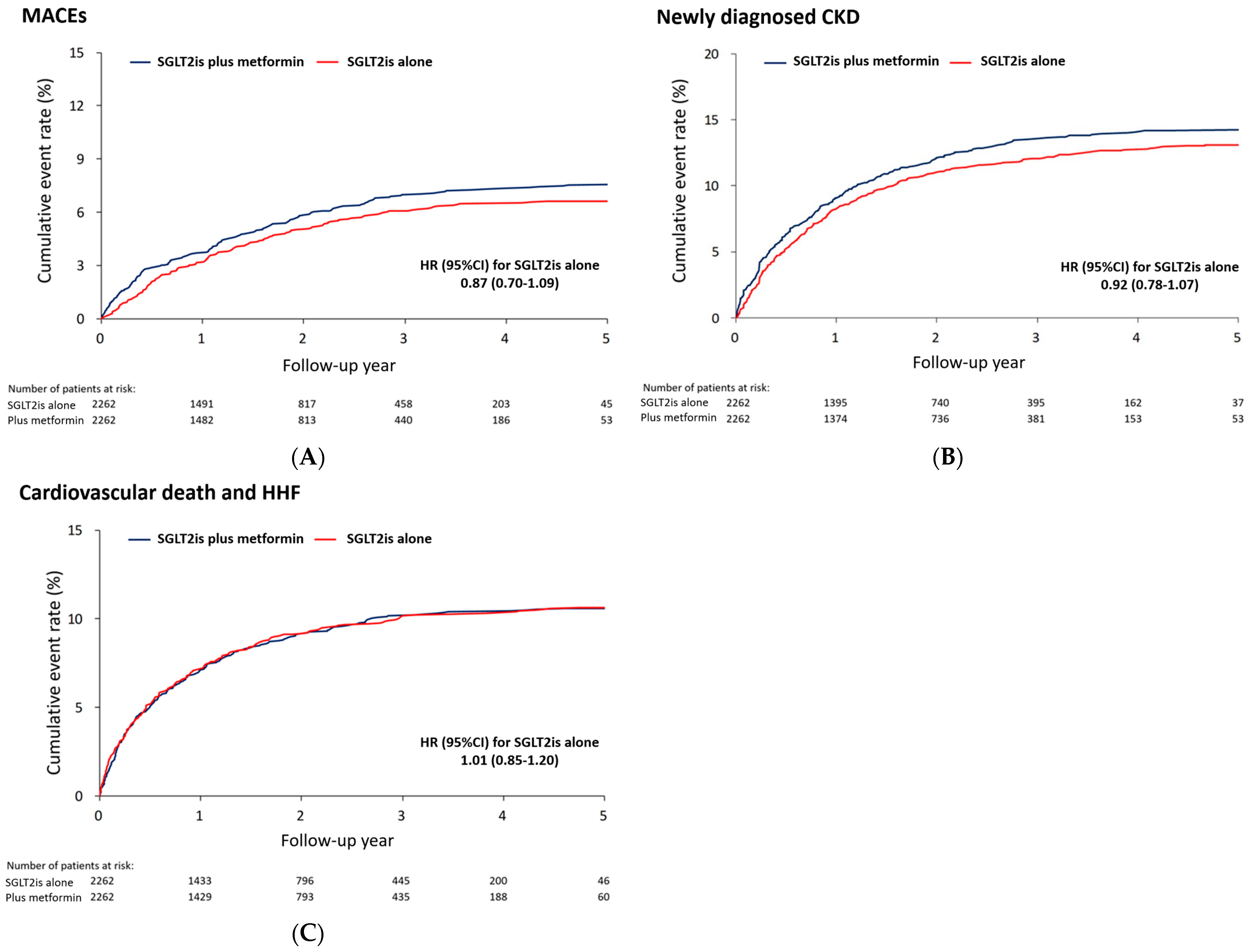
3.3. MACEs and Other Outcomes
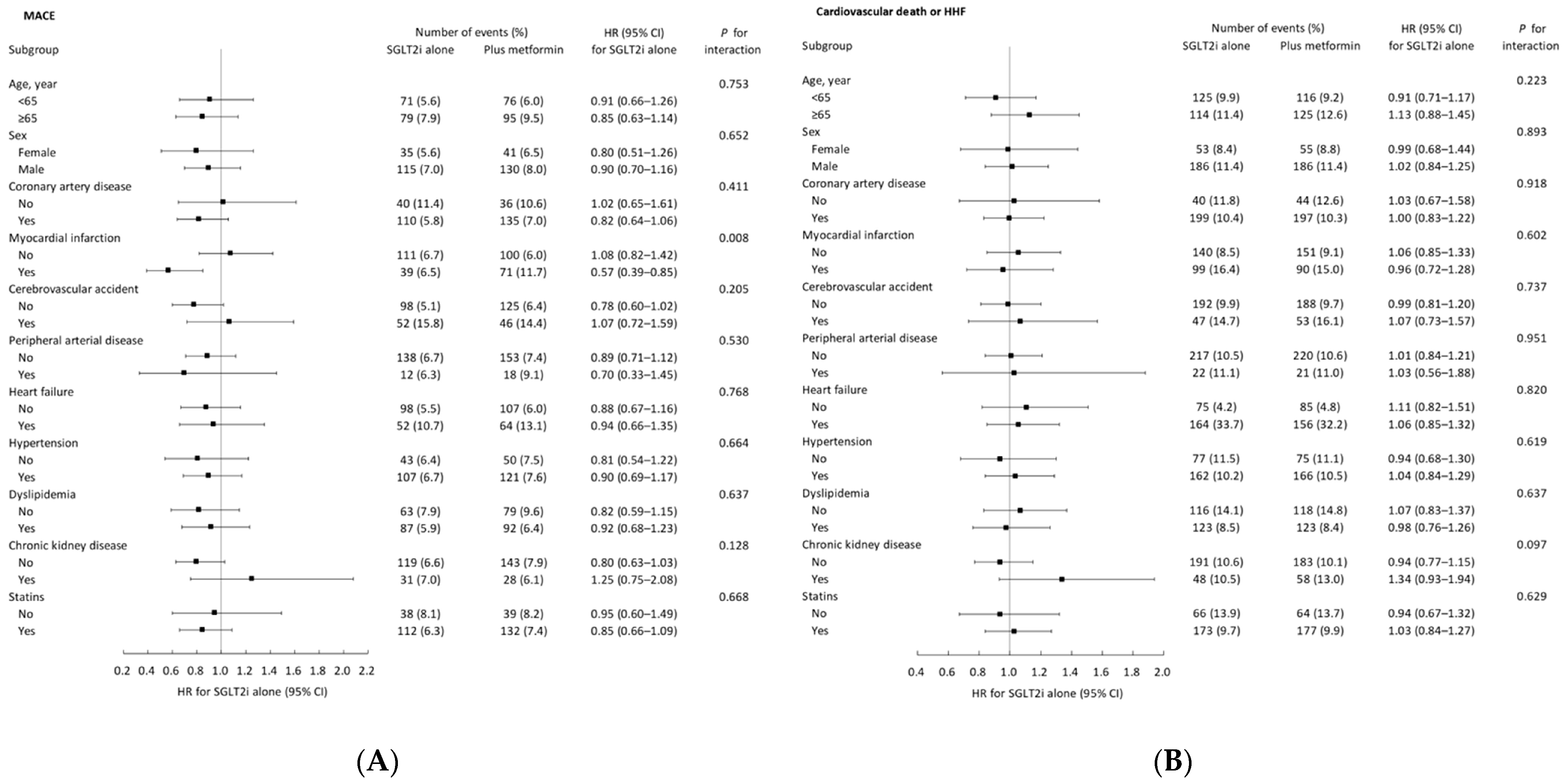
3.4. Subgroup Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braunwald, E. Gliflozins in the Management of Cardiovascular Disease. N. Engl. J. Med. 2022, 386, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Philippaert, K.; Kalyaanamoorthy, S.; Fatehi, M.; Long, W.; Soni, S.; Byrne, N.J.; Barr, A.; Singh, J.; Wong, J.; Palechuk, T.; et al. Cardiac Late Sodium Channel Current Is a Molecular Target for the Sodium/Glucose Cotransporter 2 Inhibitor Empagliflozin. Circulation 2021, 143, 2188–2204. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Hejna, J.; Green, K.; Batra, M.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin Suppresses Hepcidin And Increases Erythropoiesis. J. Clin. Endocrinol. Metab. 2020, 105, e1056–e1063. [Google Scholar] [CrossRef] [PubMed]
- Angermann, C.E.; Santos-Gallego, C.G.; Requena-Ibanez, J.A.; Sehner, S.; Zeller, T.; Gerhardt, L.M.S.; Maack, C.; Sanz, J.; Frantz, S.; Fuster, V.; et al. Empagliflozin effects on iron metabolism as a possible mechanism for improved clinical outcomes in non-diabetic patients with systolic heart failure. Nat. Cardiovasc. Res. 2023, 2, 1032–1043. [Google Scholar] [CrossRef]
- Fuchs Andersen, C.; Omar, M.; Glenthøj, A.; El Fassi, D.; Møller, H.J.; Lindholm Kurtzhals, J.A.; Styrishave, B.; Kistorp, C.; Tuxen, C.; Poulsen, M.K.; et al. Effects of empagliflozin on erythropoiesis in heart failure: Data from the Empire HF trial. Eur. J. Heart Fail. 2023, 25, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; Van Veldhuisen, D.J.; De Boer, R.A.; Van Der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef] [PubMed]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [PubMed]
- Masson, W.; Lavalle-Cobo, A.; Lobo, M.; Masson, G.; Molinero, G. Novel antidiabetic drugs and risk of cardiovascular events in patients without baseline metformin use: A meta-analysis. Eur. J. Prev. Cardiol. 2021, 28, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Arnott, C.; Perkovic, V.; Figtree, G.; de Zeeuw, D.; Fulcher, G.; Jun, M.; Jardine, M.J.; Zoungas, S.; Pollock, C.; et al. Sodium-glucose co-transporter-2 inhibitors with and without metformin: A meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes. Metab. 2021, 23, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Schütt, K.; Federici, M.; Verket, M.; Marx, N.; Müller-Wieland, D. The ‘10 commandments’ for the 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Hear. J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Lee, S.W.; Acharya, K.P. Propensity score matching for causal inference and reducing the confounding effects: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- EMPA-Kidney Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- EMPA-Kidney Collaborative Group. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Cannon, C.P.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Charbonnel, B.; Cosentino, F.; Dagogo-Jack, S.; McGuire, D.K.; Pratley, R.; Shih, W.J.; Frederich, R.; Maldonado, M.; Pong, A.; et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: An analysis from the randomised VERTIS CV trial. Diabetologia 2021, 64, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Murdolo, G.; Coiro, S.; Santucci, A.; Notaristefano, F.; Angeli, F.; Cavallini, C. Therapy of Type 2 diabetes: More gliflozines and less metformin? Eur. Heart J. Suppl. 2023, 25 (Suppl. B), B171–B176. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.L.G.; Neto, F.T.L. The use of metformin in women with polycystic ovary syndrome: An updated review. J. Assist. Reprod. Genet. 2022, 39, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Clegg, L.E.; Jing, Y.; Penland, R.C.; Boulton, D.W.; Hernandez, A.F.; Holman, R.R.; Vora, J. Author response for “Cardiovascular and renal safety of metformin in patients with diabetes and moderate or severe chronic kidney disease: Observations from the EXSCEL and SAVOR-TIMI 53 cardiovascular outcomes trials”. Diabetes Obes. Metab. 2021, 23, 1101–1110. [Google Scholar] [CrossRef]
- Kooy, A.; de Jager, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.M.; Stehouwer, C.D.A. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. JAMA Intern. Med. 2009, 169, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Gram, J.; Henriksen, J.E.; Grodum, E.; Juhl, H.; Hansen, T.B.; Christiansen, C.; Yderstræde, K.; Gjessing, H.; Hansen, H.M.; Vestergaard, V.; et al. Pharmacological treatment of the pathogenetic defects in type 2 diabetes: The randomized multicenter South Danish Diabetes Study. Diabetes Care 2011, 34, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.-P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: A meta-analysis of randomised controlled trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef]
- MacIsaac, R.J.; Jerums, G. Intensive glucose control and cardiovascular outcomes in type 2 diabetes. Heart Lung Circ. 2011, 20, 647–654. [Google Scholar] [CrossRef] [PubMed]
| Before Matching | After Matching | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Total (n = 10,151) | SGLT2is Alone (n = 2570) | Plus Metformin (n = 7581) | STD | SGLT2is Alone (n = 2262) | Plus Metformin (n = 2262) | STD |
| Age, year | 60.1 ± 12.4 | 64.9 ± 13.0 | 58.5 ± 11.8 | 0.52 | 63.0 ± 12.4 | 63.1 ± 11.7 | −0.01 |
| Male, sex | 7454 (73.4) | 1840 (71.6) | 5614 (74.1) | −0.06 | 1638 (72.4) | 1629 (72.0) | 0.01 |
| ASCVD | |||||||
| Peripheral artery disease | 798 (7.9) | 235 (9.1) | 563 (7.4) | 0.06 | 191 (8.4) | 199 (8.8) | −0.01 |
| Coronary artery disease | 8424 (83.0) | 2166 (84.3) | 6258 (82.5) | 0.05 | 1912 (84.5) | 1923 (85.0) | −0.01 |
| Myocardial infarction | 2789 (27.5) | 674 (26.2) | 2115 (27.9) | −0.04 | 599 (26.5) | 605 (26.7) | −0.01 |
| Cerebrovascular accident | 1584 (15.6) | 402 (15.6) | 1182 (15.6) | 0.00 | 329 (14.5) | 319 (14.1) | 0.01 |
| Comorbidity | |||||||
| Atrial fibrillation | 767 (7.6) | 342 (13.3) | 425 (5.6) | 0.27 | 220 (9.7) | 219 (9.7) | 0.00 |
| Hypertension | 7032 (69.3) | 1813 (70.5) | 5219 (68.8) | 0.04 | 1588 (70.2) | 1593 (70.4) | 0.00 |
| Dyslipidemia | 6593 (64.9) | 1612 (62.7) | 4981 (65.7) | −0.06 | 1466 (64.8) | 1441 (63.7) | 0.02 |
| Chronic kidney disease | 1721 (17.0) | 597 (23.2) | 1124 (14.8) | 0.22 | 446 (19.7) | 459 (20.3) | −0.01 |
| COPD | 757 (7.5) | 261 (10.2) | 496 (6.5) | 0.13 | 211 (9.3) | 206 (9.1) | 0.01 |
| Malignancy | 540 (5.3) | 191 (7.4) | 349 (4.6) | 0.12 | 148 (6.5) | 150 (6.6) | 0.00 |
| History of event | |||||||
| Heart failure | 1727 (17.0) | 673 (26.2) | 1054 (13.9) | 0.31 | 484 (21.4) | 487 (21.5) | 0.00 |
| Embolic event | 120 (1.2) | 36 (1.4) | 84 (1.1) | 0.03 | 27 (1.2) | 32 (1.4) | −0.02 |
| Venous thromboembolism | 76 (0.7) | 25 (1.0) | 51 (0.7) | 0.03 | 20 (0.9) | 23 (1.0) | −0.01 |
| Concomitant medications | |||||||
| Anti-platelet agents | |||||||
| Aspirin | 6378 (62.8) | 1326 (51.6) | 5052 (66.6) | −0.31 | 1253 (55.4) | 1278 (56.5) | −0.02 |
| Clopidogrel/Ticagrelor/Prasugrel | 3495 (34.4) | 884 (34.4) | 2611 (34.4) | 0.00 | 763 (33.7) | 763 (33.7) | 0.00 |
| Anti-coagulants | 864 (8.5) | 371 (14.4) | 493 (6.5) | 0.26 | 244 (10.8) | 233 (10.3) | 0.02 |
| Anti-diabetic medications | |||||||
| DPP4i | 1382 (13.6) | 114 (4.4) | 1268 (16.7) | −0.41 | 114 (5.0) | 112 (5.0) | 0.00 |
| GLP1RA | 21 (0.2) | 4 (0.2) | 17 (0.2) | −0.02 | 4 (0.2) | 5 (0.2) | −0.01 |
| Sulfonylurea | 1933 (19.0) | 89 (3.5) | 1844 (24.3) | −0.63 | 89 (3.9) | 93 (4.1) | −0.01 |
| Thiazolidinedione | 568 (5.6) | 20 (0.8) | 548 (7.2) | −0.33 | 20 (0.9) | 22 (1.0) | −0.01 |
| Alpha glucosidase inhibitors | 269 (2.6) | 33 (1.3) | 236 (3.1) | −0.12 | 31 (1.4) | 36 (1.6) | −0.02 |
| Glinide | 102 (1.0) | 10 (0.4) | 92 (1.2) | −0.09 | 10 (0.4) | 12 (0.5) | −0.01 |
| Insulin | 598 (5.9) | 35 (1.4) | 563 (7.4) | −0.30 | 34 (1.5) | 32 (1.4) | 0.01 |
| Other medications | |||||||
| ACEi or ARBs | 6999 (68.9) | 1844 (71.8) | 5155 (68.0) | 0.08 | 1607 (71.0) | 1599 (70.7) | 0.01 |
| Beta-blockers | 5903 (58.2) | 1525 (59.3) | 4378 (57.7) | 0.03 | 1315 (58.1) | 1319 (58.3) | 0.00 |
| DCCBs | 3239 (31.9) | 848 (33.0) | 2391 (31.5) | 0.03 | 752 (33.2) | 767 (33.9) | −0.01 |
| Statins | 8210 (80.9) | 1984 (77.2) | 6226 (82.1) | −0.12 | 1793 (79.3) | 1787 (79.0) | 0.01 |
| NSAIDs/Cox-2 | 4961 (48.9) | 1239 (48.2) | 3722 (49.1) | −0.02 | 1096 (48.5) | 1098 (48.5) | 0.00 |
| Diuretics | 1788 (17.6) | 685 (26.7) | 1103 (14.5) | 0.30 | 491 (21.7) | 489 (21.6) | 0.00 |
| Spironolactone | 1607 (15.8) | 640 (24.9) | 967 (12.8) | 0.31 | 455 (20.1) | 459 (20.3) | 0.00 |
| Follow up year | 2.0 ± 1.3 | 1.8 ± 1.3 | 2.1 ± 1.3 | −0.24 | 1.9 ± 1.3 | 1.9 ± 1.3 | −0.01 |
| SGLT2is Alone | SGLT2is with Metformin | |||||
|---|---|---|---|---|---|---|
| Outcome | Number of Events (%) | Incidence (95% CI) * | Number of Events (%) | Incidence (95% CI) * | HR/SHR (95% CI) for SGLT2is Alone | p Value |
| Cardiovascular outcome | ||||||
| Cardiovascular death | 66 (2.9) | 15.6 (11.9–19.4) | 69 (3.1) | 16.3 (12.4–20.1) | 0.96 (0.69–1.35) | 0.828 |
| Ischemic stroke | 68 (3.0) | 16.4 (12.5–20.4) | 81 (3.6) | 19.7 (15.4–24.0) | 0.84 (0.61–1.16) | 0.290 |
| Acute myocardial infarction | 32 (1.4) | 7.7 (5.0–10.3) | 44 (1.9) | 10.5 (7.4–13.6) | 0.73 (0.46–1.15) | 0.176 |
| Composite MACEs | 150 (6.6) | 36.6 (30.7–42.4) | 171 (7.6) | 42.1 (35.8–48.4) | 0.87 (0.70–1.09) | 0.222 |
| Other outcomes | ||||||
| Newly diagnosed CKD | 296 (13.1) | 77.9 (69.0–86.8) | 323 (14.3) | 85.6 (76.2–94.9) | 0.92 (0.78–1.07) | 0.261 |
| All-cause death | 94 (4.2) | 22.3 (17.8–26.8) | 114 (5.0) | 26.9 (22.0–31.9) | 0.83 (0.63–1.09) | 0.177 |
| Hospitalization for heart failure | 212 (9.4) | 53.1 (46.0–60.3) | 202 (8.9) | 50.8 (44.0–57.8) | 1.06 (0.88–1.27) | 0.572 |
| CV death and HHF | 241 (10.7) | 60.4 (52.8–68.0) | 239 (10.6) | 60.1 (52.5–67.7) | 1.01 (0.85–1.20) | 0.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-Y.; Lee, J.-K. Effects of SGLT2 Inhibitors with and without Metformin in High-Risk, Treatment-Naïve Patients with Diabetes. J. Clin. Med. 2024, 13, 1387. https://doi.org/10.3390/jcm13051387
Huang C-Y, Lee J-K. Effects of SGLT2 Inhibitors with and without Metformin in High-Risk, Treatment-Naïve Patients with Diabetes. Journal of Clinical Medicine. 2024; 13(5):1387. https://doi.org/10.3390/jcm13051387
Chicago/Turabian StyleHuang, Chen-Yu, and Jen-Kuang Lee. 2024. "Effects of SGLT2 Inhibitors with and without Metformin in High-Risk, Treatment-Naïve Patients with Diabetes" Journal of Clinical Medicine 13, no. 5: 1387. https://doi.org/10.3390/jcm13051387
APA StyleHuang, C.-Y., & Lee, J.-K. (2024). Effects of SGLT2 Inhibitors with and without Metformin in High-Risk, Treatment-Naïve Patients with Diabetes. Journal of Clinical Medicine, 13(5), 1387. https://doi.org/10.3390/jcm13051387







