Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Electronic Database Search
2.2. Eligibility Criteria
- (1)
- Evaluating the prevalence of OSA among young adults aged 18–30 years or presenting data separately for this age subgroup;
- (2)
- A healthy patient;
- (3)
- OSA diagnosis was based on a polysomnographic examination or home sleep apnea testing;
- (4)
- Published in English-language journals;
- (5)
- Only human studies and studies conducted in English were eligible;
- (6)
- studies based on age- or gender-specific subgroups such as studies on occupational subgroups and clinical subgroups.
- (1)
- Reports without sufficient reporting of OSA prevalence;
- (2)
- Narrative/systematic reviews and meta-analyses focusing on the prevalence of apnea in groups of chronic disease patients;
- (3)
- Studies relating to the adult population without reference to age groups;
- (4)
- Chronic disease as a requirement for inclusion in the study;
- (5)
- Studies based solely on questionnaires.
2.3. Data Extraction
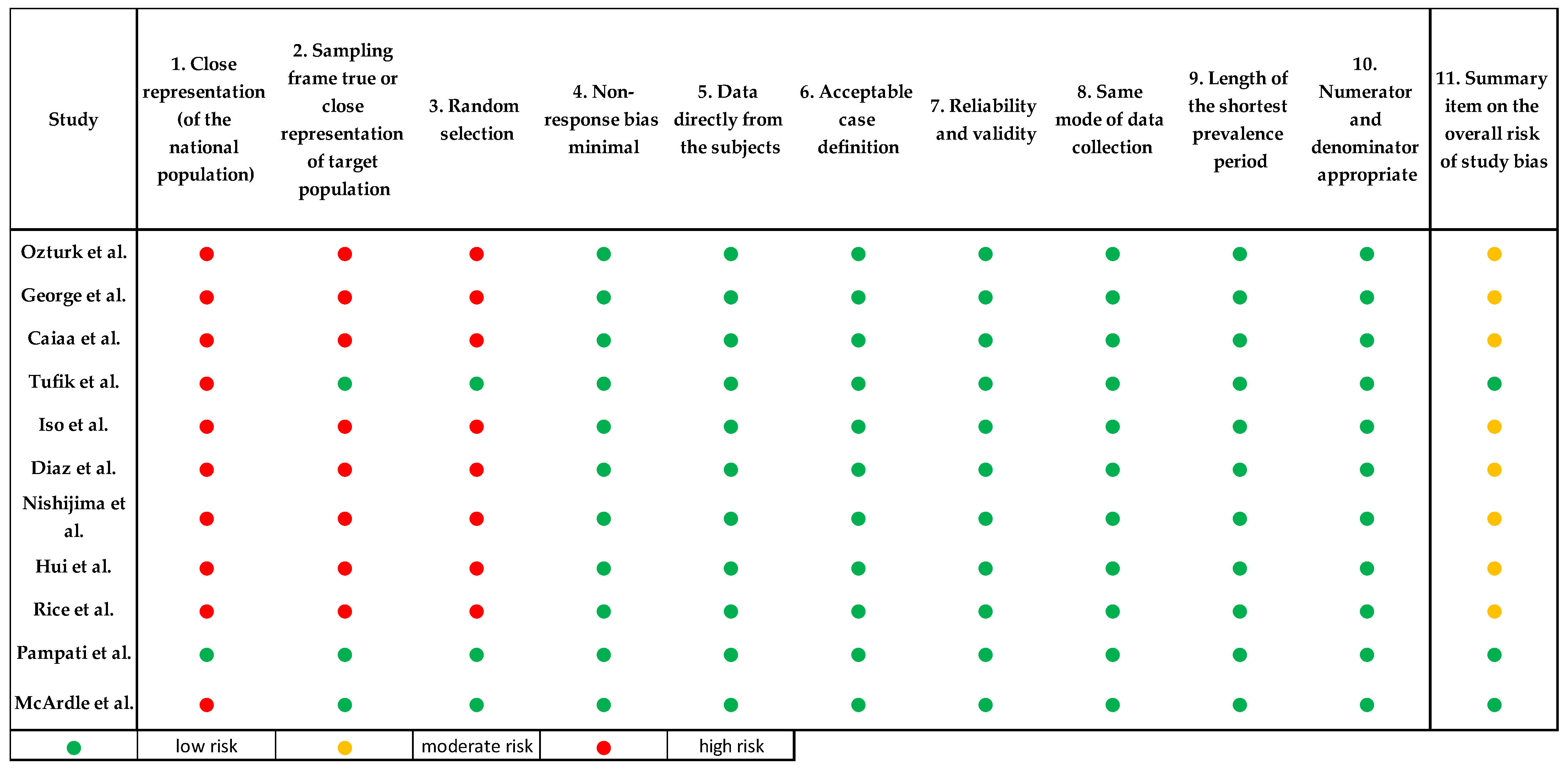
2.4. Risk of Bias Assessment of the Selected Papers
2.5. Statistical Analysis
3. Results
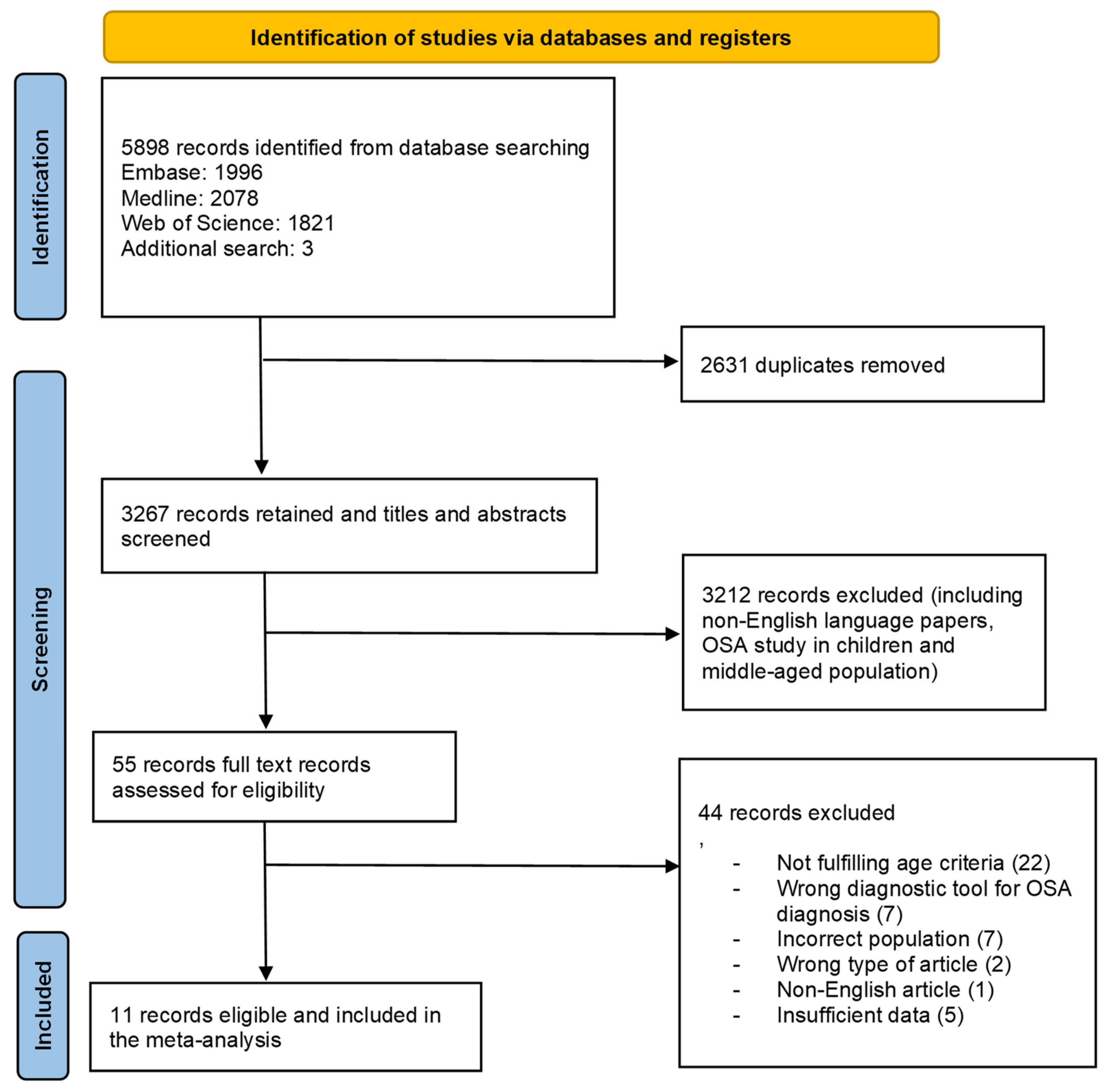
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias
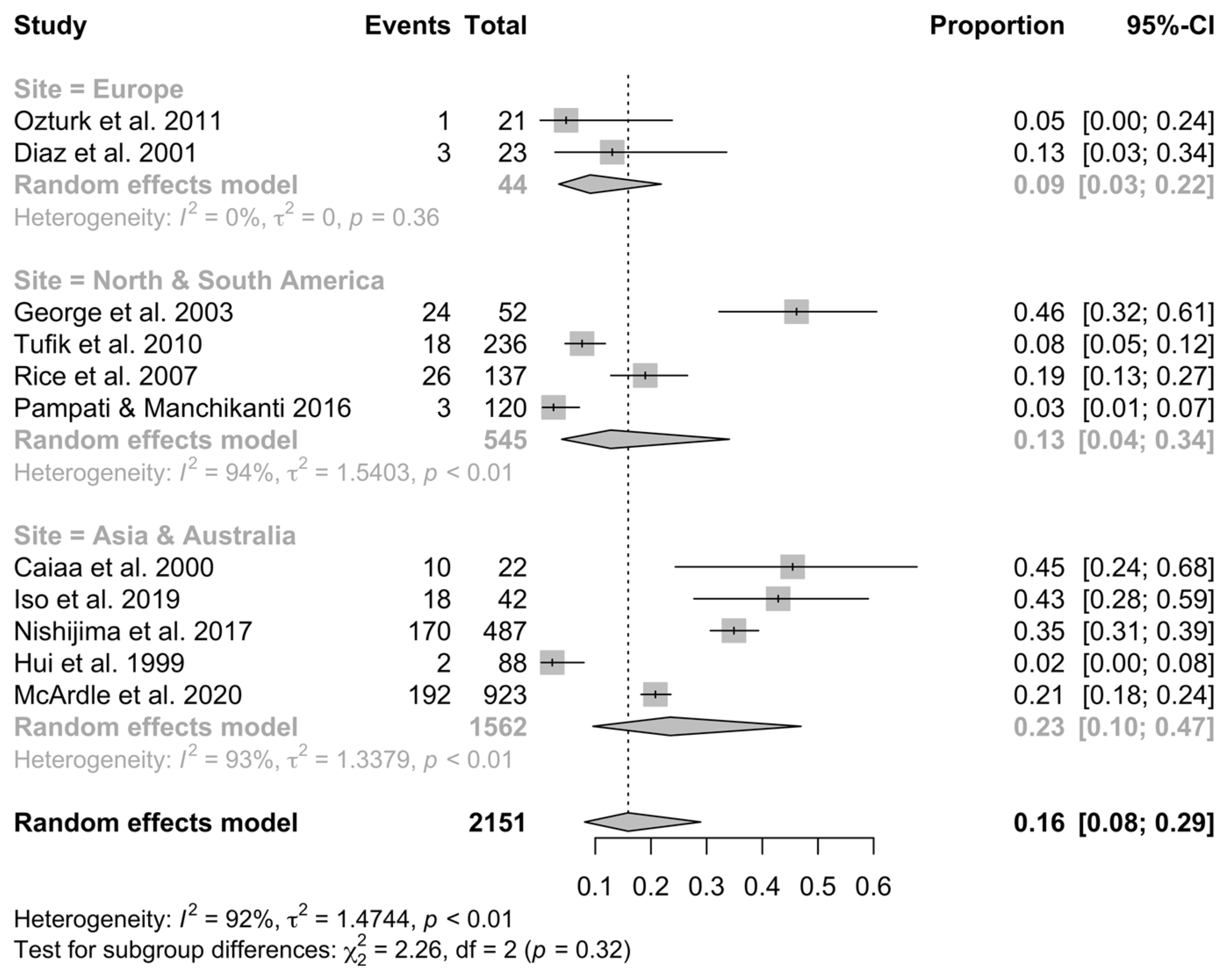
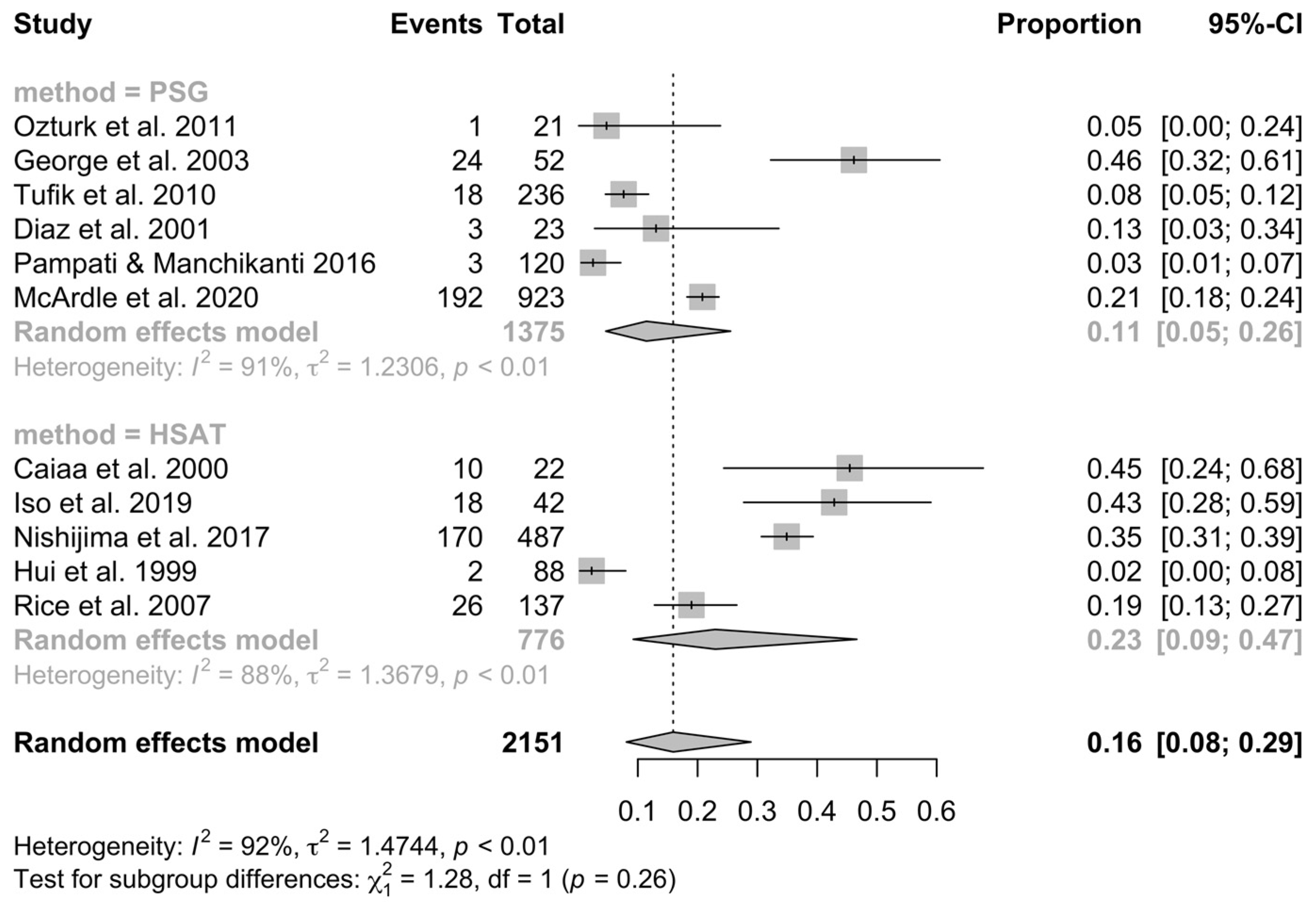
3.4. Meta-Analysis of OSA Prevalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Data Base | Strategy | Results 15 February 2024 |
|---|---|---|
| EMBASE | (((‘sleep apnea’ OR ‘sleep disordered breathing’) NEAR/3 (‘epidemiology’ OR ‘rate’ OR ‘prevalence’ OR ‘occurrence’)):ti,ab) AND [humans]/lim AND [embase]/lim | 1996 |
| MEDLINE | TI=((“sleep disordered breathing” OR “sleep apnea”) NEAR/3 (“prevalence” OR “epidemiology” OR “rate” OR “occurrence”)) OR AB=((“sleep disordered breathing” OR “sleep apnea”) NEAR/3 (“prevalence” OR “epidemiology” OR “rate” OR “occurrence”)) | 2078 |
| WOS | TI=((“sleep disordered breathing” OR “sleep apnea”) NEAR/3 (“prevalence” OR “epidemiology” OR “rate” OR “occurrence”)) OR AB=((“sleep disordered breathing” OR “sleep apnea”) NEAR/3 (“prevalence” OR “epidemiology” OR “rate” OR “occurrence”)) | 1821 |
Appendix C
| Author | Apnea and Hypopnea Definition |
|---|---|
| Ozturk et al. | NR |
| George et al. | NR |
| Caiaa et al. | NR |
| Tufik et al. | AASM 2007 |
| Iso et al. | NR |
| Diaz et al. | Apnea was defined as the cessation of airflow for ≥10 s. Hypopnea was defined as a decrease in airflow or toracoabdominal movements by ≥50% for 10 s, accompanied by oxygen desaturation ≥ 4% or microarousal |
| Nishijima et al. | AASM 2016 |
| Hui et al. | Apnea was defined as the cessation of airflow for ≥10 s, and hypopnea was defined as a reduction of airflow ≥50% for ≥10 s. |
| Rice et al. | Apnea was defined as a decrease in airflow of 80% of more from baseline for at least 10 s. Hypopnea was defined as a decrease in airflow by 50% to 80% of baseline for ≥10 s. |
| Pampati et al. | NR |
| McArdle et al. | AASM 2017 |
Appendix D
| Sample Size | AHI 5-9,9 | AHI 10< | ||||
|---|---|---|---|---|---|---|
| George et al. 2003 | All (Male) | 52 | 10 | 14 | ||
| Diaz et al. 2001 | All (Male) | 23 | 1 | 2 | ||
| Sample size | REI 5≤ | low REI 5–15 | moderate REI 15–30 | high REI 30≤ | ||
| Nishijima et al. 2017 | All | 487 | 170 | 141 | 19 | 10 |
| Male | 358 | 158 | 129 | 19 | 10 | |
| Female | 129 | 12 | 12 | 0 | 0 | |
| AHI 5≤ | AHI 5≤ AND ESS 11≤ | AHI 15≤ | ||||
| McArdle et al. 2020 | All | 923 | 192 | 26 | 34 | |
| Male | 454 | 122 | 17 | 22 | ||
| Female | 469 | 70 | 9 | 12 | ||
Appendix E
References
- Flemons, W.W.; Buysse, D.; Redline, S.; Oack, A.; Strohl, K.; Wheatley, J. Sleep-Related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research. Sleep 1999, 22, 667–689. [Google Scholar]
- Rundo, J.V. Obstructive sleep apnea basics. Cleve. Clin. J. Med. 2019, 86 (Suppl. S1), 2–9. Available online: https://pubmed.ncbi.nlm.nih.gov/31509498/ (accessed on 21 February 2023). [CrossRef] [PubMed]
- White, D.P. Sleep-related breathing disorder.2. Pathophysiology of obstructive sleep apnoea. Thorax 1995, 50, 797. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Cheung, Y.Y.; Tai, B.C.; Loo, G.; Khoo, S.M.; Cheong KY, P.; Barbe, F.; Lee, C.H. Screening for Obstructive Sleep Apnea in the Assessment of Coronary Risk. Am. J. Cardiol. 2017, 119, 996–1002. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; et al. Screening for Obstructive Sleep Apnea in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 328, 1945–1950. [Google Scholar]
- Udholm, N.; Rex, C.E.; Fuglsang, M.; Lundbye-Christensen, S.; Bille, J.; Udholm, S. Obstructive sleep apnea and road traffic accidents: A Danish nationwide cohort study. Sleep Med. 2022, 96, 64–69. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Liu, P.Y.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep Apnea as an Independent Risk Factor for All-Cause Mortality: The Busselton Health Study. Sleep 2008, 31, 1079. [Google Scholar]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Young, T.; Shahar, E.; Nieto, F.J.; Redline, S.; Newman, A.B.; Gottlieb, D.J.; Walsleben, J.A.; Finn, L.; Enright, P.; Samet, J.M. Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch. Intern. Med. 2002, 162, 893–900. [Google Scholar] [CrossRef]
- Mirrakhimov, A.E.; Sooronbaev, T.; Mirrakhimov, E.M. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm. Med. 2013, 13, 10. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Chou, K.T.; Su, K.C.; Lin, F.C.; Liu, Y.Y.; Shiao, T.H.; Chen, Y.M. Obstructive sleep apnea in young Asian adults with sleep-related complaints. Sci. Rep. 2022, 12, 20582. [Google Scholar] [CrossRef] [PubMed]
- McArdle, N.; Ward, S.V.; Bucks, R.S.; Maddison, K.; Smith, A.; Huang, R.C.; Pennell, C.E.; Hillman, D.R.; Eastwood, P.R. The prevalence of common sleep disorders in young adults: A descriptive population-based study. Sleep 2020, 43, zsaa072. [Google Scholar] [CrossRef] [PubMed]
- Athar, W.; Card, M.E.; Charokopos, A.; Akgün, K.M.; DeRycke, E.C.; Haskell, S.G.; Yaggi, H.K.; Bastian, L.A. Obstructive Sleep Apnea and Pain Intensity in Young Adults. Ann. Am. Thorac. Soc. 2020, 17, 1273–1278. [Google Scholar] [CrossRef]
- Hoy, D.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Ertuǧrul Öztürk, M. The effects of unilateral nostril breathing during the night on heart rate and sleep apnea in young sportsmen. Neurol. Psychiatry Brain Res. 2012, 18, 105–108. [Google Scholar] [CrossRef]
- George, C.F.P.; Kab, V.; Levy, A.M. Increased Prevalence of Sleep-Disordered Breathing among Professional Football Players. N. Engl. J. Med. 2003, 348, 367–368. [Google Scholar] [CrossRef]
- Caia, J.; Halson, S.L.; Scott, A.; Kelly, V.G. Obstructive sleep apnea in professional rugby league athletes: An exploratory study. J. Sci. Med. Sport. 2020, 23, 1011–1015. [Google Scholar] [CrossRef]
- Iso, Y.; Kitai, H.; Kyuno, E.; Tsunoda, F.; Nishinaka, N.; Funato, M.; Nishimura, E.; Akihiro, S.; Tanuma, H.; Yonechi, T.; et al. Prevalence and significance of sleep disordered breathing in adolescent athletes. ERJ Open Res. 2019, 5, 00029-2019. [Google Scholar] [CrossRef]
- Díaz, J.R.; Guallar, J.; Arnedo, A.; Oliva, S.; Gala, J. The prevalence of sleep apnea-hypopnea syndrome among long-haul professional drivers. Arch. Bronconeumol. 2001, 37, 471–476. [Google Scholar] [CrossRef]
- Rice, T.B.; Dunn, R.E.; Lincoln, A.E.; Tucker, A.M.; Vogel, R.A.; Heyer, R.A.; Yates, A.P.; Wilson, P.W.; Pellmen, E.J.; Allen, T.W.; et al. Sleep-Disordered Breathing in the National Football League. Sleep 2010, 33, 819. [Google Scholar] [CrossRef]
- Tufik, S.; Santos-Silva, R.; Taddei, J.A.; Bittencourt, L.R.A. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010, 11, 441–446. [Google Scholar] [CrossRef]
- Pampati, S.; Manchikanti, L. What Is the Prevalence of Symptomatic Obstructive Sleep Apnea Syndrome in Chronic Spinal Pain Patients? An Assessment of the Correlation of OSAS with Chronic Opioid Therapy, Obesity, and Smoking. Pain. Physician 2016, 19, E569. [Google Scholar] [CrossRef]
- Nishijima, T.; Kizawa, T.; Hosokawa, K.; Endo, F.; Kasai, Y.; Yamashiro, Y.; Sakurai, S. Prevalence of sleep-disordered breathing in Japanese medical students based on type-3 out-of-center sleep test. Sleep Med. 2018, 41, 9–14. [Google Scholar] [CrossRef]
- Hui, D.S.C.; Chan, J.K.W.; Ho, A.S.S.; Choy, D.K.L.; Lai, C.K.W.; Leung, R.C.C. Prevalence of snoring and sleep-disordered breathing in a student population. Chest 1999, 116, 1530–1536. [Google Scholar] [CrossRef][Green Version]
- Jonas, D.E.; Amick, H.R.; Feltner, C.; Weber, R.P.; Arvanitis, M.; Stine, A.; Lux, L.; Harris, R.P. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 415–433. [Google Scholar] [CrossRef]
- Jennum, P.; Riha, R.L. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur. Respir. J. 2009, 33, 907–914. [Google Scholar] [CrossRef]
- Faria, A.; Allen, A.H.; Fox, N.; Ayas, N.; Laher, I. The public health burden of obstructive sleep apnea. Sleep Sci. 2021, 14, 257. [Google Scholar] [PubMed]
- Liu, L.; Su, G.; Wang, S.; Zhu, B. The prevalence of obstructive sleep apnea and its association with pregnancy-related health outcomes: A systematic review and meta-analysis. Sleep Breath. 2019, 23, 399–412. [Google Scholar] [CrossRef]
- Bonsignore, M.R.; Saaresranta, T.; Riha, R.L.; Riha, R.; Bonsignore, M. Sex differences in obstructive sleep apnoea. Eur. Respir. Rev. 2019, 28, 190030. [Google Scholar] [CrossRef]
- Wimms, A.; Woehrle, H.; Ketheeswaran, S.; Ramanan, D.; Armitstead, J. Obstructive Sleep Apnea in Women: Specific Issues and Interventions. BioMed Res. Int. 2016, 2016, 1764837. [Google Scholar] [CrossRef]
- Ruehland, W.R.; Rochford, P.D.; O’Donoghue, F.J.; Pierce, R.J.; Singh, P.; Thornton, A.T. The New AASM Criteria for Scoring Hypopneas: Impact on the Apnea Hypopnea Index. Sleep 2009, 32, 150–157. [Google Scholar] [CrossRef]
- BaHammam, A.S.; Obeidat, A.; Barataman, K.; Bahammam, S.A.; Olaish, A.H.; Sharif, M.M. A comparison between the AASM 2012 and 2007 definitions for detecting hypopnea. Sleep Breath. 2014, 18, 767–773. [Google Scholar] [CrossRef]
- Duce, B.; Milosavljevic, J.; Hukins, C. The 2012 AASM Respiratory Event Criteria Increase the Incidence of Hypopneas in an Adult Sleep Center Population. J. Clin. Sleep Med. 2015, 11, 1425. [Google Scholar] [CrossRef]
- Spector, A.R.; Loriaux, D.; Farjat, A.E. The Clinical Significance of Apneas Versus Hypopneas: Is There Really a Difference? Cureus 2019, 11, e4560. [Google Scholar] [CrossRef]
- Hirotsu, C.; Haba-Rubio, J.; Andries, D.; Tobback, N.; Marques-Vidal, P.; Vollenweider, P.; Waeber, G.; Heinzer, R. Effect of Three Hypopnea Scoring Criteria on OSA Prevalence and Associated Comorbidities in the General Population. J. Clin. Sleep Med. 2019, 15, 183–194. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.L., Jr.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007; pp. 1–32. [Google Scholar]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Goyal, M.; Johnson, J. Obstructive Sleep Apnea Diagnosis and Management. Mo. Med. 2017, 114, 120. [Google Scholar]
- Abrahamyan, L.; Sahakyan, Y.; Chung, S.; Pechlivanoglou, P.; Bielecki, J.; Carcone, S.M.; Rac, V.E.; Fitzpatrick, M.; Krahn, M. Diagnostic accuracy of level IV portable sleep monitors versus polysomnography for obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2018, 22, 593–611. Available online: https://pubmed.ncbi.nlm.nih.gov/29318566/ (accessed on 20 April 2023). [CrossRef]
| Author | Year | Country | Design | Index Utilized for OSA Diagnosis | Diagnostic Tool | Population Size | Age (Years) | BMI | Sex (% Male) | Number of Patients with OSA |
|---|---|---|---|---|---|---|---|---|---|---|
| Ozturk et al. | 2011 | Turkey | Cross-sectional study | NR | Full overnight PSG: one night at the sleep laboratory for adaptation before the experimental measurements (3 nights) | 21 | 18–24 | NR | 100% | 0 |
| George et al. | 2003 | USA | Cross-sectional study | AHI ≥ 10 | Full overnight PSG | 52 | 25.5 ± 2.7 | 31.5 ± 4.6 | 100% | 24 |
| Caiaa et al. | 2020 | Australia | Cross-sectional study | AHI ≥ 5 | Home PSG: portable multi-channel device (Alice PDX, Philips Respironics, OR, USA) | 22 | 23.8 ± 3.6 | 30.0 ± 2.2 | 100% | 10 |
| Tufik et al. | 2010 | Brazil | Cross-sectional study | AHI ≥ 5 and clinical symptoms and or AHI ≥ 15 | Full overnight PSG: a digital system at the sleep laboratory (EMBLA S7000, Embla Systems, Inc., Broomfield, CO, USA) | 236 | 20–29 | NR | NR | 18 |
| Iso et al. | 2019 | Japan | Cross-sectional study | AHI ≥ 5 events/1 h over a total sleeping time of more than 3 h | Home PSG: WatchPAT-200 device- a validated surrogate for PSG (Itamar Medical Ltd., Caesarea, Israel) | 42 | 18.6 ± 0.5 | 27.7 ± 4.0 | 100% | 18 |
| Diaz et al. | 2001 | Spain | Cross-sectional study | AHI ≥ 5 | Full overnight PSG (Somnostar 4100, Sensor Medics, San Rafael, CA, USA, EE.UU.) | 23 | 20–29 | NR | 100% | 3 |
| Nishijima et al. | 2017 | Japan | Cross-sectional study | REI ≥ 5 and clinical symptoms and or REI ≥ 15 without clinical symptoms. REI is calculated from the number of total respiratory events divided by monitoring time | Home PSG: type 3 portable monitors (smartwatch PMP 300E Philips Respironics GK) | 487 | 24.6 ± 1.9 | 22.3 ± 3.2 | 74% | 170 |
| Hui et al. | 1999 | China | Cross-sectional study | RDI ≥ 3 | Home PSG: MESAM IV device (4-channel device) | 88 | 20.7 ± 2.3 | 20 ± 2.3 | 50% | 2 |
| Rice et al. | 2007 | USA | Cross-sectional study | RDI ≥ 5 | Home PSG: A single-channel screening tool measures airflow using a nasal cannula with a pressure transducer (ApneaLinkTM (AL; ResMed Corp.Poway, CA) | 137 | 27 ± 3 | 32.4 ± 4 | 100% | 26 |
| Pampati et al. | 2016 | USA | Cross-sectional study | NR | Accredited sleep laboratory | 120 | 18–24.9 | NR | NR | 3 |
| McArdle et al. | 2020 | Australia | Cross-sectional study | AHI ≥ 5 | Full overnight PSG- level 1 n-laboratory PSG study in a bedroom-like environment (GRAEL, Compumedics) | 923 | 22.2 | NR | 49% | 192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasadzińska-Stempniak, K.; Zajączkiewicz, H.; Kukwa, A. Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review. J. Clin. Med. 2024, 13, 1386. https://doi.org/10.3390/jcm13051386
Zasadzińska-Stempniak K, Zajączkiewicz H, Kukwa A. Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review. Journal of Clinical Medicine. 2024; 13(5):1386. https://doi.org/10.3390/jcm13051386
Chicago/Turabian StyleZasadzińska-Stempniak, Katarzyna, Hanna Zajączkiewicz, and Andrzej Kukwa. 2024. "Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review" Journal of Clinical Medicine 13, no. 5: 1386. https://doi.org/10.3390/jcm13051386
APA StyleZasadzińska-Stempniak, K., Zajączkiewicz, H., & Kukwa, A. (2024). Prevalence of Obstructive Sleep Apnea in the Young Adult Population: A Systematic Review. Journal of Clinical Medicine, 13(5), 1386. https://doi.org/10.3390/jcm13051386






