Abstract
Background: Atopic dermatitis (AD) is a chronic inflammatory skin disease whose incidence is increasing. Skin barrier dysfunction plays an important role in this disease. It has been observed that AD patients have higher transepidermal water loss (TEWL) and lower stratum corneum hydration (SCH); however, there is little information about skin microtopography in this pathology. The objective of this study is to evaluate skin barrier dysfunction and structural changes in patients with AD. Methods: A cross-sectional study was conducted including patients with AD. Parameters of skin barrier function were measured (TEWL, temperature, erythema, pH, skin hydration, elasticity) and also other topographical parameters (scaliness, wrinkles, smoothness, surface, contrast, variance) in both healthy skin and flexural eczematous lesions. Results: A total of 32 patients with AD were included in the study. Flexural eczematous lesions had higher erythema (369.12 arbitrary unit (AU) vs. 223.89 AU, p < 0.001), higher TEWL (27.24 g/h/m2 vs. 13.51 g/h/m2, p < 0.001), lower SCH (20.3 AU vs. 31.88 AU, p < 0.001) and lower elasticity (0.56% vs. 0.65%, p = 0.05). Regarding topographic parameters, flexural eczematous lesions presented greater scaliness (5.57 SEsc vs. 0.29 SEsc, p = 0.02), greater smoothness (316.98 SEsm vs. 220.95 SEsm p < 0.001), more wrinkles (73.33 SEw vs. 62.15 SEw p = 0.03), greater surface area (836.14% vs. 696.31%. p < 0.001), greater contrast (2.02 AU vs. 1.31 AU p = 0.01), greater variance (6.22 AU vs. 4.96 AU p < 0.001) and a lower number of cells (105.5 vs. 132.5 p < 0.001) compared to unaffected healthy skin, reflecting a decrease in skin quality in AD patients. Conclusions: Both skin barrier function and skin topography are damaged in patients with AD, with differences between healthy skin and flexural eczema.
1. Introduction
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin diseases. Recent data suggest that the prevalence among children is approximately 20% and among adults ranges from 7% to 14%, with variation between countries and higher prevalence in industrialised countries [1]. It is a chronic disease that flares with inflamed, red, itchy plaques [2]. Other signs include oedema, xerosis, erythema, oozing, erosion, crusting and lichenification [3]. A relevant clinical feature is the presence of very dry skin [4].
The skin is the largest organ of the human body [5]. Its main functions are to regulate temperature, both by isolation and sweating; to participate in nervous system functioning and water content regulation; and to protect the body from mechanical injury, micro-organisms, substances and radiation present in the environment [6]. It also acts as a physical, chemical and immunological barrier between the body and the environment [7]. This function resides mainly in the stratum corneum of the epidermis. It is important to highlight the commensal microbiome in this barrier, which due to aggression from various internal and external factors can lead to inflammation, infection or allergic or autoimmune skin diseases. Genetic predisposition, immune system dysregulation and epidermal barrier dysfunction are some of the crucial components of AD [8,9].
Patients with AD have a reduction in total ceramide levels and an alteration in their profile, hence the importance of applying topical ceramides in this condition [4]. Filaggrin, a binding protein that holds corneocytes together, becomes relevant as it helps to maintain transepidermal water loss (TEWL) within normal values [10]. Thus, factors contributing to impair the skin include decreased or mutated filaggrins [11], lipids and antimicrobial peptides.
A number of parameters such as TEWL, stratum corneum hydration (SCH), temperature, skin surface pH, erythema index, elasticity and antioxidant capacity are usually involved in determining and measuring barrier function [8,12]. Previous research on AD has shown that patients with AD have increased TEWL, erythema and temperature, as well as reduced SCH and elasticity on flexural eczematous lesions [4,12]. In addition, more severe patients had greater dysfunction of these parameters. Moreover, early changes in theses parameters could predict treatment response. Nevertheless, there is little information about skin microtopography in patients with AD.
Therefore, the aim of this study was to evaluate epidermal barrier dysfunction and skin microtopography in patients with AD.
2. Materials and Methods
Study design
A cross-sectional study was designed with patients diagnosed with AD attending the Atopic Dermatitis Clinic of the Hospital Universitario Virgen de las Nieves, Granada, Spain, who voluntarily wished to participate and who met the inclusion criteria and none of the exclusion criteria.
Inclusion and exclusion criteria
The inclusion criteria were as follows:
- –
- Patients with AD diagnosed by a dermatologist following the Hanifin and Rajka criteria of at least 6 months of evolution and who have attended the Atopic Dermatitis consultation at the Dermatology Department of the Hospital Universitario Virgen de las Nieves
- –
- Aged between 12 and 65 years of age
- –
- Have signed the informed consent required to participate in the study
Exclusion criteria include the following:
- –
- Subjects who do not wish to participate in the study and who do not sign the informed consent form
- –
- Patients with clinical infection in the area to be measured
- –
- History of cancer or immunosuppression
- –
- Any concomitant inflammatory skin pathology (psoriasis, hidradenitis suppurativa)
Variables of interest
Skin barrier function
The biophysical and objective parameters concerning the assessment of the epidermal barrier function were measured using probes with a multiprobe adapter as a non-invasive test. These measurements included temperature (°C) with the Skin-Thermometer ST 500, erythema (UA) with the Mexameter, TEWL (g/h/m2) with the Tewameter, stratum corneum hydration (SCH) (UA) with the Corneometer, pH of the skin surface with the Skin pH-Meter, skin elasticity (%) with the Cutometer, skin hardness (UA) with the Durometer, skin friction (UA) with the Frictiometer, skin gloss (UA) with the Skin-Glossymeter, skin deformability or stiffness (mm) with the Indentometer and skin antioxidant capacity (UA).
Skin microtopography
The surface evaluation of living skin (SELS) parameters smoothness (SEsm), roughness (SEr), scaliness (SEsc) and wrinkles (SEw) were assessed using the Visioscan VC 20plus (Courage + Khazaka electronic GmbH, Bilbao, Spain), high-resolution UVA light video camera. Lower SEsm means higher smoothness, lower SEr is related to higher roughness, lower SEsc means less desquamation and higher SEw is related to more wrinkles. The surface area (%), volume (mm2), contrast (UA), entropy (UA), variance (UA), homogeneity (AU) and anisotropy (AU) were also measured using the Visioscan. Entropy indicates ‘image disorganisation/disorder’ concluding that smooth and regular skin should show a lower entropy than rough skin. Variance expresses roughness, so high roughness will lead to increased variance values. High homogeneity is related to moisturised skin and high anisotropy means more aged skin. The total number of cells, the rate of desquamation and the number of corneocytes were measured using the Corneofix.
Skin barrier function and skin microtopography measurements were carried out in a room at a temperature of 23 +/−1 degree and an ambient humidity in the range of 40–50%. They were measured on a flexural eczematous lesion on the volar forearm and an uninvolved area 3 cm from the lesion.
Severity and quality of life
To evaluate the severity of the disease, we used the following scales:
- °
- Scoring Atopic Dermatitis (SCORAD), an index that evaluates the extent with a percentage of the affected surface area, the intensity of AD through six items (erythema, oedema/papules, effect of scratching, oozing/crusting, lichenification and dryness) scored from 0 (absence) to 3 (intense) and two subjective symptoms, itching and insomnia, scored from 0 to 10. The total is calculated = extent/5 + 7 × intensity/2 + symptoms of itching and insomnia. The disease is considered mild if it has a score between 0 and 25; moderate between 25 and 50; and severe >50.
- °
- The Redness, Oedema, Scratches (ROS) scale which assesses erythema, oedema and excoriations. Each item is scored from 0 to 3 and the total score, adding up each item, will give a number between 0 and 9.
- °
- The Eczema Area and Severity Index (EASI) scale, that includes the extent of disease and the percentage of body surface area in four body regions (head and neck, trunk, upper limbs and lower limbs). The disease is mild if EASI ≤ 7, moderate 7.1–21, severe >21–50 and very severe >50.
- °
- The Investigator’s Global Assessment (IGA) which uses the clinical features of erythema, infiltration, papules, oozing and crusting to classify it from 0 (clear) to 5 (very severe disease).
Patients were also provided with a series of questionnaires related to their experience with this condition. The first was the Dermatology Life Quality Index (DLQI) as an AD monitoring tool scored between 0 and 24, with a number over 7 indicating poor control. The second was the Patient-Oriented Eczema Measure (POEM) to collect patient-reported symptoms, consisting of seven questions, each weighted from 0 to 4; the score is summed and expressed as a maximum of 28. Two others were the Numerical Rating Scale (NRS) for pruritus rated from 0 (no pruritus) to 10 (most intense pruritus the patient can imagine) and the NRS for sleep, which assesses how the pathology affects sleep, also rated on a scale of 0 to 10.
Other variables
Sociodemographic variables (sex, age, occupation, level of education, residence), toxic habits, phototype, skin care in terms of moisturiser use, sun exposure and photoprotection were included. We also collected information on whether there was a family history of AD. With regard to the pathology in question, we recorded the age of onset of AD, comorbidities (triad of asthma, rhinoconjunctivitis and AD), location of the lesions, current treatment for AD and previous treatments.
Statistics
In a descriptive analysis, continuous variables were expressed as means ± standard deviations (SD) and qualitative variables as absolute and relative frequency distributions. For comparisons of continuous variables, Student’s t-test for independent samples or Student’s t-test for paired samples, as appropriate, was used. For comparisons between categorical variables, the chi-square or Fisher’s test was used as appropriate. Pearson’s correlation coefficient was calculated to test for possible correlations between continuous variables. Statistical significance was defined by a two-tailed p < 0.05. SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Ethics
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committee of the Hospital Universitario Virgen de las Nieves, Granada, Spain (code HC01/0442-N-20, impact of topical, systemic or physical treatment on skin homeostasis in patients with skin diseases (changes in skin homeostasis in dermatological patients)). Involvement in the study was explained to the participants, who decided to sign the informed consent and participate. The measurements performed on each patient were non-invasive and the confidentiality of personal data was preserved.
3. Results
3.1. Descriptive Analysis and Sociodemographic Characteristics of the Study Population
The study included thirty-two participants, of whom 37.5% (twelve) were male and 62.5% (twenty) female, with a mean age of 30.91 (14.29), Table 1. A total of 18.8% (six) smoked and 40.6% (thirteen) drank an average of 1.38 (2.08) Standard Beverage Units (SBU) per week. Regarding the use of moisturisers, 90.6% (twenty-nine) reported using cream. The mean age at onset of AD was 11.63 (19.56) years. At the time of inclusion in the study, 37.5% (twelve) of the patients were being treated with topical corticosteroids, 21.9% (seven) with a systemic drug, 18.8% (six) with a biologic and 21.9% (seven) with a JAK inhibitor (iJAK). In relation to disease severity, patients had moderate AD, reflected in a mean SCORAD of 37.37 (27.44) and an EASI of 13.54 (14.46).

Table 1.
Demographic Characteristics and Descriptive Analysis of the Participants.
3.2. Differences in Skin Barrier Function and Skin Microtopography between Healthy Skin and Flexural Eczematous Lesions
Skin barrier function and skin microtopography were different between healthy skin and flexural eczematous lesions (Table 2). Flexural eczematous lesions presented higher temperature (32.38 °C vs. 31.81 °C, p = 0.02), greater erythema (369.12 AU vs. 223.89 AU, p < 0.001), greater TEWL (27.24 g/h/m2 vs. 13.51, g/h/m2 p < 0.001), lower SCH (20.3 AU vs. 31.88 AU, p < 0.001), lower elasticity (0.56% vs. 0.65%, p = 0.05), higher hardness (15.75 AU vs. 9.63 AU, p = 0.02), lower friction (102.99 AU vs. 177.84 AU, p = 0.02), higher smoothness (316.98 SEsm vs. 220.95 SEsm, p < 0, 001), higher desquamation (5.57 SEsc vs. 0.29 SEsc p = 0.02), more wrinkles (73.33 SEw vs. 62.15 SEw p = 0.03), higher surface area (836.14% vs. 696.31% p < 0.001), higher contrast (2.02 AU vs. 1.31 AU, p = 0.01), lower entropy (1, 43 AU vs. 1.48 AU, p < 0.001), higher variance (6.22 AU vs. 4.96 AU p < 0.001), a lower total cell number (105.5 vs. 132.5, p < 0.001), a lower percentage of corneocytes (36.19% vs. 50.35%, p = 0.02) and a lower desquamation index (19.36 vs. 26.1, p = 0.04).

Table 2.
Barrier Function Parameters and Skin Topography in Healthy Skin and Eczema.
3.3. Differences between Men and Women
In an analysis grouped by sex (12 men and 20 women) (Table S1), we found a higher mean age in men without this being significant (36.83 years vs. 27.35 years, p = 0.07). In addition, women used photoprotectors (65% vs. 25%, p = 0.03) and moisturisers more frequently (100% vs. 75%, p = 0.02). In terms of severity, men had a higher severity reflected in higher values for EASI (20.08 vs. 9.62, p = 0.05), IGA (2.67 vs. 1.65, p = 0.05) and NRS sleep (5.27 vs. 2.5, p = 0.05).
In barrier function parameters, men had higher erythema in both healthy skin (260.41 AU vs. 201.97 AU, p = 0.03) and flexural eczema (415.98 AU vs. 335.65 AU, p = 0.01). Friction in eczema was lower in males (80.04 AU vs. 119.39 AU, p = 0.04), deformability/stiffness in healthy skin was lower in males (1.83 mm vs. 2.11 mm p = 0.05) and anisotropy in flexural eczema was also lower in males (25.43 AU vs. 40.69 AU, p = 0.02).
3.4. The Impact of Age in Skin Barrier Function and Skin Microtopography
Patients were divided regarding the mean age (30.91) of the population (patients ≥31 and <31) (Table S2). Patients aged ≥31 used moisturisers less frequently than those aged <31 (75% vs. 100% p = 0.02). Age at onset of AD was significantly higher in patients aged ≥31 (25.75 vs. 3.15, p = 0.01). Erythema in flexural eczema was higher in the population ≥31 years (422.54 AU vs. 347.12 AU, p = 0.03) and TEWL in flexural eczema was significantly higher (30.36 g/h/m2 vs. 19.66 g/h/m2, p = 0.04) in patients <31 years. SCH in healthy skin was higher in patients aged ≥31 (39.59 AU vs. 27.26 AU p < 0.001). Friction in healthy skin (227.9 AU vs. 147.81 AU, p = 0.05) and the healthy skin desquamation index (30.26 vs. 23.6, p = 0.05) were significantly higher in patients aged ≥31.
3.5. Differences in Skin Barrier Function and Skin Microtopography Depending on Disease Severity
Regarding disease severity, patients were divided into those having a severe AD (EASI ≥ 21) and those having a mild–moderate disease (EASI < 21) (Table S3). Those with an EASI score ≥21 had significantly less sun exposure (20% vs. 63.6%, p = 0.02) and less sun exposure time (24 min vs. 77.73 min, p = 0.05). Patients with EASI ≥ 21 had more comorbidities than patients with EASI < 21 (80% vs. 31.8%, p = 0.01). In addition, those with EASI ≥ 21 had more severe and worse controlled disease reflected in higher scores on all AD rating scales (SCORAD, ROS, IGA, POEM, DLQI, NRS pruritus, NRS sleep). Erythema on healthy skin was also higher in patients with EASI ≥ 21 (288.31 AU vs. 194.6 AU, p = 0.01). The pH in flexural eczema was higher in the group with EASI ≥ 21 (5.81 vs. 5.02, p < 0.001). The R7 elasticity in flexural eczema was lower in subjects with EASI ≥ 21 (0.48% vs. 0.61%, p = 0.01) and so was the hardness in healthy skin (7.51 AU vs. 10.59 AU, p = 0.04). The brightness in flexural eczema was lower in those with EASI ≥ 21 (5.1 AU vs. 6.44 AU, p = 0.01). Finally, the total number of cells in flexural eczema was lower in patients with EASI ≥ 21 (84 vs. 118.4, p = 0.02).
3.6. Correlations between Severity Scales and Barrier Function and Topographical Parameters
Disease severity was related to changes in skin barrier function and skin microtopography (Table 3). A positive correlation was observed between EASI and erythema on healthy skin (r = 0.56, p < 0.001), pH on flexural eczematous lesions (r = 0.66, p < 0.001), smoothness on healthy skin (r = 0.4, p = 0.02), desquamation on healthy skin (r = 0.49, p = 0.04), contrast on healthy skin (r = 0.46, p = 0.01) and homogeneity on healthy skin (r = 0.46, p = 0.01), while a negative correlation was found between EASI and brightness on flexural eczematous lesion (r = −0.48, p = 0.02) and entropy on healthy skin (r = 0.42, p = 0.02) and on the total number of cells (r = −0.42, p = 0.04), Figure 1. Other correlations were also found between SCORAD, IGA, NRS for pruritus and sleep and skin barrier function and skin microtopography.

Table 3.
Pearson’s Correlation Between Severity Scales of Atopic Dermatitis (AD) and Barrier Function and Topographic Parameters.
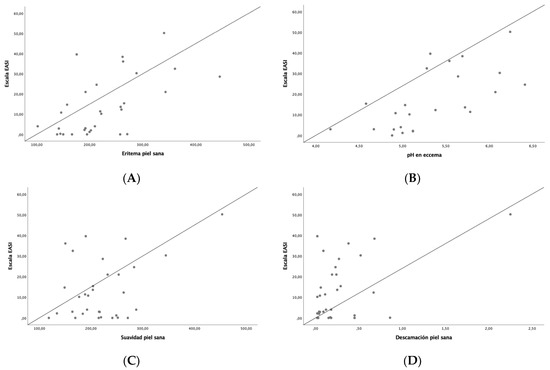
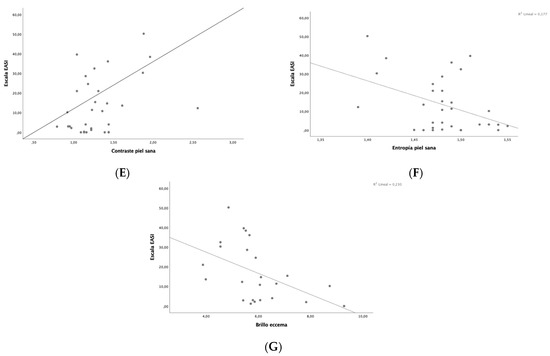
Figure 1.
Correlation between EASI and skin barrier function and skin microtopography parameters: (A) erythema on healthy skin, (B) pH on flexural eczematous lesions, (C) smoothness on healthy skin, (D) desquamation on healthy skin, (E) contrast on healthy skin, (F) entropy on healthy skin, (G) brightness on flexural eczematous lesions.
4. Discussion
This study shows that there are differences in barrier function and skin microtopography between healthy skin and flexural eczema in patients with AD and that sex, age and disease severity could impact on these parameters.
Concerning barrier function parameters, flexural eczema had higher temperature, higher erythema, higher TEWL, lower hydration and lower elasticity. These results are consistent with findings already described in previous studies [8,12,13,14,15]. The alteration of these parameters is evidence of an alteration in the barrier function of patients with AD; the increase in temperature demonstrates the inflammatory changes that occur in this pathology, as does the erythema. The increase in TEWL and the decrease in SCH reflects the dysfunction of the skin barrier due to the possible presence of mutations or alterations in the number of filaggrins, which are the main constituent proteins of the stratum corneum, which is mainly responsible for this barrier. Some studies have demonstrated the relationship between higher TEWL, greater erythema and the presence of mutations in these proteins [11]. Regarding elasticity, in the flexural eczematous lesions there was a decrease in elasticity, specifically in R7 (comparative elasticity or ratio between elastic recovery and total deformation of the skin), which may be due to an alteration in collagen and elastin, the proteins responsible for elasticity [8].
On the other hand, in our study, differences were observed between healthy skin and flexural eczematous lesions with respect to skin microtopography parameters. Flexural eczema showed an increase in the smoothness parameter (SEsm). Higher SEsm means a less smooth skin. More wrinkles (SEw) were also observed in flexural eczematous lesions, which means the flexural eczema has more visible wrinkles (wide and deep) and more desquamation (SEsc) in the stratum corneum. All these findings imply a poorer skin quality in patients with AD. These parameters have been little studied in previous studies in patients with AD. In healthy individuals, it was observed that skin barrier and microtopography differed between anatomical regions [16]. In patients with AD, the daily topical application of a nanoemulsion containing cholesterol derivatives of ceramide-like lipoamino acids during 6 weeks decreased scaliness and smoothness. In another study in children with AD, the population was divided into three groups; two received topical care with a plant-based emollient and the control group received petroleum-jelly-based emollient. After the follow-up, scaliness decreased in all the groups while smoothness only increased in one of the intervention groups [17].
Also, flexural eczematous lesions showed greater hardness, less friction and greater surface area, in relation to damaged skin. The surface area refers to the comparison of the size of the patient’s skin surface with a fully stretched skin surface. The increased surface indicates skin alteration in AD. Flexural eczema also presented higher contrast, implying poor skin quality, and higher variance, meaning high roughness [16,18].
Between both sexes, men had greater disease severity (reflected in EASI, IGA and NRS pruritus scales), greater erythema in both healthy skin and flexural eczema and less deformability in healthy skin. Less friction and anisotropy in flexural eczema was also found in male skin. These findings may be related to several factors such as males having more severe AD and females taking more care of their skin by using more sunscreen and moisturisers. A previous study found greater homogeneity and a greater number of wrinkles in the skin of women [19], reflecting higher skin quality or better skin care in women than in men, in agreement with our results.
Regarding age, older patients (≥31) showed greater erythema in flexural eczema, greater hydration in healthy skin, greater friction in healthy skin, greater desquamation in healthy skin and a greater number of corneocytes in healthy skin. In contrast, in those under 31 years of age, higher TEWL was observed in flexural eczema. One of the studies mentioned above found that older patients had higher EASI scores [8] and also correlated with lower skin hydration (SCH) [20]. The higher erythema in older patients might suggest that older patients experience more severe inflammatory responses and the higher desquamation and friction could be linked to age-related changes in skin structure and function. In contrast, younger patients (<31) exhibited higher TEWL in flexural eczema, indicating a greater impairment in skin barrier function in this group. This might suggest that younger patients experience more pronounced moisture loss through the skin, potentially leading to more acute AD flare-ups and an increased need for moisturisers, as reflected by their more frequent use of these products. Thus, while older patients seem to present with more visible skin alterations and a heightened inflammatory response, younger patients appear to have a more compromised skin barrier. These differences highlight the need for age-specific approaches to AD management, addressing the unique clinical manifestations in each age group.
The severity of AD calculated with the EASI, SCORAD and IGA scores showed a relationship with worse subjective disease control by patients with the POEM, DLQI, NRS pruritus and NRS sleep questionnaires. The more severe the disease, the more erythema was observed on healthy skin reflecting the inflammatory phenomenon of the pathology. An increase in pH was found in flexural eczema implying an alkalinisation of the skin and a disruption of this barrier function. The higher the severity was, the lower the R7 elasticity in flexural eczema, the lower the sheen in flexural eczema and the lower the number of flexural eczema cells. Previous studies have linked a higher SCORAD score (and therefore greater severity of AD) to lower SCH and higher TEWL, which, as explained above, is possible due to the presence of mutations or alterations in the number of filaggrins, proteins that make up the stratum corneum, the main barrier function [20,21].
It is also important to mention that the therapeutic paradigm in AD has rapidly shifted and new biologic drugs and JAK inhibitors have emerged that have proven their efficacy in clinical practice [22,23]. However, the patient profile that will respond best to each of them is unknown. Skin barrier function parameters and skin microtopography could help clinicians to select the right drug early in treatment [24].
This is the first study that evaluated skin microtopography in patients with AD and assessed the impact of age, sex and disease severity in these parameters. Nevertheless, it is not exempt from limitations. One of the limitations of our study was the small sample size and the lack of follow-up due to the cross-sectional design of the study. Another limitation is the impossibility of including patients recently diagnosed with AD since most of them were already on treatment, and they were also receiving different treatments, which could influence epidermal barrier function.
5. Conclusions
This study demonstrates that barrier function and skin topography are altered in patients with AD, with differences observed between flexural eczema and healthy skin. Flexural eczematous lesions showed greater desquamation, greater smoothness, more wrinkles, greater hardness, less friction, greater surface area, greater contrast, greater variance and fewer cells, reflecting a deterioration in the quality of the skin of these patients. These results may have implications when it comes to establishing a therapeutic plan or influencing the evolutionary course of this pathology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13195861/s1, Table S1. Analysis of Sociodemographic Characteristics, Severity, and Barrier Function and Topographic Parameters between Men and Women. Table S2. Analysis by average age. Table S3. Differences between patients with EASI ≥ 21 and EASI < 21.
Author Contributions
Conceptualization, T.M.-V. and S.A.-S.; methodology, C.P.-L. and R.S.-d.l.T.; software, C.P.-L. and T.M.-V.; validation, T.M.-V.; formal analysis, T.M.-V.; investigation, R.S.-d.l.T.; resources, T.M.-V.; data curation, C.P.-L.; writing—original draft preparation, C.P.-L.; writing—review and editing, T.M.-V. and S.A.-S.; visualization, T.M.-V. and S.A.-S.; supervision, T.M.-V.; project administration, S.A.-S.; funding acquisition, T.M.-V. and S.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto de Salud Carlos III (ISCIII) through the project PI23/01875. T.M.V. was supported by a postdoctoral fellowship from the ISCIII (CM22/00083) and R.S.-d.l.T. was supported by a predoctoral fellowship from Ministry of Universities (FPU21/00833).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Granada (HC01/0442-N-20, 8 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data supporting the reported results are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bylund, S.; von Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. -Venereol. 2020, 100, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Mortillo, S.; Cameli, N.; Ardigo, M.; Mariano, M. Efficacy of a Shower Cream and a Lotion with Skin-Identical Lipids in Healthy Subjects with Atopic Dry Skin. J. Cosmet. Dermatol. 2018, 17, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.; Lee, H.S.; Lee, J.Y. Skin Barrier Abnormalities and Immune Dysfunction in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Ishikawa, Y.; Numano, K.; Hirano, S.; Imokawa, G. A Nano-Emulsion Containing Ceramide-like Lipo-Amino Acid Cholesteryl Derivatives Improves Skin Symptoms in Patients with Atopic Dermatitis by Ameliorating the Water-Holding Function. Int. J. Mol. Sci. 2022, 23, 13362. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.F.; Derler, S.S.; Adlhart, C.C.; Spencer, N.D.; Rossi, R.M. The Relationship Between Skin Function, Barrier Properties, and Body-Dependent Factors. Ski. Res. Technol. 2018, 24, 165. [Google Scholar] [CrossRef]
- Lee, H.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Tercedor-Sanchez, J.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Epidermal Barrier Function and Skin Homeostasis in Atopic Dermatitis: The Impact of Age. Life 2022, 12, 132. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of Atopic Dermatitis: Clinical Implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, Pathological, and Circadian Factors Impacting Skin Hydration. Cureus 2022, 14, e27666. [Google Scholar] [CrossRef]
- Jungersted, J.M.; Scheer, H.; Mempel, M.; Baurecht, H.; Cifuentes, L.; Høgh, J.K.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T.; Weidinger, S. Stratum Corneum Lipids, Skin Barrier Function, and Filaggrin Mutations in Patients with Atopic Eczema. Allergy 2010, 65, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Sanabria-de-la-Torre, R.; Sanchez-Diaz, M.; Ureña-Paniego, C.; Molina-Leyva, A.; Arias-Santiago, S. The Impact of Dupilumab on Skin Barrier Function: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Rodriguez-Pozo, J.-A.; Diaz-Calvillo, P.; Salazar-Nievas, M.; Tercedor-Sanchez, J.; Molina-Leyva, A.; Arias-Santiago, S. Dupilumab Improves Skin Barrier Function in Adults with Atopic Dermatitis: A Prospective Observational Study. J. Clin. Med. 2022, 11, 3341. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Grube, E.; Ericksen, M.B.; Stevenson, M.D.; Lucky, A.W.; Sheth, A.P.; Assa’Ad, A.H.; Hershey, G.K.K. Intrinsically Defective Skin Barrier Function in Children with Atopic Dermatitis Correlates with Disease Severity. J. Allergy Clin. Immunol. 2008, 121, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-Aging and Brightening Effects of a Topical Treatment Containing Vitamin C, Vitamin E, and Raspberry Leaf Cell Culture Extract: A Split-Face, Randomized Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Sawatzky, S.; Schario, M.; Stroux, A.; Lünnemann, L.; Zuberbier, T.; Blume-Peytavi, U.; Bartels, N.G. Children with Dry Skin and Atopic Predisposition: Outcome Measurement with Validated Scores for Atopic Dermatitis. Ski. Pharmacol. Physiol. 2016, 29, 148–156. [Google Scholar] [CrossRef]
- Sanabria-de la Torre, R.; Ceres-Muñoz, M.; Pretel-Lara, C.; Montero-Vílchez, T.; Arias-Santiago, S. Microtopography and Barrier Function in Healthy Skin: Differences between Forearm, Cheek and Palm. Cosmetics 2024, 11, 5. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Mielcarek, A.; Nowak, I. Evaluation of Sex-Related Changes in Skin Topography and Structure Using Innovative Skin Testing Equipment. Ski. Res. Technol. 2018, 24, 614–620. [Google Scholar] [CrossRef]
- Hon, K.; Lam, P.; Ng, W.; Kung, J.; Cheng, N.; Lin, Z.; Chow, C.; Leung, T. Age, Sex, and Disease Status as Determinants of Skin Hydration and Transepidermal Water Loss among Children with and without Eczema. Hong Kong Med. J. 2020, 26, 19–26. [Google Scholar]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.; Tsang, K.Y.C.; Cheng, N.; Leung, T.F. Are Skin Equipment for Assessing Childhood Eczema Any Good? J. Dermatol. Treat. 2021, 32, 45–48. [Google Scholar] [CrossRef] [PubMed]
- De Greef, A.; Ghislain, P.D.; de Montjoye, L.; Baeck, M. Real-Life Effectiveness and Tolerance of Upadacitinib for Severe Atopic Dermatitis in Adolescents and Adults. Adv Ther. 2023, 40, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Cantafio Duò, V.L.; Vecco, C.; Gelato, F.; Giordano, S.; Roccuzzo, G.; Cavaliere, G.; Avallone, G.; Ortoncelli, M.; Ribero, S.; et al. Impact of Comorbidities in the Response of Atopic Patients Treated with Dupilumab: A Real-Life Study up to 36 Weeks. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1021–e1023. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Rodriguez-Pozo, J.A.; Cuenca-Barrales, C.; Sanabria-de-la-Torre, R.; Torres-de-Pinedo, J.M.; Arias-Santiago, S. Stratum Corneum Hydration as a Potential Marker of Response to Dupilumab in Atopic Dermatitis: A Prospective Observational Study. Dermatitis 2024, 35, 250–257. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).