Abstract
Objectives: To analyze Heart Team decisions and outcomes following failure of surgical aortic valve replacement (SAVR) prostheses. Methods: Patients undergoing re-operations following index SAVR (Redo-SAVR) and those undergoing valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) following SAVR were included in this study. Patients who underwent index SAVR and/or Redo-SAVR for endocarditis were excluded. Data are presented as medians and 25th–75th percentiles, or absolute numbers and percentages. Outcomes were analyzed in accordance to the VARC-3 criteria. Results: Between 01/2015 and 03/2021, 53 patients underwent Redo-SAVR, 103 patients ViV-TAVR. Mean EuroSCORE II was 5.7% (3.5–8.5) in the Redo-SAVR group and 9.2% (5.4–13.6) in the ViV group. In the Redo-SAVR group, 12 patients received aortic root enlargement (22.6%). Length of hospital and ICU stay was longer in the Redo-SAVR group (p < 0.001; p < 0.001), PGmax and PGmean were lower in the Redo-SAVR group as compared to the ViV-TAVR group (18 mmHg (10–30) vs. 26 mmHg (19–38), p < 0.001) (9 mmHg (6–15) vs. 15 mmHg (9–21), p < 0.001). A higher rate of paravalvular leakage was seen in the ViV-TAVR group (p = 0.013). VARC-3 Early Safety were comparable between the two populations (p = 0.343). Survival at 1 year and 5 years was 82% and 36% in the ViV-TAVR cohort and 84% and 77% in the Redo-SAVR cohort. The variables were patient age (OR 1.061; [95% CI 1.020–1.104], p = 0.004), coronary heart disease (OR 2.648; [95% CI 1.160–6.048], p = 0.021), and chronic renal insufficiency (OR 2.711; [95% CI 1.160–6.048], p = 0.021) showed a significant correlation to ViV-TAVR. Conclusions: Heart Team decisions are crucial in the treatment of patients with degenerated aortic bioprostheses and lead to a low mortality in both treatment paths thanks to patient-specific therapy planning. ViV-TAVR offers a treatment for elderly or intermediate-risk profile patients with comparable short-term mortality. However, this therapy is associated with increased pressure gradients and a high prevalence of paravalvular leakage. Redo-SAVR enables the surgical treatment of concomitant cardiac pathologies and allows anticipation for later VIV-TAVR by implanting the largest possible valve prostheses.
1. Introduction
Interdisciplinary Heart Teams have evolved considerably over the last decade and play a central role in the treatment of a wide range of cardiovascular diseases. In order to meet the individual needs of each patient in the face of constantly evolving therapeutic options, continuous self-reflection on the therapeutic decisions made and the results achieved is essential. This applies in particular to the management of aortic valve disease, which has changed dramatically over the last years. Improvement of transcatheter aortic valve replacement (TAVR) has offered an alternative strategy for treating degenerated biological surgical aortic valve prostheses [1]. Recent data have also shown that this therapy procedure is non-inferior to surgery in low- and intermediate-risk patients [2]. Valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) has emerged in recent years as a novel alternative approach to conventional Redo surgical aortic valve replacement (Redo-SAVR) resulting in an increasing trend toward implantation of biological valves at primary surgical aortic valve replacement (SAVR), even in younger patients. The rationale is that ViV-TAVR can be performed with a lower perioperative risk compared to redo-SAVR in cases of prosthetic aortic valve dysfunction and therefore increasingly eliminating the need for mechanical heart valves with a high risk for thromboembolism and lifelong use of vitamin K antagonists [3,4,5]. This increases the demands on the Heart Team, which faces lifelong therapy decisions for patients with heart valve disease although hemodynamic outcomes are difficult to predict due to multiple factors including surgical valve size, surgical valve type, patient–prosthesis mismatch, and type of transcatheter valve [6]. Data with regard to hemodynamic performance and long-term outcomes are sparse. In this study, we endeavor to analyze Heart Team decisions and outcomes following the failure of surgical aortic valve prostheses at our institution.
2. Methods
2.1. Ethics Statement
This study was approved by the institutional ethics committee of the Ludwig Maximilian University (No. 21-0423), and informed consent was obtained in case of prospective follow-up data collection. Postoperative treatment and data collection were performed as a part of routine patient care. Data acquisition was based on institutional databases and was subsequently anonymized. All procedures described in this study were in accordance with the institutional ethics boards and national data safety regulations.
2.2. Study Design
A total of 156 patients who underwent either Redo-SAVR (n = 53) or ViV-TAVR (n = 103) after Index-SAVR with subsequent prosthesis dysfunction at our institution from January 2015 to March 2021 were included. Patients with a history of native or prosthetic valve endocarditis were excluded. Patients included in the study were retrospectively reviewed in accordance with national data safety regulations. All patients were discussed in the Heart Team and all patients were evaluated individually. Patient details were collected from institutional databases and surgeon notes and were anonymized for further analysis. Follow-up was achieved by routine check-ups and patient interviews. The European System for Cardiac Operative Risk Evaluation II (EuroSCORE II) and Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) were used to predict the risk of perioperative mortality at the time of VIV-TAVR and Redo-SAVR. Endpoints were determined in accordance with the recommendations of the Valve Academic Research Consortium 3 (VARC-3) [7]. All patients were discharged to either cardiac rehabilitation centers, transferred to secondary care centers or discharged home.
2.3. Statistical Analysis
Data were analyzed using the IBM SPSS Statistics Data Editor® version 25 (IBM Corp. Released 2017. IBM SPSS Statistics, Version 25.0. Armonk, NY, USA: IBM Corp.). Data were tested for normal distribution using the Kolmogorov–Smirnov test with Lillefors correction. Categorical variables were evaluated using the Chi-Squared and Fisher’s exact method and continuous variables were evaluated using the Mann–Whitney U test. Multivariable analysis incorporated logistic regression using a forward stepwise (conditional) model, where significance for entry was set at p < 0.05 and significance for exit was p < 0.10. The regression model was verified using the regression diagnostics which included the goodness-of-fit test as well as tests for autocorrelation, multicollinearity, and heteroscedasticity. Survival analysis was performed with the Kaplan–Meier curve. Illustrations were prepared using GraphPad Prism (Version 10, GraphPad Software Inc., San Diego, CA, USA). Data are presented as medians (25–75th quartiles) or as absolute numbers (percentages) unless otherwise specified.
3. Results
3.1. Patient Characteristics and Co-Morbidities
Patient characteristics and co-morbidities are listed in Table 1. Patients in the ViV cohort were significantly older (79 (75–83) years vs. 68 (59–77) years, p < 0.001) and had a significantly higher median EuroSCORE II (9.2 (5.4–13.6) vs. 5.7 (3.5–8.5), p < 0.001) and STS PROM score (4.1 (2.6–6.8) vs. (2.0 (1.3–2.8), p < 0.001) compared to the Redo-SAVR cohort. In the Redo-SAVR cohort, index SAVR was performed as a biological valve replacement in 81.1% of cases, and as a mechanical valve replacement in 18.8% of cases. Concomitant procedures were performed in 33.9% of cases. In the ViV-TAVR cohort concomitant procedures were performed in 48.5% of cases. In the Redo-SAVR cohort, 9 (5–14.5) years, and in the ViV-TAVR cohort 10 (7–12) years elapsed until secondary valve intervention. The most common indication for repeat valve replacement in the Redo-SAVR cohort was aortic valve stenosis (AS) (23 (43.4%)), and in the ViV-TAVR cohort, mixed aortic valve disease with predominant aortic stenosis (MAVD) (43 (41.7%)).

Table 1.
Patient characteristics and co-morbidities.
The ViV-TAVR cohort had a significantly higher rate of co-morbidities such as arterial hypertension (99 (96.1%) vs. 43 (81.1%), p = 0.005), hyperuricemia (26 (25.2%) vs. 5 (9.4%), p = 0.020), and insulin-dependent diabetes mellitus (22 (21.4%) vs. 3 (5.7%), p = 0.011). The ViV-TAVR cohort had significantly higher rates of coronary artery disease (71 (68.9%) vs. 17 (32.1%), p < 0.001), preoperative coronary angioplasty or stenting (28 (27.2%) vs. 6 (11.3%), p = 0.025). Regarding other co-morbidities, the ViV-TAVR cohort had significantly higher rates of chronic renal failure (56 (54.4%) vs. 11 (20.8%), p < 0.001) and significantly higher use of new oral anticoagulants (NOAKs) (31 (30.1%) vs. 5 (9.4%), p = 0.004). In contrast, patients in the Redo-SAVR cohort had significantly higher use of vitamin K antagonists (VKAs) ((17 (32.1%)) vs. (10 (9.7%), p = 0.001).
3.2. Heart Team Decision
To identify variables that strongly influenced Heart Team decisions, we performed a multivariate analysis (Table 2). Only those variables that showed a significant difference between the cohorts in the descriptive statistics were examined. The variables were patient age (OR 1.061; [95% CI 1.020–1.104], p = 0.004), coronary heart disease (OR 2.648; [95% CI 1.160–6.048], p = 0.021), and chronic renal insufficiency (OR 2.711; [95% CI 1.160–6.048], p = 0.021) showed a significant correlation to ViV-TAVR. The use of vitamin K antagonists showed a significant correlation to Redo-SAVR (OR 0.311; [95% CI 0.107–0.906], p = 0.032). There was no significant association with insulin-dependent diabetes mellitus and the use of NOACs.

Table 2.
Multivariable analysis: Heart Team decision.
3.3. Echocardiographic Data
Echocardiographic data are listed in Table 3. Before secondary intervention, the ViV-TAVR cohort had a significantly higher incidence of severe aortic valve stenosis (p = 0.010). There were no significant differences between cohorts regarding other valve diseases such as aortic regurgitation, mitral regurgitation, tricuspid regurgitation, and LVEF. After secondary intervention, the ViV-TAVR cohort had significantly higher maximum (26 (19–38) mmHg vs. 18 (10–30) mmHg, p < 0.001) and mean (15 (9–21) mmHg vs. 9 (6–15) mmHg, p < 0.001) gradients across the aortic valve prosthesis (Figure 1). In the ViV-TAVR cohort, prosthesis gradients above 20 mmHg were also significantly more frequent (25 (24.2%) vs. 1 (1.8%), p < 0.001), as well as PVL (23 (25.3%) vs. 1 (1.9%), p = 0.013).

Table 3.
Echocardiographic data.
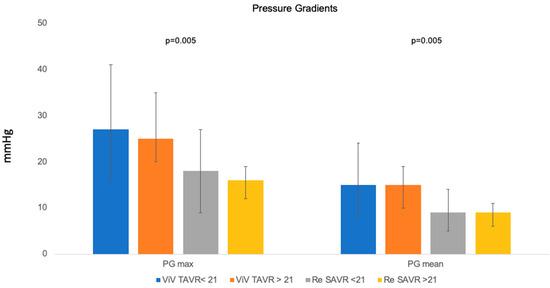
Figure 1.
Pressure gradients.
3.4. Surgical Data
Details of surgery/intervention are listed in Table 4. In the Redo-SAVR cohort, median bypass time was 156 (119–211) minutes and cross-clamp time was 103 (82–141) minutes. Concomitant procedures were performed more frequently in patients in the Redo-SAVR cohort (33 (62.2%) versus 16 (15.5%)). The most common additional procedure performed was aortic root enlargement (22.6%), followed by aortic root replacement (5.7%), aortocoronary bypass (13.2%), mitral valve replacement (24.5%), tricuspid valve repair (3.8%), and mitral valve repair (1.9%). In the ViV-TAVR cohort, 16.5% of patients underwent additional PTCA or stenting during the procedure. In the Redo-SAVR cohort, 75.4% of patients underwent biological valve replacement and 24.5% underwent mechanical valve replacement.

Table 4.
Details of surgery/intervention.
3.5. Valve Type-Based Echocardiographic Data
Echocardiographic data are listed in Table 5a,b. There were no significant differences between bioprosthetic and mechanical valves in the RS group with regard to pressure gradients and paravalvular leakage. In the ViV cohort, patients with a self-expandable prosthesis had a significantly higher incidence of paravalvular leakage compared to those with a balloon-expandable prosthesis. We observed no differences with regard to the pressure gradients.

Table 5.
(a) Echocardiographic data based on valve type. (b) Echocardiographic data based on valve type.
3.6. Postprocedural Morbidities and Outcomes
Postprocedural morbidities are listed in Table 6. Patients after Redo-SAVR had a significantly higher rate of re-explorative surgery (6 (11.3) vs. 0 (0), p = 0.001) and had a significantly higher number of packed red blood cells transfused (38 (71.6%) vs. 5 (4.8%), p < 0.001). Furthermore, patients in the Redo-SAVR group had a significantly higher rate of surgical site infections (5 (9.4%) vs. 1 (1.0%), p = 0.018). Cerebrovascular events occurred more frequently in patients after ViV-TAVR, without statistical significance between cohorts (5 (4.9%) vs. 0 (0%), p = 0.167). Patients in the Redo-SAVR group had a significantly higher rate of renal replacement therapy after valve replacement (7 (13.2%) vs. 2 (1.9%), p = 0.008).

Table 6.
Postoperative/postinterventional morbidities.
Postprocedural outcomes are listed in Table 7. Patients after RS had a significantly longer stay at the ICU (3 (3–5) days vs. 1 (1–3) days, p < 0.001) and a significantly longer overall length of stay at the hospital (15 (13–20) days vs. 11 (8–14) days, p < 0.001). Patients after ViV-TAVR achieved VARC-3 Early Safety endpoint more often, without statistical significance between cohorts (43 (87.3%) vs. 90 (81.1%), p = 0.343). The mortality at 30 days was higher in the Redo-SAVR cohort, without statistical significance (3(5.7%) vs. 3 (2.9%), p = 0.409) as well.

Table 7.
Postoperative/postinterventional outcomes.
Mean follow-up in the Redo-SAVR cohort was 2258 ± 101 days in the ViV-TAVR cohort 1937 ± 82 days (p = 0.008). At follow-up, survival at 1 year and 5 years was 82% and 36% in the ViV-TAVR cohort, and 84% and 77% in the Redo-SAVR cohort, respectively (p = 0.007).
Postprocedural morbidities and outcomes after the exclusion of Redo-SAVR patients with concomitant procedures are listed in Table 8. Patients after isolated Redo-SAVR had a significantly higher rate of atrial fibrillation (5 (17.2%) vs. 5 (4.9%), p = 0.041) and a significantly higher number of packed red blood cells transfused (18 (62.1%) vs. 5 (4.8%), p < 0.001). There was no significant difference with regard to reexplorative surgery, surgical site infections, and the need for renal replacement therapy.

Table 8.
Postoperative/postinterventional morbidities and outcomes—subgroup analysis.
4. Discussion
Following degeneration of aortic bioprostheses, treatment decisions should be made in an interdisciplinary Heart Team. This is important to ensure a patient-centric treatment plan with low mortality and morbidity as well as lifelong management of valvular heart disease [8,9]. The principal findings of the present study investigating the outcomes of Redo-SAVR and ViV-TAVR in the setting of SAVR prostheses failure may be summarized as follows:
- Redo-SAVR, being the more invasive therapy option, enables the surgical treatment of concomitant cardiac pathologies, and allows anticipation for later VIV-TAVR by implanting the largest possible valve prostheses or implantation of mechanical prostheses if mandatory.
- ViV-TAVR offers a treatment for elderly or intermediate-risk profile patients with comparable short-term mortality. However, this therapy is associated with increased pressure gradients and a high prevalence of paravalvular leakage.
4.1. Surgery or Intervention—The Heart Team Approach
The combined expertise of a Heart Team provides the basis for a more balanced appraisal of a specific case [10]. A Heart Team usually consists of cardiologists, cardiac surgeons, interventionists, imaging specialists, anesthesiologists, and mid-level providers. Furthermore, in some cases, the expert opinion of a general practitioner, geriatrician, or intensive care specialist can be of additional value [10]. Heart Teams have shown to be beneficial for patients, improving survival, and avoiding complications [10,11]. Advanced age alone should not be the main reason for intervention. Previous reports have shown that complex valve surgery may be carried out in elderly patients with good results [12,13]. Furthermore, a surgical approach offers the opportunity to therapeutically address concomitant cardiac disorders that occur in up to 30% of patients with degenerated prosthetic valves such as coronary artery disease, aortic aneurysms, hypertrophic cardiomyopathy, atrial fibrillation, as well as mitral and tricuspid valve dysfunction [14,15]. Surgery should not be immediately disregarded as a therapeutic option and should be carefully considered with a multidisciplinary approach in the Heart Team. Of vital importance is the planning of the index procedure and lifetime management of aortic valve disease. In young patients with calcific aortic valve disease, the Heart Team should plan ahead and take into consideration the need for Redo-SAVR surgery or re-interventions in the future [8,16]. At our institution, all cases are discussed in the Heart Team, and treatment options are discussed and implemented in a patient-centric, multidisciplinary fashion. This is reflected by advanced age, higher EuroSCORE II, and STS PROM in the ViV group as well as age, coronary artery disease, and chronic kidney insufficiency being associated with ViV-TAVR in the multivariate analysis.
4.2. Hemodynamic Outcomes
Since the advent of transcatheter technology, there has been an increase in the number of biological aortic valve prostheses implanted in comparison to mechanical valves, as they provide the possibility of valve re-intervention in the future. Furthermore, it has been shown that the size of valves implanted has also reduced over the years [17,18]. This is potentially dangerous at the time of reintervention, as small biological aortic valve prostheses at the index operation have been reported to be associated with a much higher 1-year mortality compared with intermediate- or large-sized biological aortic valve prostheses [18]. In the ViV-TAVR cohort, higher postinterventional maximum and mean gradients as well as higher rates of PVL were observed. It has been demonstrated that postoperative elevated gradients and PVL are not only clinically relevant but also contribute significantly to structural and nonstructural prosthetic valve degeneration [19,20]. Furthermore, other studies have reported a higher rate of aortic insufficiency in patients undergoing ViV-TAVR [21]. Studies have also shown an association between increased rehospitalization rates due to decompensated heart failure, the need for valve reintervention, and increased mortality in patients undergoing ViV-TAVR [22,23,24]. Data from the VIVID registry reports a severe prosthesis–patient mismatch in 31.8% of patients [25]. Therefore, the Heart Team often has to decide whether a patient who is actually classified for ViV-TAVR due to their age and risk profile should opt for Redo-SAVR with concomitant aortic root enlargement due to the risk of relevant PPM. According to our data, for patients at increased risk for poor hemodynamic outcome due to a small prosthesis size (<21 mm) or preexisting PPM, Redo-SAVR appears to be the superior therapy, even in cases of higher age and risk constellation, although current ESC/EACTS guidelines do not clearly recommend this [26].
4.3. Outcomes
Patients in the Redo-SAVR cohort had a significantly higher incidence of postoperative atrial fibrillation, perioperative blood transfusion, re-explorative surgery, surgical site infection, requirement of postoperative renal replacement therapy, and had significantly longer ICU and hospital stays. However, we observed no differences in the rates of myocardial infarction, new pacemaker implantation, hospital admission for cardiac reasons, major bleeding, and ischemic stroke among the groups. Despite higher invasiveness with concomitant higher surgical trauma and use of cardiopulmonary bypass, there was no difference in the rates of the VARC-3 Early Safety index. Another point that should be mentioned in this context is the high rate of concomitant procedures in the Redo-SAVR group. Exclusion of these patients shows a significant reduction in postoperative morbidities so significant differences were only found with regard to a higher rate of atrial fibrillation and a higher number of packed red blood cells transfused. Studies have shown that coronary artery obstruction occurs with an incidence of up to 3.5% during ViV-TAVR and is approximately four to six times more common compared to TAVR in native aortic valves [27]. In the ViV group, half of the cases of peri-interventional cardiopulmonary resuscitation and all cases of peri-interventional myocardial infarction were associated with coronary artery obstruction. Only one patient survived such an event. Despite advances in peri-interventional imaging, morphologic planning, and new interventional strategies, this remains a life-threatening complication that is absent with controlled removal of the degenerated prosthetic valve under visualization during Redo-SAVR [27].
In our study, the Redo-SAVR cohort had a comparable 30-day mortality to the ViV-TAVR cohort, with our results being comparable to the VIVID registry [25]. It should be noted that in the Redo-SAVR cohort, concomitant procedures were performed in more than 60% of cases, further increasing the perioperative risk due to higher invasiveness and longer cross-clamp/bypass time, and therefore biasing the comparison with interventional single-valve replacement. This should be carefully considered while interpreting the 30-day mortality. In the Redo-SAVR group, almost two-thirds of the patients underwent concomitant surgery. Although these enable holistic treatment of various cardiac pathologies, they are associated with a higher perioperative risk due to their greater complexity and invasiveness. If patients with combined procedures are excluded and thus only the isolated replacement of the valve prosthesis is considered, Patel et al. [21] reported a similar early mortality in the ViV cohort. Although the exclusion of combined interventions does not do justice to the clinical reality, it does show how important it is to consider age and risk distribution as well as the scope and complexity of the respective therapy in the assessment of mortality, especially in the short-term follow-up. In summary, the 30-day mortality in the ViV-TAVR cohort is likely to be lower in patients with a high-risk profile, but Redo-SAVR is a safe procedure with a comparable overall mortality that is due to new developments such as rapid deployment aortic valve prostheses with shorter implantation and thus operation times. However, there is further potential for improvement.
The VIVID registry reports an overall 1-year survival of 83.2% and this is comparable to our results in both the groups (Figure 2). Although associated with an initial comparable or higher mortality, several other studies have also shown an improved long-term survival rate in patients undergoing Redo-SAVR [1,14,21]. Deharo et al. [1] showed that an initial mortality advantage of the ViV cohort was lost after a median of 516 days whereas Sedeek et al. [14] reported a similar reversal of the survival curve after approximately 2 years. Whether the reduced long-term survival of the ViV cohort is due to the age of the patients or the significantly increased prosthesis gradients cannot be conclusively clarified within the scope of this analysis.
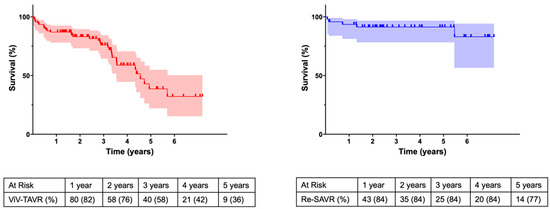
Figure 2.
Kaplan–Meier survival curve.
Careful selection of patients in the Heart Team is critical to provide optimal therapy to each individual patient. Good surgery in primary or even secondary aortic valve replacement with the avoidance of small prosthesis sizes by augmentation of the aortic root allows for the reasonable and safe use of ViV-TAVR later, when open surgery is not an option because of the risk constellation or patient age.
5. Conclusions
Heart Team decisions are crucial in the treatment of patients with degenerated aortic bioprostheses and lead to a low mortality in both treatment paths due to patient-specific therapy planning. Redo-SAVR is associated with low morbidity and mortality and allows surgical repair of multiple cardiac diseases; ViV-TAVR offers a treatment alternative with comparable morbidity and mortality for the elderly or patients with an intermediate-risk profile. However, this therapy is associated with significantly higher transvalvular pressure gradients and a high prevalence of PVL compared to conventional Redo-SAVR. Foresighted surgery for primary or even secondary aortic valve replacement with holistic management of concomitant cardiac disease and the avoidance of small prosthesis sizes by aortic root augmentation allows for the reasonable and safe use of ViV-TAVR later, when open surgery is not an option because of the risk constellation or patient age.
Limitations
This is a retrospective single-center study with the inherent limitations of such an analysis. The small number of patients is associated with a low power of statistical analyses. Further multicenter studies are required. Furthermore, there are obvious differences in the risk scores. Owing to the small sample size, propensity score matching and subgroup analyses based on valve type could not be performed.
Author Contributions
Data Curation: P.S. and A.A.; Formal analysis: P.S., S.S. (Shekhar Saha) and D.J. Investigation: P.S., K.M.H., K.R., R.K., S.M. and S.S. (Sebastian Sadoni); Methodology: M.O. and D.J.; Project administration: D.J., P.M.D. and J.B.; Resources: C.H. and S.M.; Supervision: C.H. and S.M.; Validation: C.S.M. and S.S. (Shekhar Saha); Visualisation: S.S. (Shekhar Saha) and P.S.; Writing—Original Draft: P.S., D.J. and S.S. (Shekhar Saha); Writing—review & editing: P.S., D.J. and S.S. (Shekhar Saha). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the institutional ethics committee of the Ludwig Maximilian University (No. 21-0423), and informed consent was obtained in case of prospective follow-up data collection.
Informed Consent Statement
Informed consent was obtained from all subjects involved at follow up in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to national data safety regulations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deharo, P.; Bisson, A.; Herbert, J.; Lacour, T.; Saint Etienne, C.; Jaussaud, N.; Theron, A.; Collart, F.; Bourguignon, T.; Cuisset, T.; et al. Valve-in-valve transcatheter aortic valve implantation after failed surgically implanted aortic bioprosthesis versus native transcatheter aortic valve implantation for aortic stenosis: Data from a nationwide analysis. Arch. Cardiovasc. Dis. 2021, 114, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, N.; Atti, V.; Munir, A.B.; Karam, B.; Patel, N.J.; Kumar, V.; Vemula, P.; Edla, S.; Asti, D.; Paturu, A.; et al. Valve in valve transcatheter aortic valve implantation (ViV-TAVI) versus redo-Surgical aortic valve replacement (redo-SAVR): A systematic review and meta-analysis. J. Interv. Cardiol. 2018, 31, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.H.; Yandrapalli, S.; Zaid, S.; Shetty, S.S.; Aronow, W.S.; Ahmad, H.; Tang, G.H.L. Valve-in-Valve Transcatheter Implantation Versus Redo Surgical Aortic Valve Replacement. Am. J. Cardiol. 2020, 125, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Singh, H.; Lämmer, J.; Othman, H.; Yamasaki, H.; Rosman, H.S.; Bossone, E.; Mehta, R.H.; Eggebrecht, H. Meta-Analysis of Transcatheter Valve-in-Valve Implantation Versus Redo Aortic Valve Surgery for Bioprosthetic Aortic Valve Dysfunction. Am. J. Cardiol. 2018, 121, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Kherallah, R.Y.; Koneru, S.; Krajcer, Z.; Preventza, O.; Dougherty, K.G.; Mccormack, M.L.; Costello, B.T.; Coulter, S.; Strickman, N.E.; Carlos, J.; et al. Hemodynamic outcomes after valve-in-valve transcatheter aortic valve replacement: A single-center experience. Ann. Cardiothorac. Surg. 2021, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef]
- Lee, C.; Tully, A.; Fang, J.C.; Sugeng, L.; Elmariah, S.; Grubb, K.J.; Young, M.N. Building and Optimizing the Interdisciplinary Heart Team. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101067. [Google Scholar] [CrossRef]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Sondergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interve. Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef]
- Antonides, C.F.J.; Mack, M.J.; Kappetein, A.P. Approaches to the Role of The Heart Team in Therapeutic Decision Making for Heart Valve Disease. Struct. Heart 2017, 1, 249–255. [Google Scholar] [CrossRef]
- Davierwala, P.M.; Marin-Cuartas, M.; Misfeld, M.; Borger, M.A. The value of an “Endocarditis Team”. Ann. Cardiothorac. Surg. 2019, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Varghese, S.; Al Ahmad, A.; Jebran, A.F.; Waezi, N.; Niehaus, H.; Baraki, H.; Kutschka, I. Complex Valve Surgery in Elderly Patients: Increasingly Necessary and Surprisingly Feasible. Thorac. Cardiovasc. Surg. 2020, 68, 107–113. [Google Scholar] [CrossRef]
- Peterss, S.; Fortmann, C.; Pichlmaier, M.; Bagaev, E.; Shrestha, M.L.; Hagl, C.; Haverich, A.; Khaladj, N. Advanced age: A contraindication for triple-valve surgery? J. Heart Valve Dis. 2012, 21, 641–649. [Google Scholar] [PubMed]
- Sedeek, A.F.; Greason, K.L.; Sandhu, G.S.; Dearani, J.A.; Holmes, D.R.J.; Schaff, H.V. Transcatheter Valve-in-Valve Vs Surgical Replacement of Failing Stented Aortic Biological Valves. Ann. Thorac. Surg. 2019, 108, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Woitek, F.J.; Stachel, G.; Kiefer, P.; Haussig, S.; Leontyev, S.; Schlotter, F.; Mende, M.; Hommel, J.; Crusius, L.; Spindler, A.; et al. Treatment of failed aortic bioprostheses: An evaluation of conventional redo surgery and transfemoral transcatheter aortic valve-in-valve implantation. Int. J. Cardiol. 2020, 300, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Tang, G.H.L.; Sangiorgi, G.; Pedicino, D.; Enriquez-Sarano, M.; Maisano, F.; Taramasso, M. Lifetime Management of Aortic Stenosis: Transcatheter Versus Surgical Treatment for Young and Low-Risk Patients. Circ. Cardiovasc. Interv. 2022, 15, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Edelman, J.J.; Khan, J.M.; Rogers, T.; Shults, C.; Satler, L.F.; Ben-Dor, I.I.; Waksman, R.; Thourani, V.H. Valve-in-Valve TAVR: State-of-the-Art Review. Innovations 2019, 14, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Rocha, R.V.; Wijeysundera, H.C.; Austin, P.C.; Dvir, D.; Fremes, S.E. Surgical valve selection in the era of transcatheter aortic valve replacement in the Society of Thoracic Surgeons Database. J. Thorac. Cardiovasc. Surg. 2020, 159, 416–427.e8. [Google Scholar] [CrossRef]
- Salaun, E.; Clavel, M.-A.; Rodés-Cabau, J.; Pibarot, P. Bioprosthetic aortic valve durability in the era of transcatheter aortic valve implantation. Heart 2018, 104, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, R.; Jahangiri, M.; Otto, C.M. Patient-prosthesis mismatch following aortic valve replacement. Heart 2019, 105, s28–s33. [Google Scholar] [CrossRef]
- Patel, P.M.; Chiou, E.; Cao, Y.; Binongo, J.; Guyton, R.A.; Leshnower, B.; Grubb, K.J.; Chen, E.P. Isolated Redo Aortic Valve Replacement Versus Valve-in-Valve Transcatheter Valve Replacement. Ann. Thorac. Surg. 2021, 112, 539–545. [Google Scholar] [CrossRef]
- Bleiziffer, S.; Simonato, M.; Webb, J.G.; Rodés-Cabau, J.; Pibarot, P.; Kornowski, R.; Windecker, S.; Erlebach, M.; Duncan, A.; Seiffert, M.; et al. Long-term outcomes after transcatheter aortic valve implantation in failed bioprosthetic valves. Eur. Heart J. 2020, 41, 2731–2742. [Google Scholar] [CrossRef] [PubMed]
- Dahlbacka, S.; Laakso, T.; Kinnunen, E.-M.; Moriyama, N.; Laine, M.; Virtanen, M.; Maaranen, P.; Ahvenvaara, T.; Tauriainen, T.; Husso, A.; et al. Patient-Prosthesis Mismatch Worsens Long-Term Survival: Insights From the FinnValve Registry. Ann. Thorac. Surg. 2021, 111, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.C.; Daneshvar, S.A.; Fonarow, G.C.; Stebbins, A.; Vemulapalli, S.; Desai, N.D.; Malenka, D.J.; Thourani, V.H.; Rymer, J.; Kosinski, A.S. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2018, 72, 2701–2711. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. EuroIntervention 2022, 17, e1126–e1196. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.L.M.; Dvir, D.; Rodes-Cabau, J.; Ribeiro, H.B. Valve-in-Valve Challenges: How to Avoid Coronary Obstruction. Front. Cardiovasc. Med. 2019, 6, 120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).