Abstract
One-third of adult inpatients with community-acquired pneumonia (CAP) develop acute coronary syndrome (ACS), stroke, heart failure (HF), arrhythmias, or die. The evidence linking CAP to cardiovascular disease (CVD) events is contradictory. We aimed to systematically review the role of CAP as a CVD risk factor. We registered the protocol (CRD42022352910) and searched for six databases from inception to 31 December 2022. We included 13 observational studies, 276,109 participants, 18,298 first ACS events, 12,421 first stroke events, 119 arrhythmic events, 75 episodes of new onset or worsening HF, 3379 deaths, and 218 incident CVD events. CAP increased the odds of ACS (OR 3.02; 95% CI 1.88–4.86), stroke (OR 2.88; 95% CI 2.09–3.96), mortality (OR 3.22; 95% CI 2.42–4.27), and all CVD events (OR 3.37; 95% CI 2.51–4.53). Heterogeneity was significant (I2 = 97%, p < 0.001). Subgroup analysis found differences according to the continent of origin of the study, the follow-up length, and the sample size (I2 > 40.0%, p < 0.10). CAP is a significant risk factor for all major CVD events including ACS, stroke, and mortality. However, these findings should be taken with caution due to the substantial heterogeneity and the possible publication bias.
1. Introduction
Community-acquired pneumonia (CAP) and cardiovascular disease (CVD) events are the leading causes of morbidity and mortality globally [1,2,3,4,5]. CAP is one of the most common reasons for adult hospital admissions. Over one million adults in the USA are hospitalized with pneumonia annually, and about 50,000 die from this disease [4,5,6]. Similarly, these diseases are associated with a significant social burden regarding health care resource utilization and social-economic cost [5].
There seems to exist a bidirectional relationship between pneumonia and CVD [7]. On one hand, CVDs such as coronary artery disease (CAD) and stroke increase the risk of hospitalization for pneumonia [7,8,9], but the opposite could also be true. That is, pneumonia may raise the risk of acute coronary syndrome (ACS)—myocardial infarction or unstable angina—stroke, heart failure, arrhythmias, and ev en death; acutely or even years after that [7,10,11].
However, the evidence linking CAP to cardiovascular complications is contradictory and not substantial. Most published studies included a single cohort without an adequate control group. Additionally, only two meta-analyses published in full text have included some of these studies [12,13]. Therefore, we aimed to systematically review the evidence on the role of CAP or respiratory tract infections as a risk factor for cardiovascular disease (CVD) complications.
2. Materials and Methods
We conducted this systematic review following the recommendations of the Cochrane Handbook for Systematic Reviews [14], PRISMA [15], and the AMSTAR 2 guidelines [16]. We previously registered the protocol in PROSPERO (CRD42022352910). We provide the PRISMA checklist in Figure 1. We searched for observational (cohort, case-control, and cross-sectional) studies and reviews published up until 31 December 2022, in Medline (PubMed), Google Scholar, Scopus, ScienceDirect, EMBASE, and Web of Science. We combined different keywords, controlled vocabulary terms (e.g., MeSH and Emtree terms), and free terms, according to the PECO strategy (population: “adults”; exposure: “pneumonia” OR “lower respiratory tract infection”; comparator: “no pneumonia” OR “no lower respiratory tract infection”; outcome: “acute coronary syndrome” OR “myocardial infarction” OR “unstable angina” OR “stroke” OR “mortality” OR “heart failure” OR “cardiac arrhythmia”) (Supplementary Materials). Searches were not limited by date or language. We included articles in full text and excluded case reports, case series, studies not available in full text, duplicated publications, and studies with patients aged <18 years. Two independent reviewers examined the articles, and a third researcher resolved discrepancies. References from the retrieved papers were screened for additional articles.

Figure 1.
Flowchart of the selection process of the primary studies included.
The articles found were analyzed using the terms of the PECO strategy and the inclusion and exclusion criteria. In addition, relevant data from each paper were extracted and recorded in a spreadsheet: the name of authors, year and country of publication, type of study, number of patients, number of events, the measure of association, and adjusted confounders.
In the meta-analysis, we pooled the adjusted odds ratios (OR), risk ratios (RR), or hazard ratios (HR) with 95% confidence intervals (95% CI) using the generic inverse variance method. Forest plots represented the quantitative synthesis. Heterogeneity among studies was assessed with Cochran’s Q test and Higgins I2 statistic. Heterogeneity was significant (p-value < 0.05, I2 statistics >40%), then we used a random effects model. We carried out sensitivity and subgroup analyses. The risk of bias was assessed using the Newcastle–Ottawa Scale (NOS) tool [17] and publication bias was examined using a funnel plot.
We defined “all CVD events” as a composite of first ACS or first stroke event, new or worsening episodes of heart failure, arrhythmia (atrial fibrillation or paroxysmal supraventricular tachycardia), and death. Incident CVD event was defined as a composite of ACS, stroke, and fatal CHD [18]. For studies reporting OR or RR stratified into different subgroups, we considered each subgroup analysis as a separate study.
3. Results
We collected a total of 12,796 in the primary and 31 in the secondary search. After eliminating duplicates, 83 publications were evaluated for their titles and abstracts. Subsequently, 40 articles were analyzed in full text, of which 13 papers (nine cohort and four case-control studies) were selected for qualitative and quantitative assessment (Figure 1). We only included full-text articles that reported the adjusted association measures—OR, RR, or HR—and a control group. The lack of a proper control group was the leading cause for the exclusion of most studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] (Supplementary Materials).
This study includes 276,109 participants, 18,298 first ACS events, 12,421 first stroke events, 119 arrhythmic events, 75 episodes of new onset or worsening HF, 3379 deaths, and 218 “incident CVD events” (Table 1). CAP increases the odds of ACS (OR 3.02; 95% CI 1.88–4.86), stroke (OR 2.88; 95% CI 2.09–3.96), mortality (OR 3.22; 95% CI 2.42–4.27), and all CVD events (OR 3.37; 95% CI 2.51–4.53). However, heterogeneity was significant (I2 = 97%, p < 0.001). The sensitivity analysis—with outliers excluded—did not significantly affect the overall estimate. In the subgroup analysis, we found statistically significant differences according to the continent of origin of the study (I2 = 78.2%, p < 0.10), the length of follow-up (I2 = 89.4%, p < 0.10), and the sample size (I2 = 75.1%, p < 0.10). Conversely, we did not find statistically significant differences between subgroups according to the study design (I2 = 0%, p < 0.93) (Figure 2A–G). Due to the limited data available, it was impossible to perform subgroup analysis according to other variables (i.e., the participants’ sex, the interval from exposition to the outcome, or cardiovascular risk levels). Similarly, we did not perform meta-regression analyses due to the limited number of studies.

Table 1.
General characteristics of the studies included.
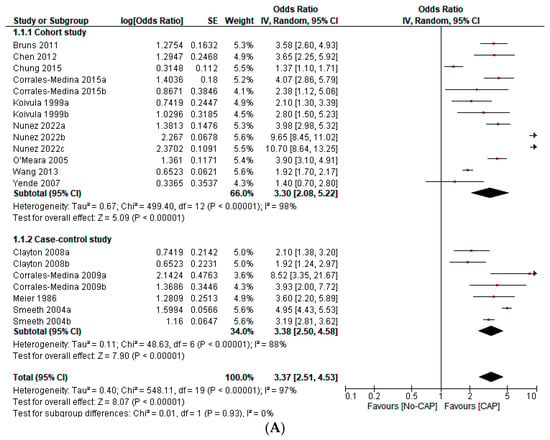
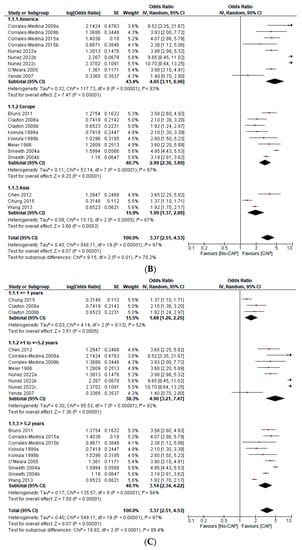
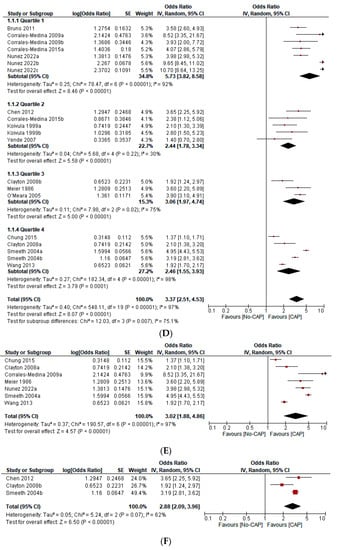
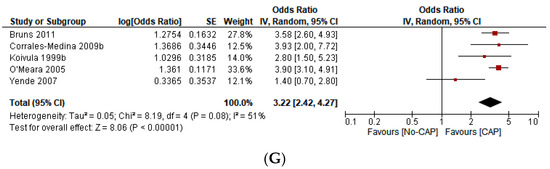
Figure 2.
(A) Forest plot on the effect of CAP on all CVD events (a composite of first ACS or first stroke event, new or worsening episodes of heart failure, arrhythmia, and death) according to the type of study design [1,2,3,4,5,6,7,8,9,10,13,15]. (B) Forest plot of the effect of CAP on all CVD events (a composite of the first ACS or first stroke event, new or worsening episodes of heart failure, arrhythmia, and death) according to the continent of origin of the study [4,8,11,12,13]. (C) Forest plot of the effect of CAP on all CVD events (a composite of first ACS or first stroke event, new or worsening episodes of heart failure, arrhythmia, and death) according to the length of follow-up in years [1,2,3,4,5,6,7,8,9,10,11,12,13]. (D) Forest plot of the effect of CAP on all CVD events (a composite of first ACS or first stroke event, new or worsening episodes of heart failure, arrhythmia, and death), according to the sample size in quartiles [1,2,3,4,5,6,7,8,9,10,11,12,13]. (E) Forest plot of the effect of CAP on the first ACS event [1,2,3,4,5,9,12]. (F) Forest plot of the effect of CAP on the first stroke event [2,3,10]. (G) Forest plot of the effect of CAP on mortality [6,7,8,12,13].
All of the studies included had a low risk of bias (Table 2). However, the funnel plot suggested publication bias (Figure 3).

Table 2.
Bias assessment of the included primary studies.

Figure 3.
Funnel plot of the effect of CAP on all CVD events (a composite of the first ACS or first stroke event, new, or worsening episodes of heart failure, arrhythmia, and death).
4. Discussion
This systematic review and meta-analysis shows that CAP significantly increases the odds of developing ACS (OR 3.02; 95% CI 1.88–4.86), stroke (OR 2.88; 95% CI 2.09–3.96), mortality (OR 3.22; 95% CI 2.42–4.27), and all CVD events—a composite of first ACS event, first stroke event, new or worsening episodes of heart failure, arrhythmia (atrial fibrillation or paroxysmal supraventricular tachycardia), and death (OR 3.37; 95% CI 2.51–4.53) (Figure 2A–E). These findings are in agreement with other primary studies [7,68,69,70,71,72,73,74,75,76,77,78] and three meta-analyses [12,13,79].
Corrales-Medina et al. performed a meta-analysis to determine the incidence of major cardiac complications in CAP patients. They searched Medline, Scopus, and EMBASE for observational studies of adults with CAP reporting the following: overall cardiac complications, incident HF, ACS, or incident cardiac arrhythmias occurring within 30 days of CAP diagnosis. They found 25 articles that met the eligibility and minimum quality criteria. Seventeen articles (68%) reported cohorts of CAP inpatients. In this group, the pooled incidence rates for overall cardiac complications (six cohorts, 2119 patients), incident HF (eights cohorts, 4215 patients), ACS (six cohorts, 2657 patients), and incident cardiac arrhythmias (six cohorts, 2596 patients) were 17.7% (95% CI 13.9–22.2, 14.1% (95% CI 9.3–20.6), 5.3% (95% CI 3.2–8.6), and 4.7% (95% CI 2.4–8.9), respectively. One article reported cardiac complications in CAP outpatients, four in low-risk (not severely ill) inpatients, and three in high-risk inpatients. The incidences for all outcomes except the overall cardiac complications were lower in the two former groups and higher in the latter. One additional study reported on CAP outpatients and low-risk inpatients without discriminating between these groups. Twelve studies (48%) asserted the evaluation of cardiac complications in their methods, but only six (24%) defined them. Only three studies, all examining ACS, carried out risk factor analysis for these events. No study analyzed the association between cardiac complications and other medical complications or their impact on other CAP outcomes. Nevertheless, the authors concluded that major cardiac complications occur in a substantial proportion of patients with CAP [12].
Tralhão et al. undertook a meta-analysis to report the incidence of overall complications, ACS, new or worsening HF, new or worsening arrhythmias, and acute stroke as well as short-term mortality outcomes. In addition, they reviewed the interplay between the two conditions (pneumonia and CVD complications). They included 39 observational studies involving 92,188 patients, divided by setting (inpatients versus outpatients) and clinical severity (low risk versus high risk). They reported that the overall cardiac complications occurred in 13.9%, ACS in 4.5%, HF in 9.2%, arrhythmias in 7.2%, and stroke in 0.71% of the pooled inpatients. Furthermore, the meta-regression analysis suggested that overall and individual cardiac complication incidence decreased. After adjusting for confounders, cardiovascular events after CAP independently increased the risk for short-term mortality (range of OR: 1.39–5.49). The authors’ findings highlighted the need for effective, large, trial-based, preventive, and therapeutic interventions in this patient population [13].
Baskaran et al. performed a meta-analysis, searching for observational studies to summarize the literature on the incidence of ACS in adults with CAP. They looked for Medline and Embase and reported that 103 studies met the inclusion criteria. The authors included 26 studies (n = 66,347 patients), most of them of good quality. This meta-analysis showed that the pooled incidence of ACS in-hospital and 30 days after CAP was 3.2% (95% CI 2.4–4.0%; n = 17 studies) and 3.5% (95% CI 2.8–4.2%; n = 25 studies), respectively. Sensitivity analysis excluding studies with selected cohorts (elderly, predominantly male, or ICU admissions only) showed a higher pooled incidence for in-hospital ACS (4%, 95% CI 2.7–5.3%, n = 13 studies) but unchanged for 30-day incidence. These researchers concluded that their meta-analysis showed a small but significant risk of ACS in patients with CAP. This study was published in 2020 only in abstract form [79].
It is worth noting that most of the primary studies published to date and included in these three previous meta-analyses [12,13,79] had significant limitations. For example, most of them did not have an adequate control group or did not control for potential confounders (Supplementary Materials), all of which may affect the real effect of the exposition or intervention [80,81,82,83]; therefore, we excluded more than 50 of these studies in our systematic review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67]. Furthermore, the meta-analyses by Corrales-Medina et al. [12], Tralhão et al. [13], and Baskaran et al. [79] are “meta-analyses of proportion”. A “proportional meta-analysis” differs significantly in its methodology from a “traditional meta-analysis”. We address these aspects later. Furthermore, the study by Baskaran et al. was published only in abstract form [79], so it is impossible to know what studies were included. Despite the limitations of the three meta-analyses described previously, their conclusions were concordant with our study [12,13,79].
The pathophysiologic mechanisms by which pneumonia can trigger CVD are probably diverse and involve several ways: (1) systemic and coronary artery inflammation increases cardiovascular risk; (2) infection and inflammation promote platelet activation and thrombosis; (3) changes in nitric oxide (NO) synthase and cyclooxygenase (COX) lead to endothelial dysfunction; (4) pneumonia impairs myocardial contractility, oxygen demand, and delivery; and additionally (5), the microorganisms may have a direct effect on cardiovascular risk [84,85,86,87,88,89]. However, most of the mechanisms above-mentioned were also observed in ischemic heart disease patients, particularly those with ACS. Thus, these findings may also affect pneumonia development after ACS, reflecting a reverse or bidirectional association [8,23,84,88].
To the best of our knowledge, this is the first “non-proportional” meta-analysis on CAP and CVD that reports ORs instead of proportions as a measure of effect size. A proportional meta-analysis is a data synthesis method that allows one to calculate a pooled, overall proportion from several individual proportions for a certain event instead of estimating an effect size, as conducted in a “traditional meta-analysis” [90,91]. That is, a proportional meta-analysis can include dichotomous data reported as a percentage. Proportional meta-analysis is encouraged when conducting systematic reviews of prevalence, incidence, and maybe, interventions and therapies where appropriate [92]. However, for dichotomous outcomes, the Cochrane Handbook does not recommend using proportional meta-analysis [14].
Furthermore, the appropriateness of conducting a proportional meta-analysis is controversial, as the individual studies contributing to such a meta-analysis commonly have been conducted in different contexts. These studies’ prevalence and cumulative incidence estimates reflect unique population characteristics [93]. This methodology raises concerns when proportional meta-analysis assumes homogeneity, and an average estimate across different populations may be of little clinical use [92,94]. However, Corrales-Medina et al. [12] and Tralhão et al. [13]. reported that they used stratification (mainly according to treatment setting and clinical severity) to minimize the influence heterogeneity in estimating the effect sizes.
This work has some limitations. (1) Although we performed subgroup analysis according to the continent of origin, the type of study design, the sample size, and the length of follow-up, we could not perform subgroup analyses according to other important variables such as age, sex, treatment setting, clinical severity, and timing after CAP because of the lack of data. (2) It is possible that a meta-regression analysis could further explain the origin of the heterogeneity, although we did not perform this analysis due to limited data. (3) We cannot rule out a possible publication bias against negative studies that did not find a significant association between CAP and CVD complications. (4) The fact that the different continent of origin explains the heterogeneity found could reflect the different forms of diagnosis, treatment, and prognosis, both for pneumonia and cardiovascular complications in each of these countries.
Overall, if the heterogeneity is high, the conclusions of the meta-analysis may be less generalizable. Nevertheless, this does not necessarily mean that the results are incorrect or do not have clinical importance. Instead, it is crucial to interpret the meta-analysis results considering the heterogeneity and possible explanations for the differences between the studies included [44,95,96,97]. In addition, there is always clinical and methodological diversity in a meta-analysis, so statistical heterogeneity is inevitable. Since systematic reviews bring together studies that are diverse both clinically and methodologically, heterogeneity in their results is to be expected [95,96,97]. In our study, heterogeneity was significant in most of the outcomes. However, we addressed this heterogeneity by implementing the recommendations of the Cochrane Handbook: (1) We verified the data correctness; (2) we explored the potential causes of heterogeneity; (3) we performed a meta-analysis with a random effects model, and (4) we performed sensitivity analyses [14].
We highlight some of the strengths of our work: (1) Our search strategy was thorough and complete; (2) we included the odds ratios instead of proportion as the effect measure; consequently, this is the first “traditional” meta-analysis, instead of proportional meta-analysis on this topic; (3) we included primary studies that specifically examined the association between pneumonia and CVD complications; (4) we excluded studies that reported a single cohort without a control group; and (5) we only included studies that reported the adjusted effect sizes. Therefore, our results are more robust than any other meta-analysis reported before.
5. Conclusions
In conclusion, our study shows that pneumonia should be considered as a new risk factor for cardiovascular complications. Furthermore, our findings support the hypothesis that inflammation triggered by acute and chronic infections such as pneumonia is crucial in the pathogenesis of atherosclerosis and cardiovascular complications. However, this conclusion should be taken with caution due to the limitations of our study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12072577/s1, S1: Search strategy; S2: Excluded primary studies.
Author Contributions
For Conceptualization, E.D.M.-R. and M.G.A.-R.; Methodology, E.D.M.-R.; Software E.D.M.-R.; Validation, E.D.M.-R., M.G.A.-R., M.J.R.-B. and G.A.V.-T.; Formal analysis, E.D.M.-R. and G.A.V.-T.; Investigation, M.G.A.-R. and G.A.V.-T.; Resources, E.D.M.-R., M.G.A.-R., M.J.R.-B. and G.A.V.-T.; Writing—original draft preparation, E.D.M.-R. and M.G.A.-R., Writing—review and editing, M.G.A.-R. and G.A.V.-T.; Visualization, E.D.M.-R.; Supervision, E.D.M.-R.; Project administration, E.D.M.-R.; Funding acquisition, E.D.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived since this was a secondary study.
Informed Consent Statement
Patient consent was waived since this was a secondary study.
Data Availability Statement
The protocol is available at https://www.crd.york.ac.uk/prospero/export_details_pdf.php (accessed on 31 January 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roger, V.L. Epidemiology of Myocardial Infarction. Med. Clin. N. Am. 2007, 91, 537–552. [Google Scholar] [CrossRef]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; De Ferranti, S.; Després, J.-P.; et al. Executive Summary: Heart Disease and Stroke Statistics—2016 Update: A Report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef]
- Storms, A.D.; Chen, J.; Jackson, L.A.; Nordin, J.; Naleway, A.L.; Glanz, J.M.; Jacobsen, S.J.; Weintraub, E.S.; Klein, N.P.; Gargiullo, P.M.; et al. Rates and risk factors associated with hospitalization for pneumonia with ICU admission among adults. BMC Pulm. Med. 2017, 17, 208. [Google Scholar] [CrossRef]
- Birnbaum, H.G.; Morley, M.; Greenberg, P.E.; Cifaldi, M.; Colice, G.L. Economic burden of pneumonia in an employed population. Arch. Intern. Med. 2001, 161, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.B.; Hayes, L.D.; Brown, K.; Hoo, E.C.; Ethier, K.A.; Centers for Disease Control and Prevention (CDC). CDC National Health Report: Leading causes of morbidity and mortality and associated behavioral risk and protective factors—United States, 2005–2013. MMWR Suppl. 2014, 63, 3–27. [Google Scholar]
- Bruns, A.H.W.; Oosterheert, J.J.; Cucciolillo, M.C.; El Moussaoui, R.; Groenwold, R.H.H.; Prins, J.M.; Hoepelman, A.I.M. Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin. Microbiol. Infect. 2011, 17, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.J.; Choi, S.; Seo, W.-W.; Lee, Y.J. Hospitalization for acute coronary syndrome increases the long-term risk of pneumonia: A population-based cohort study. Sci. Rep. 2021, 11, 9696. [Google Scholar] [CrossRef]
- Armstrong, J.R.; Mosher, B.D. Aspiration Pneumonia After Stroke: Intervention and prevention. Neurohospitalist 2011, 1, 85–93. [Google Scholar] [CrossRef]
- Singanayagam, A.; Elder, D.H.J.; Chalmers, J.D. Is community-acquired pneumonia an independent risk factor for cardiovascular disease? Eur. Respir. J. 2012, 39, 187–196. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Reyes, L.F. Pneumonia as a cardiovascular disease. Respirology 2018, 23, 250–259. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Suh, K.N.; Rose, G.; Chirinos, J.A.; Doucette, S.; Cameron, D.W.; Fergusson, D.A. Cardiac Complications in Patients with Community-Acquired Pneumonia: A Systematic Review and Meta-Analysis of Observational Studies. PLOS Med. 2011, 8, e1001048. [Google Scholar] [CrossRef]
- Tralhão, A.; Póvoa, P. Cardiovascular Events after Community-Acquired Pneumonia: A Global Perspective with Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2020, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; Updated March 2011; The Cochrane Collaboration and John Wiley & Sons Ltd.: Glasgow, UK, 2011; Available online: http://handbook-5-1.cochrane.org/ (accessed on 2 February 2023).
- Moher, D.; Liberati, M.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2013; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 July 2022).
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association Between Hospitalization for Pneumonia and Subsequent Risk of Cardiovascular Disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Musher, D.M.; Wells, G.A.; Chirinos, J.A.; Chen, L.; Fine, M.J. Cardiac complications in patients with community-acquired pneumonia: Incidence, timing, risk factors, and association with short-term mortality. Circulation 2012, 125, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.; Eurich, D.T.; Majumdar, S.R.; Jin, Y.; Marrie, T.J. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: A population-based cohort study. Medicine 2008, 8, 329–334. [Google Scholar] [CrossRef]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lazo, B.D. Complicaciones Cardiovasculares en Pacientes Hospitalizados por Neumonía en el Hospital Casimiro Ulloa-2014. Physician Degree Thesis, Faculty of Human Medicine, Ricardo Palma University, Lima, Peru, 2016. Available online: https://repositorio.urp.edu.pe/handle/20.500.14138/539 (accessed on 1 February 2023).
- Musher, D.M.; Rueda, A.M.; Kaka, A.S.; Mapara, S.M. The Association between Pneumococcal Pneumonia and Acute Cardiac Events. Clin. Infect. Dis. 2007, 45, 158–165. [Google Scholar] [CrossRef]
- Ramirez, J.; Aliberti, S.; Mirsaeidi, M.; Peyrani, P.; Filardo, G.; Amir, A.; Moffett, B.; Gordon, J.; Blasi, F.; Bordon, J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin. Infect. Dis. 2008, 47, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.M.; Coley, C.M.; Singer, D.E.; Marrie, T.J.; Obrosky, D.S.; Kapoor, W.N.; Fine, M.J. Causes of death for patients with community-acquired pneumonia: Results from the Pneumonia Patient Outcomes Research Team cohort study. Arch. Intern. Med. 2002, 162, 1059–1064. [Google Scholar] [CrossRef]
- Fuertes, J.A.G.; Molina, J.P.; Dominguez, I.J.; Mugica, M.B.L.; Mora, M.C.B.; Gomez, A.M.A.; Sierra, L.T.; Beristain, J.L.L. Acute Pneumonia and PSI: Is it posible to prevent a cardiovascular event? Eur. Respir. J. 2020, 56, 1776. [Google Scholar] [CrossRef]
- Baskaran, V.; Mckeever, T.; Shen, L.W. Incidence of Cardiac Complications after Hospitalization for Community Acquired Pneumonia: A Large Population-based Cohort Study. Eur. Respir. J. 2020, 56, 1772. [Google Scholar] [CrossRef]
- Kang, Y.; Fang, X.Y.; Wang, D.; Wang, X.J. Factors associated with acute myocardial infarction in older patients after hospitalization with community-acquired pneumonia: A cross-sectional study. BMC Geriatr. 2021, 21, 113. [Google Scholar] [CrossRef]
- Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Augello, G.; Dentali, F.; De Marzi, G.; Uomo, G.; Risaliti, F.; Morbidoni, L.; Mazzone, A.; et al. Acute cardiovascular events in patients with community acquired pneumonia: Results from the observational prospective FADOI-ICECAP study. BMC Infect. Dis. 2021, 25, 116. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Huang, X.; Zeng, M.; Chen, Y. Predictors of 30-day mortality in elderly patients with community acquired pneumonia. Eur. Respir. J. 2015, 46, PA2571. [Google Scholar] [CrossRef]
- Campos, C.C.; Liapikou, A.; Martin-Loeches, I.; Garcia-Vidal, C.; Gabarrus, A.; Ceccato, A.; Mensa, J.; Marco, F. Twenty-year Trend in Mortality among Hospitalized Patients with Pneumococcal Community-Acquired Pneumonia. Eur. Respir. J. 2018, 52, PA2626. [Google Scholar] [CrossRef]
- Allen, S.C. Lobar pneumonia in Northern Zambia: Clinical study of 502 adult patients. Thorax 1984, 39, 612–616. [Google Scholar] [CrossRef]
- Esposito, A.L. Community-acquired bacteremic pneumococcal pneumonia. Effect of age on manifestations and outcome. Arch. Intern. Med. 1984, 144, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Durant, H.; Yates, L. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev. Infect. Dis. 1989, 11, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Ortqvist, A.; Hedlund, J.; Grillner, L.; Jalonen, E.; Kallings, I.; Leinonen, M.; Kalin, M. Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur. Respir. J. 1990, 3, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Gladman, J.; Macfarlane, J.T.; Barer, D.; Berman, P.; Kinnear, W.; Finch, R.G. A hospital study of community acquired pneumonia in the elderly. Thorax 1990, 45, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Fine, M.J.; Smith, D.N.; Singer, D.E. Hospitalization decision in patients with community-acquired pneumonia: A prospective cohort study. Am. J. Med. 1990, 89, 713–721. [Google Scholar] [CrossRef]
- Leroy, O.; Santre, C.; Beuscart, C.; Georges, H.; Guery, B.; Jacquier, J.M.; Beaucaire, G. A five-year study of severe community-acquired pneumonia with emphasis on prognosis in patients admitted to an intensive care unit. Intensive Care Med. 1995, 21, 24–31. [Google Scholar] [CrossRef]
- Janssens, J.P.; Gauthey, L.; Herrmann, F.; Tkatch, L.; Michel, J.P. Community-acquired pneumonia in older patients. J. Am. Geriatr. Soc. 1996, 44, 539–544. [Google Scholar] [CrossRef]
- The British Thoracic Society Research Committee and The Public Health Laboratory Service. The aetiology, management and outcome of severe community-acquired pneumonia on the intensive care unit. Respir. Med. 1992, 86, 7–13. [Google Scholar] [CrossRef]
- Fine, M.J.; Stone, R.A.; Singer, D.E.; Coley, C.M.; Marrie, T.J.; Lave, J.R.; Hough, L.J.; Obrosky, D.S.; Schulz, R.; Ricci, E.M.; et al. Processes and outcomes of care for patients with community-acquired pneumonia: Results from the Pneumonia Patient Outcomes Research Team (PORT) cohort study. Arch Intern. Med. 1999, 159, 970–980. [Google Scholar] [CrossRef]
- Musher, D.M.; Alexandraki, I.; Graviss, E.A.; Yanbeiy, N.; Eid, A.; Inderias, L.A.; Phan, H.M.; Solomon, E. Bacteremic and nonbacteremic pneumococcal pneumonia. A prospective study. Medicine 2000, 79, 210–221. [Google Scholar] [CrossRef]
- Fernández-Sabé, N.; Carratalà, J.; Rosón, B.; Dorca, J.; Verdaguer, R.; Manresa, F.; Gudiol, F. Community-acquired pneumonia in very elderly patients: Causative organisms, clinical characteristics, and outcomes. Medicine 2003, 82, 159–169. [Google Scholar] [CrossRef]
- Fine, M.J.; Stone, R.A.; Lave, J.R.; Hough, L.J.; Obrosky, D.S.; Mor, M.K.; Kapoor, W.N. Implementation of an evidence-based guideline to reduce duration of intravenous antibiotic therapy and length of stay for patients hospitalized with community-acquired pneumonia: A randomized controlled trial. Am. J. Med. 2003, 115, 343–351. [Google Scholar] [CrossRef]
- Martínez-Moragón, E.; García Ferrer, L.; Serra Sanchis, B.; Fernández Fabrellas, E.; Gómez Belda, A.; Julve Pardo, R. La neumonía adquirida en la comunidad de los ancianos: Diferencias entre los que viven en residencias y en domicilios particulares [Community-acquired pneumonia among the elderly: Differences between patients living at home and in nursing homes]. Arch. Bronconeumol. 2004, 40, 547–552. (In Spanish) [Google Scholar] [CrossRef]
- Menéndez, R.; Torres, A.; Zalacaín, R.; Aspa, J.; Martín-Villasclaras, J.J.; Borderías, L.; Benítez-Moya, J.M.; Ruiz-Manzano, J.; de Castro, F.R.; Blanquer, J.; et al. Guidelines for the treatment of community-acquired pneumonia: Predictors of adherence and outcome. Am. J. Respir. Crit. Care Med. 2005, 172, 757–762. [Google Scholar] [CrossRef]
- Querol-Ribelles, J.M.; Tenías, J.M.; Querol-Borrás, J.M.; Labrador, T.; Nieto, A.; González-Granda, D.; Martínez, I. Levofloxacin versus ceftriaxone plus clarithromycin in the treatment of adults with community-acquired pneumonia requiring hospitalization. Int. J. Antimicrob. Agents 2005, 25, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Alvarez, M.; Callejas, C.; Rosso, R.; Schnettler, K.; Saldías, F. Cuadro clínico y factores pronósticos de la neumonía adquirida en la comunidad grave en adultos hospitalizados en la unidad de cuidados intensivos [Clinical picture and prognostic factors for severe community-acquired pneumonia in adults admitted to the intensive care unit]. Arch. Bronconeumol. 2005, 41, 20–26. (In Spanish) [Google Scholar] [CrossRef]
- Marrie, T.J.; Huang, J.Q. Low-risk patients admitted with community-acquired pneumonia. Am. J. Med. 2005, 118, 1357–1363. [Google Scholar] [CrossRef]
- McAlister, F.A.; Majumdar, S.R.; Blitz, S.; Rowe, B.H.; Romney, J.; Marrie, T.J. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005, 28, 810–815. [Google Scholar] [CrossRef]
- Becker, T.; Moldoveanu, A.; Cukierman, T.; Gerstein, H.C. Clinical outcomes associated with the use of subcutaneous insulin-by-glucose sliding scales to manage hyperglycemia in hospitalized patients with pneumonia. Diabetes Res. Clin. Pract. 2007, 78, 392–397. [Google Scholar] [CrossRef]
- Cabré, M.; Serra-Prat, M.; Force, L.; Palomera, E.; Pallarés, R. Functional status as a risk factor for mortality in very elderly patients with pneumonia. Med. Clin. 2008, 131, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Chalmers, J.D.; Choudhury, G.; Akram, A.R.; Hill, A.T. Vascular complications are associated with poor outcome in community-acquired pneumonia. QJM 2011, 104, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.W.; Pugh, M.J.V.; Waterer, G.W.; Nakashima, B.; Orihuela, C.J.; Copeland, L.A.; Restrepo, M.I.; Anzueto, A.; Mortensen, E.M. Incidence of cardiovascular events after hospital admission for pneumonia. Am. J. Med. 2011, 124, 244–251. [Google Scholar] [CrossRef]
- Griffin, A.T.; Wiemken, T.L.; Arnold, F.W. Risk factors for cardiovascular events in hospitalized patients with community-acquired pneumonia. Int. J. Infect. Dis. 2013, 17, e1125–e1129. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, S.; Ramirez, J.; Cosentini, R.; Valenti, V.; Voza, A.; Rossi, P.; Stolz, D.; Legnani, D.; Pesci, A.; Richeldi, L.; et al. Acute myocardial infarction versus other cardiovascular events in community-acquired pneumonia. ERJ Open Res. 2015, 1, 00020–02015. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Calvieri, C.; Falcone, M.; Bucci, T.; Bertazzoni, G.; Scarpellini, M.G.; Barillà, F.; Taliani, G.; Violi, F.; Battaglia, S.; et al. Relation of Cardiac Complications in the Early Phase of Community-Acquired Pneumonia to Long-Term Mortality and Cardiovascular Events. Am. J. Cardiol. 2015, 116, 647–651. [Google Scholar] [CrossRef]
- Chen, P.C.; Liao, W.I.; Wang, Y.C.; Chang, W.C.; Hsu, C.W.; Chen, Y.H.; Tsai, S.H. An Elevated Glycemic Gap is Associated With Adverse Outcomes in Diabetic Patients With Community-Acquired Pneumonia. Medicine 2015, 94, e1456. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Falcone, M.; Taliani, G.; Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Venditti, M.; Chirinos, J.A.; Corrales-Medina, V.F.; et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 64, 1486–1493. [Google Scholar] [CrossRef]
- Eurich, D.T.; Marrie, T.J.; Minhas-Sandhu, J.K.; Majumdar, S.R. Risk of heart failure after community acquired pneumonia: Prospective controlled study with 10 years of follow-up. BMJ 2017, 356, j413. [Google Scholar] [CrossRef]
- Cilli, A.; Cakin, O.; Aksoy, E.; Kargin, F.; Adiguzel, N.; Karakurt, Z.; Ergan, B.; Mersin, S.; Bozkurt, S.; Ciftci, F.; et al. Acute cardiac events in severe community-acquired pneumonia: A multicenter study. Clin. Respir. J. 2018, 12, 2212–2219. [Google Scholar] [CrossRef]
- Postma, D.F.; Spitoni, C.; van Werkhoven, C.H.; van Elden, L.J.R.; Oosterheert, J.J.; Bonten, M.J.M. Cardiac events after macrolides or fluoroquinolones in patients hospitalized for community-acquired pneumonia: Post-hoc analysis of a cluster-randomized trial. BMC Infect. Dis. 2019, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, F.; Biondo, B.; Vannucchi, V.; Falcone, M.; Antonielli, E.; De Marzi, G.; Casati, C.; Maddaluni, L.; Nozzoli, C.; Olivotto, I. Performance of the CHA2DS2-VASc score in predicting new onset atrial fibrillation during hospitalization for community-acquired pneumonia. Eur. J. Intern. Med. 2019, 62, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Calvieri, C.; Taliani, G.; Pignatelli, P.; Morelli, S.; Falcone, M.; Pastori, D.; Violi, F.; SIXTUS Study Group. Left Atrium Dilatation and Left Ventricular Hypertrophy Predispose to Atrial Fibrillation in Patients With Community-Acquired Pneumonia. Am. J. Cardiol. 2019, 124, 723–728. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Taljaard, M.; Yende, S.; Kronmal, R.; Dwivedi, G.; Newman, A.B.; Elkind, M.S.; Lyles, M.F.; Chirinos, J.A. Intermediate and long-term risk of new-onset heart failure after hospitalization for pneumonia in elderly adults. Am. Heart J. 2015, 170, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Corrales-Medina, V.F.; Taljaard, M.; Fine, M.J.; Dwivedi, G.; Perry, J.J.; Musher, D.M.; Chirinos, J.A. Risk stratification for cardiac complications in patients hospitalized for community-acquired pneumonia. Mayo Clin. Proc. 2014, 89, 60–68. [Google Scholar] [CrossRef]
- Fonseca, A.; Sá Marques, M.; Silva, E.; Shiang, T.; Vanzeller, M.; Ribeiro, C. Community acquired pneumonia: An increased risk for subsequent cardiovascular events? Eur. Respir. J. 2020, 56, 1775. [Google Scholar] [CrossRef]
- Meier, C.R.; Jick, S.S.; Derby, L.E.; Vasilakis, C.; Jick, H. Acute respiratory-tract infections and risk of first-time acute myocardial infarction. Lancet 1998, 351, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.C.; Thompson, M.; Meade, T.W. Recent respiratory infection and risk of cardiovascular disease: Case-control study through a general practice database. Eur. Heart J. 2008, 29, 96–103. [Google Scholar] [CrossRef]
- Nuñez-Delgado, R.D.P.; Tapia-Pérez, R.F.; Cachicatari-Vargas, E.; Chirinos-Lazo, R.M. Neumonía adquirida en la comunidad como factor de riesgo para enfermedades cardiovasculares. Rev. Cuerpo Med. HNAAA 2022, 15, 35–41. [Google Scholar] [CrossRef]
- Wang, C.-C.; Peng, C.-L.; Wang, G.-J.; Sung, F.-C.; Kao, C.-H. Pneumococcal pneumonia and the risk of acute coronary syndrome: A population-based cohort study. Int. J. Cardiol. 2013, 168, 4480–4481. [Google Scholar] [CrossRef]
- Koivula, I.; Stén, M.; Mäkelä, P.H. Prognosis after community-acquired pneumonia in the elderly: A population-based 12-year follow-up study. Arch. Intern. Med. 1999, 159, 1550–1555. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C. Inflammatory Markers at Hospital Discharge Predict Subsequent Mortality after Pneumonia and Sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-S.; Hsu, W.-H.; Lin, C.-L.; Kao, C.-H. Mycoplasma pneumonia increases the risk of acute coronary syndrome: A nationwide population-based cohort study. Qjm Int. J. Med. 2015, 108, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; Chen, H.-P.; Huang, Y.-S.; Huang, K.-Y.; Chou, P.; Lee, C.-C. Pneumococcal Pneumonia and the Risk of Stroke: A Population-Based Follow-Up Study. PLoS ONE 2012, 7, e51452. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Serpa, J.; Rueda, A.M.; Giordano, T.P.; Bozkurt, B.; Madjid, M.; Tweardy, D.; Musher, D.M. Acute Bacterial Pneumonia is Associated With the Occurrence of Acute Coronary Syndromes. Medicine 2009, 88, 154–159. [Google Scholar] [CrossRef]
- O’Meara, E.S.; White, M.; Siscovick, D.S.; Lyles, M.F.; Kuller, L.H. Hospitalization for Pneumonia in the Cardiovascular Health Study: Incidence, Mortality, and Influence on Longer-Term Survival. J. Am. Geriatr. Soc. 2005, 53, 1108–1116. [Google Scholar] [CrossRef]
- Baskaran, V.; Quammie, S.; Lawrence, H.; Ashton, D.; Lim, W.S.; McKeever, T. Meta-analysis of Acute Coronary Syndrome in Patients with Community-Acquired Pneumonia. Eur. Respir. J. 2020, 56 (Suppl. 64), 1778. [Google Scholar] [CrossRef]
- Haynes, R.B.; Devereaux, P.; Guyatt, G.H. Clinical expertise in the era of evidence-based medicine and patient choice. ACP J. Club 2002, 136, A11–A14. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Jager, K.; Zoccali, C.; MacLeod, A.; Dekker, F. Confounding: What it is and how to deal with it. Kidney Int. 2008, 73, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, J.; Gribsholt, S.B.; Gammelager, H.; Dekkers, O.; Luta, G. Control of confounding in the analysis pase—An overview for clinicians. Clin. Epidemiol. 2017, 9, 195–204. [Google Scholar] [CrossRef]
- Anderson, R.; Feldman, C. Review manuscript: Mechanisms of platelet activation by the pneumococcus and the role of platelets in community-acquired pneumonia. J. Infect. 2017, 75, 473–485. [Google Scholar] [CrossRef]
- Anderson, R.; Nel, J.G.; Feldman, C. Multifaceted Role of Pneumolysin in the Pathogenesis of Myocardial Injury in Community-Acquired Pneumonia. Int. J. Mol. Sci. 2018, 19, 1147. [Google Scholar] [CrossRef]
- Arroyo, A.B.; Fernández-Pérez, M.P.; del Monte, A.; Águila, S.; Méndez, R.; Hernández-Antolín, R.; García-Barber, N.; Reyes-García, A.M.D.L.; González-Jiménez, P.; Arcas, M.I.; et al. miR-146a is a pivotal regulator of neutrophil extracellular trap formation promoting thrombosis. Haematologica 2021, 106, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Brissac, T.; Shenoy, A.T.; Patterson, L.A.; Orihuela, C.J. Cell Invasion and Pyruvate Oxidase-Derived H2O2 are Critical for Streptococcus pneumoniae-Mediated Cardiomyocyte Killing. Infect. Immun. 2017, 86, e00569-17. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Pignatelli, P.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Falcone, M.; Taliani, G.; Violi, F.; Battaglia, S.; Bertazzoni, G.; et al. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J. Infect. 2016, 73, 107–114. [Google Scholar] [CrossRef]
- Feldman, C.; Normark, S.; Normark, B.H.; Anderson, R. Pathogenesis and prevention of risk of cardiovascular events in patients with pneumococcal community-acquired pneumonia. J. Intern. Med. 2019, 285, 635–652. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9780470743386 (accessed on 1 February 2023).
- Lin, L.; Chu, H. Meta-analysis of Proportions Using Generalized Linear Mixed Models. Epidemiology 2020, 31, 713–717. [Google Scholar] [CrossRef]
- Barker, T.H.; Migliavaca, C.B.; Stein, C.; Colpani, V.; Falavigna, M.; Aromataris, E.; Munn, Z. Conducting proportional meta-analysis in different types of systematic reviews: A guide for synthesisers of evidence. BMC Med. Res. Methodol. 2021, 21, 189. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int. J. Evid. -Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Sharp, S.J. Explaining heterogeneity in meta-analysis: A comparison of methods. Stat. Med. 1999, 18, 2693–2708. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Patsopoulos, N.; Evangelou, E. Heterogeneity in Meta-Analyses of Genome-Wide Association Investigations. PLoS ONE 2007, 2, e841. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).