Impact of Water Exposure and Temperature Changes on Skin Barrier Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
- Inclusion criteria: Healthy subjects, aged between 18 and 65 years who signed the informed consent form.
- Exclusion criteria: Subjects who did not sign the informed consent, subjects with an inflammatory skin disease (psoriasis, hidradenitis, atopic dermatitis) or another type of disease that may alter the epidermal barrier function and skin homeostasis; subjects receiving any topical, physical or systemic treatment which may alter the epidermal barrier function and skin homeostasis.
2.3. Study Variables Measurement
2.4. Water and Temperature Exposure
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Participants’ Characteristics
3.2. Changes in Skin Barrier Function after Water Exposure
3.3. Changes in Skin Barrier Function after Direct Contact with Different Temperatures
3.4. Differences in Skin Barrier Changes between Sexes and Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, R.A.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [Green Version]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Rosso, J.D.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J. Clin. Aesthet. Dermatol. 2016, 9, S2–S8. [Google Scholar] [PubMed]
- Elias, P.M.; Choi, E.H. Interactions among stratum corneum defensive functions. Exp. Dermatol. 2005, 14, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Kendziora, B.; Guertler, A.; Ständer, L.; Frey, S.; French, L.E.; Wollenberg, A.; Reinholz, M. Evaluation of hand hygiene and onset of hand eczema after the outbreak of SARS-CoV-2 in Munich. Eur. J. Dermatol. 2020, 30, 668–673. [Google Scholar] [CrossRef]
- Hirose, R.; Ikegaya, H.; Naito, Y.; Watanabe, N.; Yoshida, T.; Bandou, R.; Daidoji, T.; Itoh, Y.; Nakaya, T. Survival of SARS-CoV-2 and influenza virus on the human skin: Importance of hand hygiene in COVID-19. Clin. Infect. Dis. 2021, 73, e4329–e4335. [Google Scholar] [CrossRef]
- Araghi, F.; Tabary, M.; Gheisari, M.; Abdollahimajd, F.; Dadkhahfar, S. Hand Hygiene among Health Care Workers during COVID-19 Pandemic: Challenges and Recommendations. Dermatitis 2020, 31, 233–237. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Xue, X.; Mi, Z.; Wang, Z.; Pang, Z.; Liu, H.; Zhang, F. High expression of ACE2 on the keratinocytes reveals skin as a potential target for SARS-CoV-2. J. Investig. Dermatol. 2021, 141, 206–209.e1. [Google Scholar] [CrossRef] [PubMed]
- Al-Benna, S. Gene Expression of Angiotensin-Converting Enzyme 2 Receptor in Skin and the Implications for COVID-19. Adv. Skin Wound Care 2021, 34, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Cuenca-Barrales, C.; Martinez-Lopez, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin adverse events related to personal protective equipment: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1994–2006. [Google Scholar] [CrossRef] [PubMed]
- Michaels, B.; Gangar, V.; Schultz, A.; Arenas, M.; Curiale, M.; Ayers, T.; Paulson, D. Water temperature as a factor in handwashing efficacy. Food Serv. Technol. 2002, 2, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Carrico, A.; Spoden, M.; Wallston, K.A.; Vandenbergh, M.P. The environmental cost of misinformation: Why the recommendation to use elevated temperatures for handwashing is problematic. Int. J. Consum. Stud. 2013, 37, 433–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Park, J.W.; Yeon, Y.; Han, J.Y.; Kim, E. Influence of exposure to summer environments on skin properties. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2192–2196. [Google Scholar] [CrossRef]
- Jensen, D.A.; Macinga, D.R.; Shumaker, D.J.; Bellino, R.; Arbogast, J.W.; Schaffner, D.W. Quantifying the Effects of Water Temperature, Soap Volume, Lather Time, and Antimicrobial Soap as Variables in the Removal of Escherichia coli ATCC 11229 from Hands. J. Food Prot. 2017, 80, 1022–1031. [Google Scholar] [CrossRef]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737. [Google Scholar] [CrossRef]
- Schoepke, N.; Doumoulakis, G.; Maurer, M. Diagnosis of urticaria. Indian J. Dermatol. 2013, 58, 211–218. [Google Scholar]
- Kulthanan, K.; Tuchinda, P.; Chularojanamontri, L.; Kiratiwongwan, R. Cold Urticaria: Clinical Features and Natural Course in a Tropical Country. Allergy Asthma Immunol. Res. 2019, 11, 538–547. [Google Scholar] [CrossRef]
- Rodrigues-Valle, S.; Azizi, G.; Duarte-Dortas, S., Jr. TempTest(R): Un instrumento de precision en las urticarias fisicas. Rev. Alerg. Mex. 2021, 68, 2–6. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, X.; Zhao, J.; Yan, S.; Liu, Q.; Zhao, H. The Interaction of Age and Anatomical Region Influenced Skin Biophysical Characteristics of Chinese Women. Clin. Cosmet. Investig. Dermatol. 2020, 13, 911–926. [Google Scholar] [CrossRef]
- Warner, R.R.; Stone, K.J.; Boissy, Y.L. Hydration Disrupts Human Stratum Corneum Ultrastructure. J. Investig. Dermatol. 2003, 120, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firooz, A.; Aghazadeh, N.; Estarabadi, A.R.; Hejazi, P. The effects of water exposure on biophysical properties of normal skin. Skin Res. Technol. 2014, 21, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Fuse, C.; Morisaki, N.; Shima, K.; Hotta, M.; Sugata, K.; Ichihashi, T.; Oguri, M.; Yoshida, O.; Fujimura, T. Impact of water exposure on skin barrier permeability and ultrastructure. Contact Dermat. 2018, 80, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Cravello, B.; Ferri, A. Relationships between skin properties and environmental parameters. Skin Res. Technol. 2008, 14, 180–186. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.-W.; Kim, Y.-C.; Prausnitz, M.R. The effect of heat on skin permeability. Int. J. Pharm. 2008, 359, 94–103. [Google Scholar] [CrossRef]
- Plum, F.; Yüksel, Y.T.; Agner, T.; Nørreslet, L.B. Skin barrier function after repeated short-term application of alcohol-based hand rub following intervention with water immersion or occlusion. Contact Dermat. 2020, 83, 215–219. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Martinez-Lopez, A.; Cuenca-Barrales, C.; Rodriguez-Tejero, A.; Molina-Leyva, A.; Arias-Santiago, S. Impact of Gloves and Mask Use on Epidermal Barrier Function in Health Care Workers. Dermatitis 2021, 32, 57–62. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Lavender, T.; Jenerowicz, D.; Ryumina, I.; Stalder, J.; Torrelo, A.; Cork, M. Recommendations from a European Roundtable Meeting on Best Practice Healthy Infant Skin Care. Pediatr. Dermatol. 2016, 33, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Hoeger, P.H.; Enzmann, C.C. Skin Physiology of the Neonate and Young Infant: A Prospective Study of Functional Skin Parameters during Early Infancy. Pediatr. Dermatol. 2002, 19, 256–262. [Google Scholar] [CrossRef]
- Agner, T.; Serup, J. Time course of occlusive effects on skin evaluated by measurement of transepidermal water loss (TEWL) Including patch tests with sodium lauryl sulphate and water. Contact Dermat. 1993, 28, 6–9. [Google Scholar] [CrossRef]
- Jungersted, J.M.; Høgh, J.K.; Hellgren, L.I.; Jemec, G.B.E.; Agner, T. Skin barrier response to occlusion of healthy and irritated skin: Differences in trans-epidermal water loss, erythema and stratum corneum lipids. Contact Dermat. 2010, 63, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. A personal critique of diagnostic patch testing. Clin. Dermatol. 1996, 14, 35–40. [Google Scholar] [CrossRef]
- Qiu, H.; Long, X.; Ye, J.C.; Hou, J.; Senee, J.; Laurent, A.; Bazin, R.; Flament, F.; Adam, A.; Coutet, J.; et al. Influence of season on some skin properties: Winter vs. summer, as experienced by 354 Shanghaiese women of various ages. Int. J. Cosmet. Sci. 2011, 33, 377–383. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.; Rossi, R. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Waller, J.M.; Maibach, H.I. Age and skin structure and function, a quantitative approach (I): Blood flow, pH, thickness, and ultrasound echogenicity. Skin Res. Technol. 2005, 11, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Goodier, M.; Dekoven, J.G.; Maibach, H.I.; Taylor, J.S.; Sasseville, D.; Belsito, D.V.; Fowler, J.F.; Fransway, A.F.; DeLeo, V.A.; et al. Contact Dermatitis Associated With Skin Cleansers: Retrospective Analysis of North American Contact Dermatitis Group Data 2000–2014. Dermatitis 2018, 29, 32–42. [Google Scholar] [CrossRef]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.; De Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Raposo, I.; Torres, T. Palmoplantar Psoriasis and Palmoplantar Pustulosis: Current Treatment and Future Prospects. Am. J. Clin. Dermatol. 2016, 17, 349–358. [Google Scholar] [CrossRef]
- Carøe, T.K.; Ebbehøj, N.E.; Bonde, J.P.E.; Flachs, E.M.; Agner, T. Hand eczema and wet work: Dose-response relationship and effect of leaving the profession. Contact Dermat. 2018, 78, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Ramsing, D.W.; Agner, T. Effect of water on experimentally irritated human skin. Br. J. Dermatol. 1997, 136, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Ohlenschlaeger, J.; Friberg, J.; Ramsing, D.; Agner, T. Temperature dependency of skin susceptibility to water and detergents. Acta Derm. Venereol. 1996, 76, 274–276. [Google Scholar] [PubMed]
- Espinosa-Rueda, M.I.; Montero-Vilchez, T.; Martinez-Lopez, A.; Molina-Leyva, A.; Sierra-Sánchez, A.; Arias-Santiago, S.; Buendia-Eisman, A. Cutaneous homeostasis and epidermal barrier function in a young healthy Caucasian population. Eur. J. Dermatol. 2021, 31, 176–182. [Google Scholar] [CrossRef]
- Ya-Xian, Z.; Suetake, T.; Tagami, H. Number of cell layers of the stratum corneum in normal skin—Relationship to the anatomical location on the body, age, sex and physical parameters. Arch. Dermatol Res. 1999, 291, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Tagami, H. Location-related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Wa, C.V.; Maibach, H.I. Mapping the human face: Biophysical properties. Skin Res. Technol. 2010, 16, 38–54. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care Provide Health-Care Workers (HCWs). 2009. Available online: https://www.who.int/publications/i/item/9789241597906 (accessed on 15 May 2021).
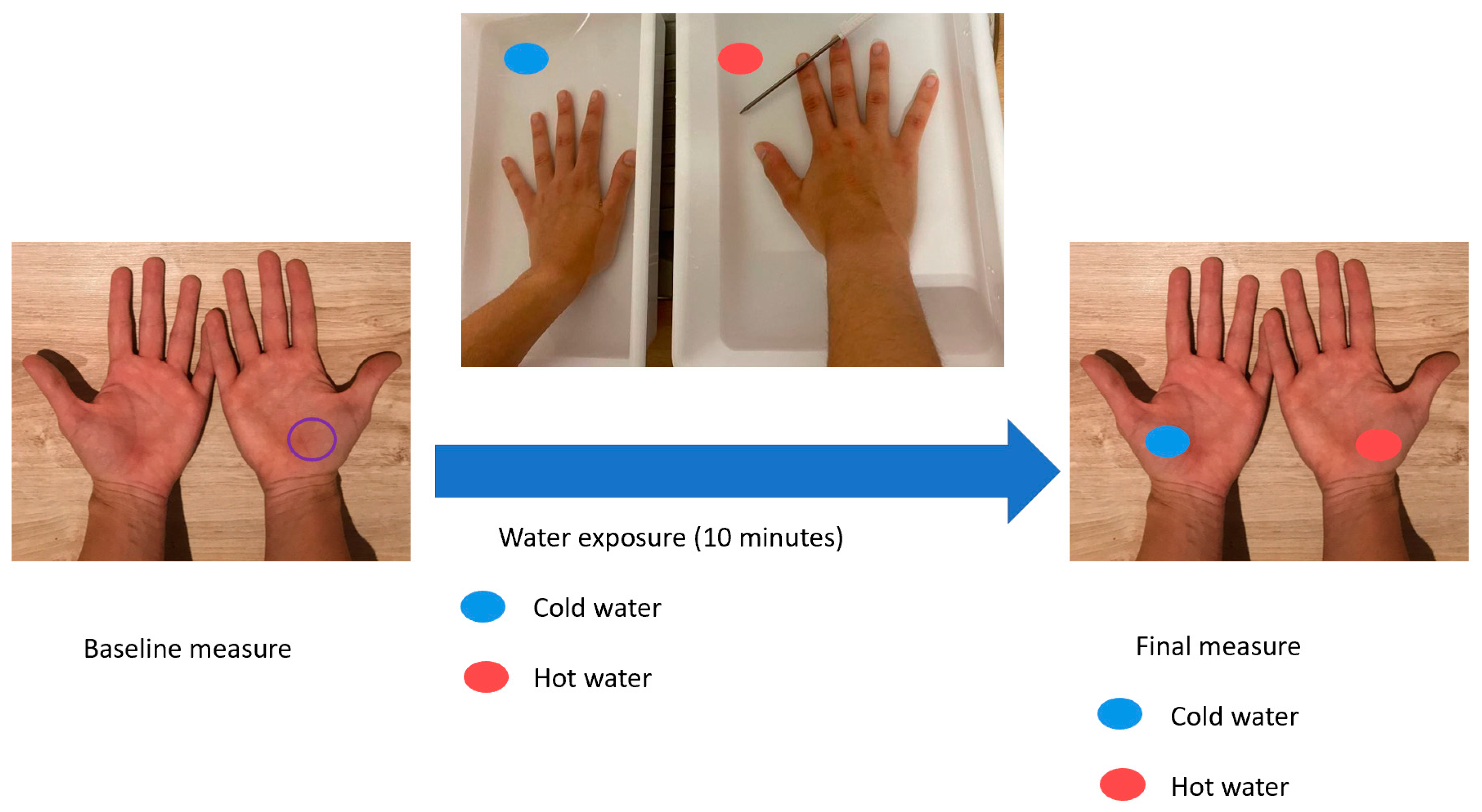
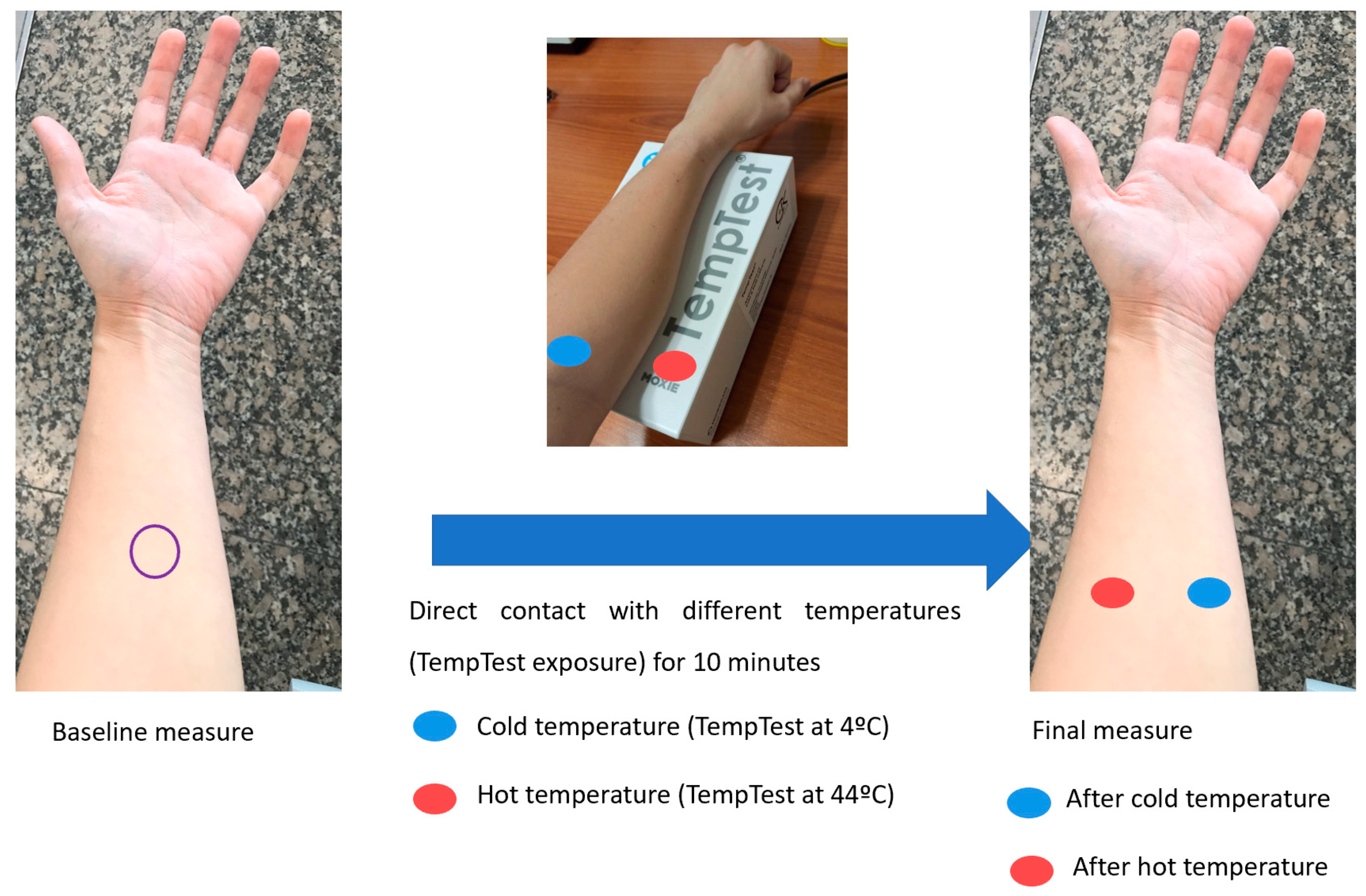

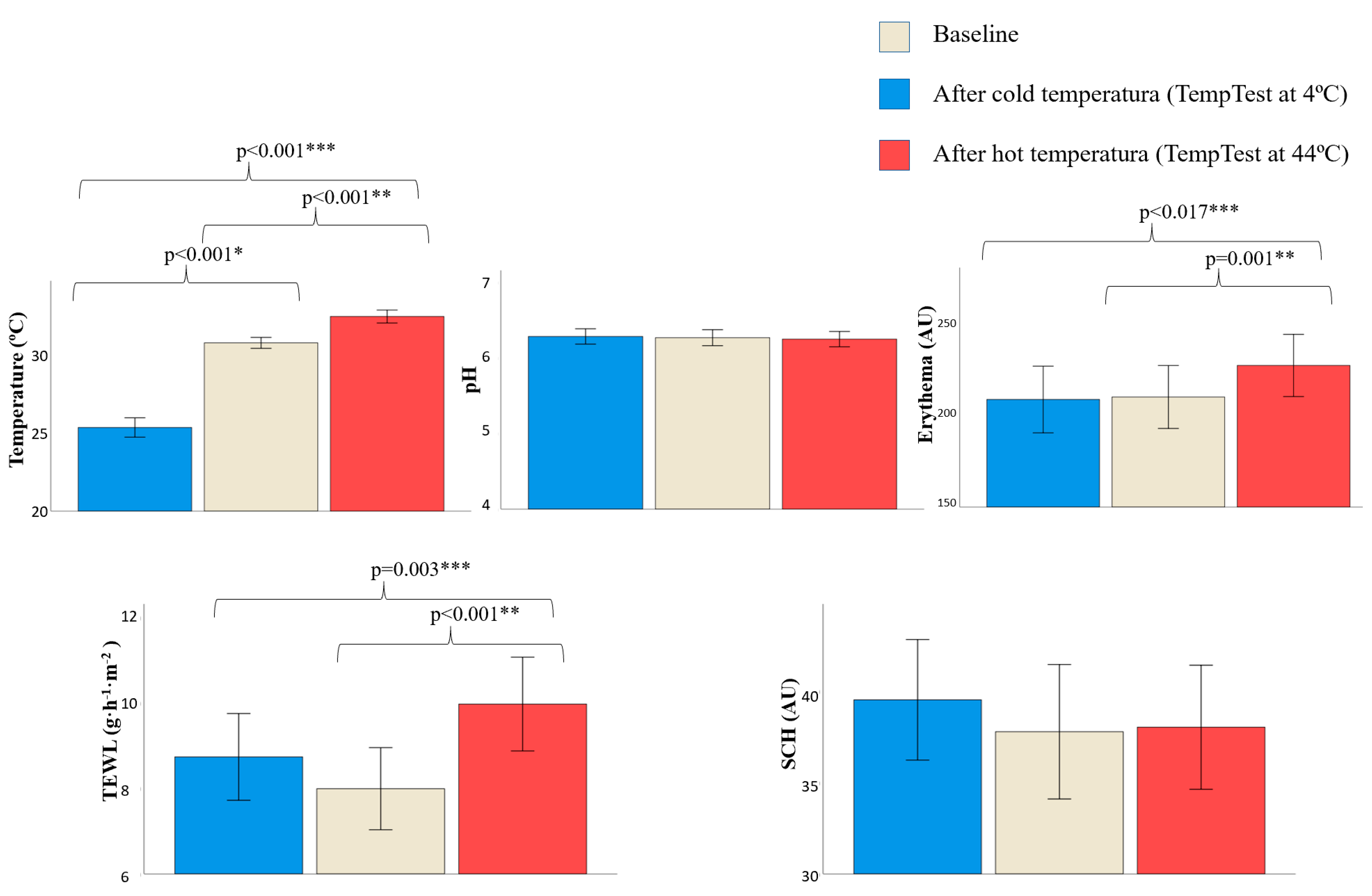
| Sociodemographic Features | Study Population (N = 50) |
|---|---|
| Age (years) | 33.3 (12.94) |
| Gender | |
| Male | 20 (40%) |
| Female | 30 (60%) |
| Phototype | |
| I | 1 (2%) |
| II | 14 (28%) |
| III | 29 (58%) |
| IV | 5 (10%) |
| VI | 1 (2%) |
| Smoking habit (yes) | 4 (8%) |
| Cigarettes per day | 7.25 (3.78) |
| Residence | |
| City | 45 (90%) |
| Country | 5 (10%) |
| Regular moisturizing | 34 (68%) |
| Regular sunlight exposure | 4 (8%) |
| Sun protection | |
| Always | 21 (42%) |
| Sometimes | 20 (40%) |
| Never | 9 (18%) |
| Occupational category | |
| Doctors | 23 (46%) |
| Nurses | 18 (36%) |
| Miscellaneous | 9 (18%) |
| Men (n = 20) | Women (n = 30) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water Exposure | |||||||||
| Basal | Change after Cold Water | Change after Hot Water | Basal | Change after Cold Water | Change after Hot Water | pB | pC | pH | |
| Temperature (°C) | 30.39 (2.04) | −5.78 (0.51) | +2.31 (0.30) | +30.03 (1.71) | −7.29 (0.37) | +2.50 (0.26) | 0.523 | 0.020 | 0.647 |
| pH | 6.30 (0.43) | +0.37 (0.37) | +0.41 (0.10) | +6.35 (0.34) | +0.23 (0.06) | 0.26 (0.06) | 0.645 | 0.165 | 0.215 |
| Erythema (AU) | 278.08 (46.99) | +10.27 (10.75) | +31.14 (10.42) | +230.37 (56.12) | +0.12 (11.31) | +40.71 (9.82) | 0.003 | 0.539 | 0.519 |
| TEWL (g·h−1·m−2) | 24.35 (10.88) | +12.21 (2.45) | +33.30 (2.36) | 26.68 (13.41) | +7.21 (2.30) | +32.51 (2.32) | 0.521 | 0.154 | 0.814 |
| SCH (AU) | 49.54 (16.51) | −1.96 (2.51) | −3.21 (2.69) | 44.80 (20.13) | +7.74 (2.31) | −1.50 (3.22) | 0.386 | 0.008 | 0.685 |
| TempTest Exposure | |||||||||
| Basal | Change after 4 °C TempTest | Change after 44 °C TempTest | Basal | Change after 4 °C TempTest | Change after 44 °C TempTest | pBt | pCt | pHt | |
| Temperature (°C) | 31.20 (1.24) | −5.96 (0.56) | +1.58 (0.28) | 30.47 (1.16) | −5.06 (0.34) | +1.76 (0.23) | 0.038 | 0.178 | 0.624 |
| pH | 6.25 (0.39) | +0.04 (0.03) | −0.03 (0.03) | 6.28 (0.37) | −0.01 (0.03) | −0.02 (0.03) | 0.787 | 0.275 | 0.830 |
| Erythema (AU) | 239.19 (63.79) | +7.68 (12.94) | +13.41 (7.16) | 191.30 (51.39) | −7.41 (8.14) | +19.95 (6.51) | 0.008 | 0.303 | 0.512 |
| TEWL (g·h−1·m−2) | 7.01 (3.29) | +1.79 (0.87) | +2.74 (0.07) | 8.65 (3.34) | +0.05 (0.58) | +1.48 (0.67) | 0.092 | 0.089 | 0.216 |
| SCH (AU) | 34.21 (10.67) | −1.69 (1.65) | +1.99 (1.47) | 40.14 (13.91) | +1.58 (1.30) | +1.53 (1.79) | 0.095 | 0.841 | 0.216 |
| Age < 30 (n = 28) | Age ≥ 30 (n = 22) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Water Exposure | |||||||||
| Basal | Cold Water | Hot Water | Basal | Cold Water | Hot Water | pB | pC | pH | |
| Temperature (°C) | 30.02 (1.80) | −5.99 (0.41) | +2.60 (0.26) | 30.37 (1.90) | −7.57 (0.44) | +2.20 (0.30) | 0.507 | 0.322 | 0.012 |
| pH | 6.29 (0.43) | +0.38 (0.07) | +0.39 (0.07) | 6.37 (0.30) | +0.18 (0.06) | +0.24 (0.08) | 0.406 | 0.153 | 0.040 |
| Erythema (AU) | 266.49 (40.45) | +2.66 (8.33) | +35.18 (8.74) | 227.88 (68.29) | +6.11 (14.99) | +39.05 (12.16) | 0.026 | 0.792 | 0.833 |
| TEWL (g·h−1·m−2) | 25.36 (12.14) | +10.79 (2.06) | +33.75 (2.06) | 26.25 (12.99) | +7.20 (2.02) | +31.65 (2.63) | 0.804 | 0.528 | 0.302 |
| SCH (AU) | 53.62 (18.46) | +1.75 (2.52) | −5.91 (3.09) | 37.87 (15.33) | +6.55 (2.61) | +2.56 (2.85) | 0.002 | 0.055 | 0.196 |
| TempTest exposure | |||||||||
| Basal | 4 °C TempTest | 44 °C TempTest | Basal | 4 °C TempTest | 44 °C TempTest | pBt | pCt | pHt | |
| Temperature (°C) | 30.89 (1.12) | −5.68 (0.48) | +1.75 (0.25) | 30.59 (1.38) | −5.21 (0.39) | +1.64 (0.25) | 0.399 | 0.778 | 0.446 |
| pH | 6.25 (0.39) | −0.03 (0.03) | −0.02 (0.03) | 6.28 (0.36) | −0.05 (0.03) | −0.02 (0.04) | 0.803 | 0.936 | 0.065 |
| Erythema (AU) | 228.80 (59.38) | −5.10 (9.42) | +19.43 (7.66) | 187.10 (55.56) | +1.54 (10.45) | +15.69 (6.26) | 0.015 | 0.705 | 0.648 |
| TEWL (g·h−1·m−2) | 7.21 (2.39) | −0.08 (0.87) | +2.50 (0.82) | 8.99 (4.19) | +1.40 (0.57) | +1.57 (0.62) | 0.087 | 0.359 | 0.163 |
| SCH (AU) | 34.76 (10.53) | +1.20 (1.68) | +0.97 (2.04) | 41.59 (14.85) | +2.18 (1.13) | −0.32 (1.63) | 0.063 | 0.618 | 0.621 |
| Study | Number of Participants | Exposure | Results |
|---|---|---|---|
| Water Exposure | |||
| Firooz et al. 2015 [24] | 20 | Water immersion at 15–20 °C for 30 min a day for five days | TEWL increased pH increased |
| Agner et al. 1993 [32] | 14 | Water patch-occlusion during 24 h:
| TEWL increased immediately and 30 min after removal of all test chambers. TEWL also increased 180 min after SLS and water patch removal. |
| Our study | 50 | Cold water exposure (11.13 (2.71 SD) °C) for 10 min | Temperature decreased by 6.69 °C pH increased by 0.29 TEWL increased by 9.21 (g·h−1·m−2) SCH increased 3.86 AU |
| Hot water exposure (41.29 (2.29 SD) °C) for 10 min | Temperature increased by 2.42 °C pH increased by by 0.32 Erythema increased by 36.88 AU TEWL increased by 32.83 (g·h−1·m−2) | ||
| Temperature exposure | |||
| Kim et al. 2019 [16] | 20 | Exposure to outdoor environment for 90 min (34.76 ± 2.79 °C, 53.13 ± 8.78% RH) | SCH on the forearm and sebum secretion on the face increased. SCH and TEWL on the cheek and greasiness on the forearm and the forehead decreased. pH decreased in the face and the forearm. |
| Exposure to indoor environment for 90 min (22.97 ± 0.74 °C, 53.14 ± 2.37% RH) | SCH in the forehead and the forearm. Sebum secretion and greasiness on the face increased. TEWL and pH in the face and the forearm decreased. | ||
| Cravello et al. 2008 [26] | 6 | Exposure to three levels of ambient temperature (20 °C, 25 °C and 30 °C) and four levels of RH (25%, 45%, 65% and 85%) | TEWL is positively correlated to ambient temperature. Skin temperature is correlated positively to ambient temperature but not to RH. SCH is strongly affected by ambient temperature and RH. |
| Qiu et al. 2011 [35] | 354 | 6 month periods, summer (35–40 °C, RH ≥ 70%) and winter (0–5 °C, RH ≥ 70%) | SCH and melanin increased in summer compared to winter. |
| Our study | 50 | Cold temperatrue exposure (4 °C) | Temperature decreased by 5.41 °C |
| Hot temperature exposure (44 °C) | Temperatrure increased by 1.69 °C Erythema increased by 17.34 AU TEWL increased by 1.98 (g·h−1·m−2) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero-Fernandez, M.; Montero-Vilchez, T.; Diaz-Calvillo, P.; Romera-Vilchez, M.; Buendia-Eisman, A.; Arias-Santiago, S. Impact of Water Exposure and Temperature Changes on Skin Barrier Function. J. Clin. Med. 2022, 11, 298. https://doi.org/10.3390/jcm11020298
Herrero-Fernandez M, Montero-Vilchez T, Diaz-Calvillo P, Romera-Vilchez M, Buendia-Eisman A, Arias-Santiago S. Impact of Water Exposure and Temperature Changes on Skin Barrier Function. Journal of Clinical Medicine. 2022; 11(2):298. https://doi.org/10.3390/jcm11020298
Chicago/Turabian StyleHerrero-Fernandez, Manuel, Trinidad Montero-Vilchez, Pablo Diaz-Calvillo, Maria Romera-Vilchez, Agustin Buendia-Eisman, and Salvador Arias-Santiago. 2022. "Impact of Water Exposure and Temperature Changes on Skin Barrier Function" Journal of Clinical Medicine 11, no. 2: 298. https://doi.org/10.3390/jcm11020298
APA StyleHerrero-Fernandez, M., Montero-Vilchez, T., Diaz-Calvillo, P., Romera-Vilchez, M., Buendia-Eisman, A., & Arias-Santiago, S. (2022). Impact of Water Exposure and Temperature Changes on Skin Barrier Function. Journal of Clinical Medicine, 11(2), 298. https://doi.org/10.3390/jcm11020298








