Lichen Planus Activity and Damage Index (LiPADI)–Creation of the Questionnaire
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Study Design
2.3. Development of Lichen Planus Area and Damage Index
2.4. Statistics
3. Results
3.1. Lichen Planus Characteristics
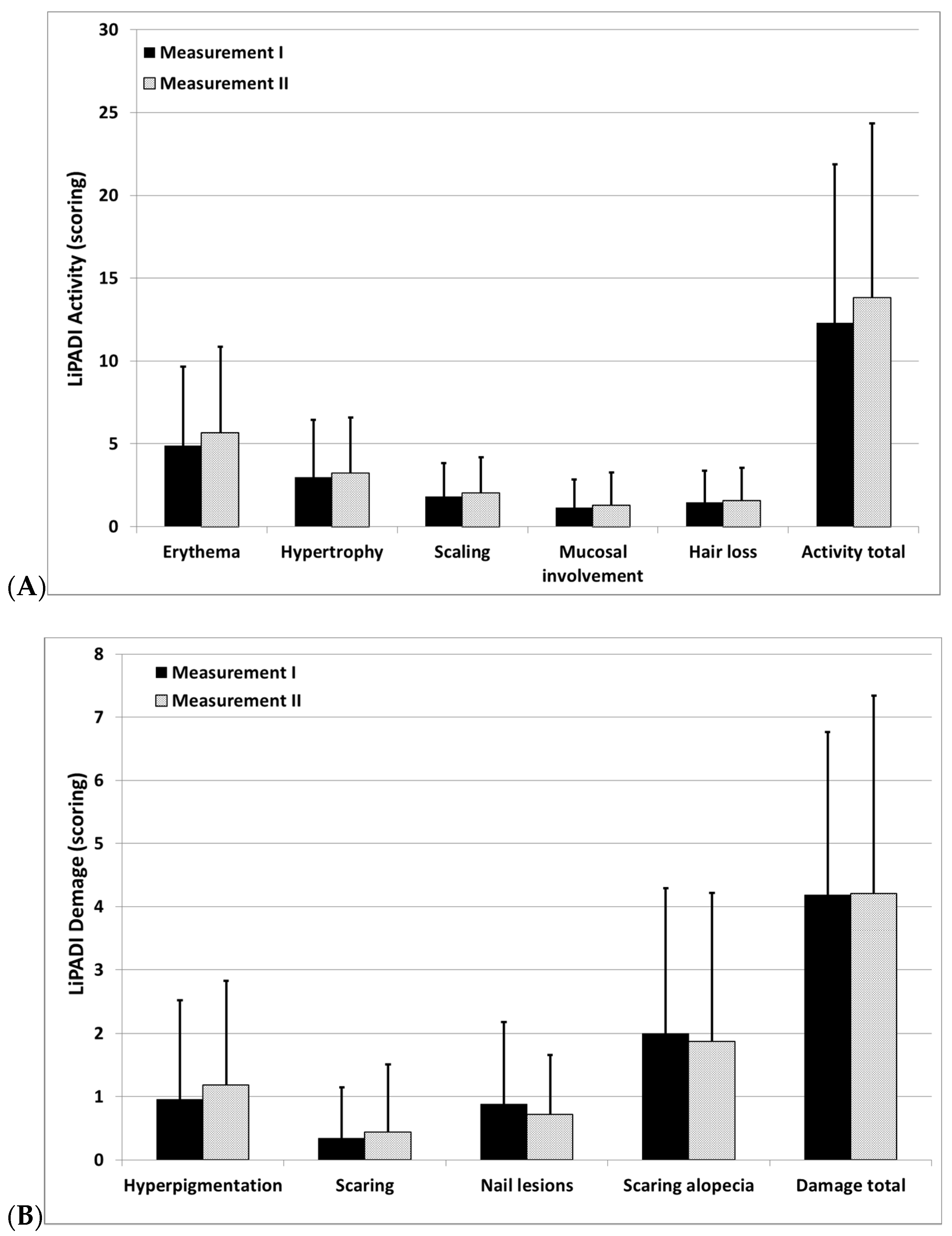
3.2. Distribution of LiPADI
3.3. Interrater Reliability and Internal Consistency of LiPADI
3.4. Discriminant and Convergent Validity of LiPADI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Lichen Planus Activity and Demage Index (LiPADI) Select the score in each anatomical location that describes the most severely affected lichen planus lesion Activity: ______________________ Damage: __________________ | ||||||
| Anatomical Location | Erythema | Hypertrophy | Scale | Hiperpigmentation | Scarring Atrophy | Anatomical Location |
| 0—absent 1—pink; faint erythema 2—red; 3—dark red; purple/violaceous | 0—absent; 1—elevation (<1 mm) 2—verrucous/hypertrophy (>1 mm) | 0—absent 1—present | 0—absent, 1—hyperpigmentation | 0—absent 1—scarring 2—severely atrophic scarring | ||
| Scalp |  | Scalp | ||||
| Face | Face | |||||
| Chest | Chest | |||||
| Abdomen | Abdomen | |||||
| Back, buttocks | Back, buttocks | |||||
| Arms | Arms | |||||
| Hands | Hands | |||||
| Legs | Legs | |||||
| Feet | Feet | |||||
| Mucous membrane lesions | Nail lesions | |||||
| 0—absent; 1—linear whitening, white papules 3—few erosions or ulcerations (max 3 lesions) 6—extensive erosions or ulcerations (more than 3 lesions or at least one lesion with a diameter of more than 5 cm) | 0—absent; 1—linear changes; 3—dystrophy | |||||
| Oral cavity | Fingernails | |||||
| Anogenital area | Toenails | |||||
| Hair: if scarring and non-scarring aspects coexist in one lesion, please score both | ||||||
| Recent Hair loss (within 30 days) | 0—No 1—Yes | |||||
| Scalp | ||||||
| Other areas (please score separately each location) | ||||||
| Divide the scalp into four quadrants as shown. The dividing line between right and left is the midline. The dividing line between frontal and occipital is the line connecting the highest points of the ear lobe. A quadrant is considered affected if there is a lesion within the quadrant. | ||||||
| Alopecia (clinically not obviously scarred) | Scarring of the scalp (judged clinically) | |||||
| 0—absent 1—diffuse; non-inflammatory 2—focal or patchy in one quadrant; 3—focal or patchy in more than one quadrant | 0—absent 3—in one quadrant 4—two quadrants 5—three quadrants 6—affects whole skull | |||||
References
- Gupta, S.; Jawanda, M. Oral Lichen Planus: An Update on Etiology, Pathogenesis, Clinical Presentation, Diagnosis and Management. Indian J. Dermatol. 2015, 60, 222–229. [Google Scholar] [CrossRef]
- Weston, G.; Payette, M. Update on Lichen Planus and Its Clinical Variants. Int. J. Womens Dermatol. 2015, 1, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhabra, S.; Saikia, U.; Dogra, S.; Minz, R.; Arora, S. Lichen Planus: A Clinical and Immuno-Histological Analysis. Indian J. Dermatol. 2014, 59, 257–261. [Google Scholar] [CrossRef]
- Errichetti, E.; Figini, M.; Croatto, M.; Stinco, G. Therapeutic Management of Classic Lichen Planopilaris: A Systematic Review. Clin. Cosmet. Investig. Dermatol. 2018, 11, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Lipner, S. Review of Nail Lichen Planus. Dermatol. Clin. 2021, 39, 221–230. [Google Scholar] [CrossRef]
- Khurana, A.; Tandon, S.; Marfatia, Y.; Madnani, N. Genital Lichen Planus: An Underrecognized Entity. Indian J. Sex. Transm. Dis. AIDS 2019, 40, 105–112. [Google Scholar] [CrossRef]
- Amsellem, J.; Skayem, C.; Duong, T.; Bagot, M.; Fouéré, S.; Dauendorffer, J. Male Genital Lichen Planus: A Retrospective Study of 89 Cases. Ann. Dermatol. Venereol. 2021, in press. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0151963821000569?via%3Dihub (accessed on 2 November 2021). [CrossRef]
- Bożek, A.; Reich, A. Assessment of Intra- and Inter-Rater Reliability of Three Methods for Measuring Atopic Dermatitis Severity: EASI, Objective SCORAD, and IGA. Dermatology 2017, 233, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Bożek, A.; Reich, A. The Reliability of Three Psoriasis Assessment Tools: Psoriasis Area and Severity Index, Body Surface Area and Physician Global Assessment. Adv. Clin. Exp. Med. 2017, 26, 851–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkachaisri, T.; Vilaiyuk, S.; Torok, K.S.; Medsger, T.A., Jr. Development and Initial Validation of the Localized Scleroderma Skin Damage Index and Physician Global Assessment of Disease Damage: A Proof-of-Concept Study. Rheumatology 2010, 49, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Stępień, K.; Reich, A. The 12-Item Pruritus Severity Scale—Determining the Severity Bands. Front. Med. 2020, 7, 614005. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Chatzigeorkidis, E.; Zeidler, C.; Osada, N.; Furue, M.; Takamori, K.; Ebata, T.; Augustin, M.; Szepietowski, J.C.; Ständer, S. Tailoring the Cut-Off Values of the Visual Analogue Scale and Numeric Rating Scale in Itch Assessment. Acta Derm. Venereol. 2017, 97, 759–760. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, J.; Werth, V. Development of the CLASI as an Outcome Instrument for Cutaneous Lupus Erythematosus. Dermatol. Ther. 2007, 20, 93–101. [Google Scholar] [CrossRef]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and Mucosal Lichen Planus: A Comprehensive Review of Clinical Subtypes, Risk Factors, Diagnosis, and Prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; van der Waal, I. Disease Scoring Systems for Oral Lichen Planus: A Critical Appraisal. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e199–e204. [Google Scholar] [CrossRef] [PubMed]
- Thongprasom, K.; Luangjarmekorn, L.; Sererat, T.; Taweesap, W. Relative Efficacy of Fluocinolone Acetonide Compared with Triamcinolone Acetonide in Treatment of Oral Lichen Planus. J. Oral Pathol. Med. 1992, 21, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Sah, D.; Cho, B.; Ochoa, B.; Price, V. Hydroxychloroquine and Lichen Planopilaris: Efficacy and Introduction of Lichen Planopilaris Activity Index Scoring System. J. Am. Acad. Dermatol. 2010, 62, 387–392. [Google Scholar] [CrossRef]
- Holmes, S.; Ryan, T.; Young, D.; Harries, M. Frontal Fibrosing Alopecia Severity Index (FFASI): A Validated Scoring System for Assessing Frontal Fibrosing Alopecia. Br. J. Dermatol. 2016, 175, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Saceda-Corralo, D.; Moreno-Arrones, Ó.; Fonda-Pascual, P.; Pindado-Ortega, C.; Buendía-Castaño, D.; Alegre-Sánchez, A.; Segurado-Miravalles, G.; Rodrigues-Barata, A.R.; Jaén-Olasolo, P.; Vaño-Galván, S. Development and Validation of the Frontal Fibrosing Alopecia Severity Score. J. Am. Acad. Dermatol. 2018, 78, 522–529. [Google Scholar] [CrossRef]
- Bishnoi, A.; Vinay, K.; Sendhil Kumaran, M.; Handa, S.; Parsad, D. Proposition of a Comprehensive Score to Assess the Disease Severity and Activity of Cutaneous Lichen Planus. Int. J. Dermatol. 2019, 58, e140–e142. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Nikam, B.; Jamale, V.; Kale, M. Lichen Planus Severity Index: A New, Valid Scoring System to Assess the Severity of Cutaneous Lichen Planus. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 169. [Google Scholar] [CrossRef] [PubMed]

| LiPADI | ||
|---|---|---|
| Activity | Damage | |
| DLQI | ρ = 0.38, p = 0.01 | ρ = −0.08, p = 0.63 |
| 12-PSS | ρ = 0.41, p = 0.01 | ρ = −0.17, p = 0.32 |
| EQ-VAS | ρ = −0.15, p = 0.34 | ρ = −0.08, p = 0.63 |
| NRS pruritus | ρ = 0.5, p = 0.001 | ρ = −0.08, p = 0.6 |
| NRS pain | ρ = 0.09, p = 0.6 | ρ = 0.1, p = 0.53 |
| Disease duration | ρ = −0.28, p = 0.06 | ρ = 0.3, p < 0.05 |
| Duration of exacerbation | ρ = −0.3, p < 0.05 | ρ = −0.02, p = 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, K.; Żabska, E.; Rahnama-Hezavah, M.; Reich, A. Lichen Planus Activity and Damage Index (LiPADI)–Creation of the Questionnaire. J. Clin. Med. 2022, 11, 23. https://doi.org/10.3390/jcm11010023
Stępień K, Żabska E, Rahnama-Hezavah M, Reich A. Lichen Planus Activity and Damage Index (LiPADI)–Creation of the Questionnaire. Journal of Clinical Medicine. 2022; 11(1):23. https://doi.org/10.3390/jcm11010023
Chicago/Turabian StyleStępień, Katarzyna, Ewa Żabska, Mansur Rahnama-Hezavah, and Adam Reich. 2022. "Lichen Planus Activity and Damage Index (LiPADI)–Creation of the Questionnaire" Journal of Clinical Medicine 11, no. 1: 23. https://doi.org/10.3390/jcm11010023
APA StyleStępień, K., Żabska, E., Rahnama-Hezavah, M., & Reich, A. (2022). Lichen Planus Activity and Damage Index (LiPADI)–Creation of the Questionnaire. Journal of Clinical Medicine, 11(1), 23. https://doi.org/10.3390/jcm11010023







