A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Selection
- (1)
- Age < 18 years, >80 years;
- (2)
- Coexisting comorbidities or medical conditions which are difficult to evaluate for pneumonia such as severe liver dysfunction, severe renal dysfunction or HIV/AIDS (severe liver dysfunction was defined as serum total bilirubin, or aspartate aminotransferase/alanine aminotransferase > the upper limit of the normal reference range × 3. Severe renal dysfunction was defined as creatinine clearance < 30 mL/min). Unassessable pulmonary diseases include viral pneumonia, pneumocystis pneumonia [19,20], mycobacterium infections, eosinophilic pneumonia and interstitial pneumonitis. Unassessable malignancies were defined as any malignancy terminated stage or the one with any metastatic lesion to the lungs and/or receiving palliative therapy. Unassessable diabetes mellitus was defined as serum-hemoglobin A1c (NGSP) ≥ 7.0%;
- (3)
- (4)
- Receiving immunosuppressive therapy due to any cause;
- (5)
- Receiving chemotherapy for malignancy;
- (6)
- Receiving hemodialysis due to any cause;
- (7)
- Poor activities of daily living (ADL) or requiring any help (Eastern Cooperative Oncology Group (ECOG) performance status (PS) ≥ 3) such as needing tube feeding or home oxygen therapy;
- (8)
- Having other complicated infection;
- (9)
- Requiring mechanical ventilation and/or requiring treatments in the intensive care unit;
- (10)
- Poor prognosis (anticipated life expectancy < 90 days or patients who are not expected to survive until the end of the trial);
- (11)
- Pregnancy.
2.3. Microbiological Evaluation
2.4. Definition of Appropriate and Inappropriate Treatment, Initial Treatment Failure
2.5. Statistical Analyses
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992, 268, 2420–2425. [Google Scholar] [CrossRef]
- Asai, N.; Sakanashi, D.; Suematsu, H.; Kato, H.; Hagihara, M.; Shiota, A.; Koizumi, Y.; Yamagishi, Y.; Mikamo, H. To What degree could clinical trials in Evidence Based Medicine reflect reality in the treatment of candidemia? J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community acquired pneumonia among adults in Europe. Thorax 2012, 67, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Asai, N.; Watanabe, H.; Shiota, A.; Kato, H.; Sakanashi, D.; Hagihara, M.; Koizumi, Y.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. Efficacy and accuracy of qSOFA and SOFA scores as prognostic tools for community-acquired and healthcare-associated pneumonia. Int. J. Infect. Dis. 2019, 84, 89–96. [Google Scholar] [CrossRef]
- Matsunuma, R.; Asai, N.; Ohkuni, Y.; Nakahsima, K.; Iwasaki, T.; Misawa, M.; Kaneko, N. I-ROAD could be efficient in predicting severity of community acquired pneumonia or healthcare-associated-pneumonia. Singapore Med. J. 2014, 55, 318–324. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- The Japanese Respiratory Society. The JRS Guidelines for the Managements of Pneumonia in Adults; Japanese Respiratory Society: Tokyo, Japan, 2017. [Google Scholar]
- Metlay, J.P.; Schulz, R.; Li, Y.H.; Singer, D.E.; Marrie, T.J.; Coley, C.M.; Hough, L.J.; Obrosky, D.S.; Kapoor, W.N.; Fine, M.J. Influence of age on symptoms at presentation in patients with community-acquired pneumonia. Arch. Intern. Med. 1997, 157, 1453–1459. [Google Scholar] [CrossRef]
- Shindo, Y.; Sato, S.; Maruyama, E.; Ohashi, T.; Ogawa, M.; Hashimoto, N.; Imaizumi, K.; Sato, T.; Hasegawa, Y. Health-care-associated pneumonia among hospitalized patients in a Japanese community hospital. Chest 2009, 135, 633–640. [Google Scholar] [CrossRef]
- Miyashita, N.; Matsushima, T.; Oka, M.; Japanese Respiratory Society. The JRS guidelines for the management of community-acquired pneumonia in adults: An update and new recommendations. Intern. Med. 2006, 45, 419–428. [Google Scholar] [CrossRef] [Green Version]
- British Thoracic Society Standards of Care Committee. BTS guidelines for the management of community acquired pneumonia in adults. Thorax 2001, 56 (Suppl. 4), 1–64. [Google Scholar] [CrossRef] [Green Version]
- Niederman, M.S.; Mandell, L.A.; Anzueto, A.; Bass, J.B.; Broughton, W.A.; Campbell, G.D.; Dean, N.; File, T.; Fine, M.J.; Gross, P.A.; et al. Guidelines for the management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med. 2001, 163, 1730–1754. [Google Scholar] [CrossRef]
- Seki, M.; Watanabe, A.; Mikasa, K.; Kadota, J.; Kohno, S. Revision of the severity rating and classifi cation of hospital-acquired pneumonia in the Japanese Respiratory Society guidelines. Respirology 2008, 13, 880–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–8300. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chron. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Kollef, M.H.; Nováček, M.; Kivistik, Ü.; Réa-Neto, Á.; Shime, N.; Martin-Loeches, I.; Timsit, J.F.; Wunderink, R.G.; Bruno, C.J.; Huntington, J.A.; et al. Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): A randomised, controlled, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2019, 19, 1299–1311. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Izumikawa, K.; Morinaga, Y.; Nakamura, S.; Kurihara, S.; Imamura, Y.; Miyazaki, T.; Tsukamoto, M.; Kakeya, H.; Yanagihara, K.; et al. Prospective randomized comparison study of piperacillin/tazobactam and meropenem for healthcare-associated pneumonia in Japan. J. Infect. Chemother. 2013, 19, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Awad, S.S.; Rodriguez, A.H.; Chuang, Y.C.; Marjanek, Z.; Pareigis, A.J.; Reis, G.; Scheeren, T.W.; Sánchez, A.S.; Zhou, X.; Saulay, M.; et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin. Infect. Dis. 2014, 59, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.F., Jr.; Limper, A.H. Pneumocystis pneumonia. N. Engl. J. Med. 2004, 350, 2487–2498. [Google Scholar] [CrossRef]
- Asai, N.; Motojima, S.; Ohkuni, Y.; Matsunuma, R.; Nakashima, K.; Iwasaki, T.; Nakashita, T.; Otsuka, Y.; Kaneko, N. Early diagnosis and treatment are crucial for the survival of Pneumocystis pneumonia patients without human immunodeficiency virus infection. J. Infect. Chemother. 2012, 18, 898–905. [Google Scholar] [CrossRef]
- Japanese Respiratory Society. Aspiration pneumonia. Respirology 2009, 14 (Suppl. 2), S59–S64. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Prina, E.; Menéndez, R.; Ceccatoo, A.; Cilloniz, C.; Méndez, R.; Gabarrus, A.; Barbeta, E.; Bassi, G.L.; Ferrer, M.; et al. New Sepsis Definition (Sepsis-3) and Community-acquired Pneumonia Mortality: A Validation and Clinical Decision-making Study. Am. J. Respir. Crit. Care Med. 2017, 10, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, N.; Ouchi, K.; Kishi, F.; Tabuchi, M.; Tsumura, N.; Bannai, H.; Iwata, S.; Tanaka, T.; Oka, M. Rapid and simple diagnosis of Chlamydophila pneumoniae pneumonia by an immunochromatographic test for detection of immunoglobulin M antibodies. Clin. Vaccine Immunol. 2008, 7, 1128–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, T.; Hashimoto, T.; Arita, M.; Osawa, M. Etiology of Community-Acquired Pneumonia in Hospitalized Patients: A 3-year Prospective Study in Japan. Chest 1998, 6, 1558–1593. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 21st informational supplement. M 100-S21; CLSI: Wayne, PA, USA, 2011. [Google Scholar]
- American Thoracic Society; Infectious Disease Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. Respir. Crit. Care 2005, 4, 388–416. [Google Scholar]
- Asai, N.; Ohkuni, Y.; Ashworth, L.; Kaneko, N. Implementation of do not attempt resuscitate orders in a Japanese nursing home. Am. J. Hosp. Palliat. Care 2014, 31, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Miyashita, N.; Kawai, Y.; Horita, N.; Yano, S.; Oka, Y.; Oda, T.; Okimoto, N. Changes in physical function after hospitalization in patients with nursing and healthcare-associated pneumonia. J. Infect. Chemother. 2016, 22, 662–666. [Google Scholar] [CrossRef]
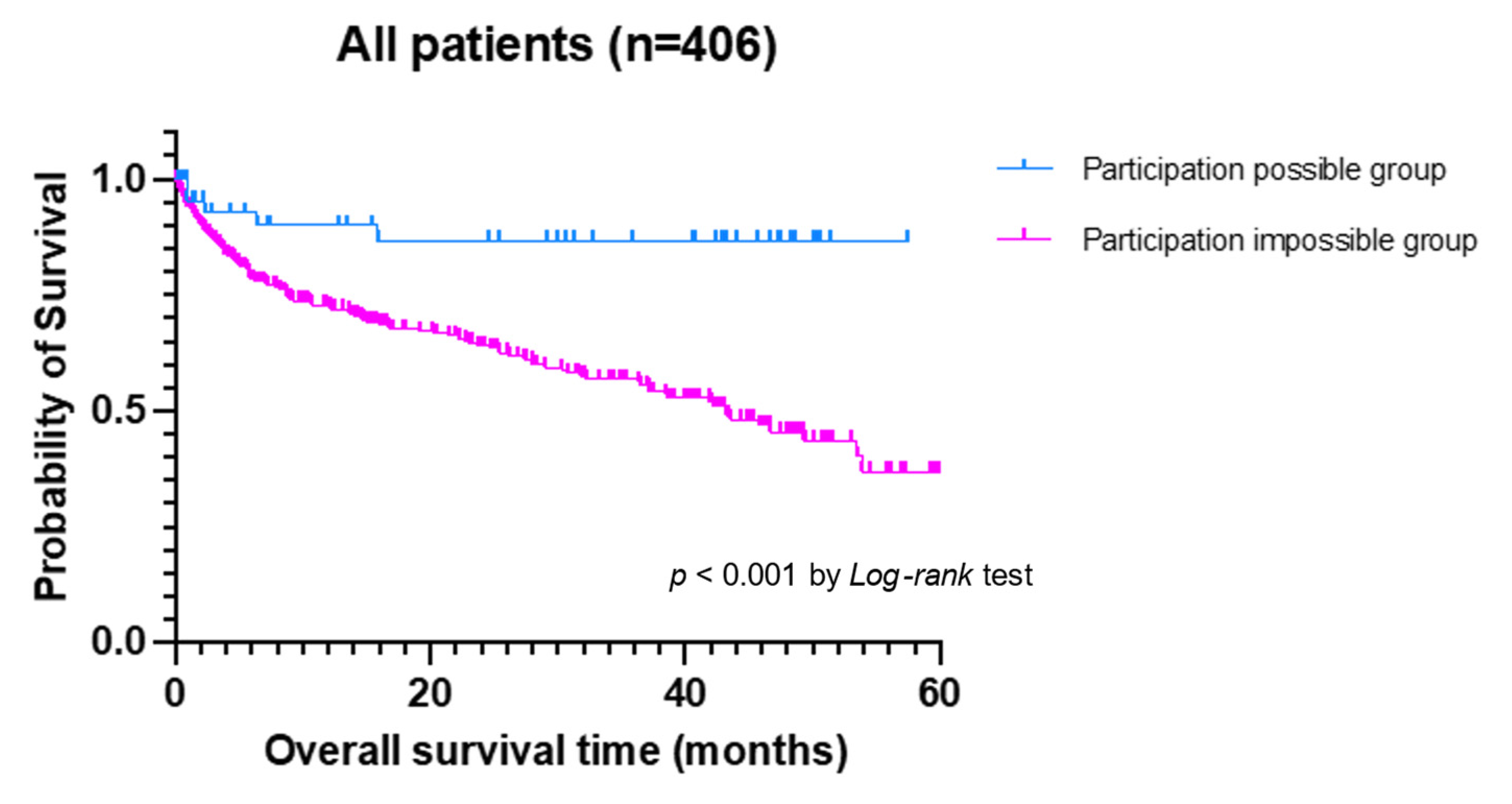

| Variables | All Patients (n = 406) | Participation-Possible Group (n = 57) | Participation-Impossible Group (n = 349) | p-Value |
|---|---|---|---|---|
| Mean age (years ± SD) | 75.4 ± 14.8 | 54.9 ± 17.6 | 78.8 ± 11.2 | <0.001 |
| Median age (years, range) | 79 (18–103) | 56 (18–79) | 81 (37–103) | - |
| Male gender (n, %) | 257 (63) | 28 (49) | 229 (66) | 0.017 |
| Smoking history (n, %) | ||||
| Current smoker | 36 (9) | 11 (19) | 25 (7) | 0.003 |
| Ex-smoker | 205 (50) | 24 (42) | 181 (52) | 0.172 |
| Never smoker | 135 (33) | 21 (37) | 114 (33) | 0.535 |
| Unknown | 30 (7) | 1 (2) | 29 (8) | 0.079 |
| Underlying diseases (n, %) | ||||
| Heart disease | 126 (31) | 4 (7) | 122 (35) | <0.001 |
| Chronic pulmonary disease | 175 (43) | 20 (35) | 155 (44) | 0.187 |
| Diabetes mellitus | 61 (15) | 1 (2) | 60 (17) | 0.001 |
| Chronic kidney disease | 51 (13) | 0 | 51 (15) | 0.002 |
| Hemodialysis | 16 (4) | 0 | 16 (5) | 0.099 |
| Hepatic disease | 14 (3) | 0 | 15 (4) | 0.111 |
| Collagen vascular disease | 41 (10) | 0 | 41 (12) | 0.006 |
| Cerebrovascular disease | 100 (25) | 0 | 100 (29) | <0.001 |
| Malignancy | 74 (18) | 0 | 75 (21) | <0.001 |
| Dementia | 74 (18) | 2 (4) | 72 (21) | 0.002 |
| Gastroesophageal reflux disease | 14 (3) | 3 (5) | 11 (3) | 0.418 |
| Proton pump inhibitor use | 122 (30) | 5 (9) | 117 (34) | <0.001 |
| Sleep agents use | 60 (15) | 0 | 60 (17) | <0.001 |
| Charlson comorbidity index (mean ± SD) | 2.1 ± 1.8 | 0.4 ± 0.5 | 2.4 ± 1.9 | <0.001 |
| Charlson comorbidity index ≥ 3 (n, %) | 120 (30) | 0 | 121 (35) | <0.001 |
| Category of pneumonia (n, %) | ||||
| Community-acquired pneumonia | 177 (44) | 51 (89) | 126 (36) | <0.001 |
| Healthcare-associated pneumonia | 229 (56) | 6 (11) | 223 (64) | |
| Severity of pneumonia (mean ± SD) | ||||
| A-DROP score | 2.0 ± 1.3 | 0.7 ± 1.0 | 2.2 ± 1.2 | <0.001 |
| CURB-65 score | 1.8 ± 1.1 | 0.6 ± 0.8 | 2.0 ± 1.0 | <0.001 |
| PSI score | 105.9 ± 42.3 | 45.9 ± 34.9 | 115.8 ± 34.8 | <0.001 |
| I-ROAD score | 2.1 ± 0.9 | 1.2 ± 0.6 | 2.3 ± 0.8 | <0.001 |
| SOFA score | 2.7 ± 1.9 | 1.3 ± 1.1 | 2.9 ± 1.9 | <0.001 |
| Conditions of the patients (mean ± SD) | ||||
| SIRS score | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.566 |
| Quick SOFA | 0.3 ± 0.5 | 0.1 ± 0.2 | 0.3 ± 0.5 | <0.001 |
| Bacteremia (n, %) * | 26 (11) | 1 (4) | 25 (13) | 0.14 |
| Treatment (n, %) | ||||
| ICU admission | 15 (4) | 3 (5) | 12 (3) | 0.471 |
| DNAR order | 77 (19) | 0 | 77 (22) | <0.001 |
| Mechanical ventilation | 19 (5) | 0 | 19 (5) | 0.071 |
| Vasopressor use | 11 (3) | 0 | 11 (3) | 0.174 |
| Initial antibiotic therapy (n, %) | ||||
| Penicillin alone | 196 (48) | 16(28) | 180 (52) | 0.001 |
| Cephems alone | 58 (14) | 7 (12) | 51 (15) | 0.641 |
| Carbapenems alone | 70 (17) | 7 (12) | 63 (18) | 0.285 |
| Fluoroquinolones alone | 26 (6) | 13 (23) | 13 (4) | <0.001 |
| Macrolides alone | 0 | 0 | 0 | - |
| β-lactams plus fluoroquinolones | 22 (5) | 10 (17) | 12 (3) | <0.001 |
| β-lactams plus macrolides | 11 (3) | 3 (5) | 8 (2) | 0.2 |
| Others | 23 (6) | 1 (2) | 22 (6) | 0.168 |
| Combination plus anti-MRSA agents | 5 (1) | 0 | 5 (1) | 0.363 |
| Any combination antibiotic therapy | 52 (13) | 15 (26) | 37 (11) | 0.001 |
| Antipseudomonal agents use (n, %) | 247 (61) | 34 (60) | 213 (61) | 0.843 |
| Route of antibiotics (n, %) | ||||
| Oral | 7 (2) | 5 (9) | 2 (1) | 0.001 |
| Intravenous | 388 (95) | 47 (82) | 341 (97) | <0.001 |
| Oral and intravenous | 12 (3) | 5 (9) | 7 (2) | 0.017 |
| Duration of | ||||
| hospital stay (mean days ± SD) | 18.6 ± 16.1 | 12.9 ± 10.2 | 19.5 ± 16.8 | 0.004 |
| antibiotics use (mean days ± SD) | 13.7 ± 10.8 | 12.5 ± 9.1 | 13.9 ± 11.1 | 0.385 |
| Outcome | ||||
| Mortality (n, %) | ||||
| 30-day mortality | 19 (5) | 0 | 19 (5) | <0.001 |
| In-hospital mortality | 23 (6) | 1 (2) | 22 (6) | <0.001 |
| Initial treatment failure (n, %) | 37 (9) | 5 (9) | 32 (9) | 0.924 |
| Inappropriate treatment (n, %) ** | 42 (22) | 1 (6) | 41 (24) | 0.32 |
| Isolating PDR pathogens (n, %) | 59 (14) | 3 (5) | 56 (16) | 0.032 |
| Gram positive (n) | *** | **** | ***** | |
| Streptococcus pneumoniae | 32 (16.3) | 4 (23.5) | 28 (15.6) | 0.831 |
| Streptococcus non-pneumonia | 19 (9.7) | 0 | 19 (9.7) | 0.381 |
| Methicillin-sensitive Staphylococcus aureus | 30 (15.3) | 1 (5.9) | 29 (16.2) | 0.083 |
| MRSA | 35 (17.9) | 0 | 35 (19.6) | 0.013 |
| Coagulase-negative Staphylococci | 1 (0.5) | 0 | 1 (0.6) | 0.689 |
| Corynebacterium species | 2 (1) | 0 | 2 (1.1) | 0.571 |
| Enterococcus species | 1 (0.5) | 0 | 1 (0.6) | 0.689 |
| Gram-negative (n) | *** | **** | ***** | |
| Haemophillus influenzae | 21 (10.7) | 6 (35.3) | 15 (8.4) | 0.042 |
| Esherichia coli | 18 (9.2) | 1 (5.9) | 17 (9.5) | 0.3 |
| Pseudomonas aeruginosa | 15 (7.7) | 1 (5.9) | 14 (7.8) | 0.416 |
| Klebsiella pneumonniae | 26 (13.3) | 1 (5.9) | 25 (14) | 0.422 |
| Klebsiella oxytoca | 4 (2) | 0 | 4 (2.2) | 0.127 |
| Moraxella catarrahis | 11 (5.6) | 2 (11.2) | 9 (5) | 0.663 |
| Serratia macescens | 5 (2.6) | 1 (5.9) | 4 (2.2) | 0.682 |
| Acinetobacter species | 3 (1.5) | 1 (5.9) | 2 (1.1) | 0.319 |
| Proteus mirabilis | 4 (2) | 0 | 4 (2.2) | 0.422 |
| Stenotrophomonas maltphilia | 2 (1) | 0 | 2 (1.1) | 0.573 |
| Legionella pneumoniae | 1 (0.5) | 1 (5.9) | 0 | 0.012 |
| Other Enterobacteriacea † | 13 (6.6) | 0 | 13 (7.3) | 0.609 |
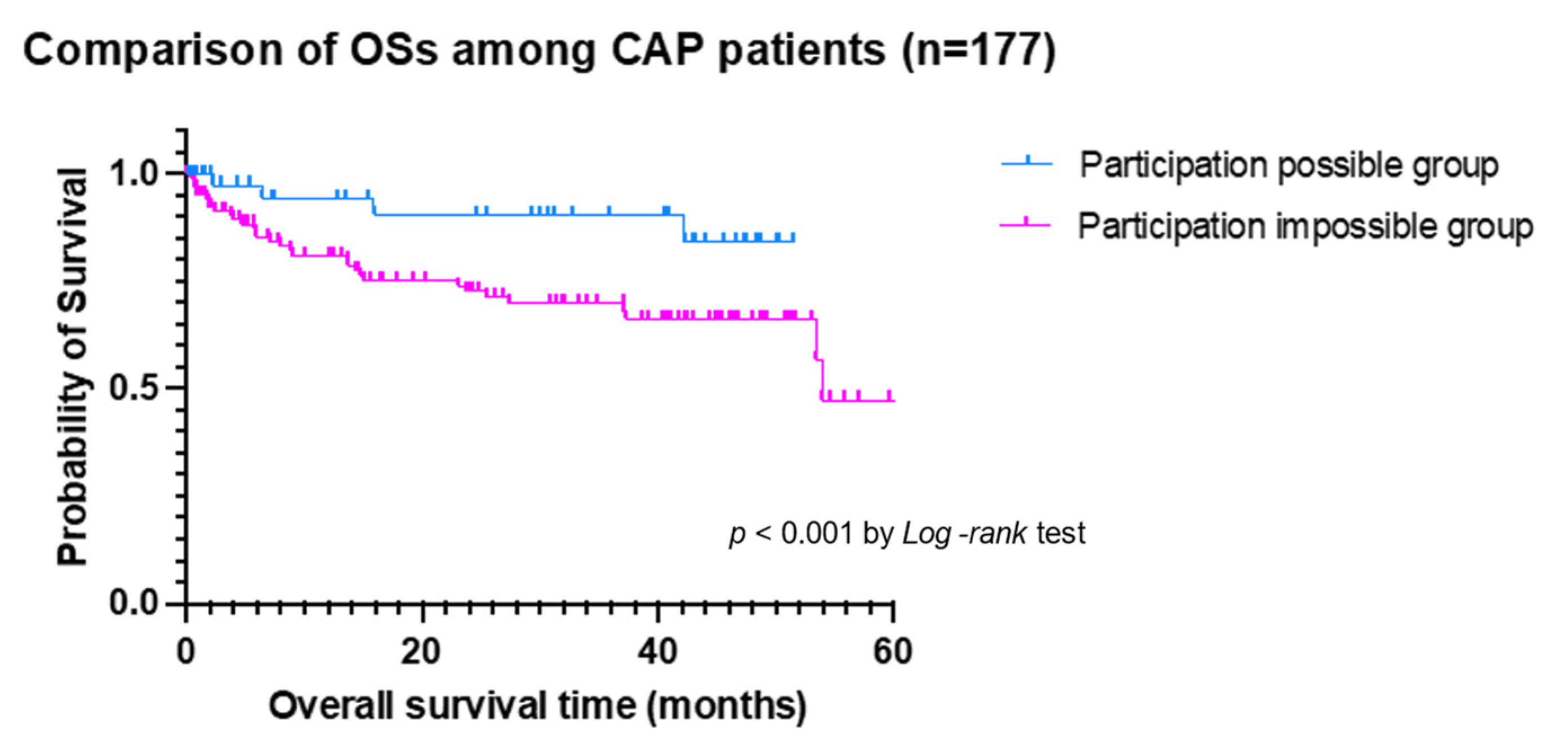
| Variables | All Patients (n = 177) | Participation-Possible Group (n = 51) | Participation-Impossible Group (n = 126) | p-Value |
|---|---|---|---|---|
| Mean age (years ± SD) | 71.9 ± 18.3 | 53.2 ± 17.7 | 79.5 ± 12.2 | <0.001 |
| Median age (years, range) | 76 (18–103) | 53 (18–79) | 82 (37–103) | - |
| Male gender (n, %) | 109 (62) | 27 (53) | 82 (65) | 0.133 |
| Smoking history (n, %) | ||||
| Current smoker | 26 (15) | 11 (22) | 15 (12) | 0.1 |
| Ex-smoker | 82 (46) | 20 (39) | 62 (49) | 0.227 |
| Never smoker | 61 (34) | 20 (39) | 41 (33) | 0.397 |
| Unknown | 8 (5) | 0 | 8 (6) | 0.066 |
| Underlying diseases (n, %) | ||||
| Heart disease | 51 (40) | 4 (8) | 47 (37) | <0.001 |
| Chronic pulmonary disease | 63 (50) | 16 (31) | 47 (37) | 0.001 |
| Diabetes mellitus | 31 (25) | 0 | 31 (25) | <0.001 |
| Chronic kidney disease | 14 (11) | 0 | 14 (11) | 0.013 |
| Hemodialysis | 0 | 0 | 0 | - |
| Hepatic disease | 4 (3) | 0 | 4 (3) | 0.198 |
| Collagen vascular disease | 1 (1) | 0 | 1 (1) | 0.523 |
| Cerebrovascular disease | 28 (22) | 0 | 28 (23) | <0.001 |
| Malignancy | 10 (8) | 0 | 10 (8) | 0.038 |
| Dementia | 23 (13) | 1 (2) | 22 (17) | 0.005 |
| Gastroesophageal reflux disease | 4 (3) | 2 (4) | 2 (2) | 0.344 |
| Proton pump inhibitor use | 37 (21) | 4 (8) | 33 (26) | <0.001 |
| Sleep agents use | 23 (13) | 0 | 23 (18) | 0.001 |
| Charlson comorbidity index (mean ± SD) | 1.2 ± 1.1 | 0.3 ± 0.5 | 1.6 ± 1.1 | <0.001 |
| Charlson comorbidity index ≥ 3 (n, %) | 23 (13) | 0 | 23 (18) | 0.001 |
| Severity of pneumonia (mean ± SD) | ||||
| A-DROP score | 1.7 ± 1.2 | 0.5 ± 0.8 | 2.1 ± 1.1 | <0.001 |
| CURB-65 score | 1.5 ± 1.1 | 0.5 ± 0.7 | 1.9 ± 1.0 | <0.001 |
| PSI score | 88.5 ± 44.4 | 42.5 ± 34.6 | 107.2 ± 33.1 | <0.001 |
| I-ROAD score | 1.8 ± 0.9 | 1.2 ± 0.6 | 2.1 ± 0.9 | <0.001 |
| SOFA score | 2.1 ± 1.5 | 1.3 ± 1.1 | 2.5 ± 1.5 | <0.001 |
| Conditions of the patients (mean ± SD) | ||||
| SIRS score | 0.6 ± 0.5 | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.208 |
| Quick SOFA | 0.2 ± 0.4 | 0.0 ± 0.2 | 0.3 ± 0.4 | <0.001 |
| Bacteremia (n, %) * | 9 (8) | 0 | 9 (11) | 0.113 |
| Treatment (n, %) | ||||
| ICU admission | 6 (3) | 3 (6) | 3 (2) | 0.23 |
| DNAR order | 23 (13) | 0 | 23 (18) | 0.001 |
| Mechanical ventilation | 7 (4) | 0 | 7 (6) | 0.086 |
| Vasopressor use | 4 (3) | 0 | 4 (3) | 0.198 |
| Initial antibiotic therapy (n, %) | ||||
| Penicillin alone | 70 (40) | 11 (22) | 59 (47) | 0.002 |
| Cephems alone | 30 (17) | 7 (14) | 23 (18) | 0.467 |
| Carbapenems alone | 26 (15) | 7 (14) | 19 (15) | 0.818 |
| Fluoroquinolones alone | 22 (12) | 13 (25) | 9 (7) | 0.001 |
| Macrolides alone | 0 | 0 | 0 | - |
| β-lactams plus fluoroquinolones | 16 (9) | 9 (18) | 7 (6) | 0.011 |
| β-lactams plus macrolides | 7 (4) | 3 (6) | 4 (3) | 0.403 |
| Others | 6 (3) | 1 (2) | 5 (4) | 0.504 |
| Combination plus anti-MRSA agents | 0 | 0 | 0 | - |
| Any combination antibiotic therapy | 27 (15) | 14 (27) | 13 (10) | 0.004 |
| Antipseudomonal agents use (n, %) | 95 (54) | 30 (59) | 65 (52) | 0.382 |
| Route of antibiotics (n, %) | ||||
| Oral | 5 (3) | 5 (10) | 0 | 0.002 |
| Intravenous | 167 (94) | 42 (82) | 125 (99) | <0.001 |
| Oral and intravenous | 5 (3) | 4 (8) | 1 (1) | 0.025 |
| Duration of | ||||
| hospital stay (mean days ± SD) | 16.3 ± 14.7 | 12.8 ± 9.6 | 13.6 ± 8.8 | 0.557 |
| antibiotics use (mean days ± SD) | 13.4 ± 9.0 | 12.4 ± 10.5 | 17.9 ± 16.0 | 0.025 |
| Outcome | ||||
| Mortality (n, %) | ||||
| 30-day mortality | 3 (2) | 0 | 3 (2) | 0.266 |
| In-hospital mortality | 5 (3) | 1 (2) | 4 (3) | 0.659 |
| Initial treatment failure (n, %) | 10 (6) | 4 (8) | 6 (5) | 0.421 |
| Inappropriate treatment (n, %) ** | 5 (7) | 1 (7) | 4 (7) | 0.988 |
| Isolating PDR pathogens (n, %) | 10 (6) | 2 (4) | 8 (6) | 0.526 |
| Gram positive (n) | *** | **** | ***** | |
| Streptococcus pneumoniae | 19 (26.8) | 4 (28.6) | 15 (26.3) | 0.415 |
| Streptococcus non-pneumonia | 5 (7) | 0 | 5 (8.8) | 0.575 |
| Methicillin-sensitive Staphylococcus aureus | 11 (15.5) | 1 (7.1) | 10 (17.5) | 0.131 |
| MRSA | 6 (8.5) | 0 | 6 (10.5) | 0.11 |
| Coagulase-negative Staphylococci | 0 | 0 | 0 | - |
| Corynebacterium species | 0 | 0 | 0 | - |
| Enterococcus species | 0 | 0 | 0 | - |
| Gram-negative (n) | ||||
| Haemophillus influenzae | 12 (16.9) | 5 (35.7) | 7 (12.3) | 0.311 |
| Esherichia coli | 4 (5.6) | 1 (7.1) | 3 (5.3) | 0.859 |
| Pseudomonas aeruginosa | 2 (2.8) | 1 (7.1) | 1 (1.8) | 0.509 |
| Klebsiella pneumonniae | 7 (9.9) | 1 (7.1) | 6 (10.5) | 0.38 |
| Klebsiella oxytoca | 2 (2.8) | 0 | 2 (3.5) | 0.363 |
| Moraxella catarrahis | 4 (5.6) | 1 (7.1) | 3 (5.3) | 0.859 |
| Serratia macescens | 2 (2.8) | 1 (7.1) | 1 (1.8) | 0.509 |
| Acinetobacter species | 0 | 0 | 0 | - |
| Proteus mirabilis | 0 | 0 | 1 (1.8) | 0.522 |
| Stenotrophomonas maltphilia | 0 | 0 | 0 | - |
| Legionella pneumoniae | 1 (1.4) | 1 (7.1) | 0 | 0.116 |
| Other Enterobacteriacea † | 7 (9.9) | 0 | 7 (12.3) | 0.332 |
| Variables | All Patients (n = 229) | Participation-Possible Group (n = 6) | Participation-Impossible Group (n = 223) | p-Value |
|---|---|---|---|---|
| Mean age (years ± SD) | 78.1 ± 10.6 | 69.5 ± 6.4 | 78.4 ± 10.6 | 0.304 |
| Median age (years, range) | 80 (42–99) | 69 (62–78) | 80 (42–99) | - |
| Male gender (n, %) | 148 (65) | 1 (17) | 147 (66) | 0.013 |
| Smoking history (n, %) | ||||
| Current smoker | 0 | 0 | 10 (4) | 0.596 |
| Ex-smoker | 123 (54) | 4 (67) | 119 (53) | 0.519 |
| Never smoker | 74 (32) | 1 (17) | 73 (33) | 0.406 |
| Unknown | 22 (10) | 1 (17) | 21 (9) | 0.552 |
| Underlying diseases (n, %) | ||||
| Heart disease | 75 (33) | 0 | 75 (34) | 0.083 |
| Chronic pulmonary disease | 112 (49) | 4 (67) | 108 (48) | 0.378 |
| Diabetes mellitus | 29 (13) | 0 | 29 (13) | 0.345 |
| Chronic kidney disease | 37 (16) | 0 | 37 (17) | 0.276 |
| Hemodialysis | 15 (7) | 0 | 15 (7) | 0.511 |
| Hepatic disease | 11 (5) | 0 | 11 (5) | 0.577 |
| Collagen vascular disease | 40 (17) | 0 | 40 (18) | 0.253 |
| Cerebrovascular disease | 72 (31) | 0 | 72 (32) | 0.093 |
| Malignancy | 65 (28) | 0 | 65 (29) | 0.118 |
| Dementia | 51 (22) | 1 (17) | 50 (22) | 0.738 |
| Gastroesophageal reflux disease | 10 (4) | 1 (17) | 11 (5) | 0.135 |
| Proton pump inhibitor use | 85 (37) | 1 (17) | 84 (38) | 0.293 |
| Sleep agents use | 37 (16) | 0 | 37 (17) | 0.273 |
| Charlson comorbidity index (mean ± SD) | 2.7 ± 2.0 | 0.8 ± 0.4 | 2.8 ± 2.1 | 0.022 |
| Charlson comorbidity index ≥ 3 (n, %) | 98 (43) | 0 | 98 (44) | 0.032 |
| Severity of pneumonia (mean ± SD) | ||||
| A-DROP score | 2.3 ± 1.3 | 1.8 ± 1.8 | 2.3 ± 1.2 | 0.154 |
| CURB-65 score | 2.1 ± 1.0 | 1.5 ± 0.8 | 2.1 ± 1.0 | 0.648 |
| PSI score | 119.4 ± 35.2 | 75.0 ± 23.3 | 120.6 ± 34.8 | 0.219 |
| I-ROAD score | 2.3 ± 0.8 | 1.5 ± 0.8 | 2.4 ± 0.8 | 0.685 |
| SOFA score | 3.2 ± 2.1 | 1.7 ± 1.4 | 3.2 ± 2.2 | 0.193 |
| Conditions of the patients (mean ± SD) | ||||
| SIRS score | 0.6 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.162 |
| Quick SOFA | 1.2 ± 0.8 | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.001 |
| Bacteremia (n, %) * | 17 (14) | 1 (50) | 16 (13) | 0.26 |
| Treatment (n, %) | ||||
| ICU admission | 9 (4) | 0 | 9 (4) | 0.615 |
| DNAR order | 54 (24) | 0 | 54 (24) | 0.168 |
| Mechanical ventilation | 12 (5) | 0 | 12 (5) | 0.559 |
| Vasopressor use | 7 (3) | 0 | 7 (3) | 0.659 |
| Initial antibiotic therapy (n, %) | ||||
| Penicillin alone | 126 (55) | 5 (83) | 121 (54) | 0.158 |
| Cephems alone | 28 (12) | 0 | 28 (13) | 0.354 |
| Carbapenems alone | 44 (19) | 0 | 44 (20) | 0.226 |
| Fluoroquinolones alone | 4 (2) | 0 | 4 (2) | 0.741 |
| Macrolides alone | 0 | 0 | 0 | - |
| β-lactams plus fluoroquinolones | 6 (3) | 1 (17) | 5 (2) | 0.029 |
| β-lactams plus macrolides | 4 (2) | 0 | 4 (2) | 0.741 |
| Others | 17 (7) | 0 | 17 (8) | 0.482 |
| Combination plus anti-MRSA agents | 5 (2) | 0 | 5 (2) | 0.711 |
| Any combination antibiotic therapy | 25 (11) | 1 (17) | 24 (11) | 0.647 |
| Antipseudomonal agents use (n, %) | 152 (66) | 4 (67) | 148 (66 | 0.998 |
| Route of antibiotics (n, %) | ||||
| Oral | 2 (1) | 0 | 2 (1) | 1.000 |
| Intravenous | 220 (96) | 5 (83) | 215 (96) | 0.216 |
| Oral and intravenous | 7 (3) | 1 (17) | 6 (3) | 0.172 |
| Duration of | ||||
| hospital stay (mean days ± SD) | 20.4 ± 16.9 | 16.7 ± 7.4 | 20.5 ± 17.2 | 0.284 |
| antibiotics use (mean days ± SD) | 14.0 ± 12.0 | 10.8 ± 4.0 | 14.0 ± 12.2 | 0.349 |
| Outcome | ||||
| Mortality (n, %) | ||||
| 30-day mortality | 16 (5) | 0 | 16 (7) | 0.456 |
| In-hospital mortality | 18 (6) | 0 | 18 (8) | 0.468 |
| Initial treatment failure (n, %) | 27 (9) | 1 (17) | 26 (12) | 0.924 |
| Inappropriate treatment (n, %) ** | 37 (31) | 0 | 37 (32) | 0.559 |
| Isolating PDR pathogens (n, %) | 49 (14) | 1 (17) | 48 (22) | 0.775 |
| Gram positive (n) | *** | **** | ***** | |
| Streptococcus pneumoniae | 13 (10.4) | 0 | 13 (10.7) | 0.584 |
| Streptococcus non-pneumonia | 14 (11.2) | 0 | 14 (11.5) | 0.571 |
| Methicillin-sensitive Staphylococcus aureus | 19 (15.2) | 0 | 19 (15.6) | 0.5 |
| MRSA | 29 (23.2) | 0 | 29 (23.8) | 0.391 |
| Coagulase-negative Staphylococci | 1 (0.8) | 0 | 1 (0.8) | 0.883 |
| Corynebacterium species | 2 (1.6) | 0 | 2 (1.6) | 0.835 |
| Enterococcus species | 1 (0.8) | 0 | 1 (0.8) | 0.883 |
| Gram-negative (n) | *** | **** | ***** | |
| Haemophillus influenzae | 9 (7.2) | 1 (35.3) | 8 (6.6) | 0.055 |
| Esherichia coli | 14 (11.2) | 0 | 14 (11.5) | 0.569 |
| Pseudomonas aeruginosa | 13 (10.4) | 0 | 13 (10.7) | 0.584 |
| Klebsiella pneumonniae | 19 (15.2) | 0 | 19 (15.6) | 0.5 |
| Klebsiella oxytoca | 2 (1.6) | 0 | 2 (1.6) | 0.835 |
| Moraxella catarrahis | 7 (5.6) | 1 (11.2) | 6 (4.9) | 0.022 |
| Serratia macescens | 3 (2.4) | 0 | 3 (2.5) | 0.798 |
| Acinetobacter species | 3 (2.4) | 1 (5.9) | 2 (1.6) | <0.001 |
| Proteus mirabilis | 3 (2.4) | 0 | 3 (2.5) | 0.798 |
| Stenotrophomonas maltphilia | 2 (1.6) | 0 | 2 (1.6) | 0.836 |
| Legionella pneumoniae | 0 | 0 | 0 | - |
| Other Enterobacteriacea † | 6 (4.8) | 0 | 6 (4.9) | 0.717 |
| Factors | n (%) |
|---|---|
| 1. Age (<18, >80 years old) | 180 (52) |
| 2. Underlying disease which could not be assessed | 254 (73) |
| Heart disease | 106 (30) |
| Pulmonary disease | 74 (21) |
| Kidney disease | 37 (11) |
| Hepatic disease | 15 (4) |
| Cerebrovascular disease | 19 (5) |
| Diabetes mellitus | 39 (12) |
| Collagen vascular disease | 41 (12) |
| Malignancy | 63 (18) |
| Mental disorder | 12 (4) |
| 3. Aspiration pneumonia | 196 (56) |
| 4. Immunosuppressor agents use Ж | 44 (13) |
| 5. Chemotherapy | 25 (7) |
| 6. Hemodialysis | 18 (5) |
| 7. Poor ADL or required any help | |
| ECOG-PS ≥ 3 | 111 (32) |
| Tube feeding | 20 (6) |
| Home oxygen therapy | 28 (8) |
| 8. Other infections complicated | 11 (3) |
| 9. Requiring mechanical ventilation and/or ICU admission | 20 (6) |
| 10. Poor life expectancy | 20 (6) |
| 11. Pregnancy | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asai, N.; Shibata, Y.; Sakanashi, D.; Kato, H.; Hagihara, M.; Yamagishi, Y.; Suematsu, H.; Mikamo, H. A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia. J. Clin. Med. 2022, 11, 297. https://doi.org/10.3390/jcm11020297
Asai N, Shibata Y, Sakanashi D, Kato H, Hagihara M, Yamagishi Y, Suematsu H, Mikamo H. A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia. Journal of Clinical Medicine. 2022; 11(2):297. https://doi.org/10.3390/jcm11020297
Chicago/Turabian StyleAsai, Nobuhiro, Yuichi Shibata, Daisuke Sakanashi, Hideo Kato, Mao Hagihara, Yuka Yamagishi, Hiroyuki Suematsu, and Hiroshige Mikamo. 2022. "A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia" Journal of Clinical Medicine 11, no. 2: 297. https://doi.org/10.3390/jcm11020297
APA StyleAsai, N., Shibata, Y., Sakanashi, D., Kato, H., Hagihara, M., Yamagishi, Y., Suematsu, H., & Mikamo, H. (2022). A Large Gap in Patients’ Characteristics and Outcomes between the Real-World and Clinical Trial Settings in Community-Acquired Pneumonia and Healthcare-Associated Pneumonia. Journal of Clinical Medicine, 11(2), 297. https://doi.org/10.3390/jcm11020297







