Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVDF Hollow Fiber Membranes
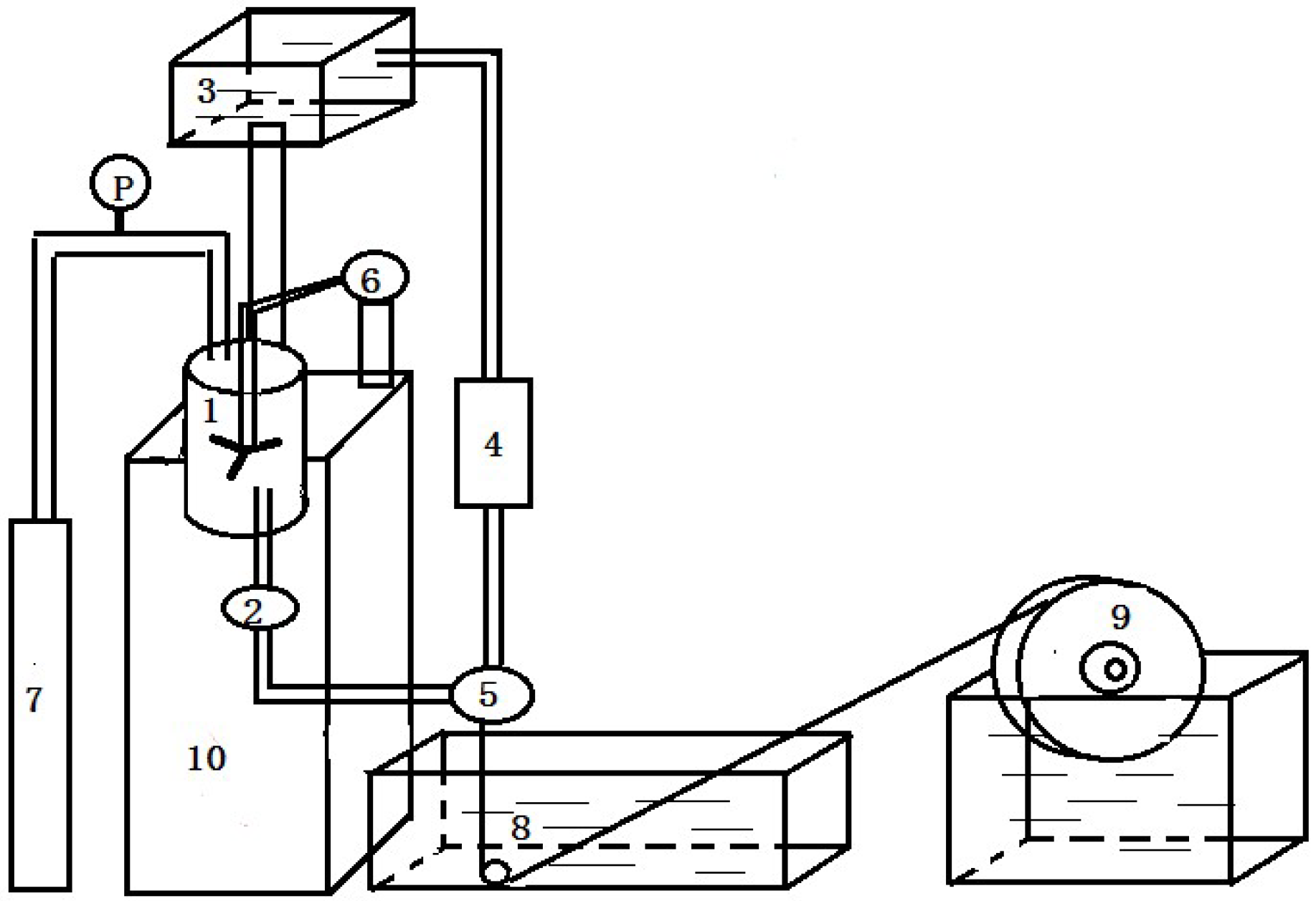
| Membrane label | PVDF (wt %) | PEG (6000 Da) (wt %) | 1,4-diethylene dioxide (wt %) | DMAc (wt %) | Viscosity mPa·s |
|---|---|---|---|---|---|
| F24-a | 24 | 14.8 | 25.2 | 36 | 2936 |
| F24-b | 24 | 16.8 | 25.2 | 34 | 4124 |
| F24-c | 24 | 18.8 | 25.2 | 32 | 4376 |
2.3. Characterization of the Hollow Fiber Membranes
2.3.1. Morphology of Membranes and Measurement of Pore Size and Porosity

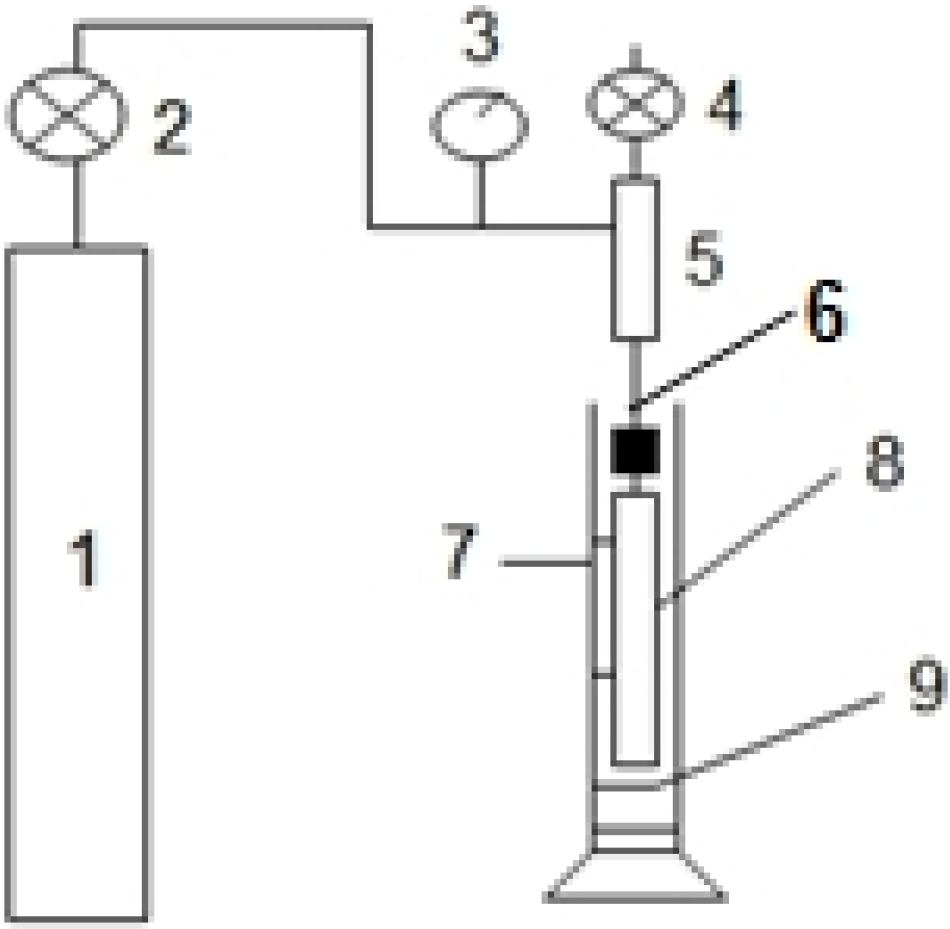

2.3.2. Mechanical Properties
2.3.3. The Pure Water Flux and Rejection of BSA

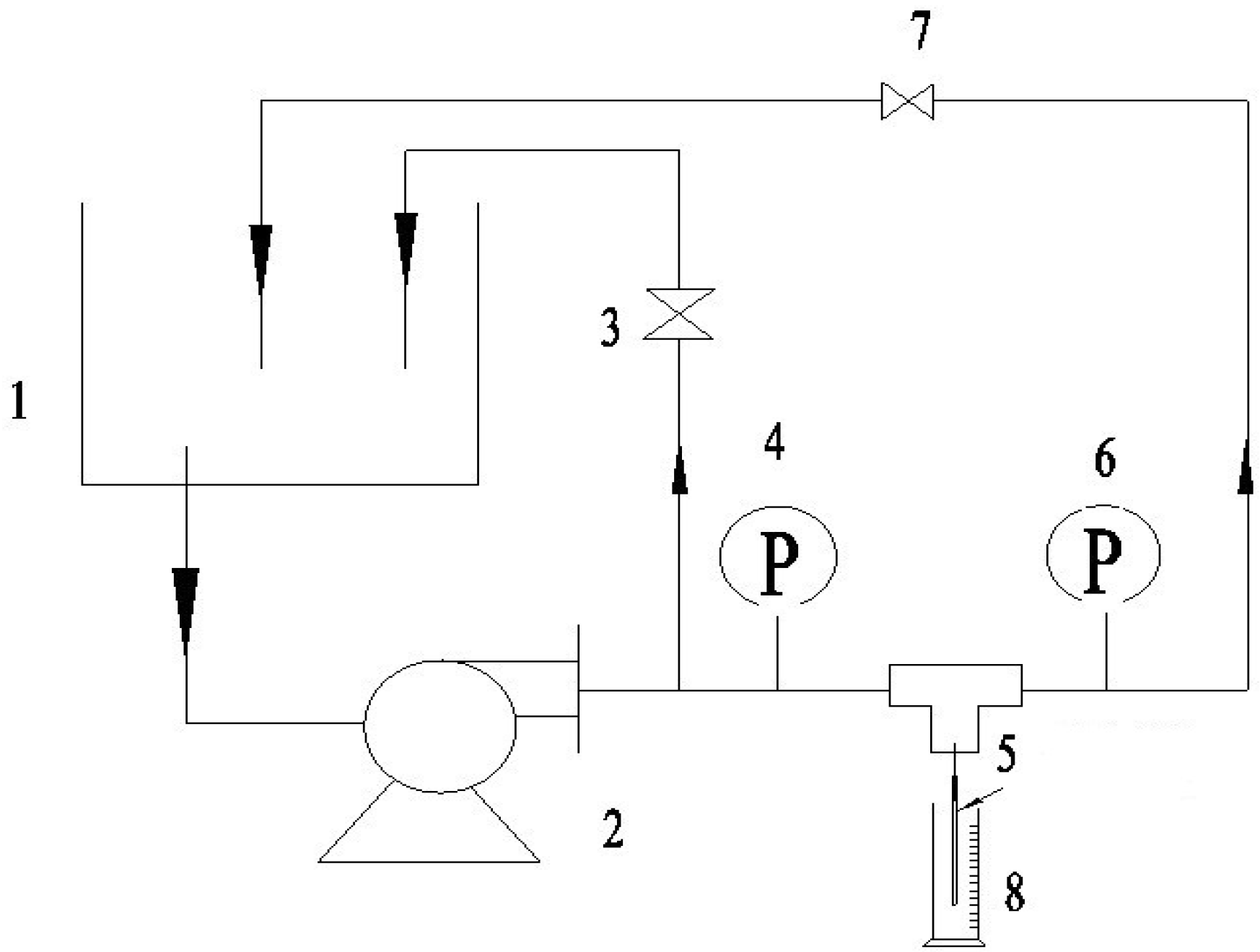

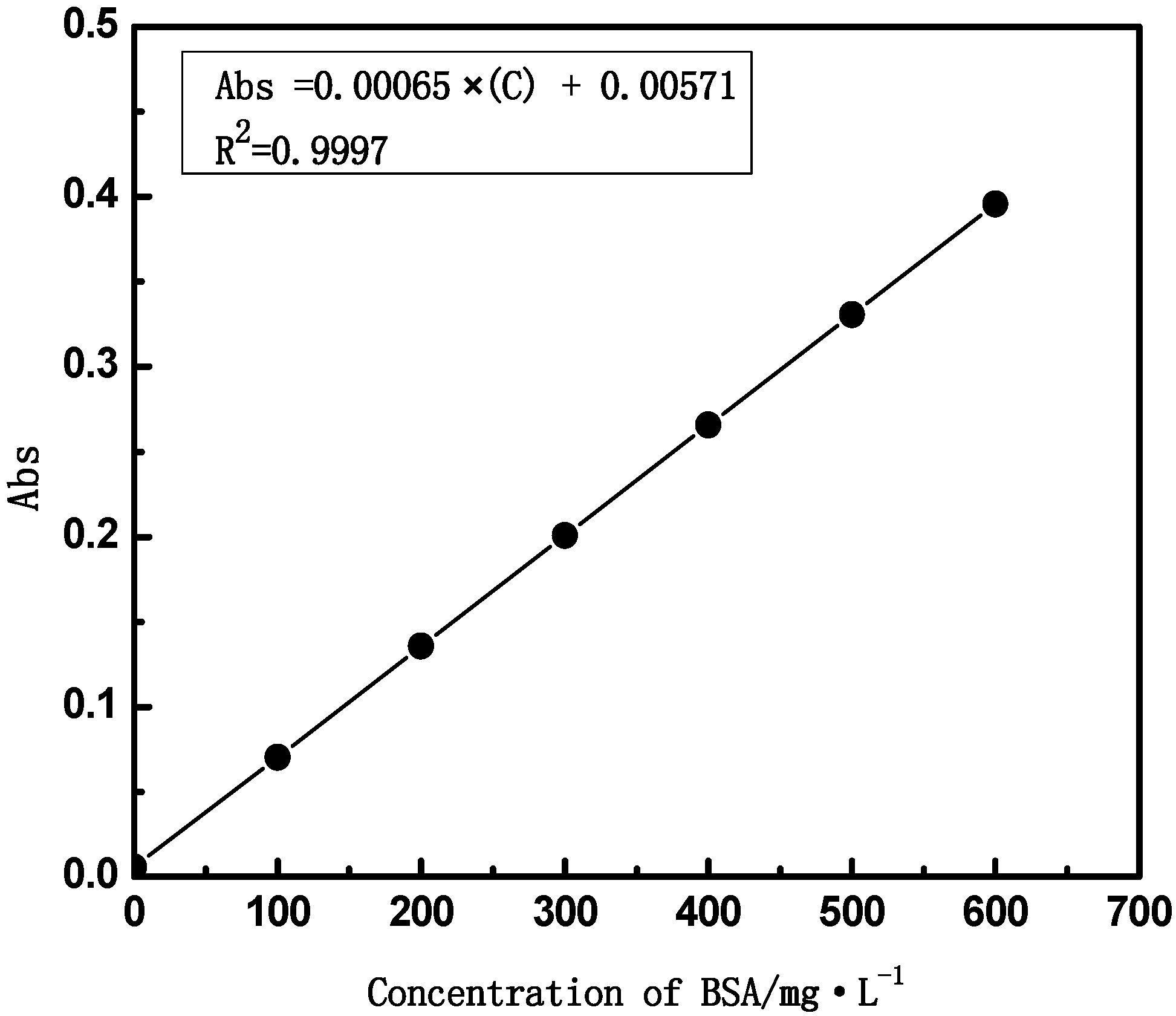
2.4. Biocompatibility of PVDF Hollow Fiber Membrane
2.4.1. Water Contact Angle
2.4.2. Protein Adsorption
3. Results and Discussion
3.1. Effect of Thickness on Membrane Characterization
3.1.1. Effect of Thickness on Membrane Morphology and Structure
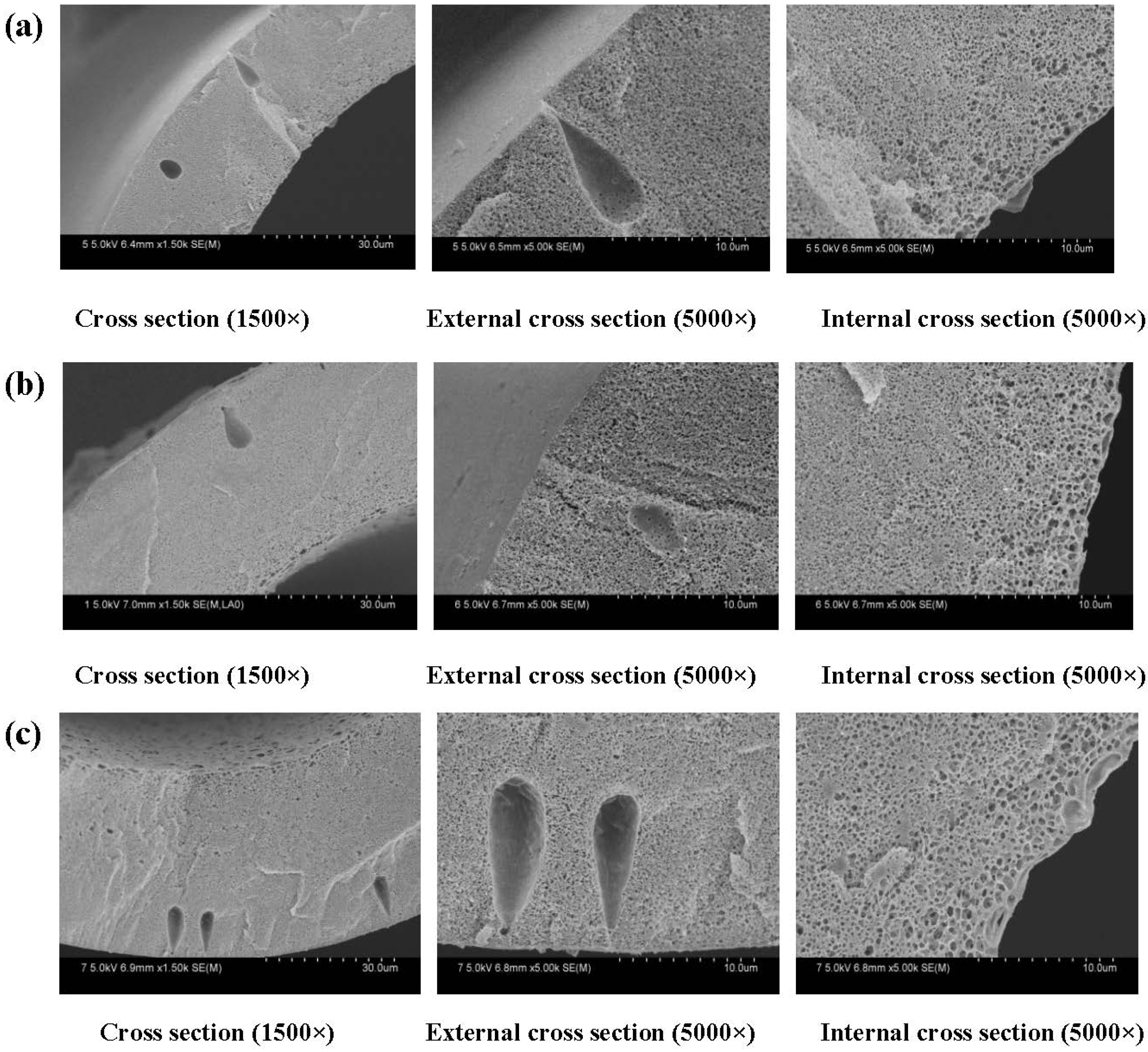
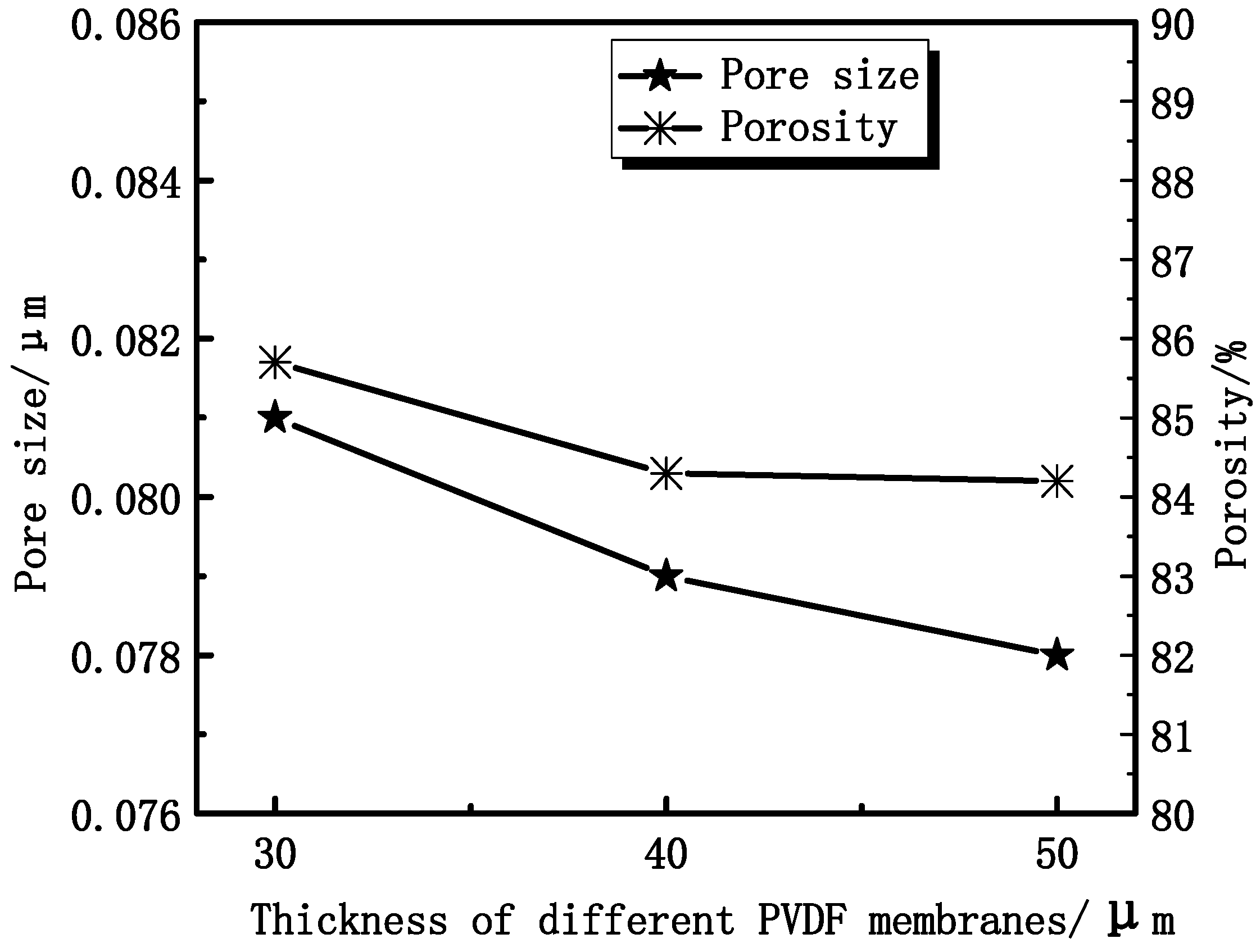
3.1.2. Effect of Thickness on Membrane Mechanical Properties
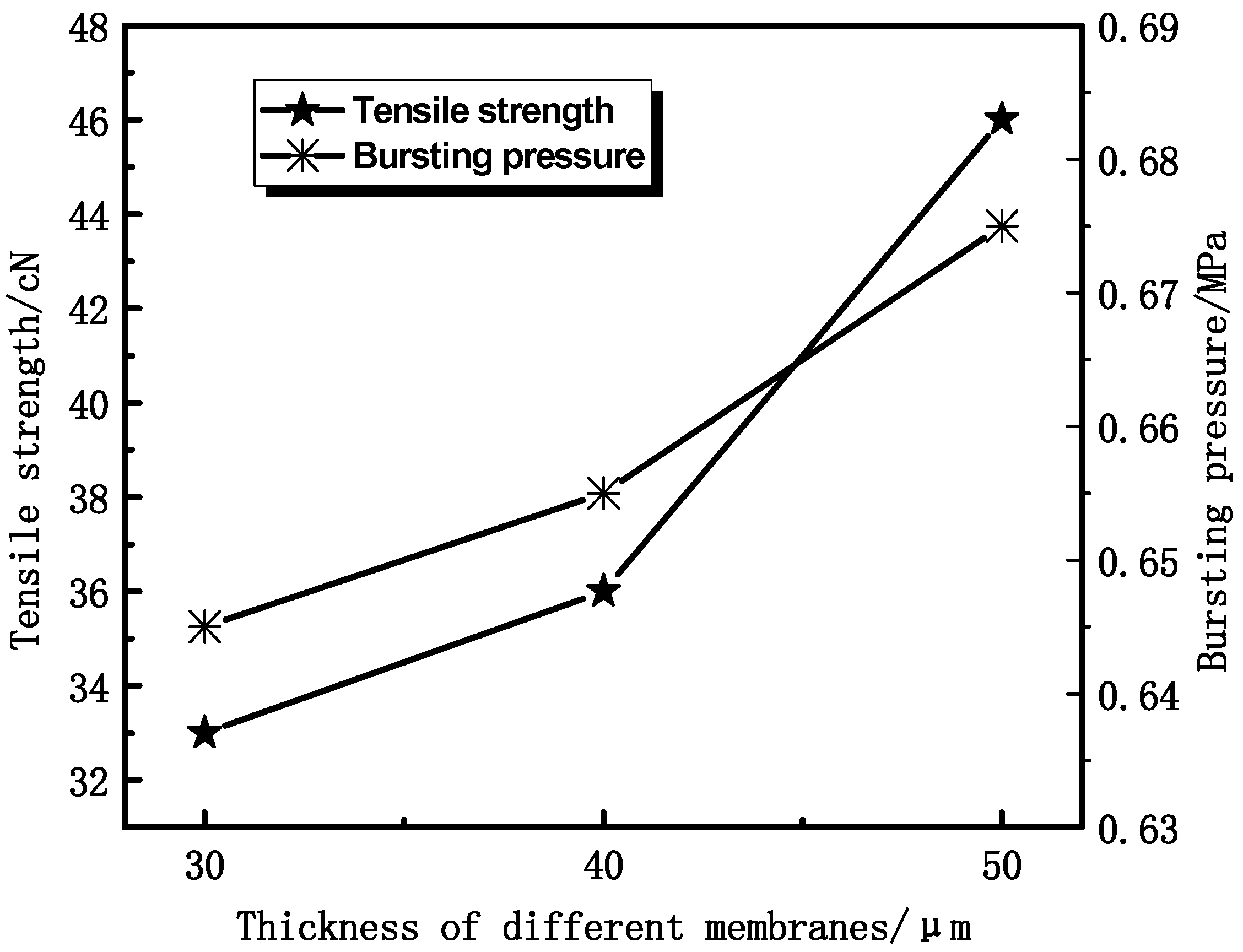
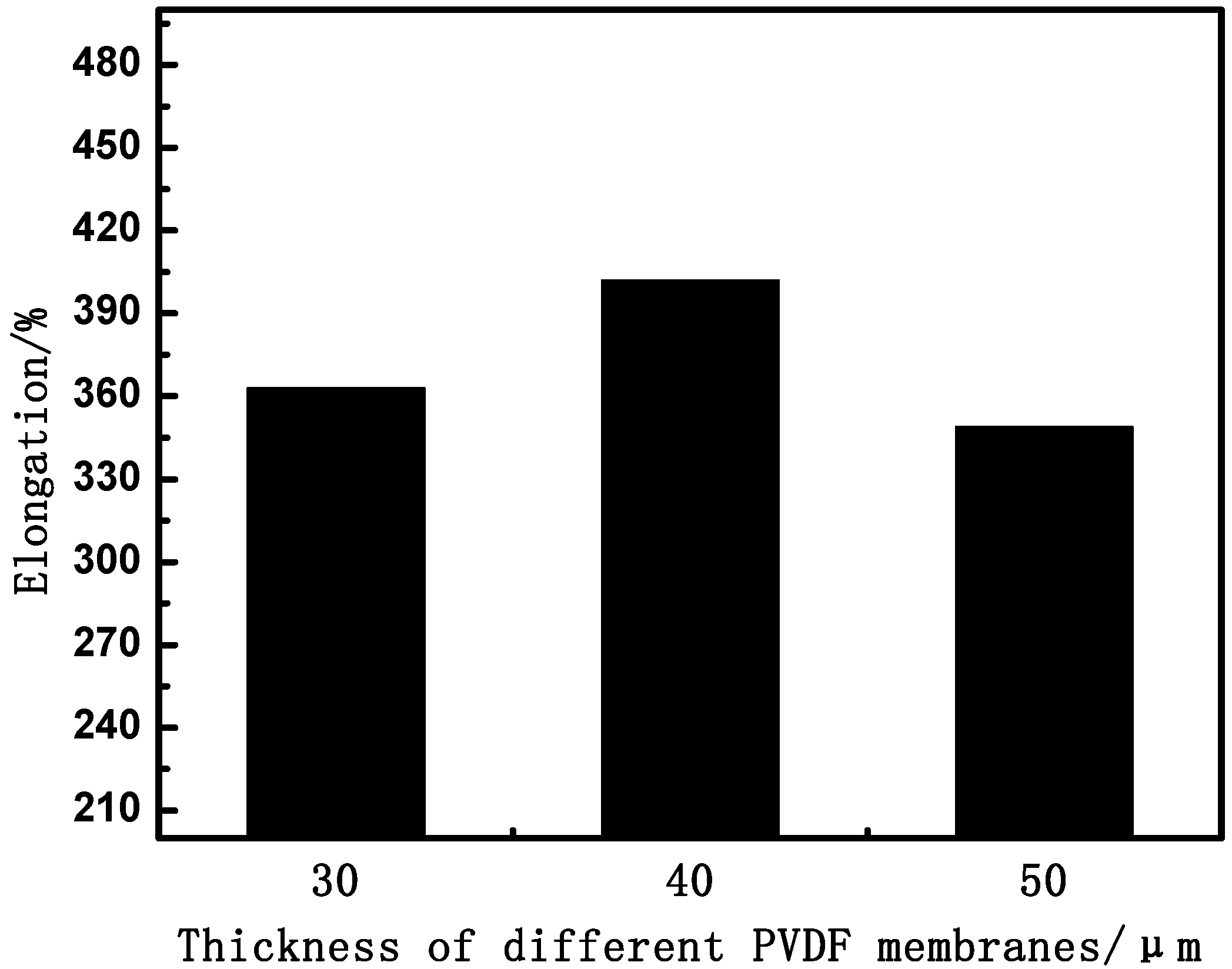
3.1.3. Effect of Thickness on Membrane Permeation Performance
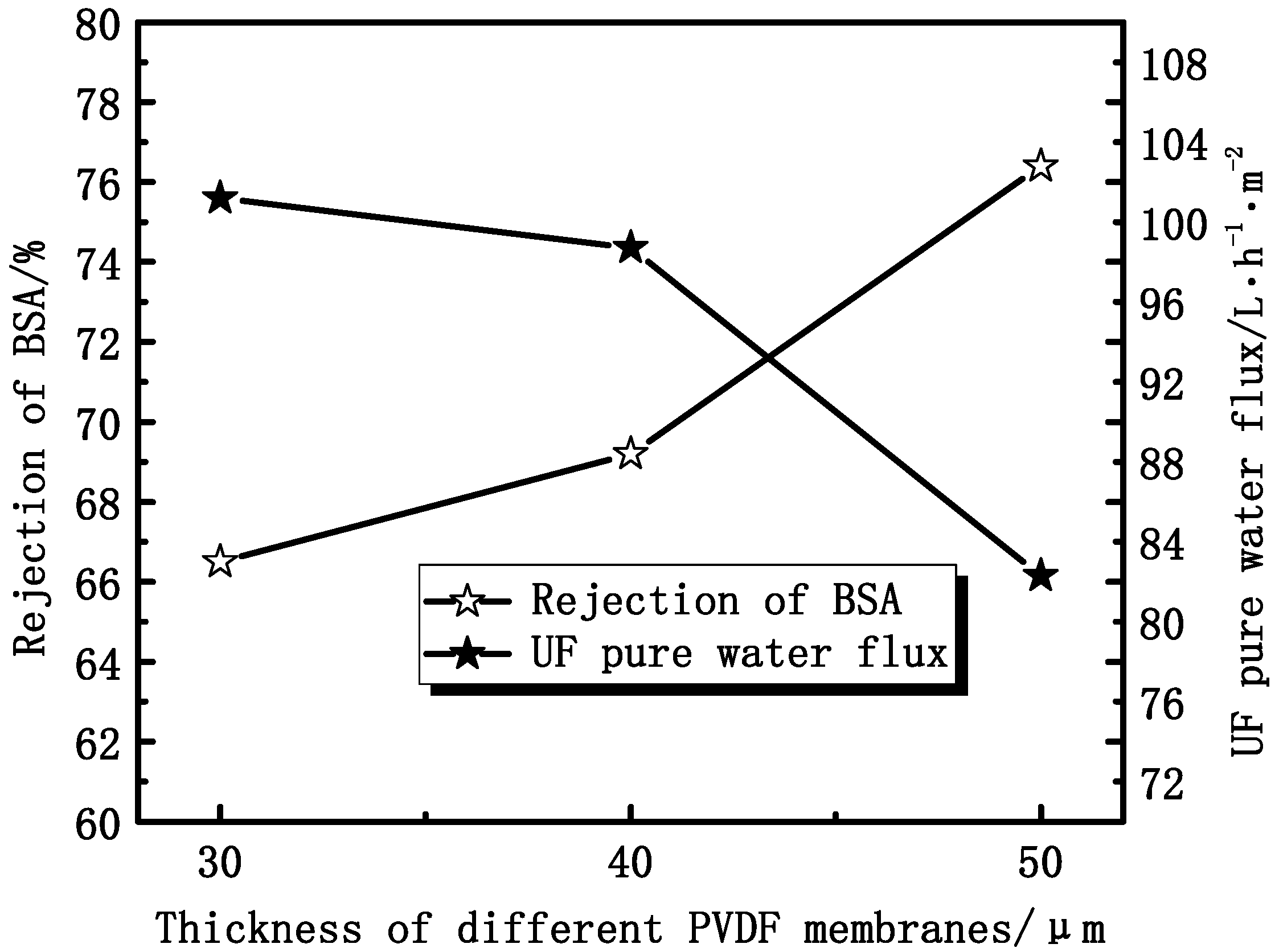
3.2. Effect of PEG Content on Membranes Performance
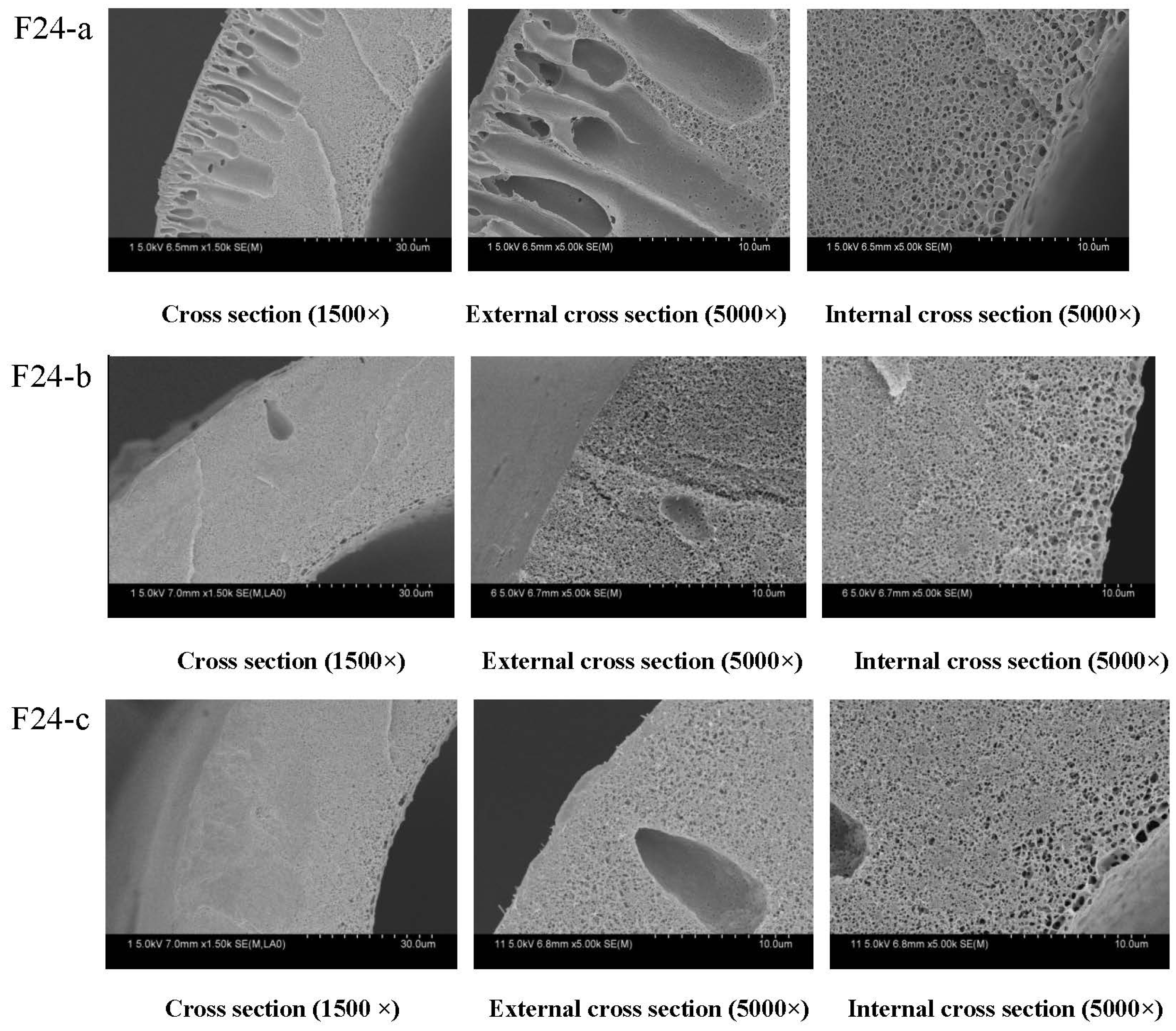
3.2.1. Effect of PEG Content on Membranes Morphology and Structure
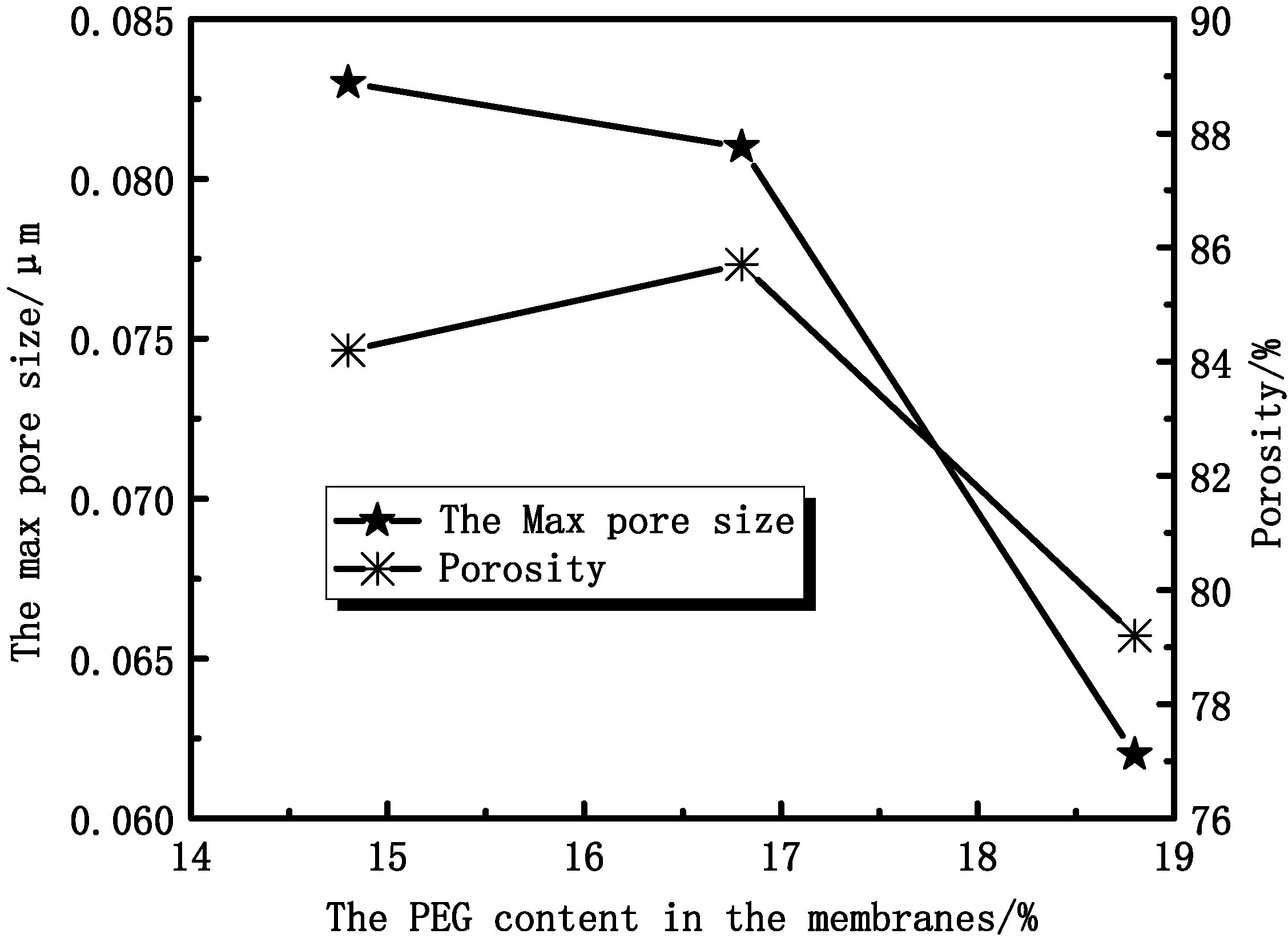
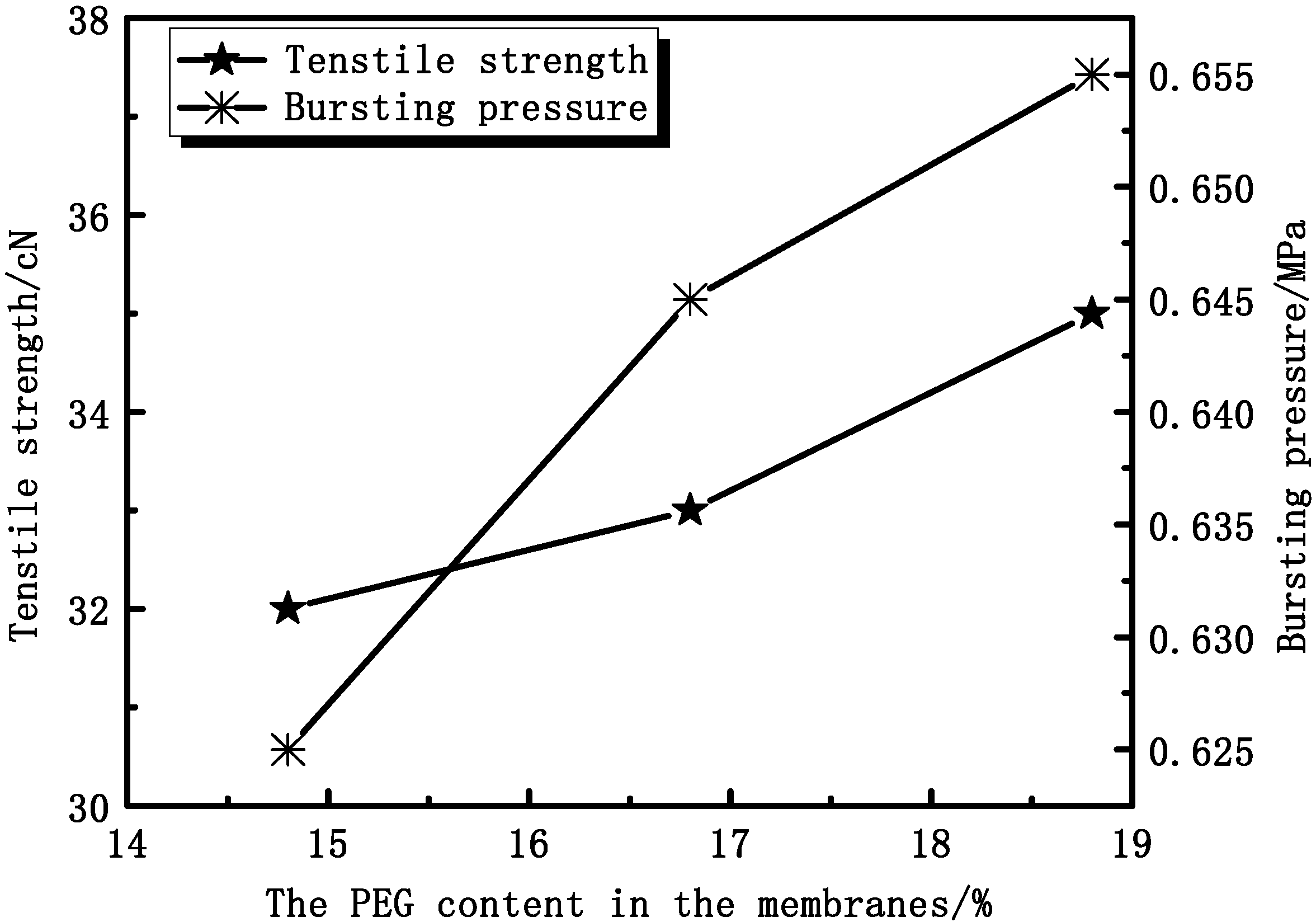
3.2.2. Effect of PEG Content on Membrane Mechanical Properties
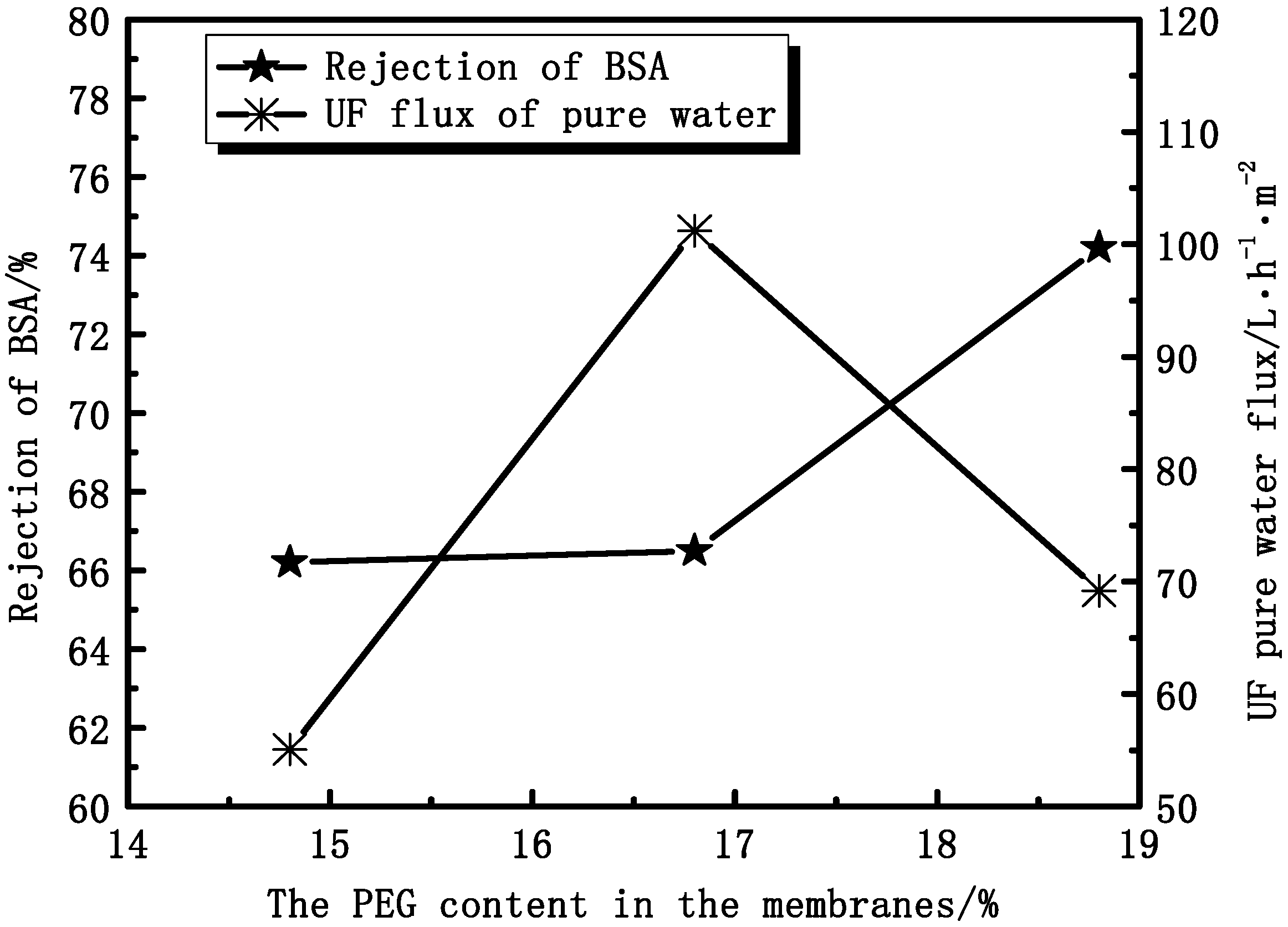
3.2.3. Effect of PEG Content on Membranes Permeation Performance
3.3. Comparison with Commercial F60S Hemodialysis Membrane
3.3.1. Mechanical and Permeation Properties

| Materials | Tensile strength cN | Elongation % | Bursting pressure MPa | Pore size μm | Porosity % | UF flux L/(h·m2) | Rejection of BSA % |
|---|---|---|---|---|---|---|---|
| PVDF | 40 | 402 | 0.645 | 0.079 | 84.3 | 98.7 | 69.2 |
| F60S | 27 | 66 | 0.475 | 0.079 | 72.3 | 72.5 | 78.2 |
3.3.2. Water Contact Angle and BSA Adsorption
| Materials | Water contact angle (°) | BSA adsorption (mg/m2) |
|---|---|---|
| PVDF | 54 ± 3 | 145 ± 3 |
| F60S | 64 ± 2 | 235 ± 2 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dahe, G.J.; Teotia, R.S.; Kadam, S.S. The biocompatibility and separation performance of antioxidative polysulfone/vitamin ETPGS composite hollow fiber membranes. Biomaterials 2011, 32, 352–365. [Google Scholar] [CrossRef]
- Yang, Q.; Chung, T.S.; Weber, M. Microscopic behavior of polyvinylpyrrolidone hydrophilizing agents on phase inversion polyethersulfone hollow fiber membranes for hemofiltration. J. Membr. Sci. 2009, 326, 322–331. [Google Scholar] [CrossRef]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Sirolli, V.; Ballone, E.; di Stante, S.; Amoroso, L.; Bonomini, M. Cell activation and cellularecellular interactions during hemodialysis: Effect of dialyzer membrane. Int. J. Artif. Organs 2002, 25, 529–537. [Google Scholar]
- Ishihara, K.; Hasegawa, T.; Watanabe, J.; Iwasaki, Y. Protein adsorption-resistant hollow fibers for blood purification. Artif. Organs 2002, 26, 1014–1019. [Google Scholar] [CrossRef]
- Hayama, M.; Yamamoto, K.; Kohori, F.; Sakai, K. How polysulfone dialysis membranes containing polyvinylpyrrolidone achieve excellent biocompatibility? J. Membr. Sci. 2004, 234, 41–49. [Google Scholar] [CrossRef]
- Matsuda, M.; Yamamoto, K.I.; Yakushiji, T.; Fukuda, M.; Miyasaka, T.; Sakai, K. Nanotechnological evaluation of protein adsorption on dialysis membrane surface hydrophilized with polyvinylpyrrolidone. J. Membr. Sci. 2008, 310, 219–228. [Google Scholar] [CrossRef]
- Park, H.H.; Deshwal, B.R.; Jo, H.D.; Choi, W.K.; Kim, W.; Lee, H.K. Absorption of nitrogen dioxide by PVDF hollow fiber membranes in a G-L contactor. Desalination 2009, 243, 52–64. [Google Scholar] [CrossRef]
- Akthakul, A.; Salinaro, R.F.; Mayes, A.M. Antifouling polymer membranes with subnanometersize selectivity. Macromolecules 2004, 37, 7663–7668. [Google Scholar] [CrossRef]
- Bai, H.L.; Wang, X.; Zhou, Y.T.; Zhang, L.P. Preparation and characterization of poly (vinylidene fluoride) composite membranes blended with nano-crystalline cellulose. Prog. Nat. Sci. Mater. Int. 2007, 106, 1482–1495. [Google Scholar]
- Li, Y.J.; Tian, K.P.; Lu, X.L. Preparation of polyvinylidene fluoride blood separate hollow fiber membrane. Membr. Sci. Technol. 2011, 31, 35–40. [Google Scholar]
- Zhang, J.; Guo, J.H.; Lu, X.L.; Wu, C.R.; Jia, Y.; Wang, X.; Chen, H.Y.; Gao, Q.J. Study of polyvinylidene fluoride hollow fiber dialyzers. Int. J. Biomed. Eng. 2011, 34, 11–15. [Google Scholar]
- Laroche1, G.; Marois1, Y.; Guidoin, R.; King, M.W.; Martin, L.; How, T.; Douville, Y. Polyvinylidene fluoride (PVDF) as a biomaterial: From polymeric raw material to monofilament vascular suture. J. Biomed. Mater. Res. 1996, 29, 1525–1536. [Google Scholar]
- Chan, R.; Chen, V. Characterization of protein fouling on membranes: Opportunities and challenges. J. Membr. Sci. 2004, 242, 169–188. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Wee, K.H.; Bai, R.B. Highly hydrophilic and low-protein-fouling polypropylene membrane prepared by surface modification with sulfobetaine-based zwitterionic polymer through a combined surface polymerization method. J. Membr. Sci. 2010, 362, 326–333. [Google Scholar] [CrossRef]
- Lu, X.L. Discuss about the measure methods for performance of hollow fiber porous membranes. Membr. Sci. Technol. 2011, 32, 1–6. [Google Scholar]
- Xu, Z.L. Study on high-porosity poly (vinylidene fluoride) hollow fiber ultrafiltration membrane. Membr. Sci. Technol. 2000, 20, 13–18. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Q.; Lu, X.; Zhao, L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes 2014, 4, 81-95. https://doi.org/10.3390/membranes4010081
Zhang Q, Lu X, Zhao L. Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes. 2014; 4(1):81-95. https://doi.org/10.3390/membranes4010081
Chicago/Turabian StyleZhang, Qinglei, Xiaolong Lu, and Lihua Zhao. 2014. "Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes" Membranes 4, no. 1: 81-95. https://doi.org/10.3390/membranes4010081
APA StyleZhang, Q., Lu, X., & Zhao, L. (2014). Preparation of Polyvinylidene Fluoride (PVDF) Hollow Fiber Hemodialysis Membranes. Membranes, 4(1), 81-95. https://doi.org/10.3390/membranes4010081




