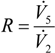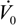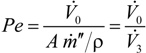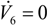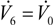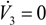Improvement of Membrane Performances to Enhance the Yield of Vanillin in a Pervaporation Reactor
Abstract
:1. Introduction
2. Materials and Methods
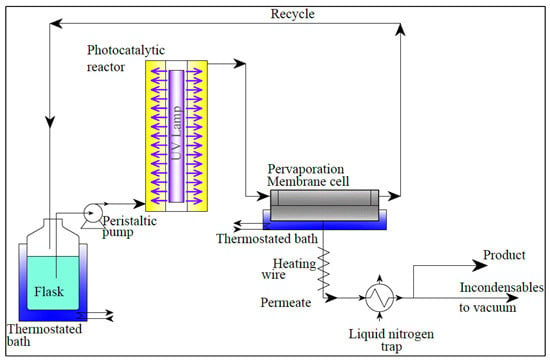
2.1. Experimental
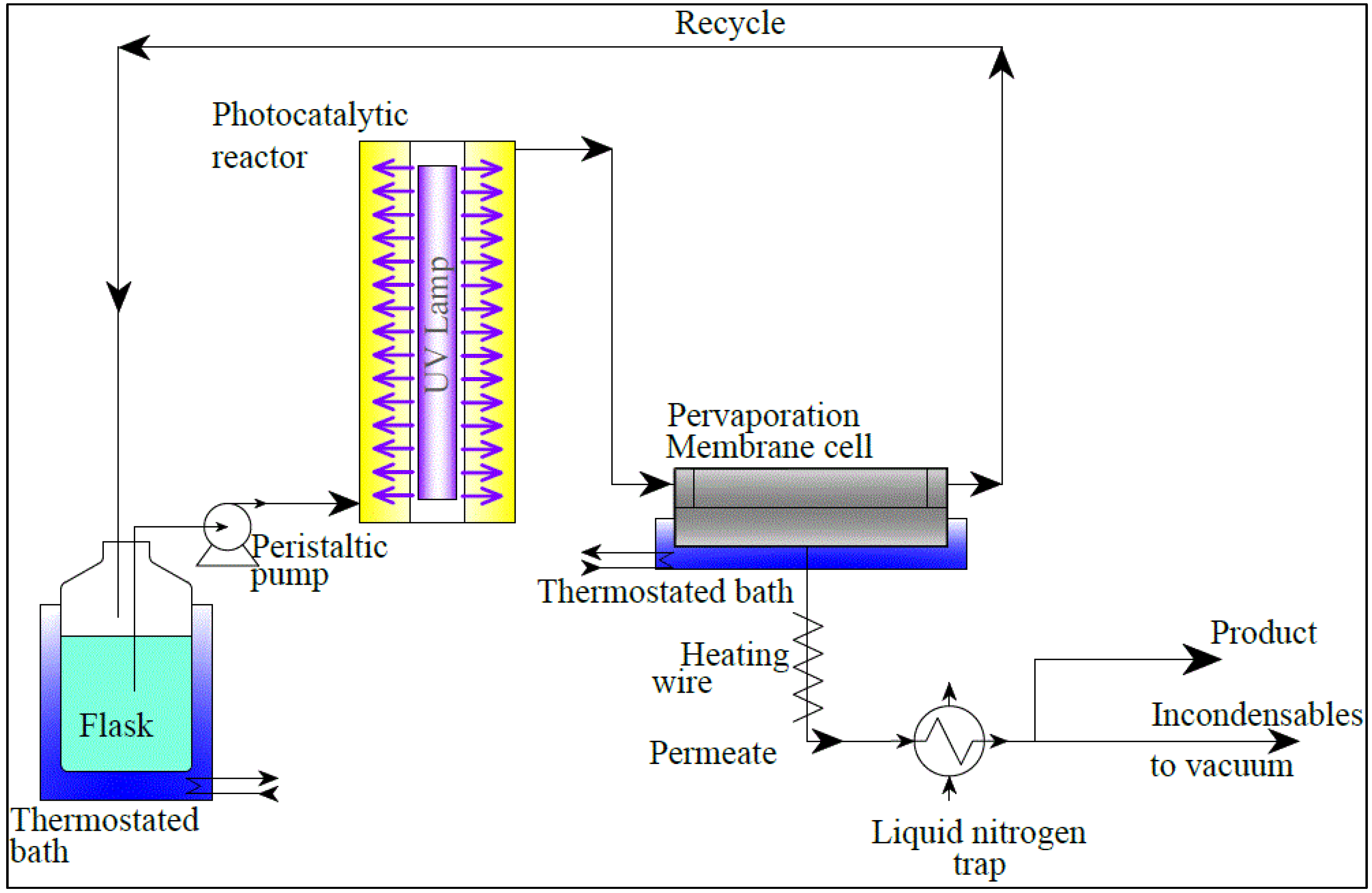
2.2. Process Simulation
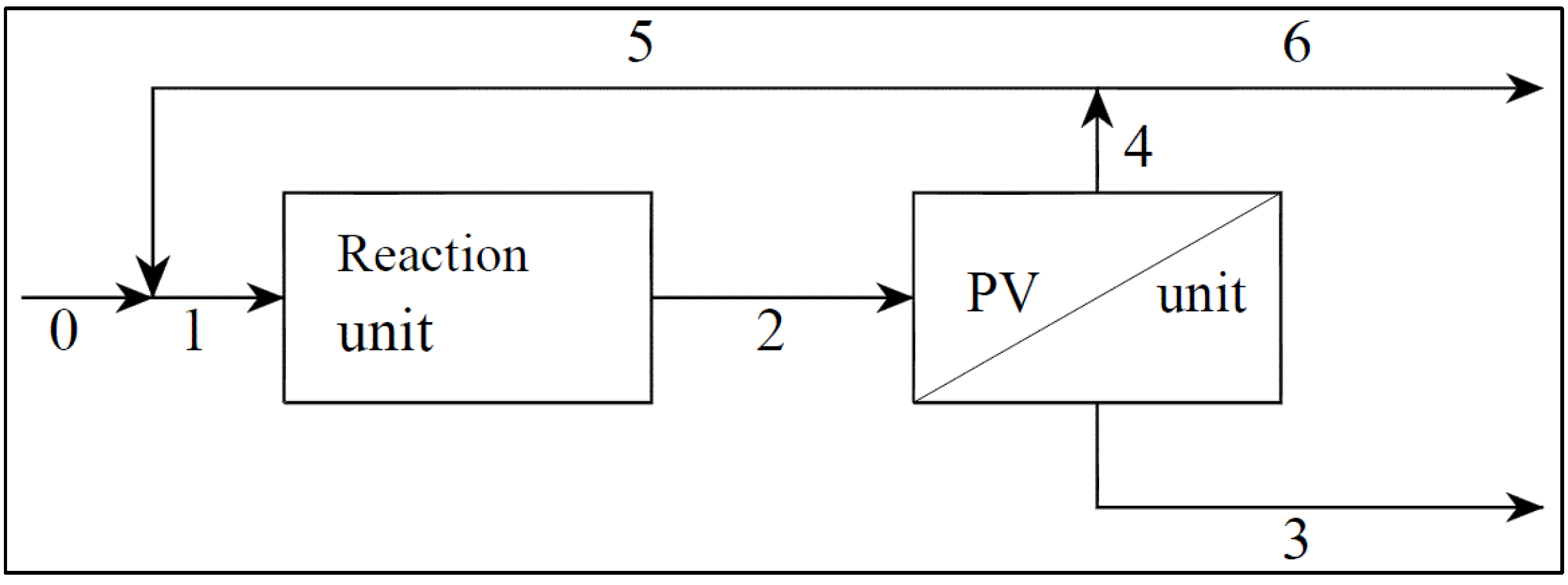
- S→P Reaction 1, which produces the desired product P (vanillin) from the substrate, S (ferulic acid), by photocatalytic partial oxidation with a reaction rate R1 = K1 CS;
- S→B Reaction 2, which, in parallel, produces the unwanted Product B with a reaction rate R2 = K2 CS;
- P→C Reaction 3, which degrades vanillin by a further oxidation with a reaction rate R3 = K3 CP.

- the ratios, R2 = k2/k1 and R3 = k3/k1, between kinetic constants;
- the enrichment factors of the membrane, βS = CS,permeate/CS and βP = CP,permeate/CP , where Ci is the concentration of the permeating compound upstream of the membrane and Ci,permeate is the concentration in the condensed permeate;
- the recycle ratio,
(0 ≤ R < 1), of the flow rate of Stream 5 to the flow rate of Stream 2;
- the Damköhler number,
, where Vr is the volume of the reactor and
is the volumetric flow rate of stream 0;
- the Péclet number,
, where A is the membrane area, ρ is the density of the condensed permeate and
is the total mass flux of the permeate through the membrane. The present definition of the Péclet number is consistent with the one commonly adopted in membrane reactors (see, e.g., [34,35]), with Pe representing the ratio of the convective transport to the permeation rate through the membrane. The highest allowable value of the reciprocal of Pe, 1/Pe, is 1, and it is reached when the membrane area is so high as to attain a flow rate of the permeate that equals the flow rate of the fresh feed to the system (
and
), while the lowest value is 0, which represents the case of a photocatalytic reactor without pervaporation (
and
).
3. Results and Discussion
3.1. Results Obtained by the Process Simulation
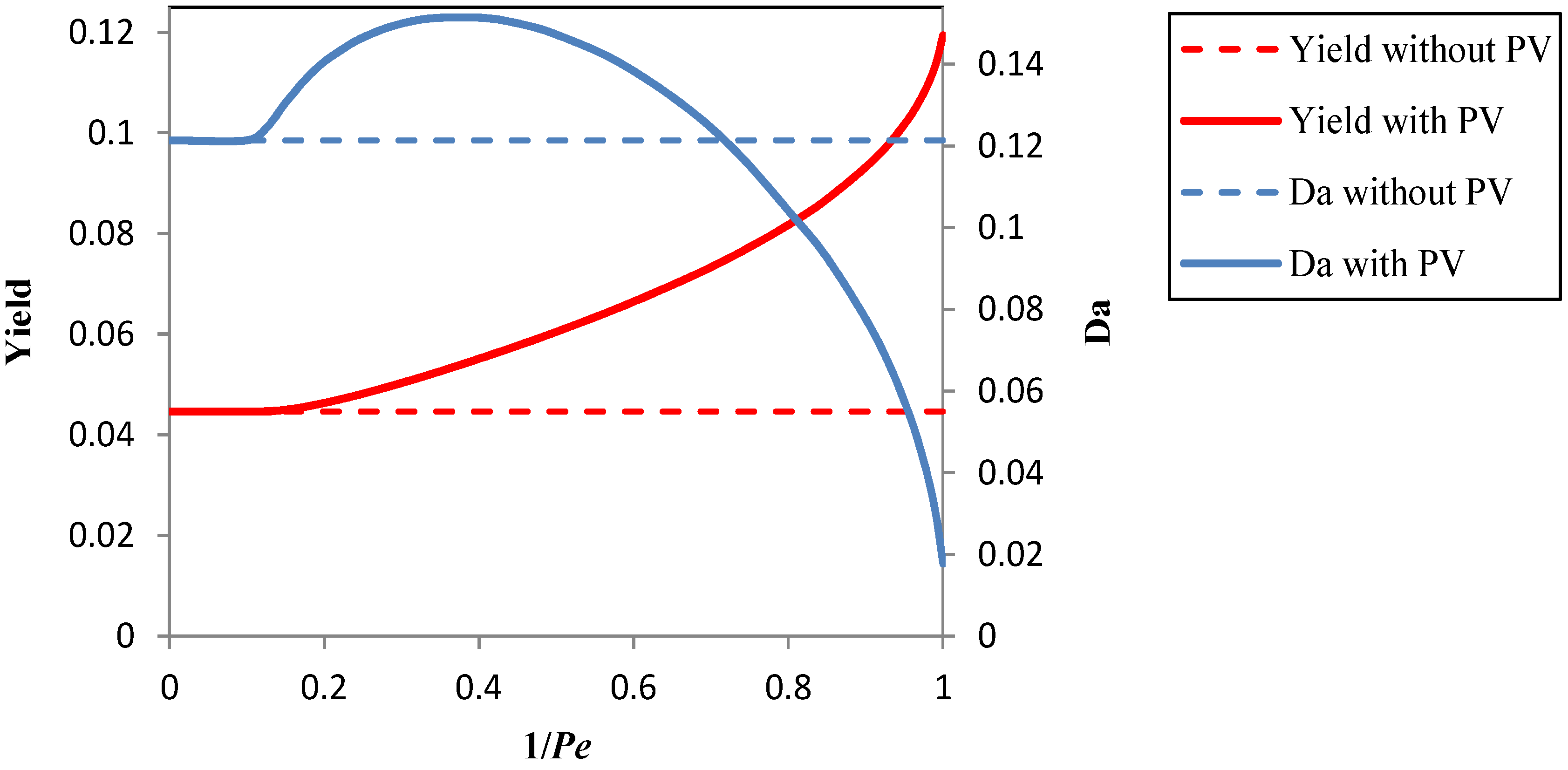
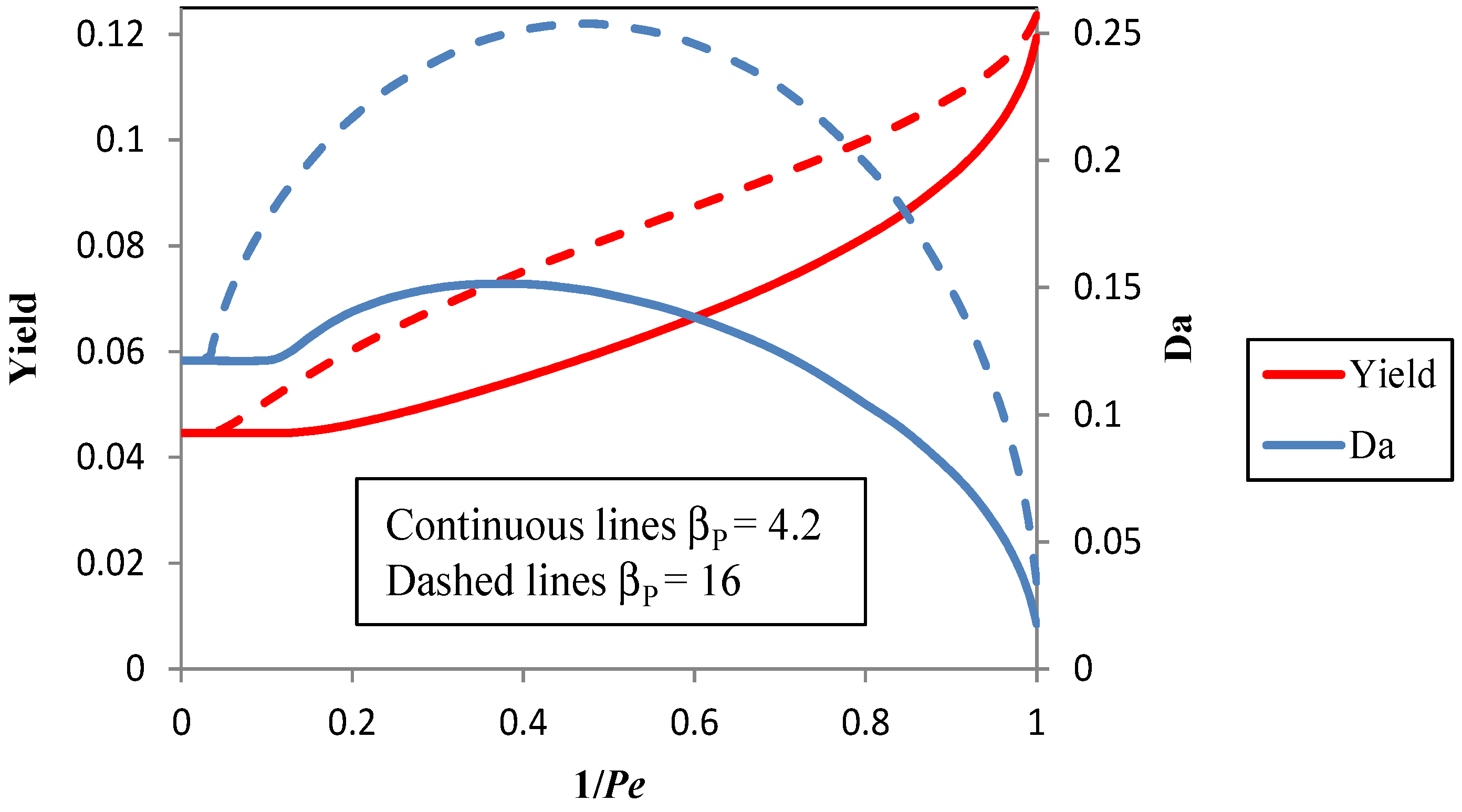
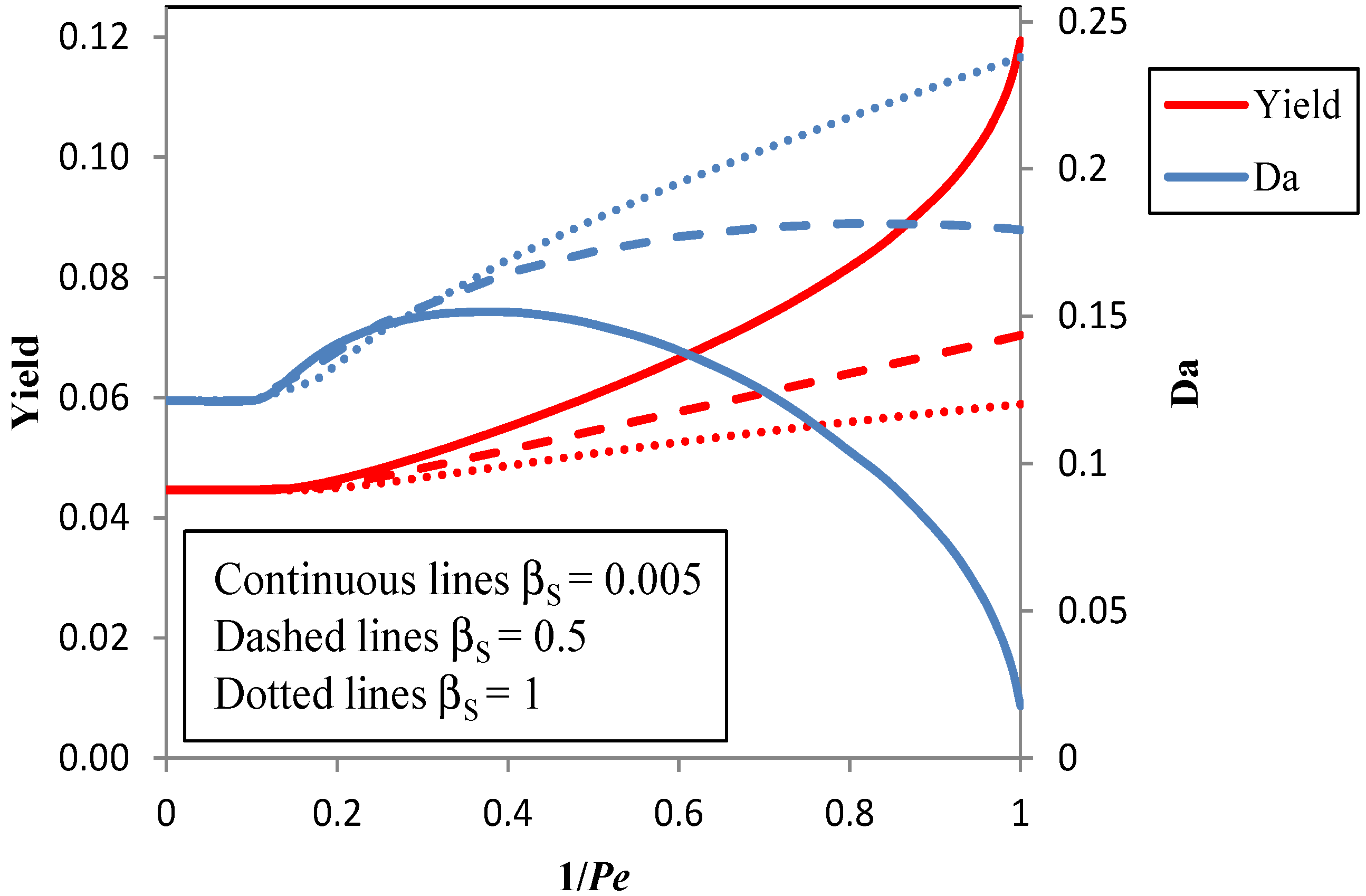
3.2. Improvement of the Pervaporation Performances of PEBA Membranes
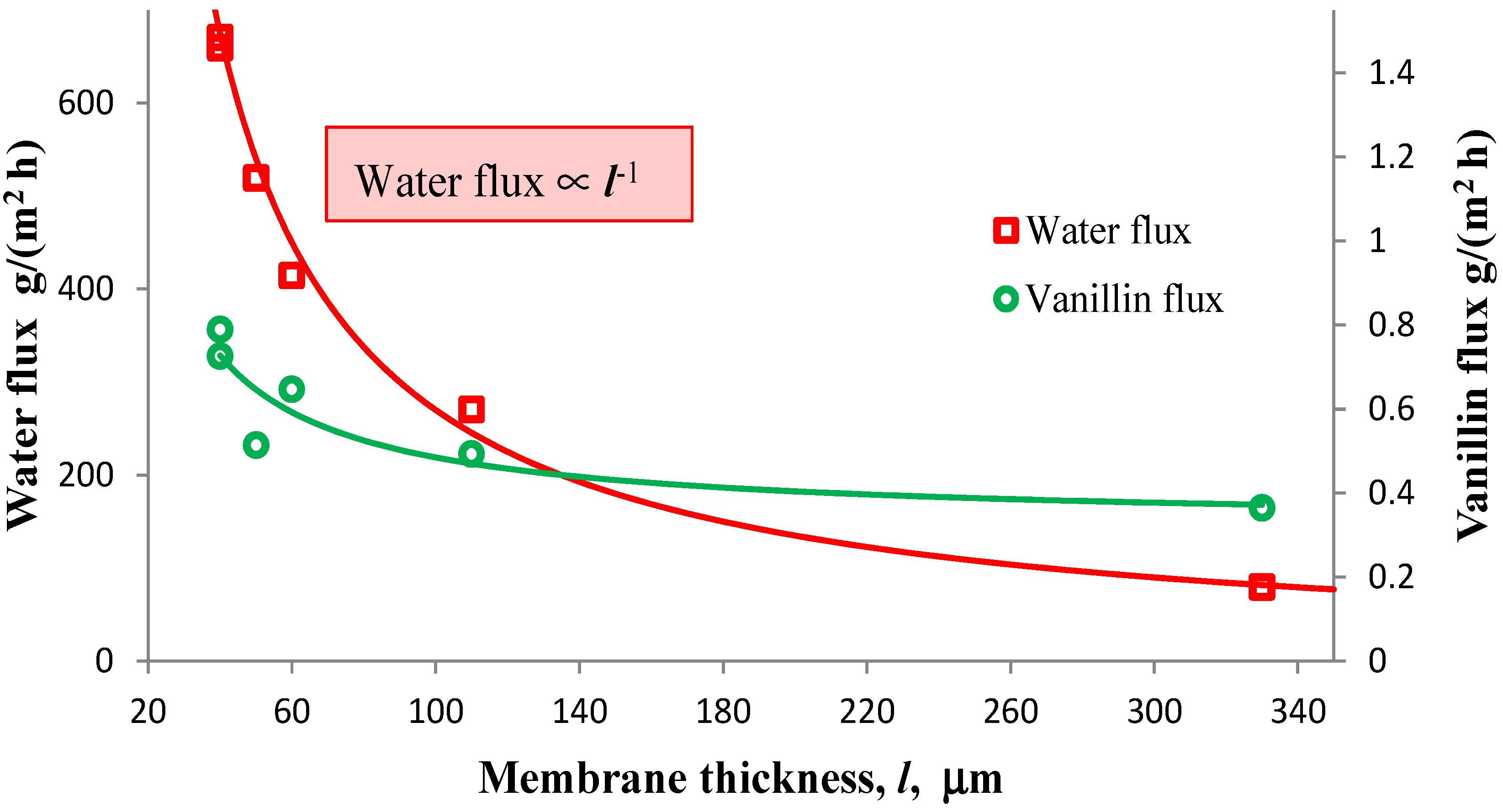
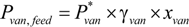 , where
, where 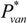 is the vapor pressure of vanillin and γvan is the activity coefficient of vanillin.
is the vapor pressure of vanillin and γvan is the activity coefficient of vanillin.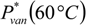 = 0.1105 mbar. The activity coefficient, computed with the UNIFAC group contribution method, is γvan = 47.18 at T = 60 °C and xvan = 1.18 × 10−4.
= 0.1105 mbar. The activity coefficient, computed with the UNIFAC group contribution method, is γvan = 47.18 at T = 60 °C and xvan = 1.18 × 10−4.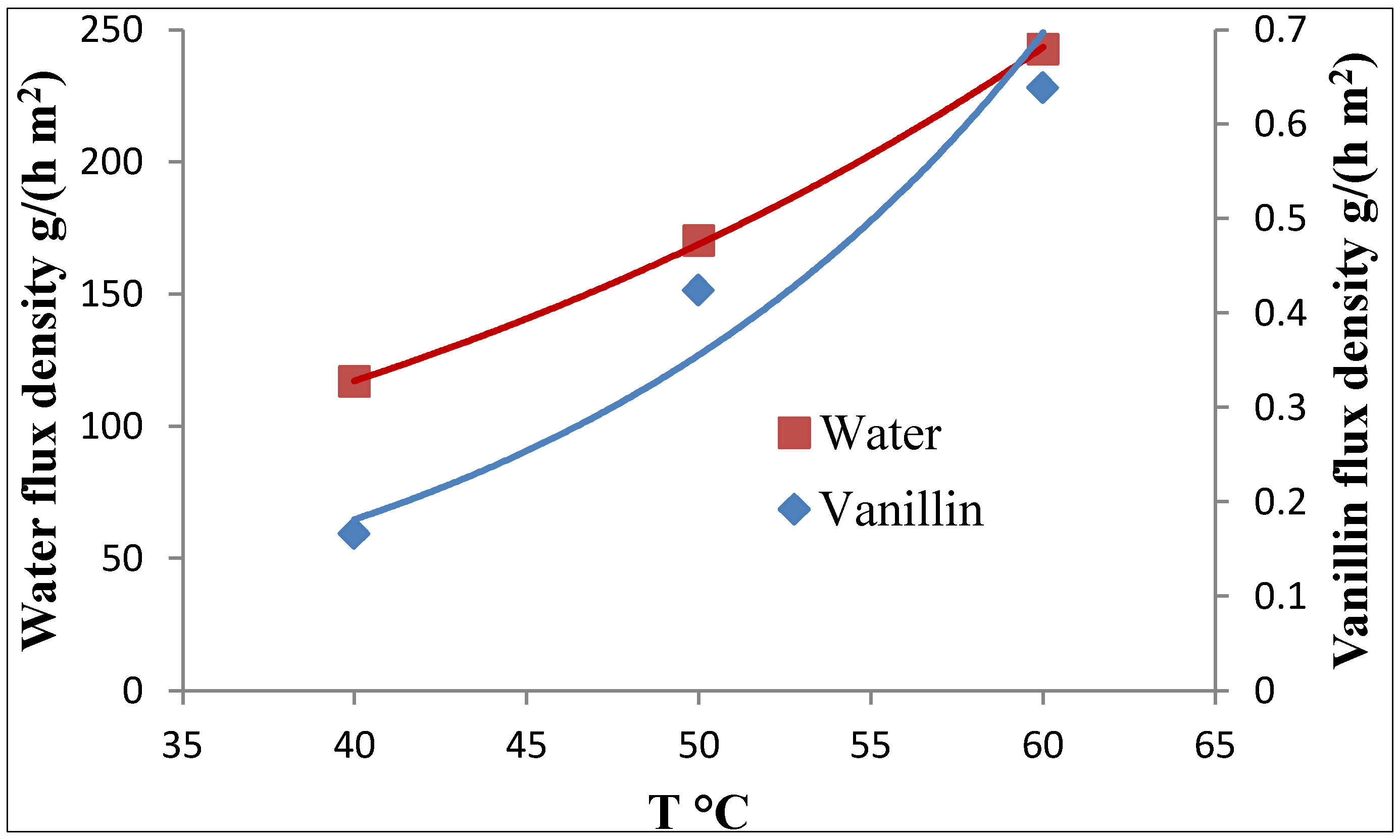
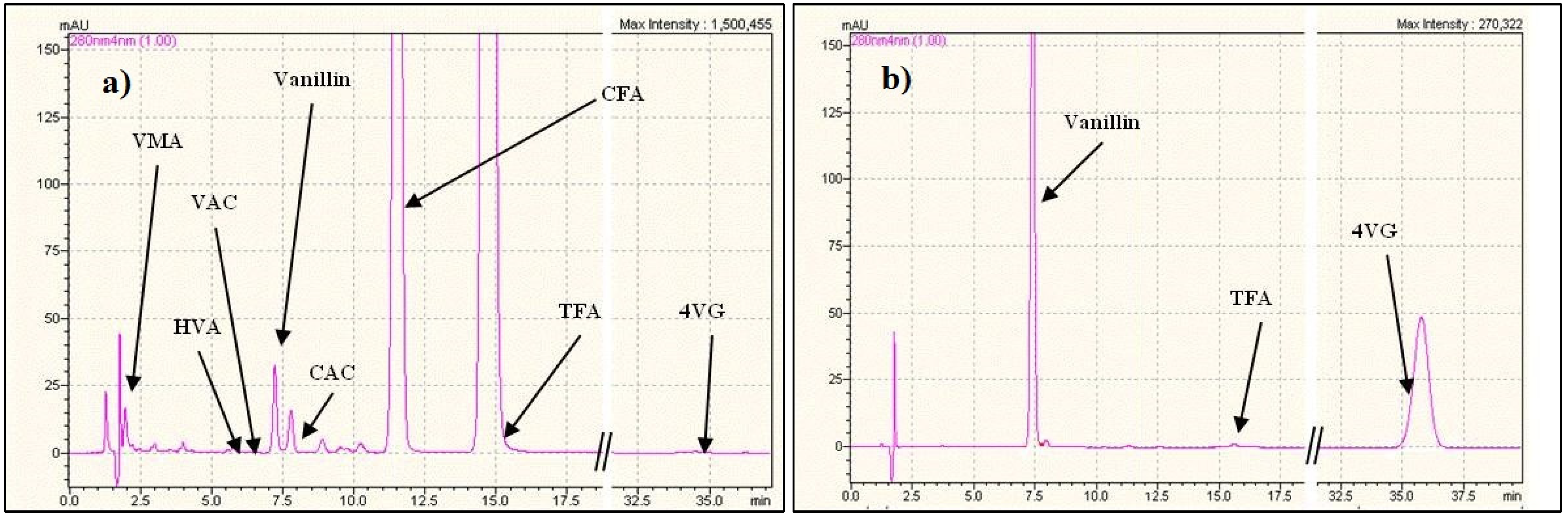
| pH | Feed concentrations | Flux vanillin | Flux TFA |
|---|---|---|---|
| ppm | g/(h m2) | g/(h m2) | |
| 3 | 609 Van + 777 TFA | 0.64 | 2.74 × 10−3 |
| 4.5 | 600 Van + 388 TFA | 0.64 | 4.50 × 10−4 |
| 7.9 | 540 Van + 660 TFA | 0.28 | 3.20 × 10−4 |
| 10.3 | 609 Van + 774 TFA | 0.01 | 1.63 × 10−4 |
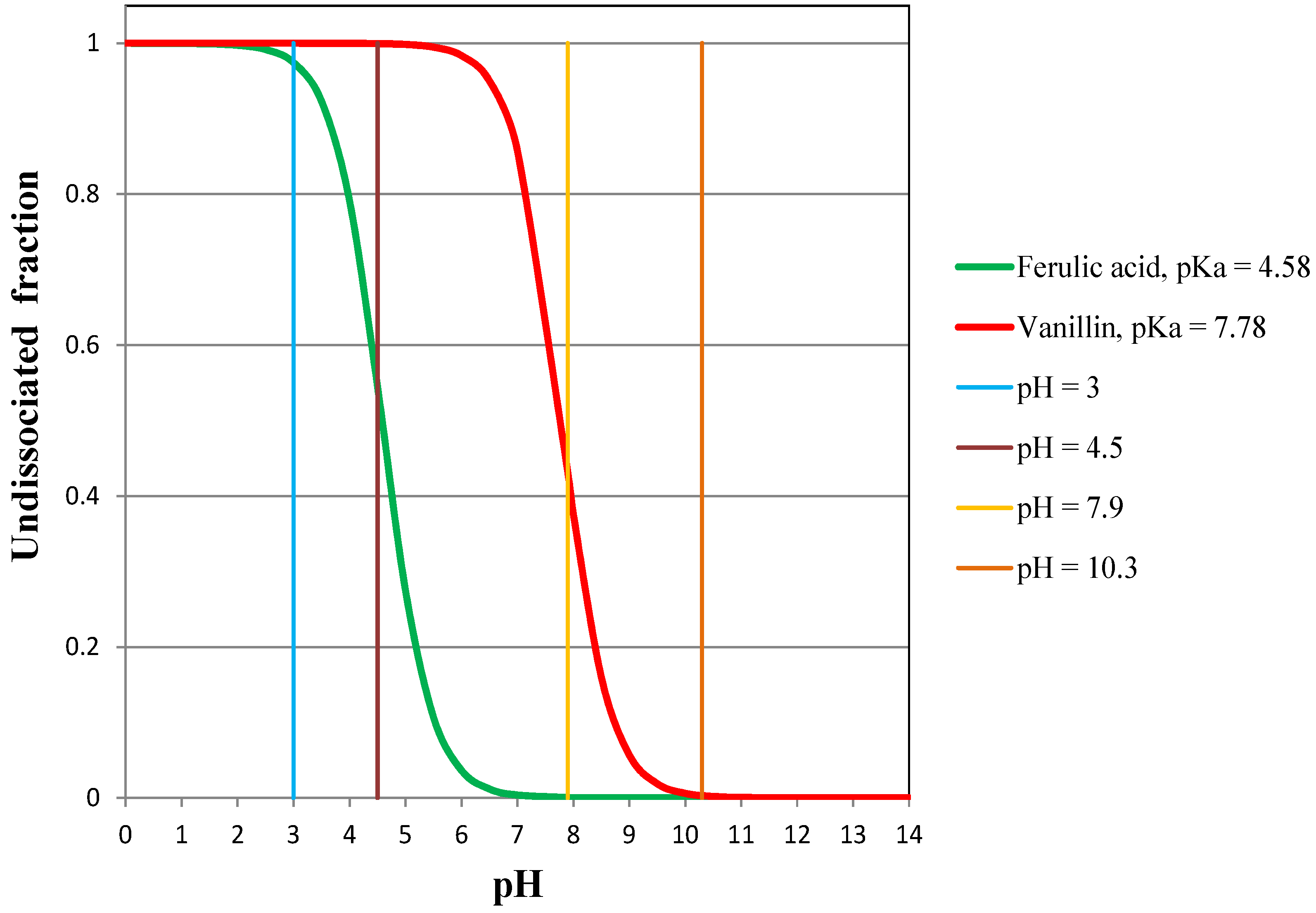
- the flux of vanillin does not change as long as it is totally undissociated (pH = 3 and pH = 4.5);
- the flux of vanillin is reduced to a certain degree when it is partly dissociated (pH = 7.9);
- the flux of vanillin is drastically reduced when it is dissociated (pH = 10.3);
- the higher the pH (or the higher the dissociated fraction of TFA), the lower the permeate flux of TFA;
- when TFA is totally undissociated, its flux is highly enhanced (at pH = 3, the flux is one order of magnitude higher than at pH = 10.3), but it still remains absolutely negligible.
4. Conclusions
Conflicts of Interest
References
- Havkin-Frenkel, D.; Belanger, F.C. Handbook of Vanilla Science and Technology; Wiley-Blackwell: Chichester, UK, 2011. [Google Scholar]
- Dignum, M.J.W.; Kerler, J.; Verpoorte, R. Vanilla production: Technological, chemical and biosynthetic aspects. Food Rev. Int. 2000, 17, 119–120. [Google Scholar]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Esposito, L.; Formanek, K.; Kientz, K.G.; Mauger, F.; Maureaux, V.; Robert, G.; Truchet, F. Vanillin. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 1997; Volume 24, pp. 812–825. [Google Scholar]
- Korthou, H.; Verpoorte, R. Vanilla. In Flavours and Fragrances; Springer: Heidelberg, Germany, 2007; pp. 203–217. [Google Scholar]
- Ramachandra, R.S.; Ravishankar, G.A. Vanilla flavour: production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Cheetham, P.S.J. Combining the technical push and the business pull for natural flavours. Adv. Biochem. Eng. Biotechnol. 1997, 55, 1–49. [Google Scholar]
- Straughan, R.D.; Roberts, J.A. Environmental segmentation alternatives: A look at green consumer behavior in the new millennium. J. Consum. Market. 1999, 16, 558–575. [Google Scholar] [CrossRef]
- Laroche, M.; Bergeron, J.; Barbaro-Forleo, G. Targeting consumers who are willing to pay more for environmentally friendly products. J. Consum. Market. 2001, 18, 503–520. [Google Scholar] [CrossRef]
- Pickett-Baker, J.; Ozaki, R. Pro-environmental products: Marketing influence on consumer purchase decision. J. Consum. Market. 2008, 25, 281–293. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef]
- Longo, M.A.; Sanromán, M.A. Production of food aroma compounds: Microbial and enzymatic methodologies. Food Technol. Biotechnol. 2006, 44, 335–353. [Google Scholar]
- Muheim, A.; Lerch, K. Towards a high-yield bioconversion of ferulic acid to vanillin. Appl. Microbiol. Biotechnol. 1999, 51, 456–461. [Google Scholar] [CrossRef]
- Gounaris, Y. Biotechnology for the production of essential oils, flavours and volatile isolates. A review. Flavour Frag. J. 2010, 25, 367–386. [Google Scholar] [CrossRef]
- Vandamme, E.J.; Soetaert, W. Bioflavours and fragrances via fermentation and biocatalysis. J. Chem. Technol. Biotechnol. 2002, 77, 1323–1332. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Singh, O.V. Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. J. Ind. Microbiol. Biotechnol. 2008, 35, 377–391. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology of flavours—The next generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Parrino, F.; Puma, M.A. Synthesis of vanillin in water by TiO2 photocatalysis. Appl. Catal. B Environ. 2012, 111–112, 555–561. [Google Scholar]
- Stankiewicz, A.; Moulijn, J.N. Process intensification transforming chemical engineering. Chem. Eng. Progr. 2000, 96, 22–34. [Google Scholar]
- Converti, A.; Aliakbarian, B.; Domìnguez, J.M.; Bustoz Vàsquez, G.; Perego, P. Microbial production of vanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Bengston, G.; Bode, E. Pervaporation of low volatility aromatics from water. J. Membr. Sci. 1990, 53, 143–158. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Bengston, G.; Pingel, H.; Dozel, S. Pervaporation of high boilers using heated membranes. Desalination 1993, 90, 249–257. [Google Scholar] [CrossRef]
- Böddeker, K.W.; Gatfield, I.L.; Jähnig, J.; Schorm, C. Pervaporation at the vapor pressure limit: Vanillin. J. Membr. Sci. 1997, 137, 155–158. [Google Scholar] [CrossRef]
- Brazhina, C.; Barbosa, B.; Crespo, G.J. Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Santarelli, F.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Photocatalytic process intensification by coupling with pervaporation. Catal. Today 2011, 161, 209–213. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Cardillo, A.; Loddo, V.; Palmisano, G.; Palmisano, L. A pervaporation photocatalytic reactor for the green synthesis of vanillin. Chem. Eng. J. 2013, 224, 136–143. [Google Scholar] [CrossRef]
- Camera-Roda, G.; Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Production of Aldehydes by Oxidation in Aqueous Medium with Recovery of the Product by Means of Pervaporation. U.S. Patent 20130123546 A1, 10 June 2011. [Google Scholar]
- Sanchez Marcano, J.G.; Tsotsis, T.T. Pervaporation Membrane Reactors. In Catalytic Membranes and Membrane Reactors; Wiley-VCH: Weinheim, Germany, 2002; pp. 97–132. [Google Scholar]
- Camera-Roda, G.; Augugliaro, V.; Loddo, V.; Palmisano, L. Pervaporation Membrane Reactors. In Handbook of Membrane Reactors, 1st ed.; Basile, A., Ed.; Woodhead Publishing: Cambridge, UK, 2013; Volume 1, pp. 107–151. [Google Scholar]
- Schembecker, G.; Tlatlik, S. Process synthesis for reactive separations. Chem. Eng. Process. 2003, 42, 179–189. [Google Scholar] [CrossRef]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reactor Engineering; Springer Science: New York, NY, USA, 2005. [Google Scholar]
- Camera Roda, G.; Santarelli, F. Design of a pervaporation photocatalytic reactor for process intensification. Chem. Eng. Technol. 2012, 35, 1221–1228. [Google Scholar] [CrossRef]
- Battersby, P.W.; Teixeira, P.W.; Beltramini, J.; Duke, M.C.; Rudolph, V.; Diniz Da Costa, J.C. An analysis of the Péclet and Damköhler numbers for dehydrogenation reactions using molecular sieve silica (MSS) membrane reactors. Catal. Today 2006, 116, 12–17. [Google Scholar] [CrossRef]
- Moon, W.S.; Park, S.B. Design guide of a membrane for a membrane reactor in terms of permeability and selectivity. J. Membr. Sci. 2000, 170, 43–51. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Böddeker, K.W. Liquid Separation with Membranes, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008; pp. 1–146. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Groß, A.; Heintz, A. Diffusion coefficients of aromatics in nonporous PEBA membranes. J. Membr. Sci. 2000, 168, 233–242. [Google Scholar] [CrossRef]
- Vane, L.M. A review of pervaporation for product recovery from biomass fermentation processes. J. Chem. Technol. Biotechnol. 2005, 80, 603–629. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Camera-Roda, G.; Cardillo, A.; Loddo, V.; Palmisano, L.; Parrino, F. Improvement of Membrane Performances to Enhance the Yield of Vanillin in a Pervaporation Reactor. Membranes 2014, 4, 96-112. https://doi.org/10.3390/membranes4010096
Camera-Roda G, Cardillo A, Loddo V, Palmisano L, Parrino F. Improvement of Membrane Performances to Enhance the Yield of Vanillin in a Pervaporation Reactor. Membranes. 2014; 4(1):96-112. https://doi.org/10.3390/membranes4010096
Chicago/Turabian StyleCamera-Roda, Giovanni, Antonio Cardillo, Vittorio Loddo, Leonardo Palmisano, and Francesco Parrino. 2014. "Improvement of Membrane Performances to Enhance the Yield of Vanillin in a Pervaporation Reactor" Membranes 4, no. 1: 96-112. https://doi.org/10.3390/membranes4010096
APA StyleCamera-Roda, G., Cardillo, A., Loddo, V., Palmisano, L., & Parrino, F. (2014). Improvement of Membrane Performances to Enhance the Yield of Vanillin in a Pervaporation Reactor. Membranes, 4(1), 96-112. https://doi.org/10.3390/membranes4010096