Nanocomposites for Improved Physical Durability of Porous PVDF Membranes
Abstract
:1. Introduction
1.1. The Water Issue and Role of Membrane Technology
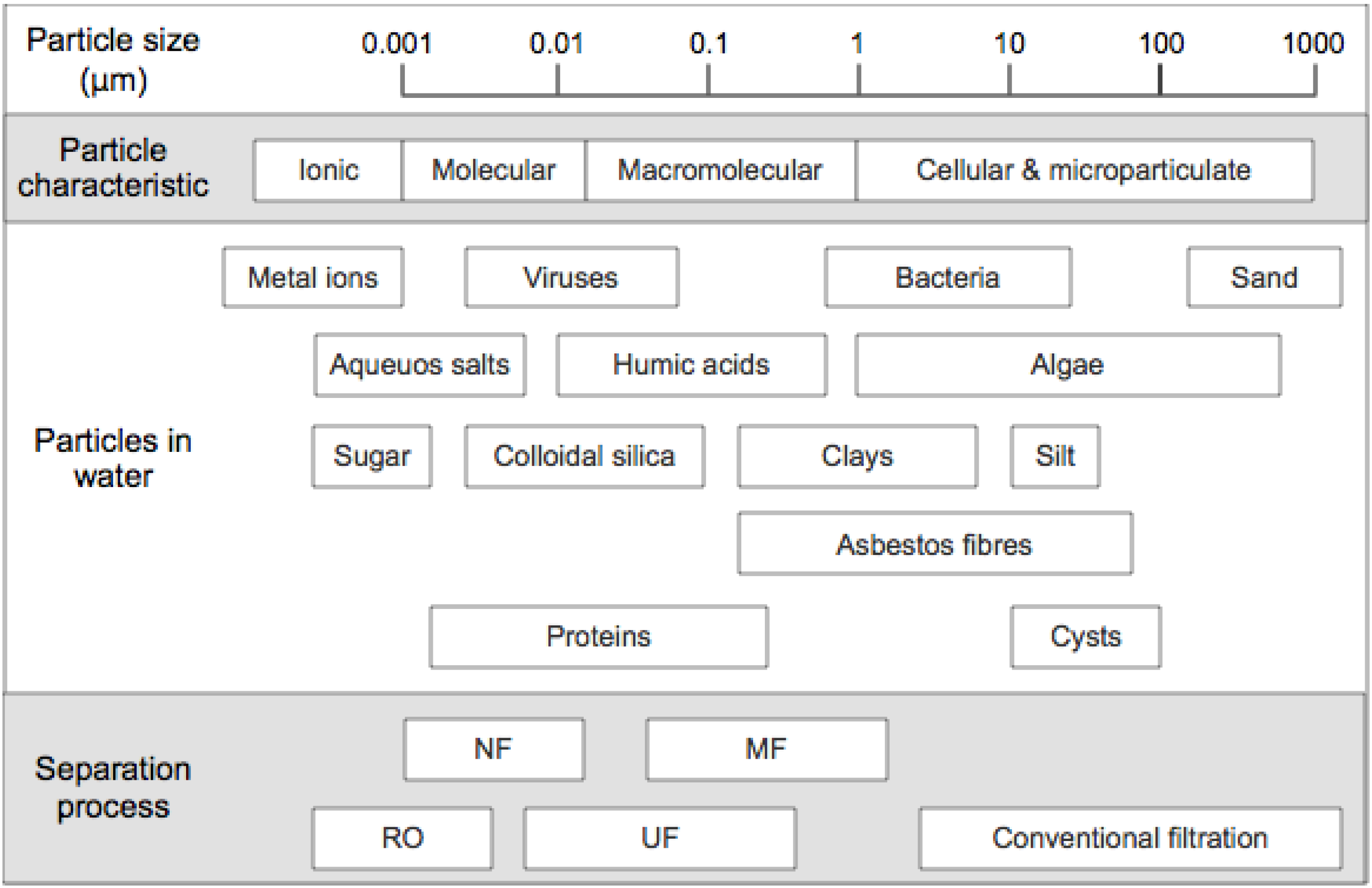
1.2. Current Performance Issues
1.2.1. Membrane Fouling
| Fouling type | Foulants | Mechanism | Mitigation |
|---|---|---|---|
Particulate deposition (Reprinted with permission from [17]. Copyright 2001 Elsevier) | Inorganic particles and colloids from weathering of rocks (e.g., silts and clays) | Deposition of particles and colloids forms cake layer on top of membrane which become compressed and reduce flux | Backwashing or air scrubbing is often effective to remove the cake |
Organic fouling (Reprinted with permission from [18]. Copyright 2013 Elsevier) | Natural organic matters (NOM) including humic acids, fulvic acids, proteins, amino sugars, polysaccharides, polyoxyaromatics | Negative charged foulants have an affinity for charged membrane surface which forms layer reducing flux and salt rejection | Chemical cleaning with caustic and/or chlorine is used to control organic fouling |
Inorganic fouling (Reprinted with permission from [19]. Copyright 2013 Elsevier) | Inorganic precipitates such as metal hydroxides | Accumulation of inorganic precipitates causes scaling on membrane surface or within pore structure | Cleaning with acids and chelating agents can remove scales and metal dioxides from fouling layers |
Biofouling (Reprinted with permission from [20]. Copyright 2007 Elsevier) | Microorganism including bacteria, algae and fungi | Microbial activities lead to formation of biofilms on the membrane | Biofouling is commonly controlled using chlorine (including chloramine) and biocide cleans |
1.2.2. Physical Durability

1.3. Polymer Composite and Nanocomposite
2. Nanocomposite Membranes
2.1. Membrane Materials
PVDF Crystalline Phases
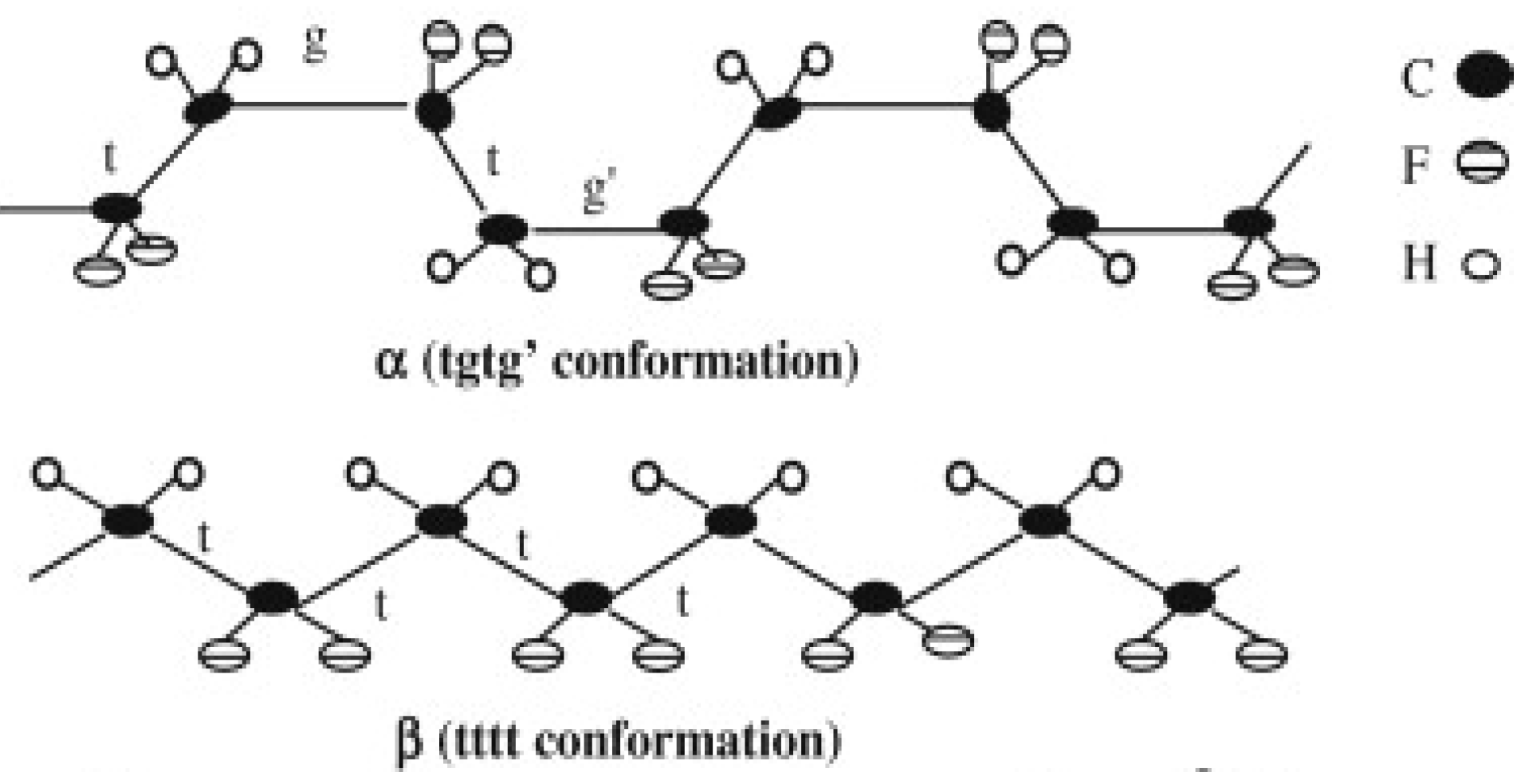
2.2. Nanofillers
2.2.1. Nanoparticles

2.2.1.1. Mechanical Enhancement
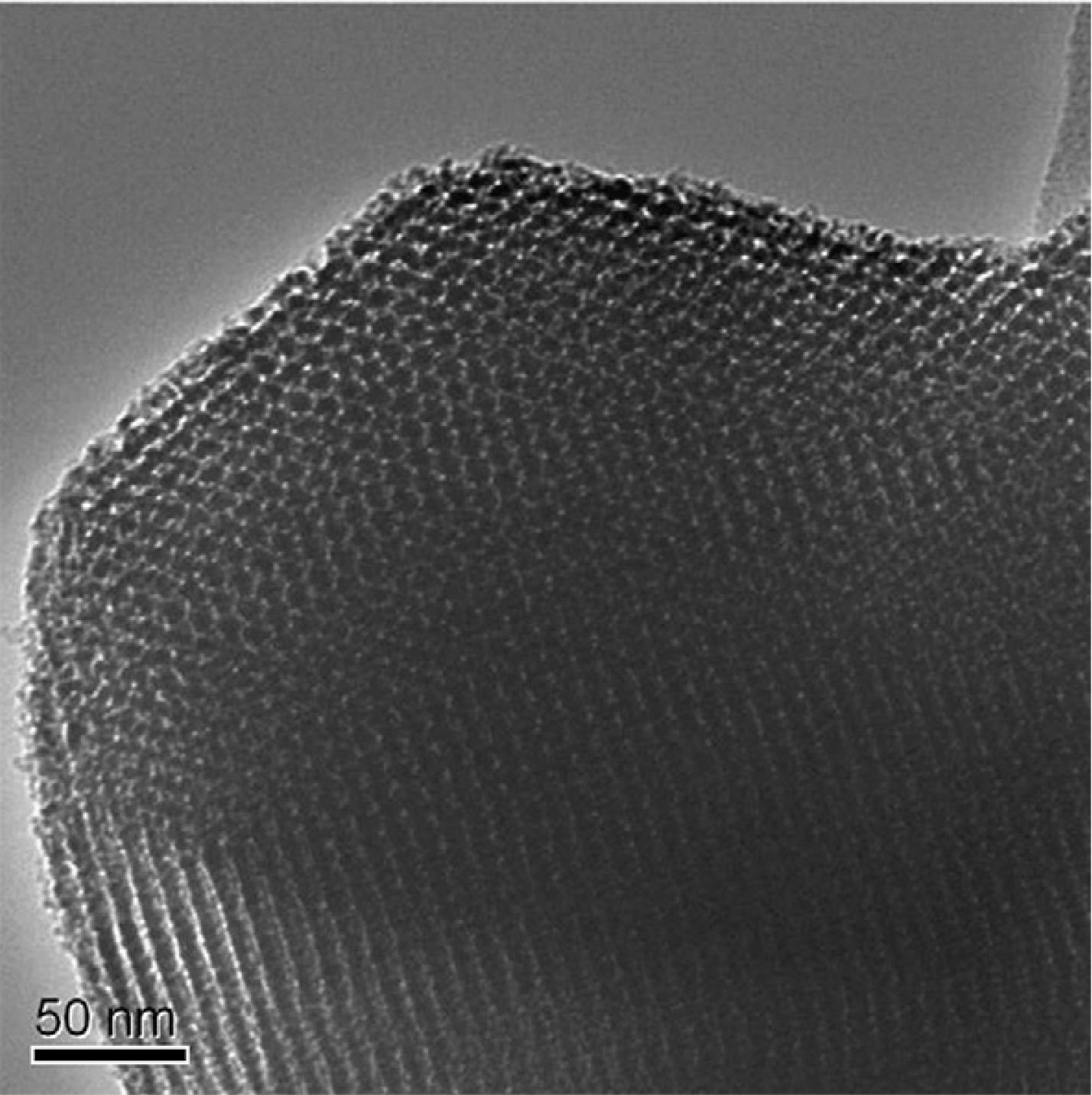
2.2.1.2. Hydraulic Performance
| Membrane No. | SiO2 concentration (wt % in dope) | Contact angle (°) | Pure water flux (L/m2·h) |
|---|---|---|---|
| MTEOS-3 | 3 | 53.4 | 301 |
| MTEOS-2 | 2 | 64.4 | 255 |
| MTEOS-4 | 4 | 67.7 | 210 |
| MTEOS-1 | 1 | 78.5 | 185 |
| MTEOS-5 | 5 | 76.3 | 125 |
| MTEOS-0 | 0 | 82.9 | 80 |
2.2.1.3. Fouling Resistance
2.2.2. CNT
2.2.3. Nanoclay
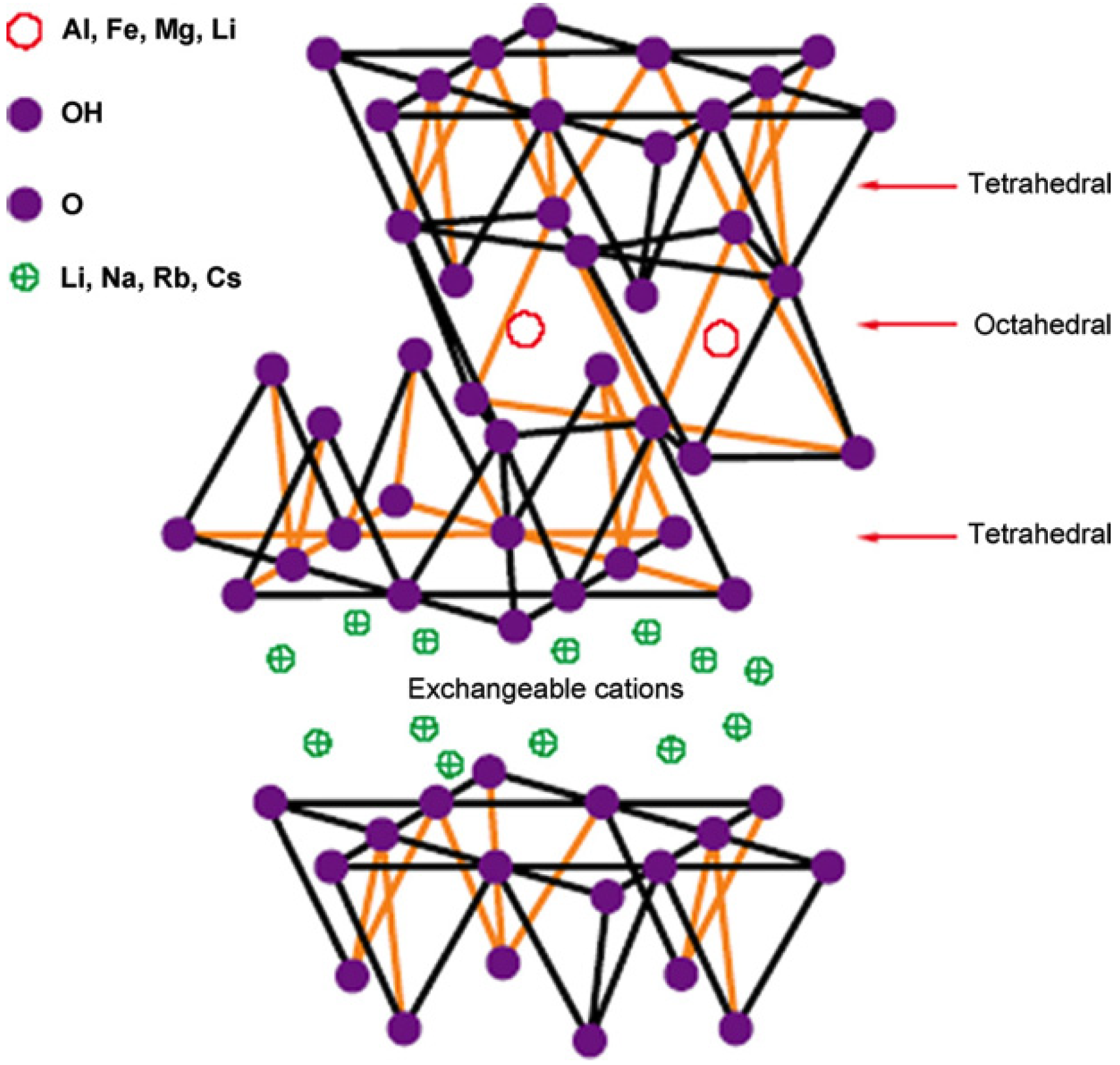

2.2.3.1. Mechanical Enhancement
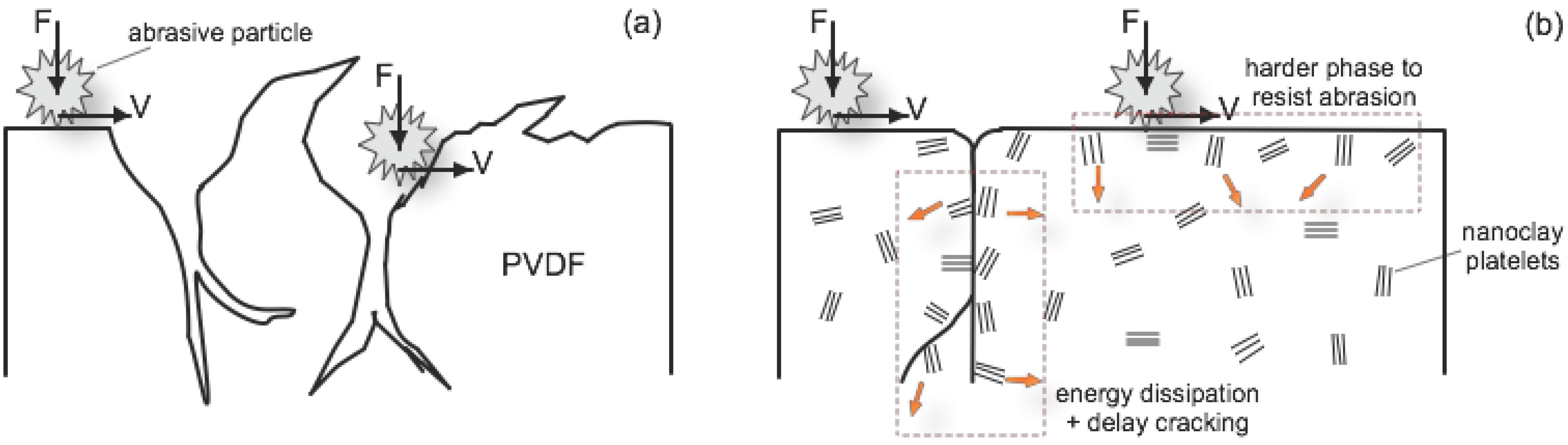
2.2.3.2. Abrasion Resistance
2.2.3.3. Flux Performance
2.2.3.4. Fouling Resistance
| Nanofiller added | Type | Application | Casting condition | Observed changes | Ref. |
|---|---|---|---|---|---|
| 40% TiO2 by weight of PVDF | Flat sheet | Mechanical support for composite membrane | PVDF dissolved in DMAc with LiCl then mixed with TiO2 Quench bath medium: water |
| [66] |
| TiO2, SiO2 and Al2O3 Ratio of dope: PVDF/DMAc/NMP/nanoparticles/PVP (18/59.2/14.8/3/5) | Hollow fibre | UF | 24 h of mechanical stirring of PVDF and nanoparticles in DMAc/NMP/PVP at 25 °C then 1 h of ultrasonic stirring. Internal coagulant: 40 wt % ethanol aqueous solution at 60 °C External coagulant: water at 60°C |
| [67] |
| 0.12–0.72 wt % SBA-15 by weight of PVDF | Flat sheet | UF | PVDF dissolved in DMAc and mixed with PVP and SBA-15 at 60 °C Quench bath medium: water |
| [22] |
| 1 wt % of Cloisite® Na+ or 1 wt % of Cloisite® 15A or 1 wt % of Cloisite® 20A, or 1 wt % of Cloisite® 30B by weight of PVDF | Flat sheet | Lithium-ion battery | PVDF dissolved in DMF at 70 °C then mixed with clay/DMF suspensions. Air retention time: 30 s or 60 s Quench bath medium: water |
| [83] |
| Cloisite® 20A Ratio of dope: PVDF/NMP/Cloisite® 20A/EG (10.0/74.7/3.3/12.0) | Hollow fibre | DCMD | PVDF stirred with clay in NMP and EG mixture. Internal and external coagulants: water |
| [84] |
| 0.88–5.08 wt % of Cloisite® 30B or 0.88–5.08 wt % of Nanomer® I.44P by weight of PVDF | Hollow fibre | MF/UF | PVDF mixed with pre-dispersed nanoclay (dispersed with ultrasonication and a high shear hydrodynamic dispersion process) in NMP at 90 °C for 48 h and extruded with dry-wet spinning at 60 °C |
| [86] |
3. Conclusions
Abbreviations
| BSA | bovine serum albumin |
| CNF | carbon nanofibers |
| CNT | carbon nanotubes |
| DCMD | direct contact membrane distillation |
| EG | ethylene glycol |
| MF | microfiltration |
| MFI | modified fouling index |
| MMT | montmorillonite |
| MOF | metal-organic frameworks |
| MWCNT | multi-walled nanotubes |
| NF | nanofiltration |
| NMP | 1-methyl-2-pyrrolidinone |
| NOM | natural organic matters |
| PEG | poly(ethylene glycol) |
| PP | polypropylene |
| PSf | polysulfone |
| PTFE | polytetrafluoroethylene |
| PVDF | poly(vinylidene fluoride) |
| PVP | poly(vinyl pyrrolidone) |
| RO | reverse osmosis |
| SBA-15 | Santa Barbara Amorphous No. 15 |
| SWCNT | single-walled nanotubes |
| UF | ultrafiltration |
| XRD | X-ray powder diffraction |
Acknowledgments
Conflicts of Interest
References
- Strathmann, H.; Giorno, L.; Drioli, E. An Introduction to Membrane Science and Technology; Wiley: Rome, Italy, 2011. [Google Scholar]
- Graham, T. On the law of the diffusion of gases. J. Membr. Sci. 1995, 100, 17–21. [Google Scholar] [CrossRef]
- Graham, T. Notice of the singular inflation of a bladder. J. Membr. Sci. 1995, 100, 9. [Google Scholar] [CrossRef]
- Reid, C.E.; Breton, E.J. Water and ion flow across cellulosic membranes. J. Appl. Polym. Sci. 1959, 1, 133–143. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. In Saline Water Conversion II; American Chemical Society: Ottawa, Canada, 1963; Volume 38, pp. 117–132. [Google Scholar]
- Kurihara, M.; Himeshima, Y.; Uemura, T. Preprints of ICOM, Tokyo, 1987; The Aseanian Membrane Society: Tokyo, Japan, 1987; p. 428. [Google Scholar]
- American Water Works Association. Microfiltration and Ultrafiltration Membranes for Drinking Water (M53); American Water Works Association: Denver, CO, USA, 2011. [Google Scholar]
- Hammer, M.J. Water and Wastewater Technology; Prentice Hall/Pearson Education International: Upper Saddle River, NJ, USA, 2004; Volume 5, p. 540. [Google Scholar]
- Chen, J.P.; Mou, H.; Wang, L.K.; Matsuura, T. Membrane Filtration. In Advanced Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2006; pp. 203–259. [Google Scholar]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons, Ltd.: Chichester, UK, 2007; p. 306. [Google Scholar]
- Weber, R.; Chmiel, H.; Mavrov, V. Characteristics and application of new ceramic nanofiltration membranes. Desalination 2003, 157, 113–125. [Google Scholar] [CrossRef]
- Funk, C.V.; Lloyd, D.R. Zeolite-filled microporous mixed matrix (ZeoTIPS) membranes: Prediction of gas separation performance. J. Membr. Sci. 2008, 313, 224–231. [Google Scholar] [CrossRef]
- Wetterau, G.E.; Clark, M.M.; Anselme, C. A dynamic model for predicting fouling effects during the ultrafiltration of a groundwater. J. Membr. Sci. 1996, 109, 185–204. [Google Scholar] [CrossRef]
- Kennedy, M.D.; Kamanyi, J.; Rodríguez, S.G.S.; Lee, N.H.; Schippers, J.C.; Amy, G. Water Treatment by Microfiltration and Ultrafiltration. In Advanced Membrane Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 131–170. [Google Scholar]
- Baker, J.S.; Dudley, L.Y. Biofouling in membrane systems—A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Parida, V.; Ng, H.Y. Forward osmosis organic fouling: Effects of organic loading, calcium and membrane orientation. Desalination 2013, 312, 88–98. [Google Scholar] [CrossRef]
- Boo, C.; Elimelech, M.; Hong, S. Fouling control in a forward osmosis process integrating seawater desalination and wastewater reclamation. J. Membr. Sci. 2013, 444, 148–156. [Google Scholar] [CrossRef]
- Herzberg, M.; Elimelech, M. Biofouling of reverse osmosis membranes: Role of biofilm-enhanced osmotic pressure. J. Membr. Sci. 2007, 295, 11–20. [Google Scholar] [CrossRef]
- Contreras, A.E.; Steiner, Z.; Miao, J.; Kasher, R.; Li, Q. Studying the role of common membrane surface functionalities on adsorption and cleaning of organic foulants using QCM-D. Environ. Sci. Technol. 2011, 45, 6309–6315. [Google Scholar] [CrossRef]
- Liao, C.; Zhao, J.; Yu, P.; Tong, H.; Luo, Y. Synthesis and characterization of SBA-15/poly (vinylidene fluoride) (PVDF) hybrid membrane. Desalination 2010, 260, 147–152. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, N.; Lee, Y.T. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J. Membr. Sci. 2009, 345, 13–20. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B. Preparation of poly(vinylidene fluoride) (PVDF) ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer 2005, 46, 7701–7706. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Xu, Z.-L.; Shen, H.-M.; Yang, H. Preparation and characterization of PVDF–SiO2 composite hollow fiber UF membrane by sol-gel method. J. Membr. Sci. 2009, 337, 257–265. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Shen, H.-M.; Xu, Z.-L. PVDF-TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol-gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Wang, P.; Ma, J.; Wang, Z.; Shi, F.; Liu, Q. Enhanced separation performance of PVDF/PVP-g-MMT nanocomposite ultrafiltration membrane based on the NVP-grafted polymerization modification of montmorillonite (MMT). Langmuir 2012, 28, 4776–4786. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, K.; Mo, Y.; Huang, X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J. Membr. Sci. 2012, 394–395, 184–192. [Google Scholar] [CrossRef]
- Gray, S.R.; Semiat, R.; Duke, M.; Rahardianto, A.; Cohen, Y. Seawater Use and Desalination Technology. In Treatise on Water Science; Wilderer, P., Ed.; Academic Press: Oxford, UK, 2011; Volume 4, pp. 73–109. [Google Scholar]
- Voutchkov, N. Pretreatment Technologies for Membrane Seawater Desalination; Australian Water Association: St Leonards, Australia, 2008. [Google Scholar]
- Knops, F.; van Hoof, S.; Futselaar, H.; Broens, L. Economic evaluation of a new ultrafiltration membrane for pretreatment of seawater reverse osmosis. Desalination 2007, 203, 300–306. [Google Scholar] [CrossRef]
- Busch, M.; Chu, R.; Rosenberg, S. Novel trends in dual membrane systems for seawater desalination: Minimum primary pretreatment and low environmental impact treatment schemes. IDA J. Desalin. Water Reuse 2010, 2, 56–71. [Google Scholar] [CrossRef]
- Henthorne, L. Evaluation of Membrane Pretreatment for Seawater Reverse Osmosis Desalination; U.S. Department of the Interior, Bureau of Reclamation: Denver, CO, USA, 2007.
- Gasia-Bruch, E.; Sehn, P.; Garcia-Molina, V.; Busch, M.; Raize, O.; Negrin, M. Field experience with a 20,000 m3/d integrated membrane seawater desalination plant in Cyprus. Desalin. Water Treat. 2011, 31, 178–189. [Google Scholar] [CrossRef]
- Voutchkov, N. Conventional or Membrane Filtration for Seawater RO? In Asian Water; SHP Media Sdn. Bhd: Kuala Lumpur, Malaysia, 2008. [Google Scholar]
- American Membrane Technology Association. Membrane Filtration (MF/UF); American Membrane Technology Association: Stuart, FL, USA, 2007. [Google Scholar]
- Stear, R.M.; Parr, J.; Smith, M.D. Decreasing Freshwater Demand: Dual supplies. In Proceedings of the 23rd WEDC Conference on Water and Sanitation for All, Durban, South Africa, 1–5 September 1997.
- Chesters, S.P.; Pena, N.; Gallego, S.; Fazel, M.; Armstrong, M.W.; del Vigo, F. Results from 99 Seawater RO Membrane Autopsies; IDA World Congress: Perth, Western Australia, 2011. [Google Scholar]
- Voutchkov, N. Considerations for selection of seawater filtration pretreatment system. Desalination 2010, 261, 354–364. [Google Scholar] [CrossRef]
- Sheldon, R.W.; Praksh, A.; Sutcliffe, W.H., Jr. The size distribution of particles in the ocean. Limnol. Oceanogr. 1972, 17, 327–340. [Google Scholar] [CrossRef]
- McCave, I.N. Size spectra and aggregation of suspended particles in the deep ocean. Deep Sea Res. 1984, 31, 329–352. [Google Scholar] [CrossRef]
- Guo, H.; Wyart, Y.; Perot, J.; Nauleau, F.; Moulin, P. Low-pressure membrane integrity tests for drinking water treatment: A review. Water Res. 2010, 44, 41–57. [Google Scholar] [CrossRef]
- Gijsbertsen-Abrahamse, A.J.; Cornelissen, E.R.; Hofman, J.A.M.H. Fiber failure frequency and causes of hollow fiber integrity loss. Desalination 2006, 194, 251–258. [Google Scholar] [CrossRef]
- Childress, A.E.; Le-Clech, P.; Daugherty, J.L.; Chen, C.; Leslie, G.L. Mechanical analysis of hollow fiber membrane integrity in water reuse applications. Desalination 2005, 180, 5–14. [Google Scholar] [CrossRef]
- Pochiraju, K.V.; Tandon, G.P.; Schoeppner, G.A. Long-Term Durability of Polymeric Matrix Composites; Springer: New York, NY, USA, 2011. [Google Scholar]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Takahara, A.; Magnome, T.; Kajiyama, T. Effect of glass fiber-matrix polymer interaction on fatigue characteristics of short glass fiber-reinforced poly(butylene terephthalate) based on dynamic viscoelastic measurement during the fatigue process. J. Polym. Sci. B Polym. Phys. 1994, 32, 839–849. [Google Scholar] [CrossRef]
- Unal, H.; Mimaroglu, A.; Alkan, M. Mechanical properties and morphology of nylon-6 hybrid composites. Polym. Int. 2004, 53, 56–60. [Google Scholar] [CrossRef]
- Li, W.; Sun, X.; Wen, C.; Lu, H.; Wang, Z. Preparation and characterization of poly (vinylidene fluoride)/TiO2 hybrid membranes. Front. Environ. Sci. Eng. 2013, 7, 492–502. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Basu, S.; Maes, M.; Cano-Odena, A.; Alaerts, L.; de Vos, D.E.; Vankelecom, I.F.J. Solvent resistant nanofiltration (SRNF) membranes based on metal-organic frameworks. J. Membr. Sci. 2009, 344, 190–198. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.J.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Silver nanoparticles immobilized on thin film composite polyamide membrane: Characterization, nanofiltration, antifouling properties. Polym. Adv. Technol. 2007, 18, 562–568. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic: Dordrecht, The Netherlands; Boston, MA, USA, 1996. [Google Scholar]
- Kubota, N.; Hashimoto, T.; Mori, Y. Microfiltration and Ultrafiltration. In Advanced Membrane Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 101–129. [Google Scholar]
- Shah, D.; Maiti, P.; Gunn, E.; Schmidt, D.F.; Jiang, D.D.; Batt, C.A.; Giannelis, E.P. Dramatic enhancements in toughness of polyvinylidene fluoride nanocomposites via nanoclay-directed crystal structure and morphology. Adv. Mater. 2004, 16, 1173–1177. [Google Scholar] [CrossRef]
- Lovinger, A.J. Poly(vinylidene fluoride). In Development in Crystalline Polymers; Applied Science Publishers: London, UK, 1982; Volume 1. [Google Scholar]
- Mago, G.; Kalyon, D.M.; Fisher, F.T. Membranes of polyvinylidene fluoride and PVDF nanocomposites with carbon nanotubes via immersion precipitation. J. Nanomater. 2008. [Google Scholar] [CrossRef]
- Buonomenna, M.G.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Ameduri, B. From vinylidene fluoride (VDF) to the applications of VDF-containing polymers and copolymers: Recent developments and future trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef]
- Kawai, H. The piezoelectricity of poly(vinylidene fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Bharti, V.; Zhao, X. Giant electrostriction and relaxor ferroelectric behavior in electron-irradiated poly(vinylidene fluoride-trifluoroethylene) copolymer. Science 1998, 280, 2101–2104. [Google Scholar] [CrossRef]
- Peng, Q.-Y.; Cong, P.-H.; Liu, X.-J.; Liu, T.-X.; Huang, S.; Li, T.-S. The preparation of PVDF/clay nanocomposites and the investigation of their tribological properties. Wear 2009, 266, 713–720. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, J.; Wang, X.; Ma, W. Crystallization behavior of PVDF in PVDF-DMP system via thermally induced phase separation. J. Appl. Polym. Sci. 2006, 102, 3714–3719. [Google Scholar] [CrossRef]
- Belouadah, R.; Kendil, D.; Bousbiat, E.; Guyomar, D.; Guiffard, B. Electrical properties of two-dimensional thin films of the ferroelectric material Polyvinylidene Fluoride as a function of electric field. Phys. B Condens. Matter 2009, 404, 1746–1751. [Google Scholar] [CrossRef]
- Luo, M.-L.; Zhao, J.-Q.; Tang, W.; Pu, C.-S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Ebert, K.; Fritsch, D.; Koll, J.; Tjahjawiguna, C. Influence of inorganic fillers on the compaction behaviour of porous polymer based membranes. J. Membr. Sci. 2004, 233, 71–78. [Google Scholar] [CrossRef]
- Han, L.F.; Xu, Z.L.; Yu, L.Y.; Wei, Y.M.; Cao, Y. Performance of PVDF/multi-nanoparticles composite hollow fibre ultrafiltration membranes. Iran. Polym. J. 2010, 19, 553–565. [Google Scholar]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Effect of modified PVDF hollow fiber submerged ultrafiltration membrane for refinery wastewater treatment. Desalination 2011, 283, 214–220. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; D’Asti, V.; Piaggio, P. Preparation and properties of novel organic-inorganic porous membranes. Sep. Purif. Technol. 2001, 22–23, 269–275. [Google Scholar] [CrossRef]
- Ribeiro, C.; Panadero, J.A.; Sencadas, V.; Lanceros-Méndez, S.; Tamaño, M.N.; Moratal, D.; Salmerón-Sánchez, M.; Ribelles, J.L.G. Fibronectin adsorption and cell response on electroactive poly(vinylidene fluoride) films. Biomed. Mater. 2012, 7, 035004:1–035004:10. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Chichester, UK, 2004. [Google Scholar]
- Huang, W.; Edenzon, K.; Fernandez, L.; Razmpour, S.; Woodburn, J.; Cebe, P. Nanocomposites of poly(vinylidene fluoride) with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2010, 115, 3238–3248. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, W.-T.; Yu, W.-X.; Hua, L.-G.; Zhang, Y.-J.; Zhao, Z.-D. Crystallization behavior and mechanical properties of poly (vinylidene fluoride)/multi-walled carbon nanotube nanocomposites. Chem. Res. Chin. Univ. 2010, 26, 491–495. [Google Scholar]
- Causin, V.; Carraro, M.L.; Marega, C.; Saini, R.; Campestrini, S.; Marigo, A. Structure and morphology of solution blended poly(vinylidene fluoride)/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2008, 109, 2354–2361. [Google Scholar] [CrossRef]
- Patro, T.U.; Mhalgi, M.V.; Khakhar, D.V.; Misra, A. Studies on poly(vinylidene fluoride)-clay nanocomposites: Effect of different clay modifiers. Polymer 2008, 49, 3486–3499. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Dayma, N.; Satapathy, B.K.; Patnaik, A. Structural correlations to sliding wear performance of PA-6/PP-g-MA/nanoclay ternary nanocomposites. Wear 2011, 271, 827–836. [Google Scholar] [CrossRef]
- Pan, B.; Xing, Y.; Zhang, C.; Zhang, Y. Study on erosion wear behavior of PDCPD/MMT nanocomposite. Adv. Mater. Res. 2010, 123–125, 231–234. [Google Scholar] [CrossRef]
- Beyer, G. Nanocomposites: A new class of flame retardants for polymers. Plast. Addit. Compd. 2002, 4, 22–28. [Google Scholar] [CrossRef]
- Dillon, D.R.; Tenneti, K.K.; Li, C.Y.; Ko, F.K.; Sics, I.; Hsiao, B.S. On the structure and morphology of polyvinylidene fluoride-nanoclay nanocomposites. Polymer 2006, 47, 1678–1688. [Google Scholar] [CrossRef]
- Yu, W.; Zhao, Z.; Zheng, W.; Long, B.; Jiang, Q.; Li, G.; Ji, X. Crystallization behavior of poly(vinylidene fluoride)/montmorillonite nanocomposite. Prog. Polym. Sci. 2009, 49, 491–498. [Google Scholar]
- Hwang, H.-Y.; Kim, D.-J.; Kim, H.-J.; Hong, Y.-T.; Nam, S.-Y. Effect of nanoclay on properties of porous PVdF membranes. Trans. Nonferrous Met. Soc. China 2011, 21, 141–147. [Google Scholar] [CrossRef]
- Wang, K.Y.; Foo, S.W.; Chung, T.S. Mixed matrix PVDF hollow fiber membranes with nanoscale pores for desalination through direct contact membrane distillation. Ind. Eng. Chem.Res. 2009, 48, 4474–4483. [Google Scholar] [CrossRef]
- Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Investigation of the dispersion of nanoclays into PVDF for enhancement of physical membrane properties. Desalin. Water Treat. 2011, 34, 251–256. [Google Scholar] [CrossRef]
- Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Enhanced abrasion resistant PVDF/nanoclay hollow fibre composite membranes for water treatment. J. Membr. Sci. 2014, 449, 146–157. [Google Scholar] [CrossRef]
- Priya, L.; Jog, J.P. Polymorphism in intercalated poly(vinylidene fluoride)/clay nanocomposites. J. Appl. Polym. Sci. 2003, 89, 2036–2040. [Google Scholar] [CrossRef]
- Sinha, S.K.; Briscoe, B.J. Surface Mechanical Damage and Wear of Polymers. In Encyclopedia of Polymer Science and Technology, Concise; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Nanocomposites for Improved Physical Durability of Porous PVDF Membranes. Membranes 2014, 4, 55-78. https://doi.org/10.3390/membranes4010055
Lai CY, Groth A, Gray S, Duke M. Nanocomposites for Improved Physical Durability of Porous PVDF Membranes. Membranes. 2014; 4(1):55-78. https://doi.org/10.3390/membranes4010055
Chicago/Turabian StyleLai, Chi Yan, Andrew Groth, Stephen Gray, and Mikel Duke. 2014. "Nanocomposites for Improved Physical Durability of Porous PVDF Membranes" Membranes 4, no. 1: 55-78. https://doi.org/10.3390/membranes4010055
APA StyleLai, C. Y., Groth, A., Gray, S., & Duke, M. (2014). Nanocomposites for Improved Physical Durability of Porous PVDF Membranes. Membranes, 4(1), 55-78. https://doi.org/10.3390/membranes4010055






