Characterization of Antimicrobial Composite Edible Film Formulated from Fermented Cheese Whey and Cassava Peel Starch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cassava Peel Starch Production
2.2. Composite Edible Film Production
2.3. Physical Characterization of Edible Film
2.4. Edible Film Antimicrobial Activity Identification
3. Results
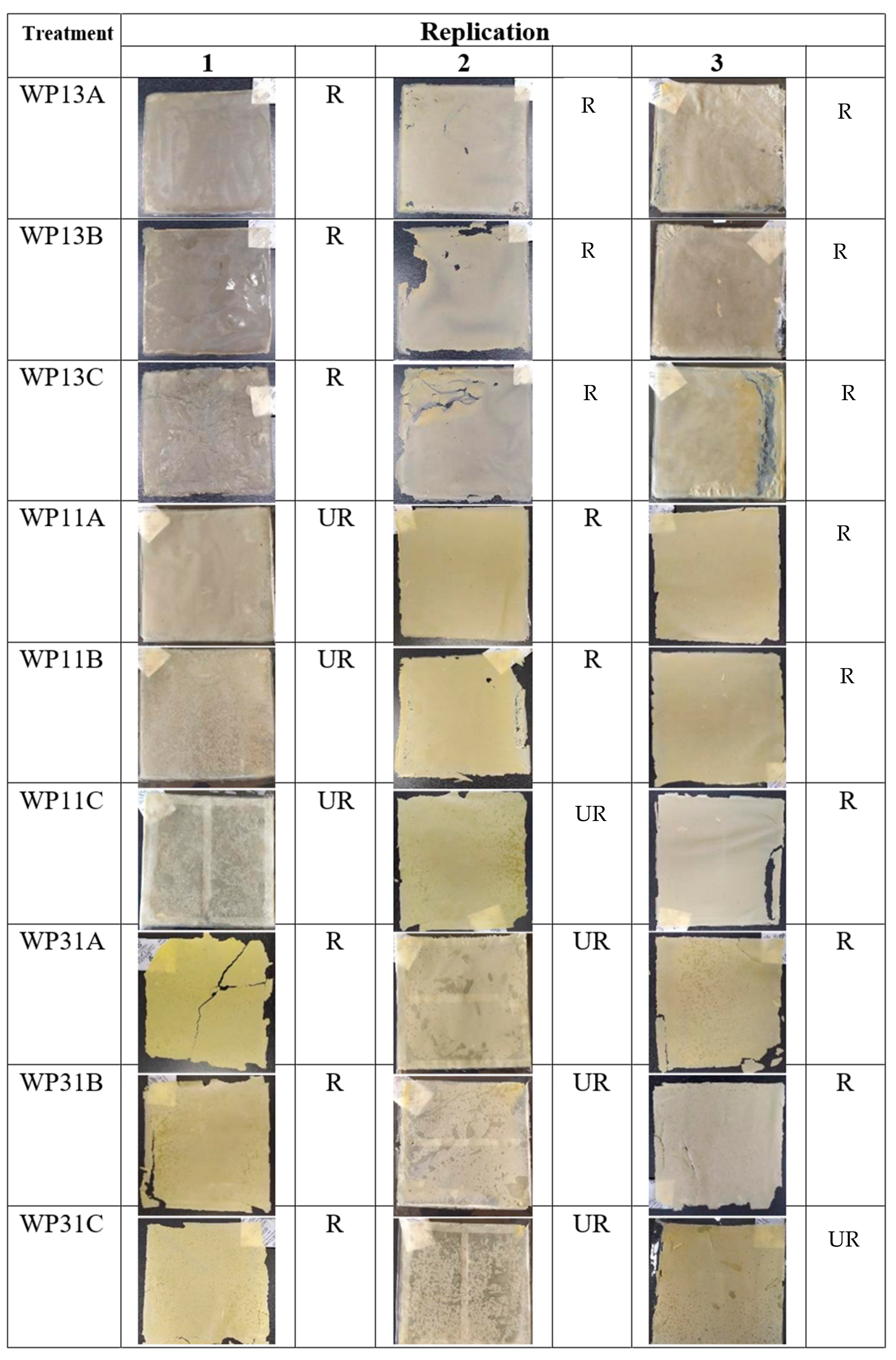
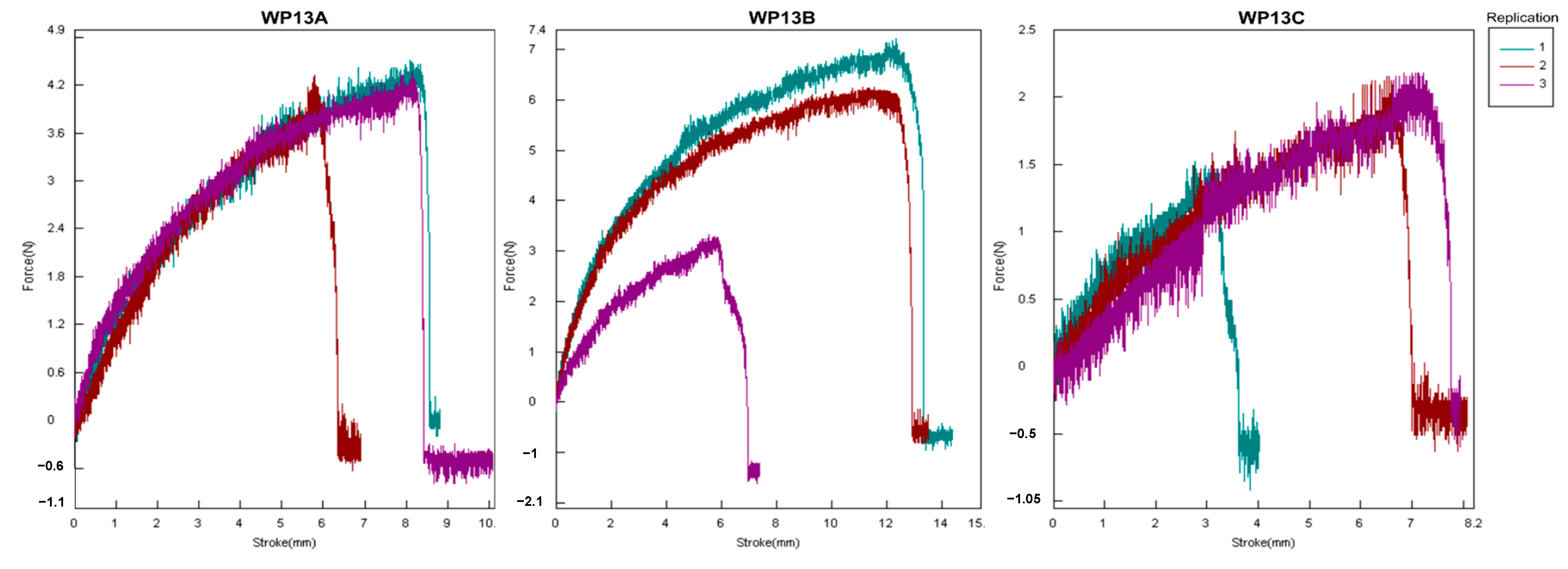
3.1. Physical Characteristics of Antimicrobial Composite Edible Film
3.2. Antimicrobial Characteristics of Antimicrobial Composite Edible Film
4. Discussion
4.1. Physical Characteristics of Antimicrobial Composite Edible Film
4.2. Antimicrobial Characteristics of Antimicrobial Composite Edible Film
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinika, I.; Nurhadi, B.; Masruchin, N.; Utama, G.L.; Balia, R.L. The Roles of Candida Tropicalis Toward Peptide and Amino Acid Changes in Cheese Whey Fermentation. IJTech 2019, 10, 1533. [Google Scholar] [CrossRef] [Green Version]
- Utama, G.L.; Januaramadhan, I.; Dinika, I.; Balia, R.L. Bioconversions of Cheese-Making Wastes to Bioethanol and Their Link to Sustainability. IOP Conf. Ser.: Earth Environ. Sci. 2019, 306, 012017. [Google Scholar] [CrossRef]
- Suwandi Outlook Komoditas Pertanian Tanaman Pangan Ubi Kayu; Pusat Data dan Sistem Informasi Pertanian, Kementerian Pertanian: Jakarta, Indonesia, 2016.
- Akbar, F.; Anita, Z.; Harahap, H. Pengaruh waktu simpan film plastik biodegradasi dari pati kulit singkong terhadap sifat mekanikalnya. J. Tek. Kim. 2013, 2. [Google Scholar] [CrossRef] [Green Version]
- Dinkci, N. Whey, Waste or Value? WJASS 2021, 6, 1–5. [Google Scholar] [CrossRef]
- Kuliahsari, D.E.; Sari, I.N.I.; Estiasih, T. Cyanide Detoxification Methods in Food: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012099. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A. Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows. Vet. Sci 2020, 7, 98. [Google Scholar] [CrossRef]
- Dinika, I.; Verma, D.K.; Balia, R.; Utama, G.L.; Patel, A.R. Potential of Cheese Whey Bioactive Proteins and Peptides in the Development of Antimicrobial Edible Film Composite: A Review of Recent Trends. Trends Food Sci. Technol. 2020, 103, 57–67. [Google Scholar] [CrossRef]
- Utama, G.L.; Utba, F.; Lestari, W.D.; Balia, R. Microbiological Approach for Environmentally Friendly Dairy Industry Waste Utilization. In Microbiology for Food and Health; Apple Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-429-27617-0. [Google Scholar]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods – Biotechnological Facilitation of Industrial Scale-Up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Utama, G.L.; Utba, F.; Cahyana, Y.; Balia, R.L. Mozzarella Whey Indigenous Yeasts and Their Potential in Amino Acid and Peptide Production through Fermentation. Syst. Rev. Pharm. 2021, 12, 9. [Google Scholar]
- Morgan, N.K.; Choct, M. Cassava: Nutrient Composition and Nutritive Value in Poultry Diets. Anim. Nutr. 2016, 2, 253–261. [Google Scholar] [CrossRef]
- Susanti, S.; Karoma, D.A.; Mulyani, D.; Masruri, M. Physical Properties and Characterization of Cassava Peel Waste Modified by Esterification. J. Pure Appl. Chem. Res. 2017, 6, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Jha, P.; Dharmalingam, K.; Nishizu, T.; Katsuno, N.; Anandalakshmi, R. Effect of Amylose–Amylopectin Ratios on Physical, Mechanical, and Thermal Properties of Starch-Based Bionanocomposite Films Incorporated with CMC and Nanoclay. Starch Stärke 2020, 72, 1900121. [Google Scholar] [CrossRef]
- Yuliarie, W.; Noor, H. Warkoyo Characterization of Edible Film from Starch of Taro (Colocasia Esculenta (L.) Schott) with Addition of Chitosan on Dodol Substituted Seaweed (Eucheuma Cottonii L.). Food Technol. Halal Sci. 2019, 1, 22. [Google Scholar] [CrossRef]
- Andrade Martins, Y.A.; Ferreira, S.V.; Silva, N.M.; Sandre, M.F.B.; Filho, J.G.O.; Leão, P.V.T.; Leão, K.M.; Nicolau, E.S.; Plácido, G.R.; Egea, M.B.; et al. Edible Films of Whey and Cassava Starch: Physical, Thermal, and Microstructural Characterization. Coatings 2020, 10, 1059. [Google Scholar] [CrossRef]
- Dinika, I.; Utama, G.L. Cheese Whey as Potential Resource for Antimicrobial Edible Film and Active Packaging Production. Foods Raw Mater. 2019, 229–239. [Google Scholar] [CrossRef]
- Cortés-Rodríguez, M.; Villegas-Yépez, C.; Gil González, J.H.; Rodríguez, P.E.; Ortega-Toro, R. Development and Evaluation of Edible Films Based on Cassava Starch, Whey Protein, and Bees Wax. Heliyon 2020, 6, e04884. [Google Scholar] [CrossRef]
- Yan, Q.; Hou, H.; Guo, P.; Dong, H. Effects of Extrusion and Glycerol Content on Properties of Oxidized and Acetylated Corn Starch-Based Films. Carbohydr. Polym. 2012, 87, 707–712. [Google Scholar] [CrossRef]
- Ministry of Environment and Forestry National Plastic Waste Reduction Strategic Actions for Indonesia. 2020. Available online: http://resp.llas.ac.cn/C666/handle/2XK7JSWQ/282027 (accessed on 16 May 2021).
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Tan, J. Single-Use Plastics in the Food Services Industry: Can It Be Sustainable? Mater. Circ. Econ. 2021, 3, 16. [Google Scholar] [CrossRef]
- Youssef, A.M. Utilization of Edible Films and Coatings as Packaging Materials for Preservation of Cheeses. J. Packag. Technol. Res. 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Basiak, E.; Galus, S.; Lenart, A. Characterisation of Composite Edible Films Based on Wheat Starch and Whey-Protein Isolate. Int. J. Food Sci. Technol. 2015, 50, 372–380. [Google Scholar] [CrossRef]
- Tulamandi, S.; Rangarajan, V.; Rizvi, S.S.H.; Singhal, R.S.; Chattopadhyay, S.K.; Saha, N.C. A Biodegradable and Edible Packaging Film Based on Papaya Puree, Gelatin, and Defatted Soy Protein. Food Packag. Shelf Life 2016, C, 60–71. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Olsen, C.W.; Bilbao-Sáinz, C.; McHugh, T.H. Mechanical and Water Barrier Properties of Isolated Soy Protein Composite Edible Films as Affected by Carvacrol and Cinnamaldehyde Micro and Nanoemulsions. Food Hydrocoll. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci Anim Resour 2015, 35, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, E752. [Google Scholar] [CrossRef]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga Pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia Ficus-Indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Indah, H.; Putri, F.; Utama, G.L. Preliminary Studies of Halophilic Yeasts Antimicrobial Activities Isolated from Cocoa Bean Pulp towards E. Coli and Salmonella spp. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S. Major Factors Affecting the Characteristics of Starch Based Biopolymer Films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Ebrahimi, S.E.; Koocheki, A.; Milani, E.; Mohebbi, M. Interactions between Lepidium Perfoliatum Seed Gum – Grass Pea (Lathyrus Sativus) Protein Isolate in Composite Biodegradable Film. Food Hydrocoll. 2016, 54, 302–314. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta Arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Hammam, A.R.A. Technological, Applications, and Characteristics of Edible Films and Coatings: A Review. SN Appl. Sci. 2019, 1, 632. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Pascual, N.; Gómez-Estaca, J. Production and Processing of Edible Packaging Stability and Applications. In Edible Food Packaging; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-37317-1. [Google Scholar]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of Glycerol and Thymol on Physical, Mechanical, and Thermal Properties of Corn Starch Films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Vieira, M.G.A.; da Silva, M.A.; dos Santos, L.O.; Beppu, M.M. Natural-Based Plasticizers and Biopolymer Films: A Review. Eur. Polym. J. 2011, 47, 254–263. [Google Scholar] [CrossRef] [Green Version]
- de Vries, A.; Wesseling, A.; van der Linden, E.; Scholten, E. Protein Oleogels from Heat-Set Whey Protein Aggregates. J. Colloid Interface Sci. 2017, 486, 75–83. [Google Scholar] [CrossRef]
- Wijayanti, H.B.; Bansal, N.; Deeth, H.C. Stability of Whey Proteins during Thermal Processing: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1235–1251. [Google Scholar] [CrossRef]
- Nandane, A.S.; Jain, R. Study of Mechanical Properties of Soy Protein Based Edible Film as Affected by Its Composition and Process Parameters by Using RSM. J. Food Sci. Technol. 2015, 52, 3645–3650. [Google Scholar] [CrossRef] [Green Version]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.T.; Zempleni, J. Riboflavin12. Adv. Nutr. 2016, 7, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, S.; Lasekan, O. Effects of Glycerol and Stearic Acid on the Performance of Chickpea Starch-Based Coatings Applied to Fresh-Cut Papaya. CyTA J. Food 2019, 17, 365–374. [Google Scholar] [CrossRef] [Green Version]
- Al-Hassan, A.A.; Norziah, M.H. Starch–Gelatin Edible Films: Water Vapor Permeability and Mechanical Properties as Affected by Plasticizers. Food Hydrocoll. 2012, 26, 108–117. [Google Scholar] [CrossRef]
- Filla, J.M.; Stadler, M.; Heck, A.; Hinrichs, J. Assessing Whey Protein Sources, Dispersion Preparation Method and Enrichment of Thermomechanically Stabilized Whey Protein Pectin Complexes for Technical Scale Production. Foods 2021, 10, 715. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Ghanbari, R.; Ebrahimpour, A. Separation and Identification of Bromelain-Generated Antibacterial Peptides from Actinopyga Lecanora. Food Sci. Biotechnol. 2017, 27, 591–598. [Google Scholar] [CrossRef]
- Al-Baarri, A.N.; Legowo, A.M.; Arum, S.K.; Hayakawa, S. Extending Shelf Life of Indonesian Soft Milk Cheese (Dangke) by Lactoperoxidase System and Lysozyme. Int. J. Food Sci. 2018, 2018, 4305395. [Google Scholar] [CrossRef]
- Hwang, C.-F.; Chen, Y.-A.; Luo, C.; Chiang, W.-D. Antioxidant and Antibacterial Activities of Peptide Fractions from Flaxseed Protein Hydrolysed by Protease from Bacillus Altitudinis HK02. Int. J. Food Sci. Technol. 2016, 51, 681–689. [Google Scholar] [CrossRef]
- Christofi, T.; Panayidou, S.; Dieronitou, I.; Michael, C.; Apidianakis, Y. Metabolic Output Defines Escherichia Coli as a Health-Promoting Microbe against Intestinal Pseudomonas Aeruginosa. Sci. Rep. 2019, 9, 14463. [Google Scholar] [CrossRef] [Green Version]
- Karagözlü, N.; Karagözlü, C.; Ergönül, B. Survival Characteristics of E. Coli O157:H7, S. Typhimurium and S. Aureus during Kefir Fermentation. Czech. J. Food Sci. 2008, 25, 202–207. [Google Scholar] [CrossRef] [Green Version]
| Whey: Starch Ratio (v/v) | ||||
|---|---|---|---|---|
| 1:3 | 1:1 | 3:1 | ||
| Glycerol Ratio (v/v) | 20% | WP13A | WP11A | WP31A |
| 33% | WP13B | WP11B | WP31B | |
| 45% | WP13C | WP11C | WP31C | |
| Whey Volume (mL) | Whey Total Solid (g) | Starch Volume (mL) | Starch Total Solid (g) | Glycerol Volume (mL) | ||
|---|---|---|---|---|---|---|
| WP13 | A | 50 | 2.5 | 150 | 7.5 | 1 |
| B | 50 | 2.5 | 150 | 7.5 | 2.5 | |
| C | 50 | 2.5 | 150 | 7.5 | 4 | |
| WP11 | A | 100 | 5 | 100 | 5 | 1 |
| B | 100 | 5 | 100 | 5 | 2.5 | |
| C | 100 | 5 | 100 | 5 | 4 | |
| WP31 | A | 150 | 7.5 | 50 | 2.5 | 1 |
| B | 150 | 7.5 | 50 | 2.5 | 2.5 | |
| C | 150 | 7.5 | 50 | 2.5 | 4 |
| Treatment | Thickness (mm) | Tensile Strength (N/mm2) | Elongation at Break (%) |
|---|---|---|---|
| WP13A | 0.23 ± 0.01 | 0.97 ± 0.04 | 13.86 ± 2.36 |
| WP13B | 0.21 ± 0.00 | 0.81 ± 0.29 | 19.62 ± 7.20 |
| WP13C | 0.26 ± 0.01 | 0.22 ± 0.04 | 10.56 ± 4.53 |
| Treatment | WVP (RH 100%–35%) (10−10·g/m·s·Pa) | WVP (RH 75%–35%) (10−10·g/m·s·Pa) |
|---|---|---|
| WP13A | 3.56 ± 1.28 | 12.18 ± 2.04 |
| WP13B | 3.41 ± 1.13 | 9.84 ± 1.50 |
| WP13C | 4.84 ± 1.80 | 14.59 ± 1.43 |
| Treatment | L * | a * | b * |
|---|---|---|---|
| WP13A | 94.21 ± 2.30 | 1.64 ± 0.17 | 9.65 ± 0.76 |
| WP13B | 96.15 ± 0.57 | 1.43 ± 0.19 | 8.55 ± 1.00 |
| WP13C | 94.27 ± 0.76 | 1.58 ± 0.23 | 9.51 ± 0.65 |
| Treatment | Clear Zone (mm) | |||
|---|---|---|---|---|
| P. aeruginosa | S. aureus | S. thypimurium | E. coli | |
| Whey | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Unfermented whey | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Fermented whey | 18.50 ± 8.73 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| WP13A solution | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| WP13B solution | 5.11 ± 0.71 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
| WP13C solution | 7.00 ± 1.73 | 5.00 ± 0.00 | 5.00 ± 0.00 | 5.00 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utama, G.L.; Dinika, I.; Nurmilah, S.; Masruchin, N.; Nurhadi, B.; Balia, R.L. Characterization of Antimicrobial Composite Edible Film Formulated from Fermented Cheese Whey and Cassava Peel Starch. Membranes 2022, 12, 636. https://doi.org/10.3390/membranes12060636
Utama GL, Dinika I, Nurmilah S, Masruchin N, Nurhadi B, Balia RL. Characterization of Antimicrobial Composite Edible Film Formulated from Fermented Cheese Whey and Cassava Peel Starch. Membranes. 2022; 12(6):636. https://doi.org/10.3390/membranes12060636
Chicago/Turabian StyleUtama, Gemilang Lara, Isfari Dinika, Siti Nurmilah, Nanang Masruchin, Bambang Nurhadi, and Roostita Lobo Balia. 2022. "Characterization of Antimicrobial Composite Edible Film Formulated from Fermented Cheese Whey and Cassava Peel Starch" Membranes 12, no. 6: 636. https://doi.org/10.3390/membranes12060636
APA StyleUtama, G. L., Dinika, I., Nurmilah, S., Masruchin, N., Nurhadi, B., & Balia, R. L. (2022). Characterization of Antimicrobial Composite Edible Film Formulated from Fermented Cheese Whey and Cassava Peel Starch. Membranes, 12(6), 636. https://doi.org/10.3390/membranes12060636






