Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials
2.3. Preparation of CpG ODN-Loaded Cationic Liposomes
2.4. Immunization Schedule and Blocking Effect of Anti-IL-6R Antibody on the Mucosal Adjuvanticity of CpG ODN-Loaded Cationic Liposomes
2.5. Expression of IL-6 and Transforming Growth Factor-Beta (TGF-β) in Leukocytes
2.6. Cytokine Assay
2.7. Evaluation of OVA-Specific Antibody by Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Cell Isolation and Flow Cytometric Analysis
2.9. Statistics
3. Results
3.1. IL-6 Expression in the Nasal Mucosa of Mice Nasally Administered CpG ODN-Loaded Cationic Liposomes
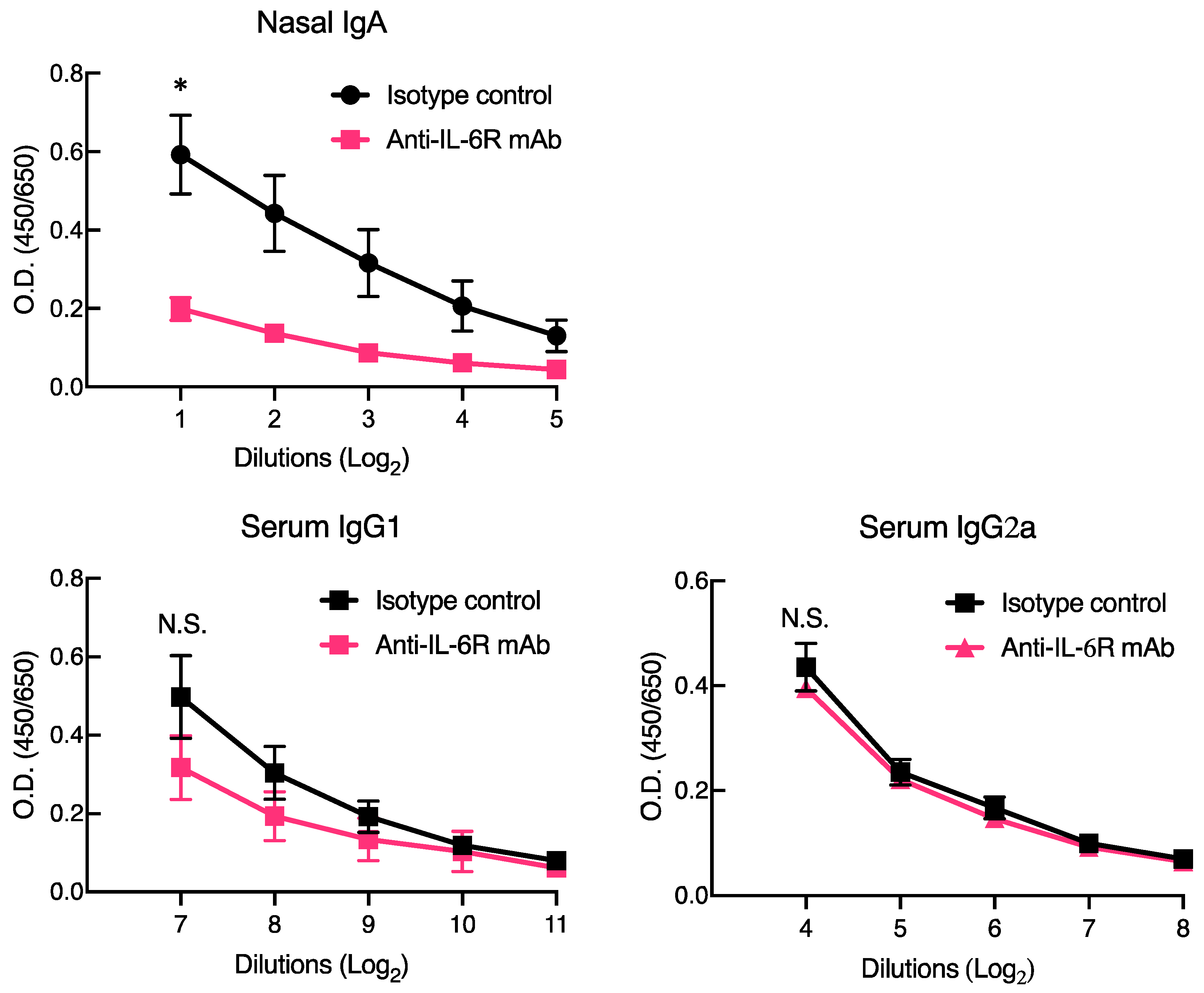
3.2. Anti-IL-6R Blocking Antibody Ameliorated Antigen-Specific Mucosal IgA, but Not Systemic IgG, by CpG ODN-Loaded Cationic Liposomes
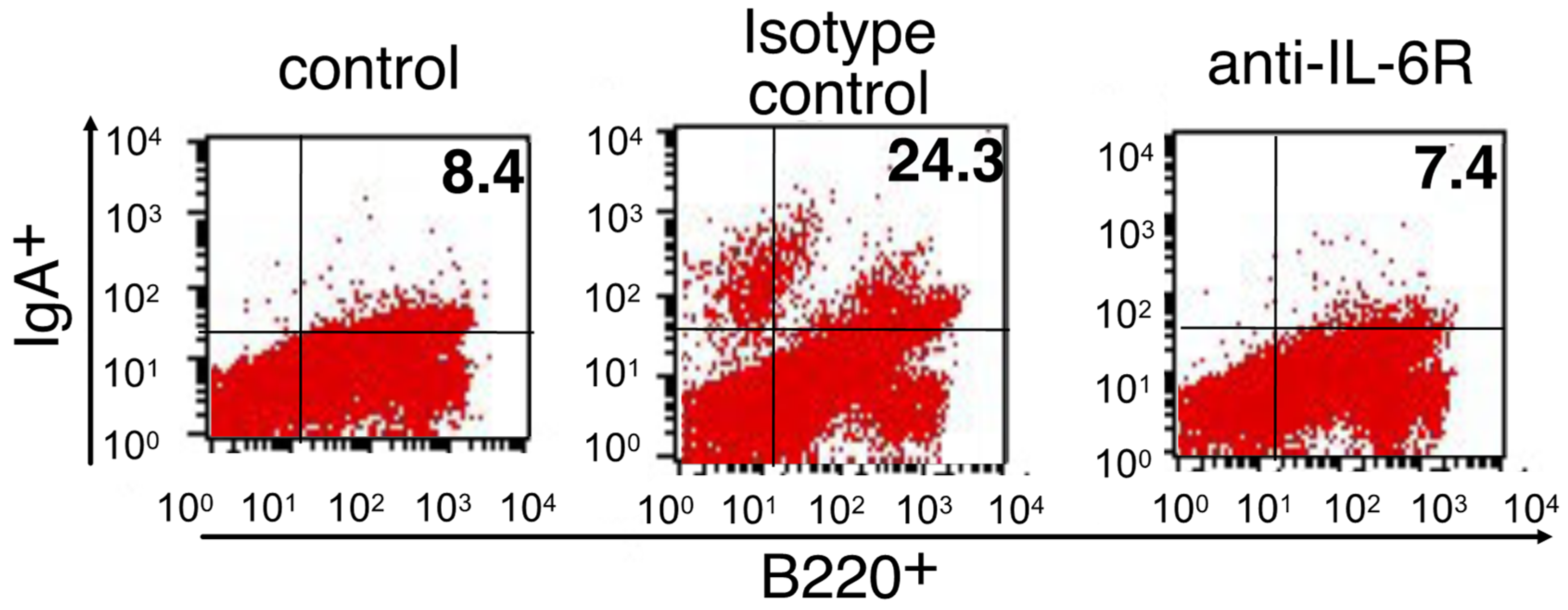
3.3. Role of IL-6 in the Differentiation into IgA-Secreting Plasma Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fauci, A.S. Infectious Diseases: Considerations for the 21st Century. Clin. Infect. Dis. 2001, 32, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Fauci, A.S.; Touchette, N.A.; Folkers, G.K. Emerging Infectious Diseases: A 10-Year Perspective from the National Institute of Allergy and Infectious Diseases. Emerg. Infect. Dis. 2005, 11, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Momin, T.; Kansagra, K.; Patel, H.; Sharma, S.; Sharma, B.; Patel, J.; Mittal, R.; Sanmukhani, J.; Maithal, K.; Dey, A.; et al. Safety and Immunogenicity of a DNA SARS-CoV-2 Vaccine (ZyCoV-D): Results of an Open-Label, Non-Randomized Phase I Part of Phase I/II Clinical Study by Intradermal Route in Healthy Subjects in India. Eclinicalmedicine 2021, 38, 101020. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kondoh, M.; Yagi, K.; Kiyono, H.; Kunisawa, J. The Development of Mucosal Vaccine Using Bacterial Function for Targeting Mucosal Tissues. Yakuga Zasshi 2014, 134, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Borges, O.; Lebre, F.; Bento, D.; Borchard, G.; Junginger, H.E. Mucosal Vaccines: Recent Progress in Understanding the Natural Barriers. Pharm. Res. 2010, 27, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jang, Y.-S. The Development of Mucosal Vaccines for Both Mucosal and Systemic Immune Induction and the Roles Played by Adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Aoshi, T. Modes of Action for Mucosal Vaccine Adjuvants. Viral Immunol. 2017, 30, 463–470. [Google Scholar] [CrossRef]
- Rhee, J.H.; Lee, S.E.; Kim, S.Y. Mucosal Vaccine Adjuvants Update. Clin. Exp. Vaccine Res. 2012, 1, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Hidaka, A.; Iwase, N.; Takahashi, S.; Yamakita, Y.; Iwata, T.; Muto, S.; Sato, E.; Takayama, N.; Honjo, E.; et al. Intranasal Immunization with DOTAP Cationic Liposomes Combined with DC-Cholesterol Induces Potent Antigen-Specific Mucosal and Systemic Immune Responses in Mice. PLoS ONE 2015, 10, e0139785. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Muto, S.; Iwata, T.; Hidaka, A.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Attachment of Class B CpG ODN onto DOTAP/DC-Chol Liposome in Nasal Vaccine Formulations Augments Antigen-Specific Immune Responses in Mice. BMC Res. Notes 2017, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: Discovery of a Pleiotropic Cytokine. Arthritis Res. Ther. 2006, 8, S2. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A. The Regulation of IgA Class Switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Ohshima, A.; Tanazawa, Y.; Ohmi, A.; Takahashi, S.; Kiyono, H.; Kunisawa, J.; Aramaki, Y.; Negishi, Y. Essential Role of Host Double-Stranded DNA Released from Dying Cells by Cationic Liposomes for Mucosal Adjuvanticity. Vaccines 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Tada, R.; Hidaka, A.; Tanazawa, Y.; Ohmi, A.; Muto, S.; Ogasawara, M.; Saito, M.; Ohshima, A.; Iwase, N.; Honjo, E.; et al. Role of Interleukin-6 in Antigen-Specific Mucosal Immunoglobulin A Induction by Cationic Liposomes. Int. Immunopharmacol. 2021, 101, 108280. [Google Scholar] [CrossRef]
- Tada, R.; Suzuki, H.; Ogasawara, M.; Yamanaka, D.; Adachi, Y.; Kunisawa, J.; Negishi, Y. Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus Pneumoniae Infections in Mice. Pharmaceutics 2021, 13, 585. [Google Scholar] [CrossRef]
- Tada, R.; Ogasawara, M.; Yamanaka, D.; Sakurai, Y.; Negishi, Y.; Kiyono, H.; Ohno, N.; Kunisawa, J.; Aramaki, Y. Enzymatically Polymerised Polyphenols Prepared from Various Precursors Potentiate Antigen-Specific Immune Responses in Both Mucosal and Systemic Compartments in Mice. PLoS ONE 2021, 16, e0246422. [Google Scholar] [CrossRef]
- Tada, R.; Yamanaka, D.; Ogasawara, M.; Saito, M.; Ohno, N.; Kiyono, H.; Kunisawa, J.; Aramaki, Y. Polymeric Caffeic Acid Is a Safer Mucosal Adjuvant That Augments Antigen-Specific Mucosal and Systemic Immune Responses in Mice. Mol. Pharm. 2018, 15, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Sato, A.; Fukuyama, S.; Sagara, H.; Nagatake, T.; Kong, I.G.; Goda, K.; Nochi, T.; Kunisawa, J.; Sato, S.; et al. The Airway Antigen Sampling System: Respiratory M Cells as an Alternative Gateway for Inhaled Antigens. J. Immunol. 2011, 186, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Staats, H.F. Cytokines: The Future of Intranasal Vaccine Adjuvants. Clin. Dev. Immunol 2011, 2011, 289597. [Google Scholar] [CrossRef] [PubMed]
- Boyaka, P.N.; McGhee, J.R. Cytokines as Adjuvants for the Induction of Mucosal Immunity. Adv. Drug Deliv. Rev. 2001, 51, 71–79. [Google Scholar] [CrossRef]
- Boyaka, P.N.; Marinaro, M.; Jackson, R.J.; Menon, S.; Kiyono, H.; Jirillo, E.; McGhee, J.R. IL-12 Is an Effective Adjuvant for Induction of Mucosal Immunity. J. Immunol. 1999, 162, 122–128. [Google Scholar] [PubMed]
- Cazac, B.B.; Roes, J. TGF-β Receptor Controls B Cell Responsiveness and Induction of IgA In Vivo. Immunity 2000, 13, 443–451. [Google Scholar] [CrossRef]
- Ramsay, A.; Husband, A.; Ramshaw, I.; Bao, S.; Matthaei, K.; Koehler, G.; Kopf, M. The Role of Interleukin-6 in Mucosal IgA Antibody Responses In Vivo. Science 1994, 264, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Bromander, A.K.; Ekman, L.; Kopf, M.; Nedrud, J.G.; Lycke, N.Y. IL-6-Deficient Mice Exhibit Normal Mucosal IgA Responses to Local Immunizations and Helicobacter Felis Infection. J. Immunol. 1996, 156, 4290–4297. [Google Scholar]
- Mihara, M.; Takagi, N.; Takeda, Y.; Ohsugi, Y. IL-6 Receptor Blockage Inhibits the Onset of Autoimmune Kidney Disease in NZB/W F1 Mice. Clin. Exp. Immunol. 1998, 112, 397–402. [Google Scholar] [CrossRef]
- Okazaki, M.; Yamada, Y.; Nishimoto, N.; Yoshizaki, K.; Mihara, M. Characterization of Anti-Mouse Interleukin-6 Receptor Antibody. Immunol. Lett. 2002, 84, 231–240. [Google Scholar] [CrossRef]
- Mora, J.R.; Andrian, U.H. von Differentiation and Homing of IgA-Secreting Cells. Mucosal. Immunol. 2008, 1, 96–109. [Google Scholar] [CrossRef]
- Kunisawa, J.; Gohda, M.; Kiyono, H. Uniqueness of the Mucosal Immune System for the Development of Prospective Mucosal Vaccine. Yakuga Zasshi 2007, 127, 319–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bianchi, M.E. DAMPs, PAMPs and Alarmins: All We Need to Know about Danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Adjuvants and Adjuvanticity. Vaccines Vet. 2021, 7, 75–86.e1. [Google Scholar] [CrossRef]
- Greene, C.J.; Hu, J.C.; Vance, D.J.; Rong, Y.; Mandell, L.; King-Lyons, N.; Masso-Welch, P.; Mantis, N.J.; Connell, T.D. Enhancement of Humoral Immunity by the Type II Heat-Labile Enterotoxin LT-IIb Is Dependent upon IL-6 and Neutrophils. J. Leukoc. Biol. 2016, 100, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gallichan, W.S.; Woolstencroft, R.N.; Guarasci, T.; McCluskie, M.J.; Davis, H.L.; Rosenthal, K.L. Intranasal Immunization with CpG Oligodeoxynucleotides as an Adjuvant Dramatically Increases IgA and Protection against Herpes Simplex Virus-2 in the Genital Tract. J. Immunol. 2001, 166, 3451–3457. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tada, R.; Honjo, E.; Muto, S.; Takayama, N.; Kiyono, H.; Kunisawa, J.; Negishi, Y. Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes. Membranes 2022, 12, 635. https://doi.org/10.3390/membranes12060635
Tada R, Honjo E, Muto S, Takayama N, Kiyono H, Kunisawa J, Negishi Y. Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes. Membranes. 2022; 12(6):635. https://doi.org/10.3390/membranes12060635
Chicago/Turabian StyleTada, Rui, Emi Honjo, Shoko Muto, Noriko Takayama, Hiroshi Kiyono, Jun Kunisawa, and Yoichi Negishi. 2022. "Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes" Membranes 12, no. 6: 635. https://doi.org/10.3390/membranes12060635
APA StyleTada, R., Honjo, E., Muto, S., Takayama, N., Kiyono, H., Kunisawa, J., & Negishi, Y. (2022). Role of Interleukin-6 in the Antigen-Specific Mucosal Immunoglobulin A Responses Induced by CpG Oligodeoxynucleotide-Loaded Cationic Liposomes. Membranes, 12(6), 635. https://doi.org/10.3390/membranes12060635





