Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dope Solution Preparation and Casting Method
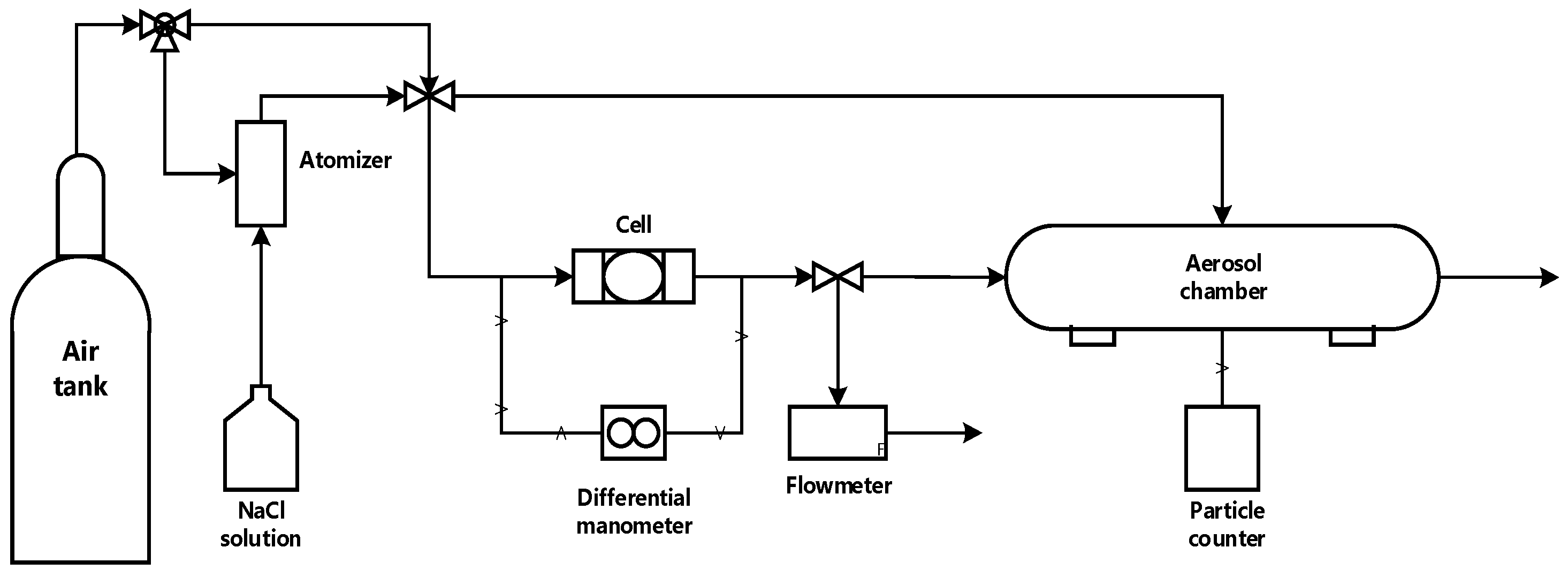
2.2. Airflow, Pressure Drop and Filtration Efficiency (FE) Test
2.3. Tensile Test, Membrane Wettability and Cloud Point Measurements
2.4. Porosity and Viscosity Measurements
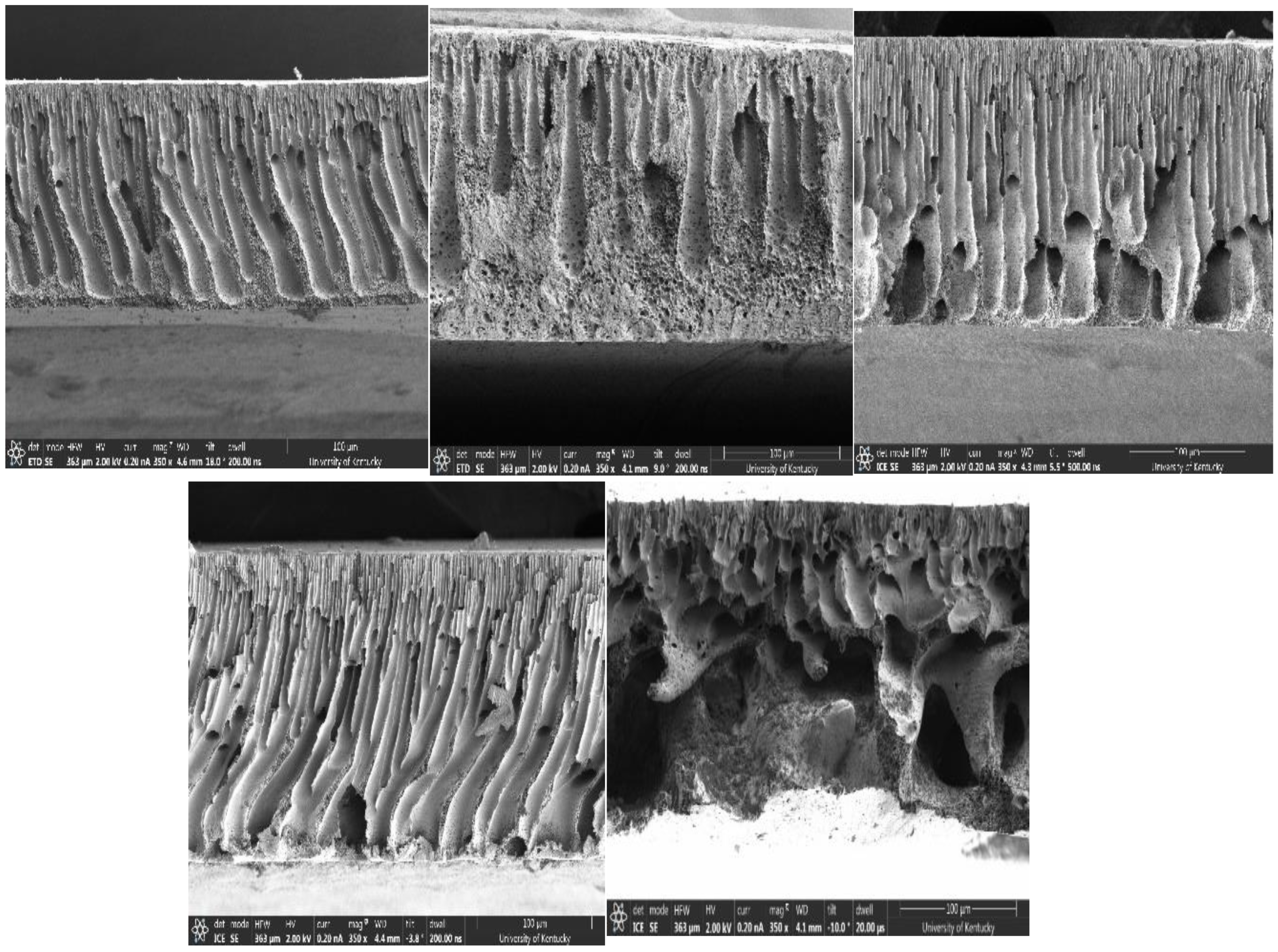
2.5. X-ray Photoelectron Spectroscopy (XPS), Scanning Electron Microscope (SEM), and Surface Pore Analysis
3. Results and Discussion
3.1. Membrane Composition
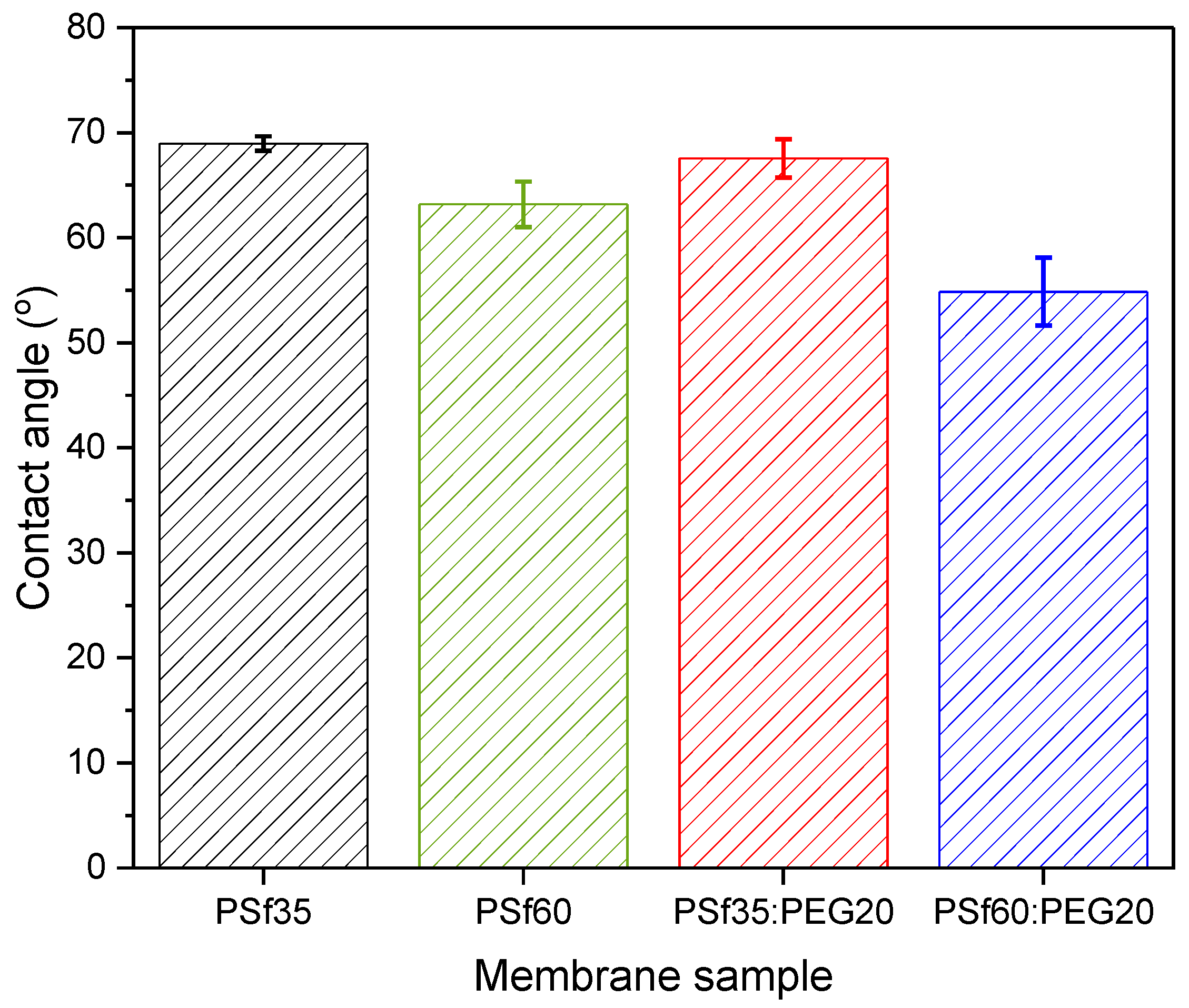
3.2. Membrane Wettability
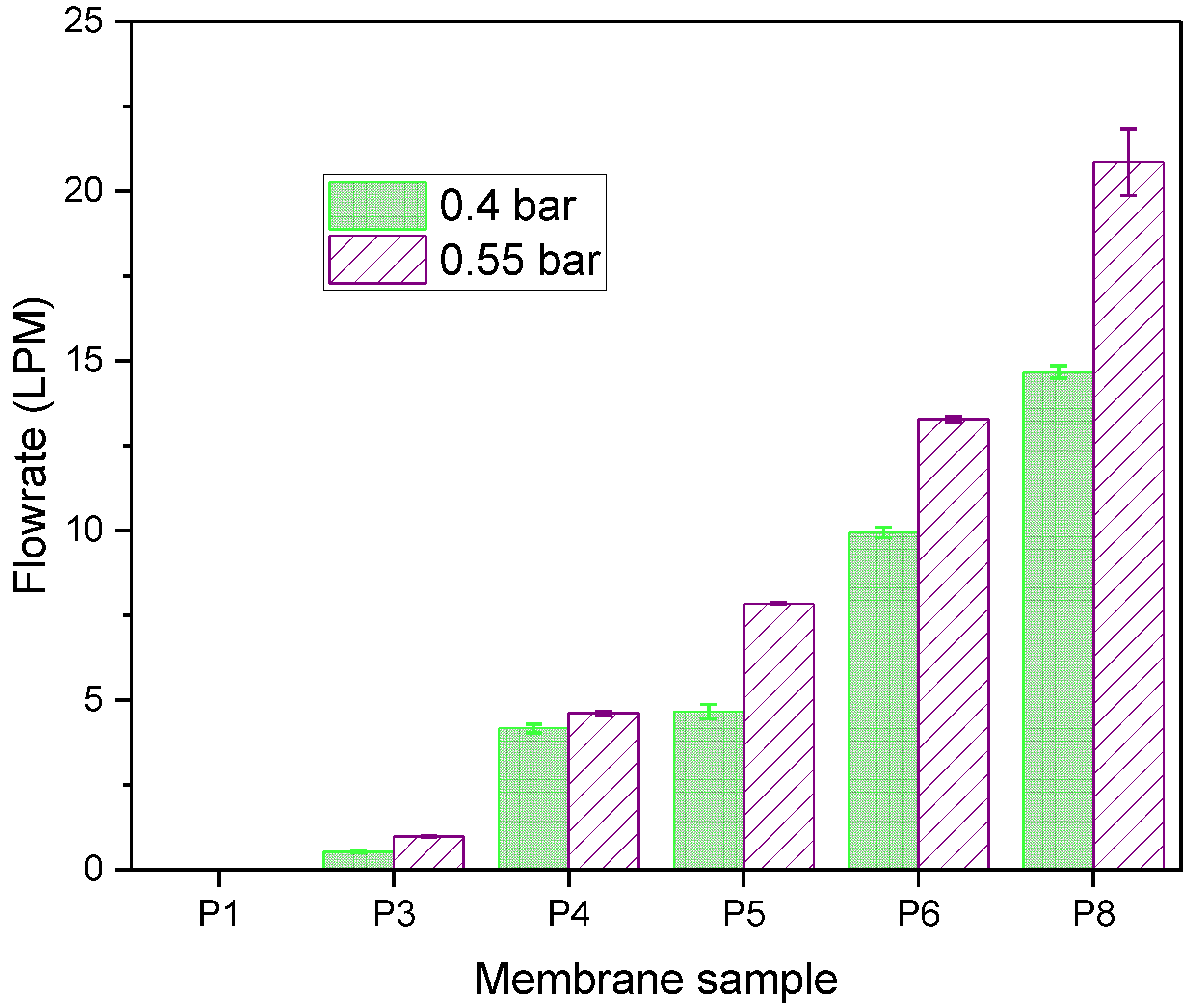
3.3. Effect of Pore Formers and Pore Former Molecular Weight on Air Permeability
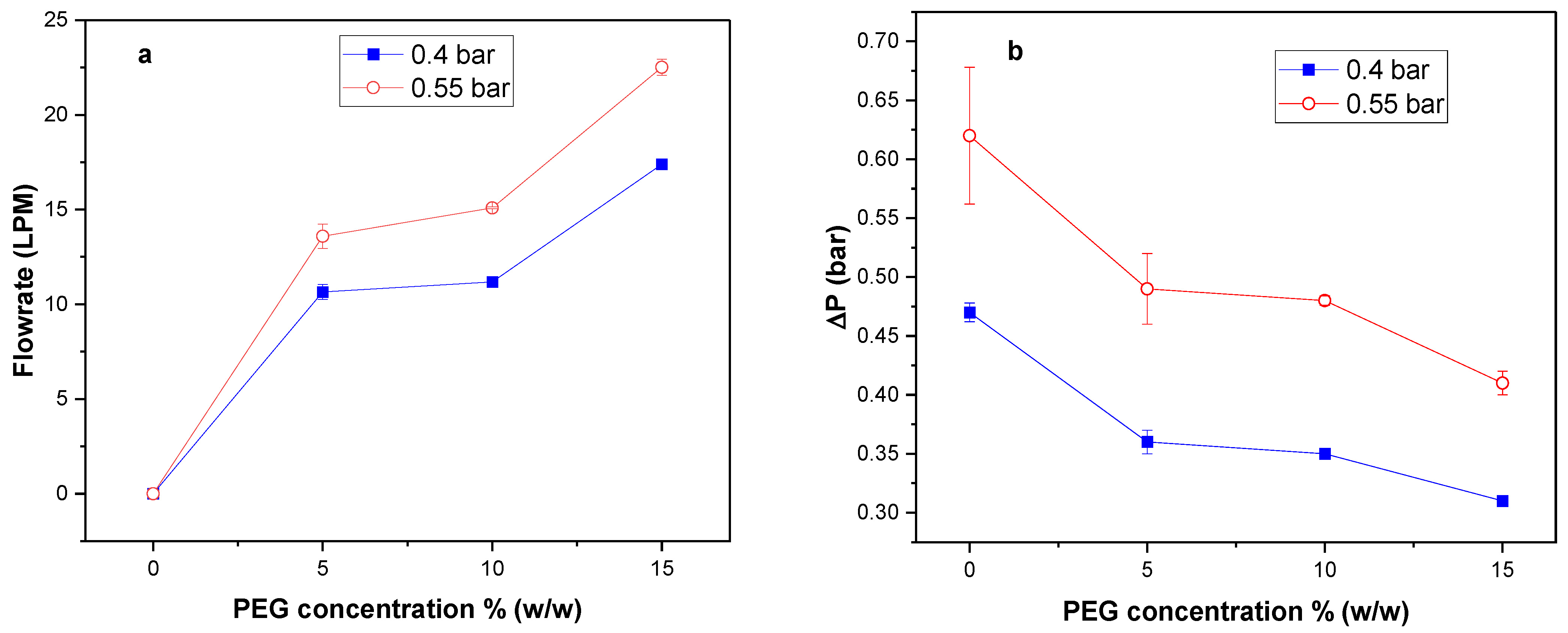
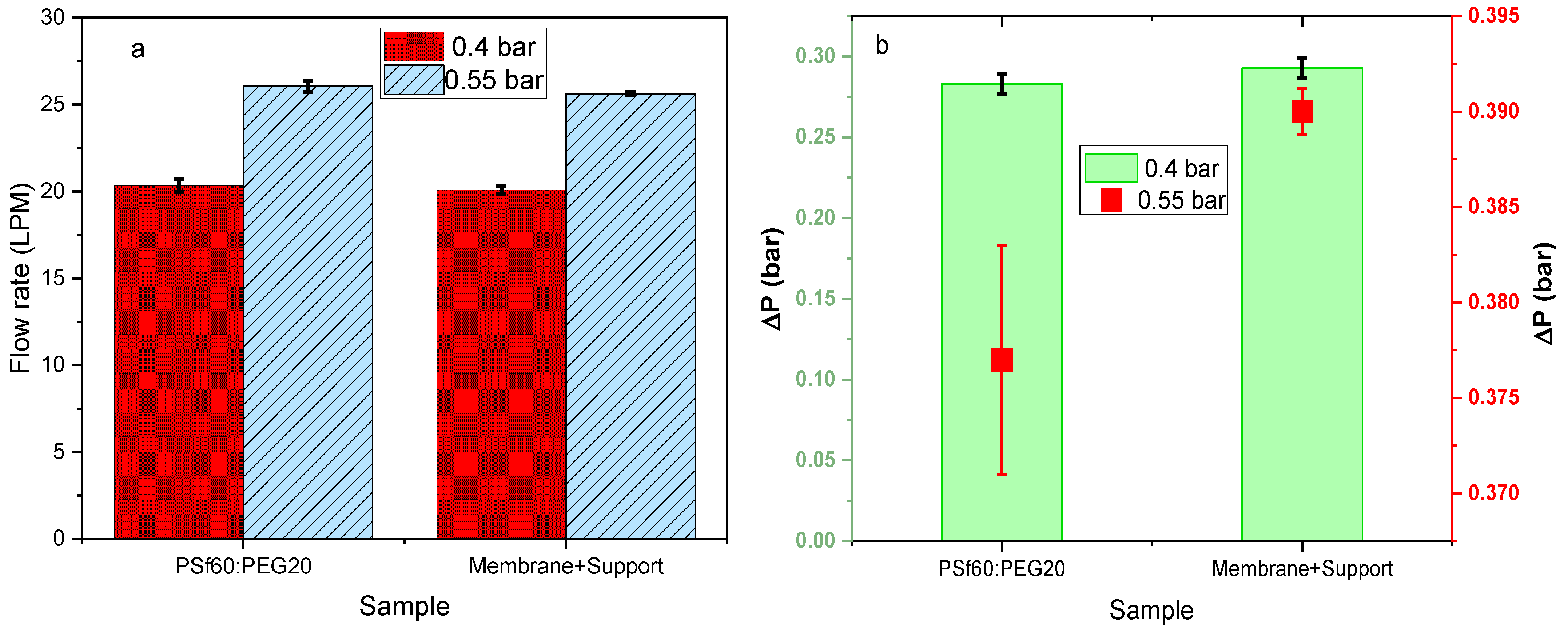
3.4. Effect of Pore Former Concentration on Airflow Rate and Pressure Drop
3.5. Effect of Polysulfone Molecular Weight on Airflow Rate and Pressure Drop
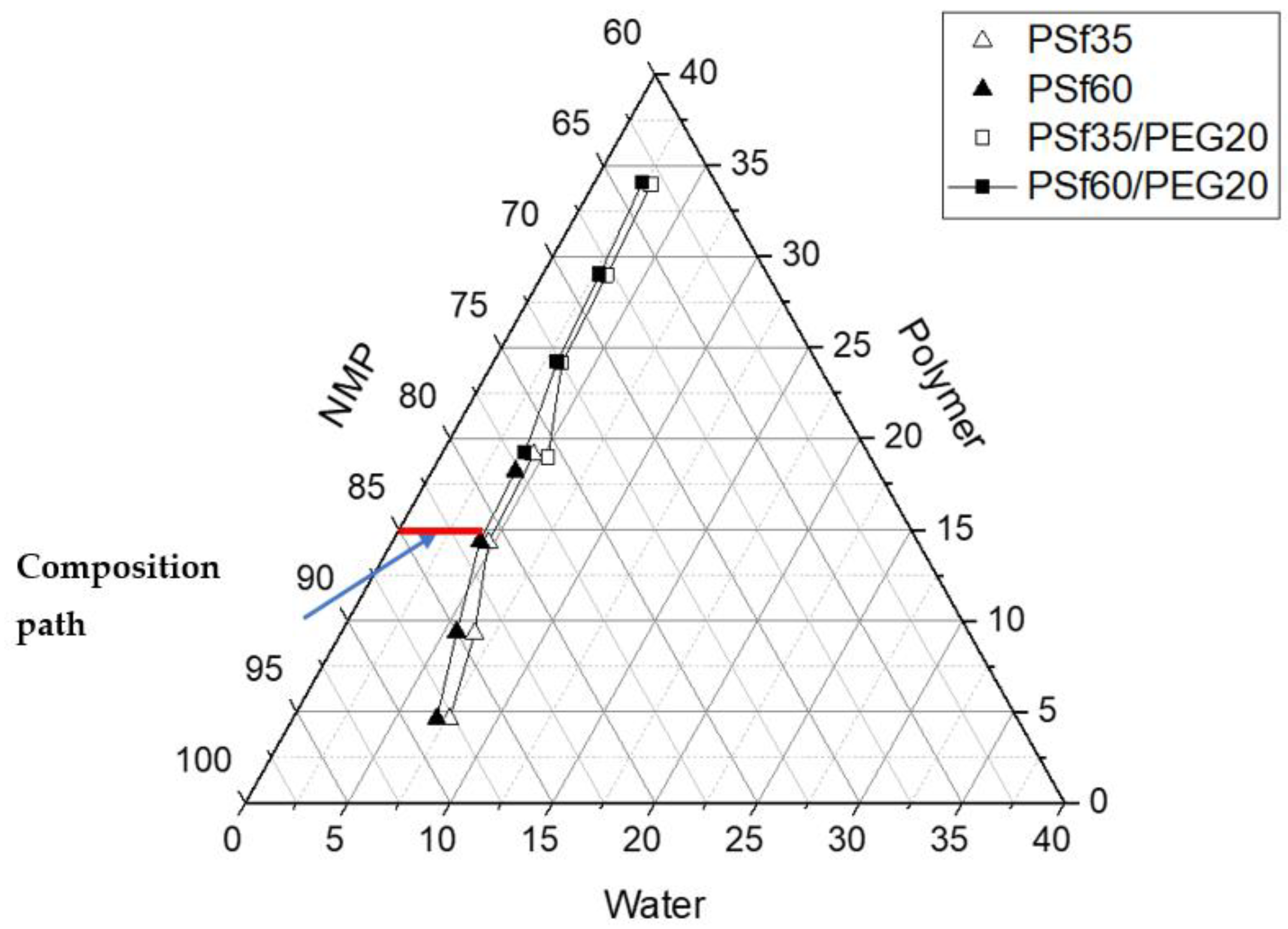
3.6. Ternary Phase Diagram
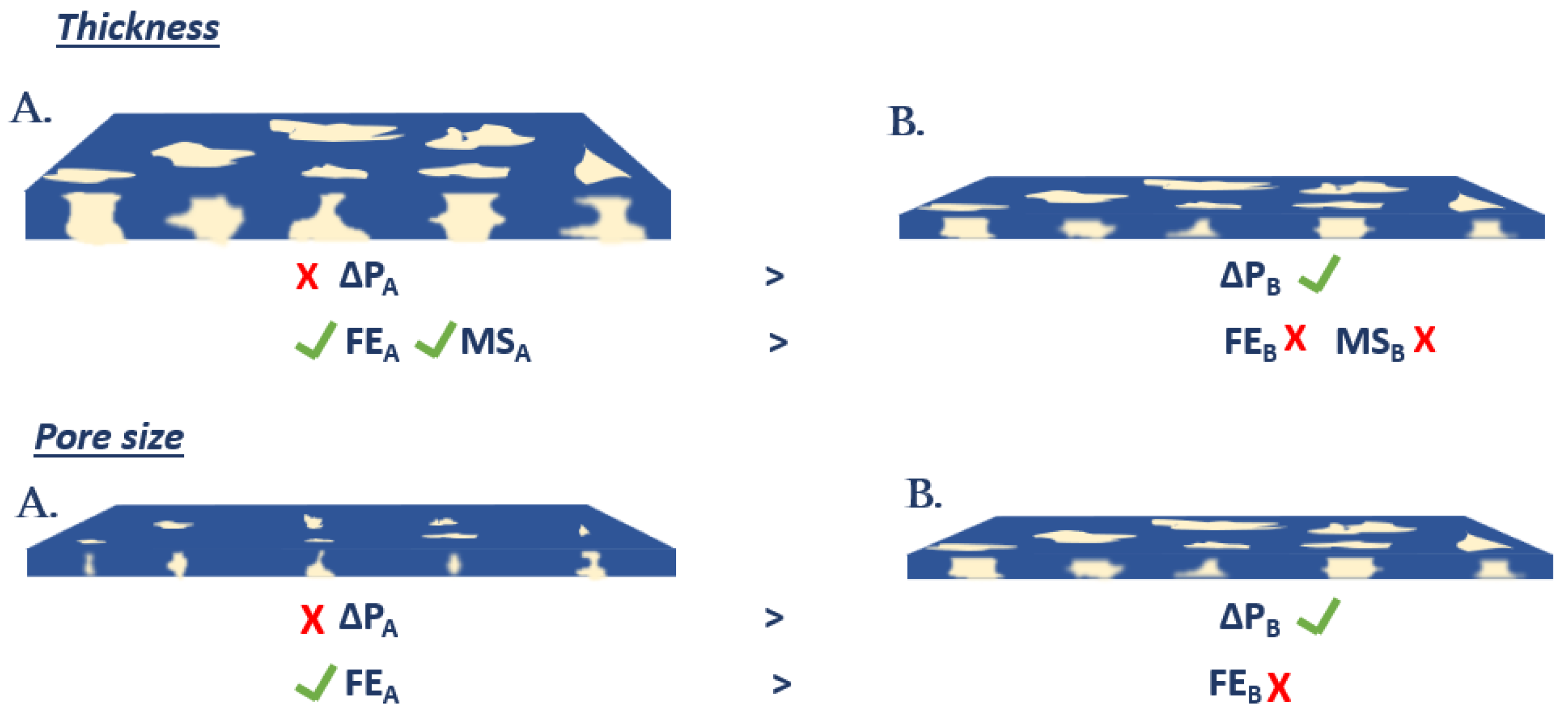
3.7. Membrane Mechanical Strength and Filtration Efficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pure Air Systems. Medical, Commercial, Institutional, Industrial, and Military Applications. Available online: https://www.pureairsystems.com/air-filter-applications/ (accessed on 5 April 2022).
- Howard, J.; Huang, A.; Li, Z.; Tufekci, Z.; Zdimal, V.; van der Westhuizen, H.-M.; von Delft, A.; Price, A.; Fridman, L.; Tang, L.-H. An evidence review of face masks against COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2014564118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, H.; Zhang, L.; Zhang, M.; Guo, D.; Wu, W.; Zhang, X.; Kan, G.L.; Jia, L.; Huo, D. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: A cohort study in Beijing, China. BMJ Glob. Health 2020, 5, e002794. [Google Scholar] [CrossRef] [PubMed]
- Chuaybamroong, P.; Chotigawin, R.; Supothina, S.; Sribenjalux, P.; Larpkiattaworn, S.; Wu, C.Y. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air 2010, 20, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. NIOSH-Approved N95 Particulate Filtering Facepiece Respirators. 2022. Available online: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html (accessed on 16 February 2022).
- Abbasi, S.A.; Khalil, A.B.; Arslan, M. Extensive use of face masks during COVID-19 pandemic: (micro-) plastic pollution and potential health concerns in the Arabian Peninsula. Saudi J. Biol. Sci. 2020, 27, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Ogbuoji, E.A.; Zaky, A.M.; Escobar, I.C. Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review. Polymers 2021, 13, 1998. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A. Textiles for Protection; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Adanur, S.; Jayswal, A. Filtration mechanisms and manufacturing methods of face masks: An overview. J. Ind. Text. 2020, 1528083720980169. [Google Scholar] [CrossRef]
- Payen, J.; Vroman, P.; Lewandowski, M.; Perwuelz, A.; Calle-Chazelet, S.; Thomas, D. Influence of fiber diameter, fiber combinations and solid volume fraction on air filtration properties in nonwovens. Text. Res. J. 2012, 82, 1948–1959. [Google Scholar] [CrossRef]
- Mittal, R.; Ni, R.; Seo, J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894, F2. [Google Scholar] [CrossRef]
- Ou, Q.; Pei, C.; Kim, S.C.; Abell, E.; Pui, D.Y. Evaluation of decontamination methods for commercial and alternative respirator and mask materials–view from filtration aspect. J. Aerosol Sci. 2020, 150, 105609. [Google Scholar] [CrossRef]
- Drewnick, F.; Pikmann, J.; Fachinger, F.; Moormann, L.; Sprang, F.; Borrmann, S. Aerosol filtration efficiency of household materials for homemade face masks: Influence of material properties, particle size, particle electrical charge, face velocity, and leaks. Aerosol Sci. Technol. 2021, 55, 63–79. [Google Scholar] [CrossRef]
- El-Atab, N.; Qaiser, N.; Badghaish, H.; Shaikh, S.F.; Hussain, M.M. Flexible nanoporous template for the design and development of reusable anti-COVID-19 hydrophobic face masks. ACS Nano 2020, 14, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.W.-F.; Sun, Q. Charged PVDF multilayer nanofiber filter in filtering simulated airborne novel coronavirus (COVID-19) using ambient nano-aerosols. Sep. Purif. Technol. 2020, 245, 116887. [Google Scholar] [CrossRef] [PubMed]
- Galka, N.; Saxena, A. High efficiency air filtration: The growing impact of membranes. Filtr. Sep. 2009, 46, 22–25. [Google Scholar] [CrossRef]
- Baldridge, K.C.; Edmonds, K.; Dziubla, T.; Hilt, J.Z.; Dutch, R.E.; Bhattacharyya, D. Demonstration of Hollow Fiber Membrane-Based Enclosed Space Air Remediation for Capture of an Aerosolized Synthetic SARS-CoV-2 Mimic and Pseudovirus Particles. ACS Environ. Sci. Technol. EST Eng. 2022, 2, 251–262. [Google Scholar] [CrossRef]
- Simmons, R.; Price, D.; Noble, J.; Crow, S.; Ahearn, D. Fungal colonization of air filters from hospitals. Am. Ind. Hyg. Assoc. J. 1997, 58, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Reponen, T.; Willeke, K.; Grinshpun, S.A.; Choi, K.-J. Collection of fungal spores on air filters and spore reentrainment from filters into air. J. Aerosol Sci. 2000, 31, 969–978. [Google Scholar] [CrossRef]
- Goswami, T.; Hingorani, S.; Greist, H.; Goswami, D.; Block, S. Photocatalytic system to destroy bioaerosols in air. J. Adv. Oxid. Technol. 1999, 4, 185–188. [Google Scholar]
- Tuñón-Molina, A.; Takayama, K.; Redwan, E.M.; Uversky, V.N.; Andrés, J.; Serrano-Aroca, Á. Protective Face Masks: Current Status and Future Trends. ACS Appl. Mater. Interfaces 2021, 13, 56725–56751. [Google Scholar] [CrossRef]
- Jung, S.; Yang, J.-Y.; Byeon, E.-Y.; Kim, D.-G.; Lee, D.-G.; Ryoo, S.; Lee, S.; Shin, C.-W.; Jang, H.W.; Kim, H.J. Copper-coated polypropylene filter face mask with SARS-COV-2 antiviral ability. Polymers 2021, 13, 1367. [Google Scholar] [CrossRef]
- Podder, S.; Halder, S.; Roychowdhury, A.; Das, D.; Ghosh, C.K. Superb hydroxyl radical-mediated biocidal effect induced antibacterial activity of tuned ZnO/chitosan type II heterostructure under dark. J. Nanoparticle Res. 2016, 18, 294. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zhong, W.-H.; Pan, S. Hierarchically structured all-biomass air filters with high filtration efficiency and low air pressure drop based on pickering emulsion. ACS Appl. Mater. Interfaces 2019, 11, 14266–14274. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A. Composite nonwovens in filters: Applications. In Composite Non-Woven Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 164–210. [Google Scholar]
- Yang, Y.; Rana, D.; Matsuura, T.; Zheng, S.; Lan, C.Q. Criteria for the selection of a support material to fabricate coated membranes for a life support device. Rsc Adv. 2014, 4, 38711–38717. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Hamzah, H.H.; Shafiee, S.A.; Abdalla, A.; Patel, B.A. 3D printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem. Commun. 2018, 96, 27–31. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M. Effect of molecular weight of PEG on membrane morphology and transport properties. J. Membr. Sci. 2008, 309, 209–221. [Google Scholar] [CrossRef]
- Technologies, A. Membrane Synthesis. Available online: https://www.analytical-online.com/membrane-synthesis.html (accessed on 21 March 2022).
- Tortora, G.J.; Derrickson, B.H. Principles of Anatomy and Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Lindh, W.Q.; Pooler, M.; Tamparo, C.D.; Dahl, B.M.; Morris, J. Delmar’s Comprehensive Medical Assisting: Administrative and Clinical Competencies; Cengage Learning: Boston, MA, USA, 2013. [Google Scholar]
- Ray, S.S.; Park, Y.-I.; Park, H.; Nam, S.-E.; Kim, I.-C.; Kwon, Y.-N. Surface innovation to enhance anti-droplet and hydrophobic behavior of breathable compressed-polyurethane masks. Environ. Technol. Innov. 2020, 20, 101093. [Google Scholar] [CrossRef]
- Wijmans, J.; Kant, J.; Mulder, M.; Smolders, C. Phase separation phenomena in solutions of polysulfone in mixtures of a solvent and a nonsolvent: Relationship with membrane formation. Polymer 1985, 26, 1539–1545. [Google Scholar] [CrossRef] [Green Version]
- Alayande, A.B.; Obaid, M.; Yu, H.-W.; Kim, I.S. High-flux ultrafiltration membrane with open porous hydrophilic structure using dual pore formers. Chemosphere 2019, 227, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Song, H.-M.; Wang, G.; Zeng, Z.-X.; Zhao, C.-T.; Xue, Q.-J.; Guo, X.-P. Microstructures and performances of pegylated polysulfone membranes from an in situ synthesized solution via vapor induced phase separation approach. J. Colloid Interface Sci. 2018, 515, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Su, Y.; Zhao, X.; Li, Y.; Zhang, R.; Ma, T.; Liu, Y.; Jiang, Z. Manipulating the segregation behavior of polyethylene glycol by hydrogen bonding interaction to endow ultrafiltration membranes with enhanced antifouling performance. J. Membr. Sci. 2016, 499, 56–64. [Google Scholar] [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Bustam, M.A.; Huda, N.; Jaafar, J.; Narkkun, T.; Faungnawakij, K. Development of polysulfone membrane via vapor-induced phase separation for oil/water emulsion filtration. Polymers 2020, 12, 2519. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R. Insight into the role of amphiphilic pluronic block copolymer as pore-forming additive in PVDF membrane formation. J. Membr. Sci. 2013, 446, 492–503. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Y.; Zhou, J.; Song, L.; Hu, X.; Larbot, A. FAS grafted superhydrophobic ceramic membrane. Appl. Surf. Sci. 2009, 255, 9092–9099. [Google Scholar] [CrossRef]
- Suk, D.E.; Chowdhury, G.; Matsuura, T.; Narbaitz, R.M.; Santerre, P.; Pleizier, G.; Deslandes, Y. Study on the kinetics of surface migration of surface modifying macromolecules in membrane preparation. Macromolecules 2002, 35, 3017–3021. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Application of surface modifying macromolecules for the preparation of membranes for membrane distillation. Desalination 2003, 158, 51–56. [Google Scholar] [CrossRef]
- Prince, J.; Singh, G.; Rana, D.; Matsuura, T.; Anbharasi, V.; Shanmugasundaram, T. Preparation and characterization of highly hydrophobic poly (vinylidene fluoride)–Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 2012, 397, 80–86. [Google Scholar] [CrossRef]
- Nasirian, D.; Salahshoori, I.; Sadeghi, M.; Rashidi, N.; Hassanzadeganroudsari, M. Investigation of the gas permeability properties from polysulfone/polyethylene glycol composite membrane. Polym. Bull. 2020, 77, 5529–5552. [Google Scholar] [CrossRef]
- Saljoughi, E.; Amirilargani, M.; Mohammadi, T. Effect of PEG additive and coagulation bath temperature on the morphology, permeability and thermal/chemical stability of asymmetric CA membranes. Desalination 2010, 262, 72–78. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Young, T.-H.; Chen, L.-W. A diffusion-controlled model for wet-casting membrane formation. J. Membr. Sci. 1991, 59, 169–181. [Google Scholar] [CrossRef]
- Plisko, T.; Bildyukevich, A.; Usosky, V.; Volkov, V. Influence of the concentration and molecular weight of polyethylene glycol on the structure and permeability of polysulfone hollow fiber membranes. Pet. Chem. 2016, 56, 321–329. [Google Scholar] [CrossRef]
- Han, M.-J.; Nam, S.-T. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Membr. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Strathmann, H.; Kock, K. The formation mechanism of phase inversion membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Huang, D.-H.; Ruaan, R.-C.; Lai, J.-Y. Mechanical properties of asymmetric polysulfone membranes containing surfactant as additives. Ind. Eng. Chem. Res. 2001, 40, 5917–5922. [Google Scholar] [CrossRef]
- Ebewele, R.O. Polymer Science and Technology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Young, R.J.; Lovell, P.A. Introduction to Polymers; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]





| Membranes | Concentrations (% w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PSF35 | PSF60 | PEG 1K | PEG 4K | PEG 8K | PEG 10K | PEG 20K | GLY | NMP | |
| P1 | 15 | - | - | - | - | - | - | - | 85 |
| P2 | - | 15 | - | - | - | - | - | - | 85 |
| P3 | 15 | - | - | - | - | - | - | 10 | 75 |
| P4 | 15 | - | 10 | - | - | - | - | - | 75 |
| P5 | 15 | - | - | 10 | - | - | - | - | 75 |
| P6 | 15 | - | - | - | 10 | - | - | - | 75 |
| P7 | 15 | - | - | - | - | - | 15 | - | 70 |
| P8 | 15 | - | - | - | - | 10 | - | - | 75 |
| P9 | - | 15 | - | - | - | - | 15 | - | 70 |
| P7-5 | 15 | - | - | - | - | - | 5 | - | 80 |
| P7-10 | 15 | - | - | - | - | - | 10 | - | 75 |
| Membrane | Surface Elemental (mol%) | |||
|---|---|---|---|---|
| C1s | O1s | S2p | O1s/C1s | |
| P1 | 86.10 ± 0.95 | 12.74 ± 0.68 | 1.16 ± 0.27 | 0.148 |
| P2 | 86.88 ± 0.3 | 12.23 ± 0.29 | 0.89 ± 0.01 | 0.141 |
| P7 | 82.83 ± 1.95 | 15.55 ± 1.7 | 1.62 ± 0.28 | 0.188 |
| P9 | 82.18 ± 1.5 | 15.75 ± 1.05 | 2.07 ± 0.4 | 0.192 |
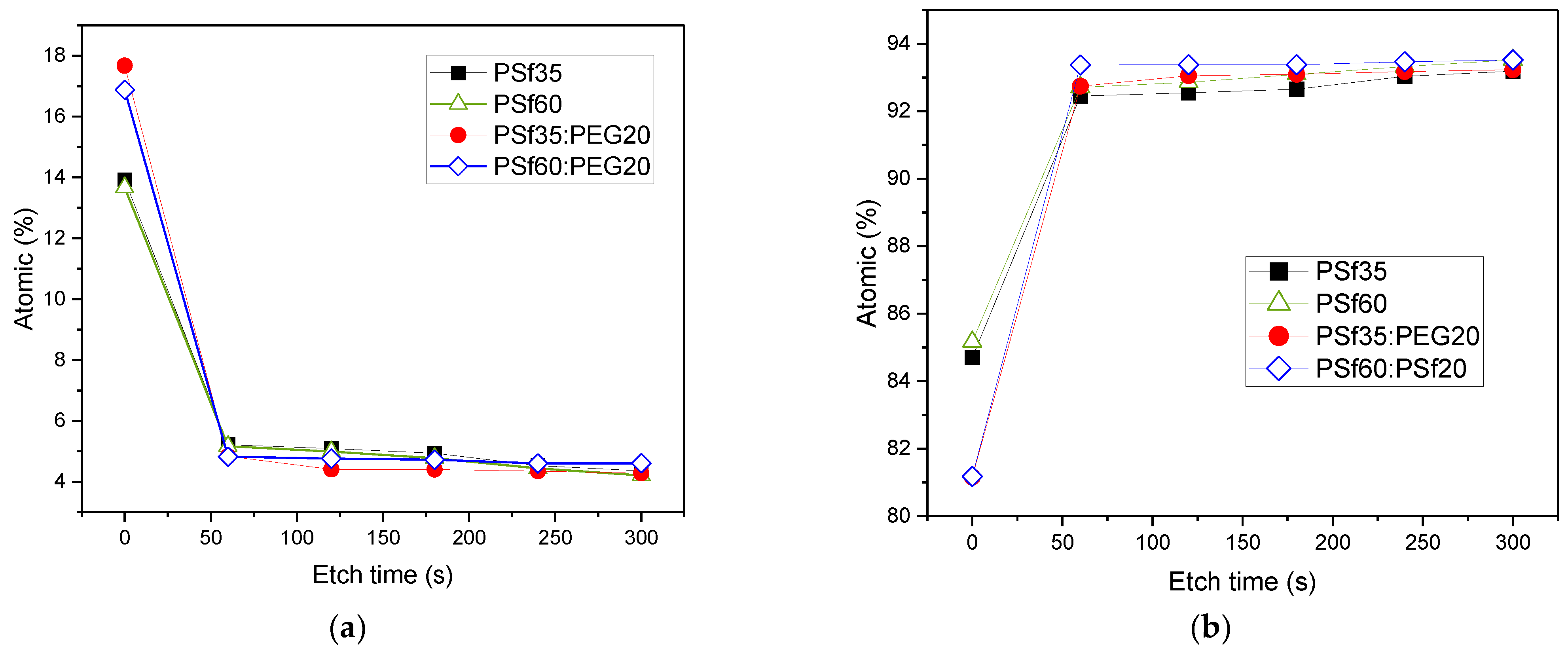
| Membrane | Oxygen Concentration (%mol) | Carbon Concentration (%mol) | ||||
|---|---|---|---|---|---|---|
| Top Surface | 1st Etched Surface | % Decrease | Top Surface | 1st Etched Surface | % Increase | |
| P1 | 13.92 | 5.22 | 62.5 | 84.70 | 92.45 | 9.2 |
| P2 | 13.67 | 5.17 | 62.2 | 85.17 | 92.71 | 8.9 |
| P7 | 16.88 | 4.83 | 71.4 | 81.16 | 92.74 | 14.3 |
| P9 | 17.67 | 4.84 | 72.6 | 81.18 | 93.37 | 15 |
| Membrane Sample | Viscosity (Pa.s) |
|---|---|
| P1 | 3.8 ± 0.63 |
| P2 | 4.0 ± 0.77 |
| P4 | 8.9 ± 1.96 |
| P7 | 16.1 ± 3.54 |
| P8 | 11.1 ± 1.43 |
| P9 | 21.1 ± 4.21 |
| Membrane | Mean Pore Size (nm) | Max Pore Size (nm) | Min Pore Size (nm) |
|---|---|---|---|
| PSf 35 (P1) | 383 ± 45 | 1151 | 82 |
| PSf60 (P2) | 392 ± 23 | 878 | 66 |
| PSf35:PEG20 (P7) | 1433 ± 675 | 18,051 | 292 |
| PSf60:PEG20 (P9) | 2207 ± 808 | 19,366 | 412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbuoji, E.A.; Stephens, L.; Haycraft, A.; Wooldridge, E.; Escobar, I.C. Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications. Membranes 2022, 12, 637. https://doi.org/10.3390/membranes12070637
Ogbuoji EA, Stephens L, Haycraft A, Wooldridge E, Escobar IC. Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications. Membranes. 2022; 12(7):637. https://doi.org/10.3390/membranes12070637
Chicago/Turabian StyleOgbuoji, Ebuka A., Lauren Stephens, Amber Haycraft, Eric Wooldridge, and Isabel C. Escobar. 2022. "Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications" Membranes 12, no. 7: 637. https://doi.org/10.3390/membranes12070637
APA StyleOgbuoji, E. A., Stephens, L., Haycraft, A., Wooldridge, E., & Escobar, I. C. (2022). Non-Solvent Induced Phase Separation (NIPS) for Fabricating High Filtration Efficiency (FE) Polymeric Membranes for Face Mask and Air Filtration Applications. Membranes, 12(7), 637. https://doi.org/10.3390/membranes12070637







