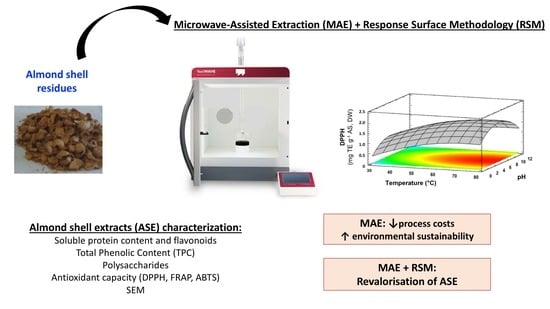
Extraction and Characterization of Antioxidant Compounds in Almond (Prunus amygdalus) Shell Residues for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
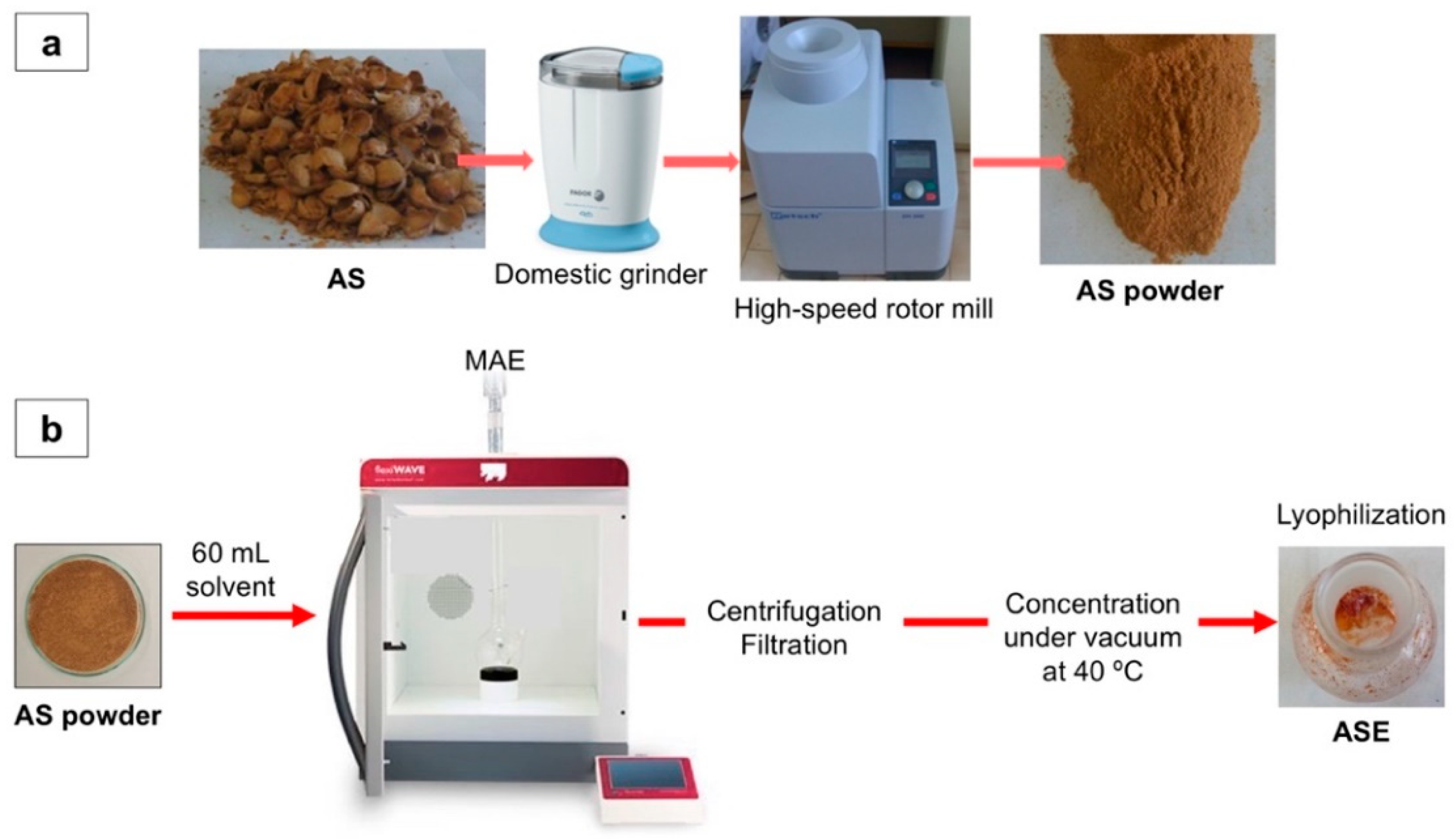
2.2. Microwave-Assisted Extraction (MAE)
2.3. MAE Optimization
2.4. Characterization of AS Extracts
2.4.1. Antioxidant Activity
2.4.2. Total Phenolic Content
2.4.3. Extraction Yield
2.4.4. Flavonoid Content
2.4.5. Soluble Protein Content
2.4.6. Total Polysaccharide Content
2.4.7. Morphological Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fitting the Models
∗ D − 0.000470702 ∗ A2 + 0.000294677 ∗ A ∗ B + 0.000199397 ∗ A ∗ C −
0.0000259385 ∗ A ∗ D − 0.000300107 ∗ B2 + 0.000262823 ∗ B ∗ C + 0.000219005
∗ B ∗ D − 0.0200391 ∗ C2 − 0.0000210329 ∗ C ∗ D − 0.00017416 ∗ D2
∗ D − 0.000689 ∗ A2 + 0.000198274 ∗ A ∗ B + 0.00247619 ∗ A ∗ C + 0.00079343
∗ A ∗ D + 0.00027336 ∗ B2 + 0.003072 ∗ B ∗ C − 0.00010136 ∗ B ∗ D − 0.0274489
∗ C2 + 0.0009920 ∗ C ∗ D − 0.0007529 ∗ D2
D − 0.00001184 ∗ A2 + 0.0001335 ∗ A ∗ B − 0.000396 ∗ A ∗ C + 0.0015187 ∗ A ∗
D − 0.001335 ∗ B2 − 0.0064199 ∗ B ∗ C − 0.0005226 ∗ B ∗ D − 0.0230505 ∗ C2 +
0.001150 ∗ C ∗ D − 0.0006496 ∗ D2
3.2. Optimal MAE Conditions
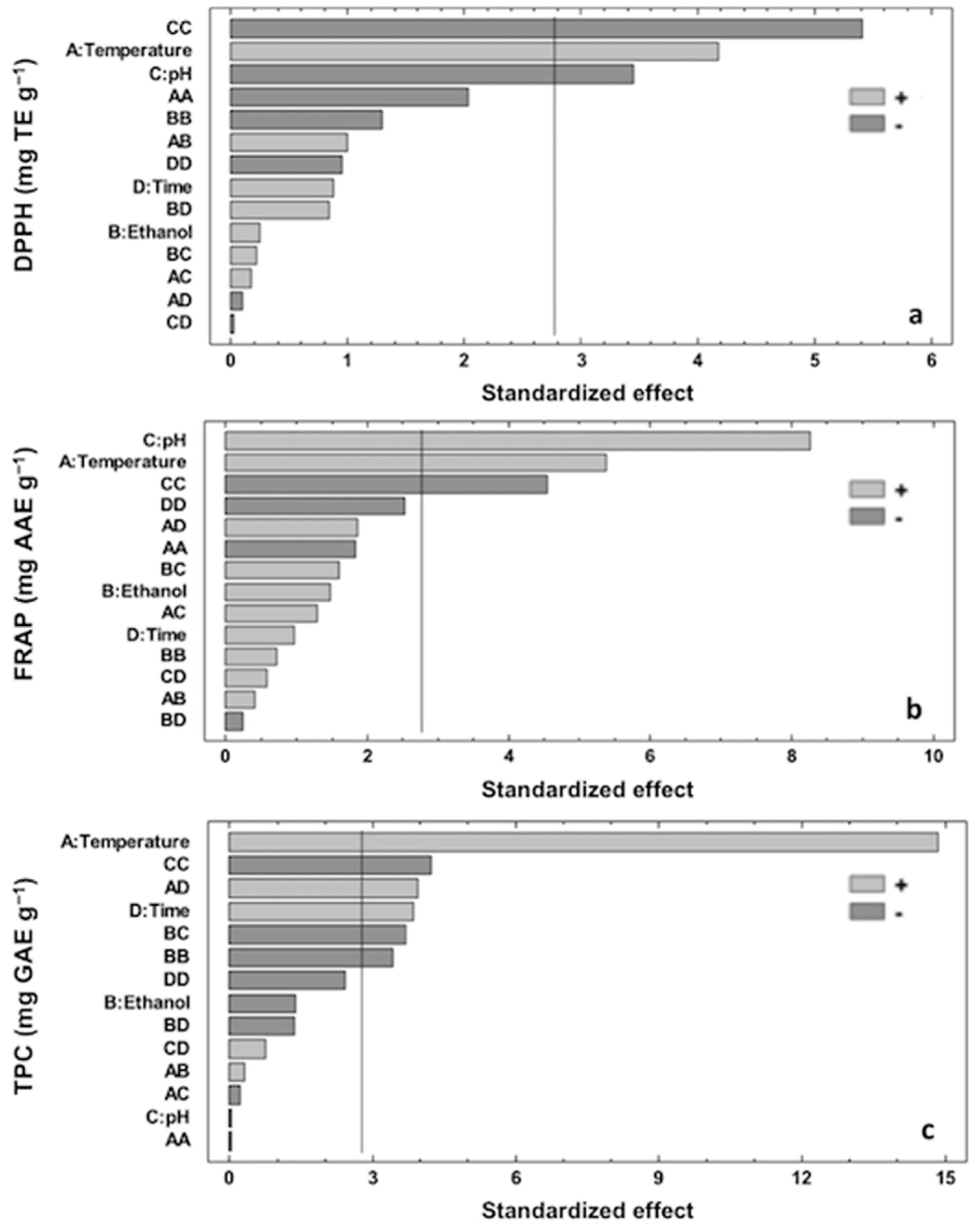
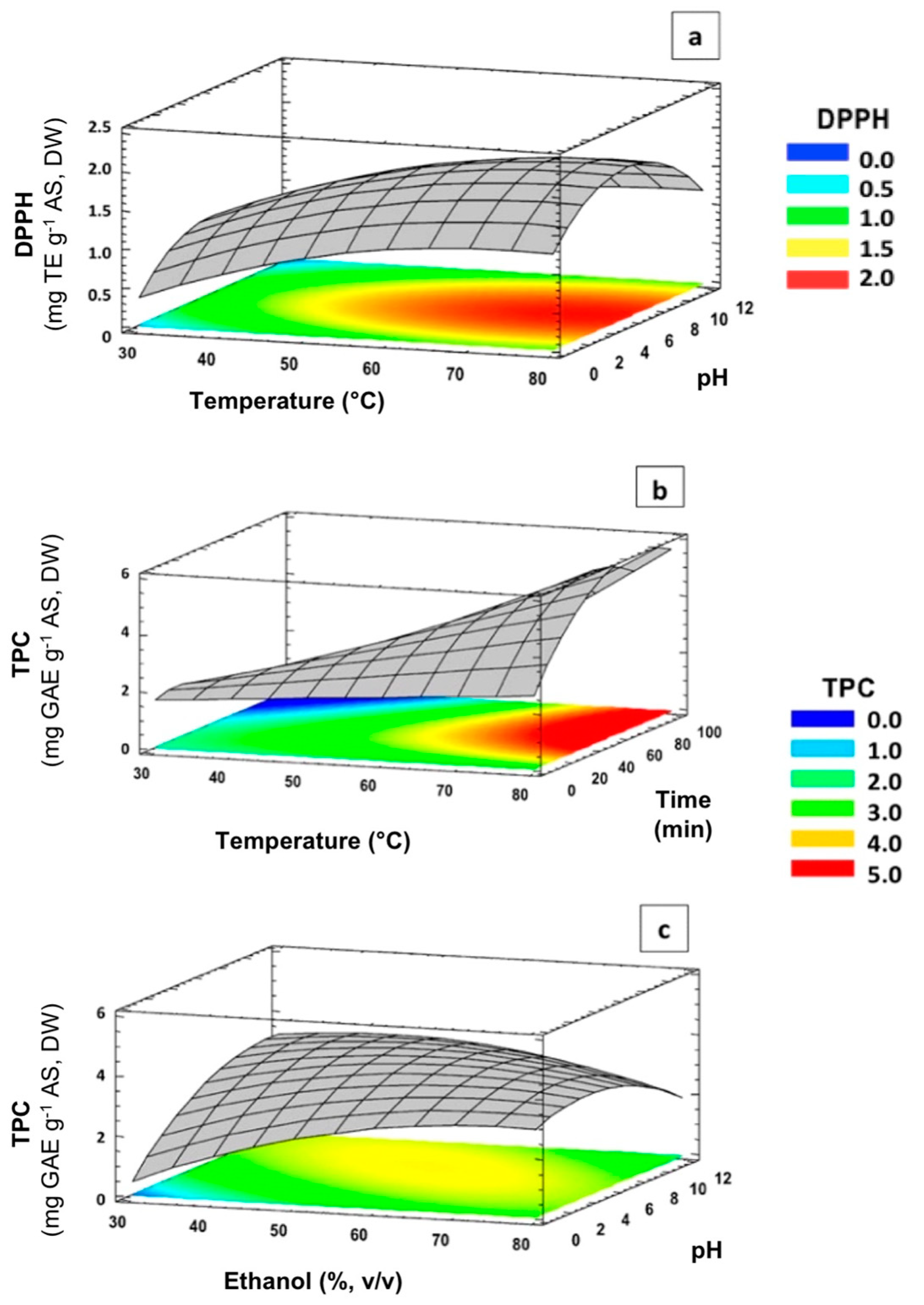
3.3. Effect of Extraction Variables on Antioxidant Capacity (DPPH and FRAP)
3.4. Effect of Extraction Variables on TPC
3.5. Chemical Characterization of ASE Obtained under Optimal Conditions
3.6. Morphological Characterization of Optimized ASE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Proc. Eng. 2021, 44, e13629. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Biores. Bioproc. 2017, 4, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Pirayesh, H.; Khazaeian, A. Using Almond (Prunus amygdalus L.) Shell as a Bio-Waste Resource in Wood Based Composite. Compos. B Eng. 2012, 43, 1475–1479. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Vecchia, S.B.D.; Giovine, F.; Kbaier, H.B.H.; Bouzouita, N.; Pereira, L.B.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef] [Green Version]
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food Industry Waste: Mine of Nutraceuticals. Int. J. Sci. Environ. 2015, 4, 205–229. [Google Scholar]
- Rodríguez, S.L.; Raghavan, V. Green Extraction Techniques from Fruit and Vegetable Waste to Obtain Bioactive Compounds—A Review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6446–6466. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to Be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural Additives and Agricultural Wastes in Biopolymer Formulations for Food Packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Juárez, N.; Beltrán, A.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active Edible Films Fortified with Natural Extracts: Case Study with Fresh-Cut Apple Pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef]
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804. [Google Scholar] [CrossRef]
- Weligama, V.T.; Karim, M.A. A comprehensive review on the properties and functionalities of biodegradable and semibiodegradable food packaging materials. Compr. Rev. Food Sci. 2022, 21, 689–718. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.B.M.; Nor, M.S.M. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J. Biores. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Henry, C.; Chinomso, M.; Ewulonu, I.C.; Nwuzor, C.M.; Obele, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Biores. Bioprod. 2021, 6, 168–185. [Google Scholar]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Pascual-Seva, N.; Barros, A.I.R.N.A. A Box-Behnken Design for Optimal Extraction of Phenolics from Almond By-Products. Food Anal. Met. 2019, 12, 2009–2024. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T. Antioxidant Constituents of Almond [Prunus dulcis (Mill.) D.A. Webb] Hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Bottone, A.; Masullo, M.; Montoro, P.; Pizza, C.; Piacente, S. HR-LC-ESI-Orbitrap-MS Based Metabolite Profiling of Prunus dulcis Mill. (Italian Cultivars Toritto and Avola) Husks and Evaluation of Antioxidant Activity. Phytochem. Anal. 2019, 30, 415–423. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of Antioxidant Phenolics from Almond Hulls (Prunus amygdalus) and Pine Sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Bolling, B.W.; Dolnikowski, G.; Blumberg, J.B.; Chen, C.Y.O. Polyphenol Content and Antioxidant Activity of California Almonds Depend on Cultivar and Harvest Year. Food Chem. 2010, 122, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Dahuja, A.; Tiwari, S.; Punia, S.; Tak, Y.; Amarowicz, R.; Bhoite, A.G.; Singh, S.; Joshi, S.; Panesar, P.S.; et al. Recent Trends in Extraction of Plant Bioactives Using Green Technologies: A Review. Food Chem. 2021, 353, 129431. [Google Scholar] [CrossRef]
- Quiroz, J.Q.; Torres, A.C.; Ramirez, L.M.; Garcia, M.S.; Gomez, G.C.; Rojas, J. Optimization of the Microwave-Assisted Extraction Process of Bioactive Compounds from Annatto Seeds (Bixa orellana L.). Antioxidants 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Doulabi, M.; Golmakani, M.T.; Ansari, S. Evaluation and Optimization of Microwave-Assisted Extraction of Bioactive Compounds from Eggplant Peel by-Product. J. Food Proc. Preserv. 2020, 44, 405–415. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The Effect of Microwave Assisted Extraction on the Isolation of Anthocyanins and Phenolic Acids from Sour Cherry Marasca (Prunus cerasus Var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-Assisted Extraction of Phenolic Compounds from Almond Skin Byproducts (Prunus amygdalus): A Multivariate Analysis Approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of Ultrasound-Assisted Extraction (UAE) Process for the Recovery of Bioactive Compounds from Bitter Gourd Using Response Surface Methodology (RSM). Food Bioprod. Proc. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Feki, F.; Klisurova, D.; Masmoudi, M.A.; Choura, S.; Denev, P.; Trendafilova, A.; Chamkha, M.; Sayadi, S. Optimization of Microwave Assisted Extraction of Simmondsins and Polyphenols from Jojoba (Simmondsia chinensis) Seed Cake Using Box-Behnken Statistical Design. Food Chem. 2021, 356, 129670. [Google Scholar] [CrossRef]
- Nishad, J.; Dutta, A.; Saha, S.; Rudra, S.G.; Varghese, E.; Sharma, R.R.; Tomar, M.; Kumar, M.; Kaur, C. Ultrasound-Assisted Development of Stable Grapefruit Peel Polyphenolic Nano-Emulsion: Optimization and Application in Improving Oxidative Stability of Mustard Oil. Food Chem. 2021, 334, 127561. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of Microwave-Assisted Extraction of Cocoa Bean Shell Waste and Evaluation of Its Antioxidant, Physicochemical and Functional Properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, S.C.; Hu, C.C.; Shyu, Y.S.; Hsu, C.Y.; Yang, D.J. Effects of Roasting Temperature and Duration on Fatty Acid Composition, Phenolic Composition, Maillard Reaction Degree and Antioxidant Attribute of Almond (Prunus dulcis) Kernel. Food Chem. 2016, 190, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Salcedo, C.L.; López de Mishima, B.A.; Nazareno, M.A. Walnuts and Almonds as Model Systems of Foods Constituted by Oxidisable, pro-Oxidant and Antioxidant Factors. Food Res. Int. 2010, 43, 1187–1197. [Google Scholar] [CrossRef]
- Milutinović, M.; Radovanović, N.; Ćorović, M.; Šiler-Marinković, S.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. Optimisation of Microwave-Assisted Extraction Parameters for Antioxidants from Waste Achillea Millefolium Dust. Ind. Crops Prod. 2015, 77, 333–341. [Google Scholar] [CrossRef]
- Pérez, L.; Conde, E.; Domínguez, H. Microwave Hydrodiffusion and Gravity Processing of Sargassum Muticum. Process Biochem. 2014, 49, 981–988. [Google Scholar] [CrossRef]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of Microwave-Assisted Extraction of Polyphenols from Quercus Bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Reuse Potential of Vegetable Wastes (Broccoli, Green Bean and Tomato) for the Recovery of Antioxidant Phenolic Acids and Flavonoids. Int. J. Food Sci. Technol. 2017, 52, 98–107. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Fernández-Bolaños, J.; García-Borrego, A.; Lama-Muñoz, A.; Rodríguez-Gutiérrez, G. Influence of PH on the Antioxidant Phenols Solubilised from Hydrothermally Treated Olive Oil By-Product (Alperujo). Food Chem. 2017, 219, 339–345. [Google Scholar] [CrossRef]
- Zapata, I.C.; Álzate, A.F.; Zapata, K.; Arias, J.P.; Puertas, M.A.; Rojano, B. Effect of PH, Temperature and Time of Extraction on the Antioxidant Properties of Vaccinium Meridionale Swartz. J. Berry Res. 2019, 9, 39–49. [Google Scholar] [CrossRef]
- Wiyono, T.; Nurhayati, R.; Herawati, E.R.N.; Laila, U. The Effect of Time, PH and Solvent Composition on Cocoa Shell Polyphenol Extraction and Its Antioxidant Activity: Response Surface Method Approach. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Tangerang, Indonesia, 23–24 October 2019; Institute of Physics Publishing: Bristol, UK, 2020; Volume 462. [Google Scholar]
- Quiles-Carrillo, L.; Mellinas, C.; Garrigos, M.C.; Balart, R.; Torres-Giner, S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds with Antioxidant Activity from Carob Pods. Food Anal. Met. 2019, 12, 2480–2490. [Google Scholar] [CrossRef]
- Hashim, M.Y.; Rsolan, M.N.; Amin, A.M.; Zaidi, A.M.A.; Ariffin, S. Mercerization Treatment Parameter Effect on Natural Fibre Reinforced Polymer Matrix Composite: A Brief Review. World Acad. Sci. Eng. Technol. 2012, 6, 1382–1388. [Google Scholar]
- Urruzola, I.; Robles, E.; Serrano, L.; Labidi, J. Nanopaper from Almond (Prunus dulcis) Shell. Cellulose 2014, 21, 1619–1629. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, Andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of Phenolic Antioxidants from Green Kiwifruit Peel Using Subcritical Water Extraction. Food Bioprod. Proc. 2020, 122, 136–144. [Google Scholar] [CrossRef]
- Zeynep, C.; İsmail, Ş.; Selin, S.; Gökben, K. Optimization and Kinetic Studies of Ultrasound-Assisted Extraction on Polyphenols from Satsuma Mandarin (Citrus unshiu Marc.) Leaves. Iran. J. Chem. Chem. Eng. 2017, 36, 163–171. [Google Scholar]
- Librán, C.; Mayor, L.; Garcia-Castello, E.; Vidal-Brotons, D. Polyphenol Extraction from Grape Wastes: Solvent and PH Effect. Agric. Sci. 2013, 4, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Labuckas, D.; Maestri, D.; Lamarque, A. Molecular Characterization, Antioxidant and Protein Solubility-Related Properties of Polyphenolic Compounds from Walnut (Juglans regia). Nat. prod. Commun. 2016, 11, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Švarc-Gajić, J.; Stojanović, Z.; Segura Carretero, A.; Arráez Román, D.; Borrás, I.; Vasiljević, I. Development of a Microwave-Assisted Extraction for the Analysis of Phenolic Compounds from Rosmarinus Officinalis. J. Food Eng. 2013, 119, 525–532. [Google Scholar] [CrossRef]
- Hughey, C.A.; Janusziewicz, R.; Minardi, C.S.; Phung, J.; Huffman, B.A.; Reyes, L.; Wilcox, B.E.; Prakash, A. Distribution of Almond Polyphenols in Blanch Water and Skins as a Function of Blanching Time and Temperature. Food Chem. 2012, 131, 1165–1173. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of Extraction Conditions on Ultrasound-Assisted Recovery of Bioactive Phenolics from Blueberry Pomace and Their Antioxidant Activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [Green Version]
- Morales, A.; Hernández-Ramos, F.; Sillero, L.; Fernández-Marín, R.; Dávila, I.; Gullón, P.; Erdocia, X.; Labidi, J. Multiproduct Biorefinery Based on Almond Shells: Impact of the Delignification Stage on the Manufacture of Valuable Products. Biores. Technol. 2020, 315, 123896. [Google Scholar] [CrossRef]
- Maaloul, N.; Arfi, R.B.; Rendueles, M.; Ghorbal, A.; Diaz, M. Dialysis-Free Extraction and Characterization of Cellulose Crystals from Almond (Prunus dulcis) Shells. J. Mater. Environ. Sci. 2017, 11, 4171–4181. [Google Scholar]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar]
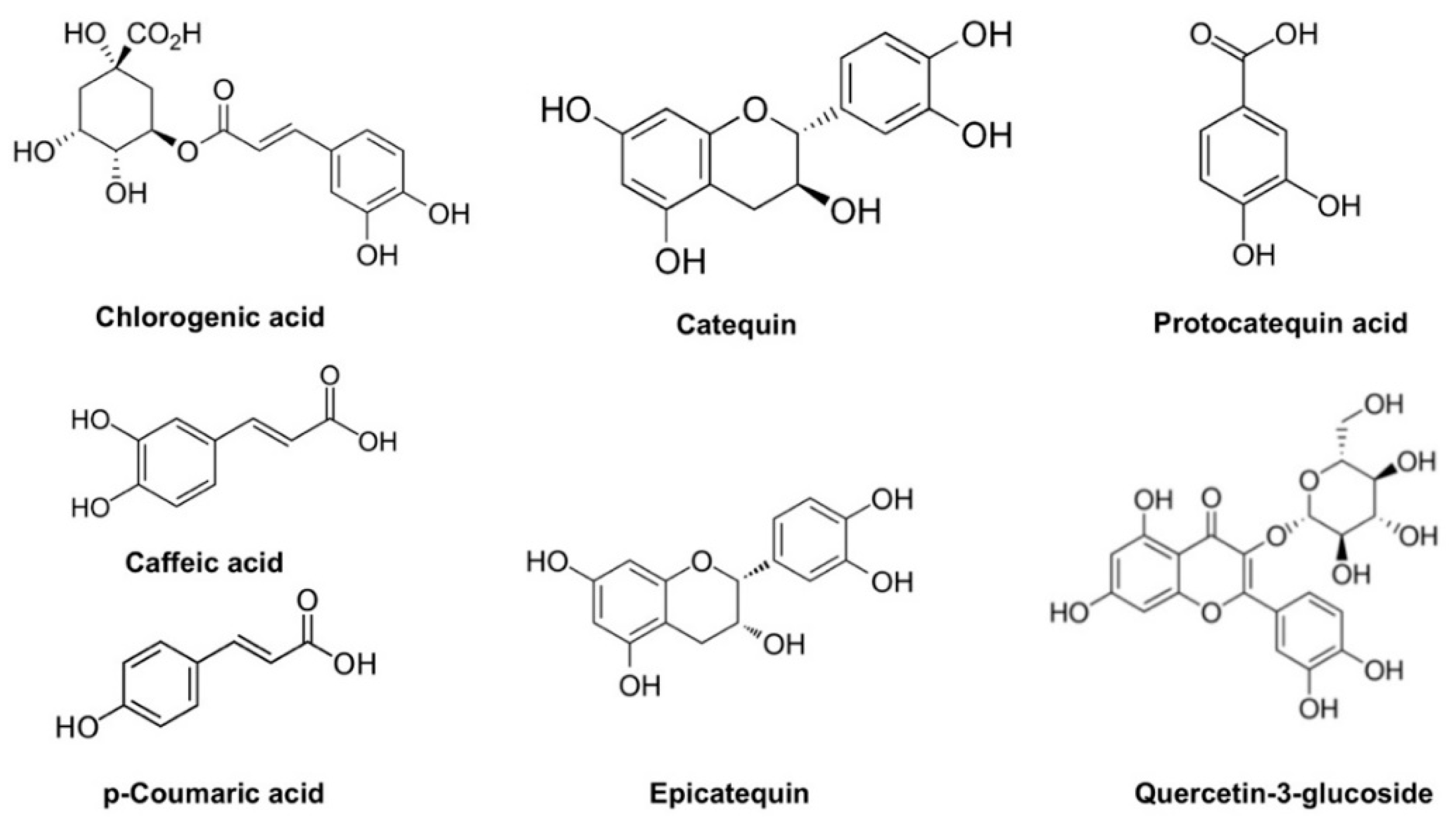
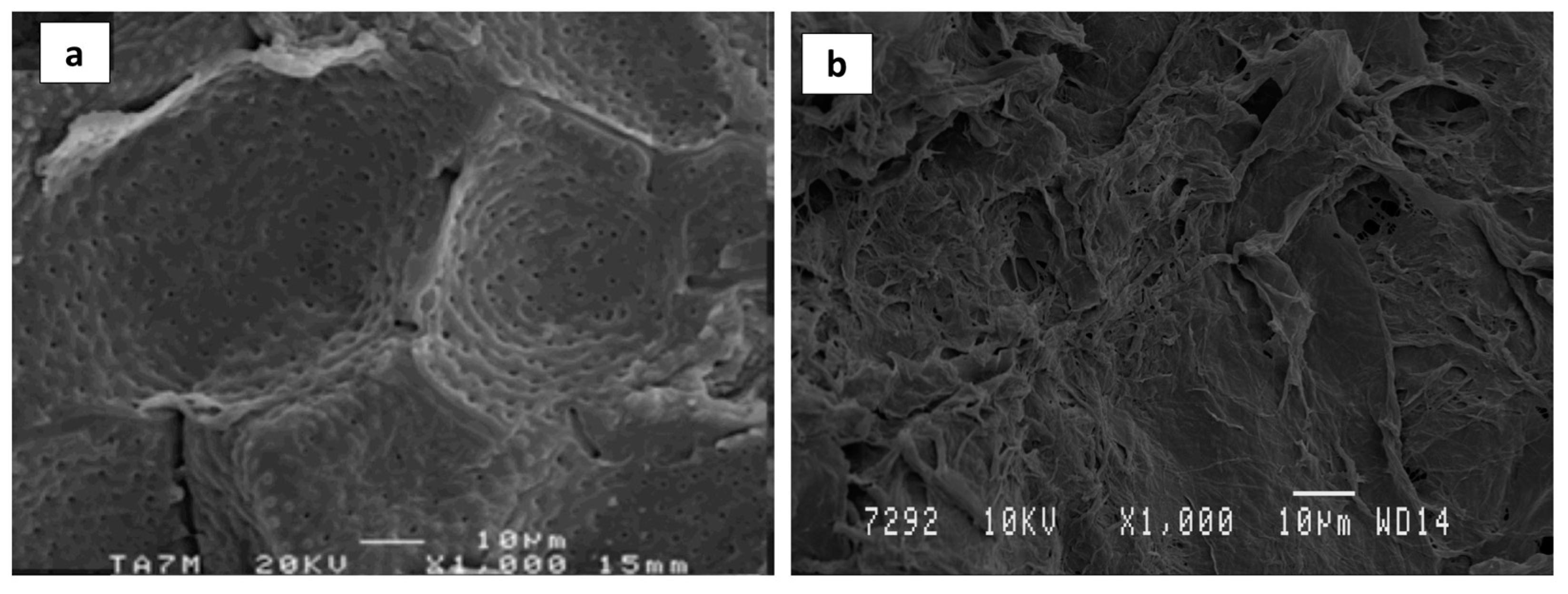
| Run | Temperature (°C) | Ethanol (%, v/v) | pH | Time (min) | DPPH (mg TE g−1) | FRAP (mg AAE g−1) | TPC (mg GAE g−1) |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 60 | 7 | 37.5 | 1.57 | 2.28 | 3.68 |
| 2 | 60 | 60 | 12 | 60.0 | 1.01 | 1.78 | 2.97 |
| 3 | 60 | 60 | 7 | 37.5 | 1.57 | 1.66 | 3.25 |
| 4 | 40 | 60 | 2 | 37.5 | 0.85 | 0.27 | 1.51 |
| 5 | 60 | 80 | 2 | 37.5 | 1.42 | 0.54 | 2.96 |
| 6 | 60 | 60 | 7 | 37.5 | 1.97 | 2.01 | 3.93 |
| 7 | 80 | 80 | 7 | 37.5 | 1.77 | 2.58 | 5.07 |
| 8 | 60 | 80 | 7 | 15.0 | 1.51 | 1.71 | 2.97 |
| 9 | 40 | 60 | 7 | 15.0 | 1.32 | 1.25 | 2.15 |
| 10 | 60 | 60 | 2 | 15.0 | 1.42 | 0.38 | 2.94 |
| 11 | 60 | 80 | 12 | 37.5 | 1.09 | 4.15 | 1.52 |
| 12 | 60 | 40 | 7 | 15.0 | 1.68 | 1.53 | 2.30 |
| 13 | 40 | 60 | 7 | 60.0 | 1.42 | 0.81 | 1.99 |
| 14 | 60 | 80 | 7 | 60.0 | 1.76 | 1.82 | 3.12 |
| 15 | 40 | 40 | 7 | 37.5 | 1.46 | 1.32 | 1.57 |
| 16 | 80 | 40 | 7 | 37.5 | 1.54 | 2.38 | 4.85 |
| 17 | 40 | 80 | 7 | 37.5 | 1.21 | 1.21 | 1.58 |
| 18 | 60 | 40 | 7 | 60.0 | 1.54 | 1.82 | 3.40 |
| 19 | 60 | 40 | 2 | 37.5 | 1.38 | 0.31 | 2.81 |
| 20 | 60 | 60 | 7 | 37.5 | 1.96 | 2.23 | 3.84 |
| 21 | 80 | 60 | 7 | 15.0 | 1.71 | 2.16 | 3.67 |
| 22 | 40 | 60 | 12 | 37.5 | 0.42 | 1.04 | 2.17 |
| 23 | 60 | 60 | 12 | 15.0 | 0.79 | 1.39 | 2.24 |
| 24 | 60 | 60 | 2 | 60.0 | 1.64 | 0.32 | 3.16 |
| 25 | 80 | 60 | 12 | 37.5 | 1.48 | 2.29 | 4.73 |
| 26 | 80 | 60 | 2 | 37.5 | 1.83 | 0.52 | 4.23 |
| 27 | 60 | 60 | 7 | 37.5 | 2.04 | 2.70 | 4.18 |
| 28 | 80 | 60 | 7 | 60.0 | 1.76 | 3.14 | 6.25 |
| 29 | 60 | 40 | 12 | 37.5 | 0.94 | 2.69 | 3.94 |
| DPPH | |||||
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
| A | 0.974067 | 1 | 0.974067 | 17.50 | 0.0139 * |
| B | 0.00345051 | 1 | 0.00345051 | 0.06 | 0.8156 |
| C | 0.662551 | 1 | 0.662551 | 11.90 | 0.0261 * |
| D | 0.0425043 | 1 | 0.0425043 | 0.76 | 0.4315 |
| AA | 0.229943 | 1 | 0.229943 | 4.13 | 0.1119 |
| AB | 0.0555739 | 1 | 0.0555739 | 1.00 | 0.3743 |
| AC | 0.00159037 | 1 | 0.00159037 | 0.03 | 0.8740 |
| AD | 0.000544973 | 1 | 0.000544973 | 0.01 | 0.9259 |
| BB | 0.0934723 | 1 | 0.0934723 | 1.68 | 0.2648 |
| BC | 0.00276304 | 1 | 0.00276304 | 0.05 | 0.8346 |
| BD | 0.0388503 | 1 | 0.0388503 | 0.70 | 0.4505 |
| CC | 1.62797 | 1 | 1.62797 | 29.24 | 0.0057 ** |
| CD | 0.0000223956 | 1 | 0.0000223956 | 0.00 | 0.9850 |
| DD | 0.0504238 | 1 | 0.0504238 | 0.91 | 0.3951 |
| Lack of fit | 0.367673 | 10 | 0.0367673 | 0.66 | 0.7296 |
| Pure error | 0.222669 | 4 | 0.0556673 | ||
| Total (corr.) | 4.06348 | 28 | |||
| R2 | 0.8547 | ||||
| Adjusted R2 | 0.7094 | ||||
| CV (%) | 12.95 | ||||
| FRAP | |||||
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
| A | 4.26738 | 1 | 4.26738 | 28.85 | 0.0058 ** |
| B | 0.324264 | 1 | 0.324264 | 2.19 | 0.2128 |
| C | 10.1047 | 1 | 10.1047 | 68.31 | 0.0012 ** |
| D | 0.136747 | 1 | 0.136747 | 0.92 | 0.3908 |
| AA | 0.493357 | 1 | 0.493357 | 3.34 | 0.1418 |
| AB | 0.0251601 | 1 | 0.0251601 | 0.17 | 0.7012 |
| AC | 0.24526 | 1 | 0.24526 | 1.66 | 0.2673 |
| AD | 0.50992 | 1 | 0.50992 | 3.45 | 0.1369 |
| BB | 0.0775534 | 1 | 0.0775534 | 0.52 | 0.5091 |
| BC | 0.377365 | 1 | 0.377365 | 2.55 | 0.1855 |
| BD | 0.00832178 | 1 | 0.00832178 | 0.06 | 0.8242 |
| CC | 3.05449 | 1 | 3.05449 | 20.65 | 0.0105 * |
| CD | 0.0498219 | 1 | 0.0498219 | 0.34 | 0.5928 |
| DD | 0.94252 | 1 | 0.94252 | 6.37 | 0.0651 |
| Lack of fit | 4.08977 | 10 | 0.408977 | 2.76 | 0.1696 |
| Pure error | 0.591696 | 4 | 0.147924 | ||
| Total (corr.) | 24.8943 | 28 | |||
| R2 | 0.8119 | ||||
| Adjusted R2 | 0.6239 | ||||
| CV (%) | 16.68 | ||||
| TPC | |||||
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
| A | 26.4646 | 1 | 26.4646 | 220.39 | 0.0001 *** |
| B | 0.230731 | 1 | 0.230731 | 1.92 | 0.2380 |
| C | 0.000250063 | 1 | 0.000250063 | 0.00 | 0.9658 |
| D | 1.78016 | 1 | 1.78016 | 14.82 | 0.0183 * |
| AA | 0.000145457 | 1 | 0.000145457 | 0.00 | 0.9739 |
| AB | 0.0114068 | 1 | 0.0114068 | 0.09 | 0.7733 |
| AC | 0.00629103 | 1 | 0.00629103 | 0.05 | 0.8302 |
| AD | 1.8682 | 1 | 1.8682 | 15.56 | 0.0169 * |
| BB | 1.40154 | 1 | 1.40154 | 11.67 | 0.0269 * |
| BC | 1.64864 | 1 | 1.64864 | 13.73 | 0.0207 * |
| BD | 0.221111 | 1 | 0.221111 | 1.84 | 0.2463 |
| CC | 2.15403 | 1 | 2.15403 | 17.94 | 0.0133 * |
| CD | 0.0669926 | 1 | 0.0669926 | 0.56 | 0.4966 |
| DD | 0.701689 | 1 | 0.701689 | 5.84 | 0.0730 |
| Lack of fit | 2.3358 | 10 | 0.23358 | 1.95 | 0.2726 |
| Pure error | 0.480317 | 4 | 0.120079 | ||
| Total (corr.) | 38.5406 | 28 | |||
| R2 | 0.9269 | ||||
| Adjusted R2 | 0.8539 | ||||
| CV (%) | 9.18 |
| Response | Content |
|---|---|
| Extraction yield (wt%) | 35.2 ± 0.07 |
| Flavonoids (mg CE g−1) | 1.42 ± 0.05 |
| Soluble proteins (mg BSA g−1) | 0.43 ± 0.08 |
| Total polysaccharides (mg glucose g−1) | 1.59 ± 0.05 |
| DPPH (mg TE g−1) | 4.21 ± 0.44 |
| DPPH RSA (%) | 78 ± 7 |
| DPPH IC50 (mg mL−1) | 64.96 ± 1.17 |
| FRAP (mg AAE acid g−1) | 3.85 ± 0.54 |
| TPC (mg GAE g−1) | 6.59 ± 0.25 |
| ABTS (mg TE g−1) | 6.20 ± 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdés, A.; Garrigós, M.C.; Jiménez, A. Extraction and Characterization of Antioxidant Compounds in Almond (Prunus amygdalus) Shell Residues for Food Packaging Applications. Membranes 2022, 12, 806. https://doi.org/10.3390/membranes12080806
Valdés A, Garrigós MC, Jiménez A. Extraction and Characterization of Antioxidant Compounds in Almond (Prunus amygdalus) Shell Residues for Food Packaging Applications. Membranes. 2022; 12(8):806. https://doi.org/10.3390/membranes12080806
Chicago/Turabian StyleValdés, Arantzazu, María Carmen Garrigós, and Alfonso Jiménez. 2022. "Extraction and Characterization of Antioxidant Compounds in Almond (Prunus amygdalus) Shell Residues for Food Packaging Applications" Membranes 12, no. 8: 806. https://doi.org/10.3390/membranes12080806
APA StyleValdés, A., Garrigós, M. C., & Jiménez, A. (2022). Extraction and Characterization of Antioxidant Compounds in Almond (Prunus amygdalus) Shell Residues for Food Packaging Applications. Membranes, 12(8), 806. https://doi.org/10.3390/membranes12080806