Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Constructs
2.2. Fish
2.3. Vaccination and SAV Cohabitation Challenge
| Treatment | Total # of fish | Analysis (# of fish) | |||||
|---|---|---|---|---|---|---|---|
| Immune gene expression 12 and 48 hpv | nsP1 RT-qPCR 3 wpc 1 | Histopathology 5 and 6 wpc 2 | nAb assay | ||||
| 6 wpv | 3 wpc 1 | 6wpc 2 | |||||
| SAV Ag | 74 + 1 | 8 + 8 | 10 | 15 + 15 | 13 | 15 | 15 |
| SAV Ag oil | 64 + 1 | 8 + 8 | 10 | 15 + 15 | 8 | 10 | 15 |
| SAV Ag CpG (50 µg)/poly I:C (50 µg) | 74 + 1 | 8 + 8 | 10 | 15 + 15 | 13 | 15 | 15 |
| SAV Ag PcDNA3-vhsG (10 µg) | 68 + 1 | 10 + 10 | 10 | 15 + 15 | 8 | 10 | 15 |
| SAV Ag oilPcDNA3-vhsG (10 µg) | 68 + 1 | 10 + 10 | 10 | 15 + 15 | 8 | 10 | 15 |
| SAV Ag CpG (50µg)/poly I:C (50 µg) PcDNA3-vhsG (10 µg) | 68 + 1 | 10 + 10 | 10 | 15 + 15 | 8 | 10 | 15 |
| PcDNA3-vhsG (10 µg) | 68 + 1 | 10 + 10 | 10 | 15 + 15 | 8 | 10 | 15 |
| Saline 0.9% | 78 + 1 | 10 + 10 | 10 | 15 + 15 | 13 | 15 | 15 |
2.4. RT-qPCR of Immune Gene Expression: RNA Isolation, cDNA Synthesis and RT-qPCR
2.5. SAV Neutralizing Ab Responses

2.6. SAV nsP1 RT-qPCR Detection
2.7. Histopathology
2.8. Data Analyses
| GENES | ASSAY | PRIMERS/PROBE | SEQUENCE (5'–3') | PCR EFFICIENCY | ACCESSION NO. |
|---|---|---|---|---|---|
| CD4-1 | Fw/Rev 200 nM Probe 200 nM | Forward Reverse Probe | GAATCTGCCGCTGCAAAGAC AGGGATTCCGGTCTGTATGATATCT [6FAM]CCCAAACCAAAAGGATTC[BHQ1] | 1.78 | EU409792 |
| CD4-2a | SYBR® Green 3.75 µM | Forward Reverse | TGCAAAGAAGGCGCAGAT GAAAACCTTTAATTTAACAGG | 1.76 | EU409793 |
| CD8a | Fw/Rev 450 nM Probe 250 nM | Forward Reverse Probe | CGTCTACAGCTGTGCATCAATCAA GGCTGTGGTCATTGGTGTAGTC [6FAM]CTGGGCCAGCCCCTAC[BHQ1] | 1.74 | AY693391 |
| EF1aB | Fw/Rev 900 nM Probe 250 nM SYBR Green* 3.75 µM | Forward * Reverse * Probe | TGCCCCTCCAGGATGTCTAC CACGGCCCACAGGTACTG [6FAM]AAATCGGCGGTATTGG[BHQ1] | 2.09/2.16 * | BG933897 |
| IFNa1 | SYBR Green 3.75 µM | Forward Reverse | CCTTTCCCTGCTGGACCA TGTCTGTAAAGGGATGTTGGGAAAA | 2.0 | AY2169594 AY2169595 |
| IFNγ | Fw/Rev 900 nM Probe 250 nM | Forward Reverse Probe | AAGGGCTGTGATG TGTTTCTGTGTACTGAGCGGCATTACTCC [6FAM]TTGATGGGCTGGATGACTTTAGGA[BHQ1] | 2.0 | AY795563 |
| mIgM | Fw/Rev 200 nM Probe 200 nM | Forward Reverse Probe | CCTACAAGAGGGAGACCGA GATGAAGGTGAAGGCTGTTTT [6FAM]TGACTGACTGTCCATGCAGCAACACC[BHQ1] | 2.0 | |
| Mx1/2 | Fw/Rev 900 nM Probe 250 nM | Forward Reverse Probe | GATGCTGCACCTCAAGTCCTATTA CGGATCACCATGGGAATCTGA [6FAM]CAGGATATCCAGTCAACGTT[BHQ1] | 1.96 | U66475/U66476 |
| PAX5 | Fw/Rev 200 nM Probe 200 nM | Forward Reverse Probe | CCACTGCCAGGTCGA GAGTCAGCGAGGAGGTGGAGTA [6FAM]CCCCGGCTATCCACCACACG[BHQ1] | 1.85 | |
| sIgM | Fw/Rev 900 nM Probe 250 nM | Forward Reverse Probe | CTACAAGAGGGAGACCGGAG AGGGTCACCGTATTATCACTAGTTT TCCACAGCGTCCATCTGTCTTTC | 1.94 | BT060420 |
| Vig-1 | Fw/Rev 900 nM Probe 250 nM | Forward Reverse Probe | AGCAATGGCAGCATGATCAG TGGTTGGTGTCCTCGTCAAAG [6FAM]AGTGGTTCCAAACGTATGGCGAATACCTG[BHQ1] | 1.94 | BT047610 |
| Q_nsP1 | Fw/Rev 900 nM Probe 130 nM | Forward Reverse Probe | CCGGCCCTGAACCAGTT GTAGCCAAGTGGGAGAAAGCT [6FAM]CTGGCCACCACTTCGA[BHQ1] | - | AY604235 |
3. Results
3.1. Immune Genes
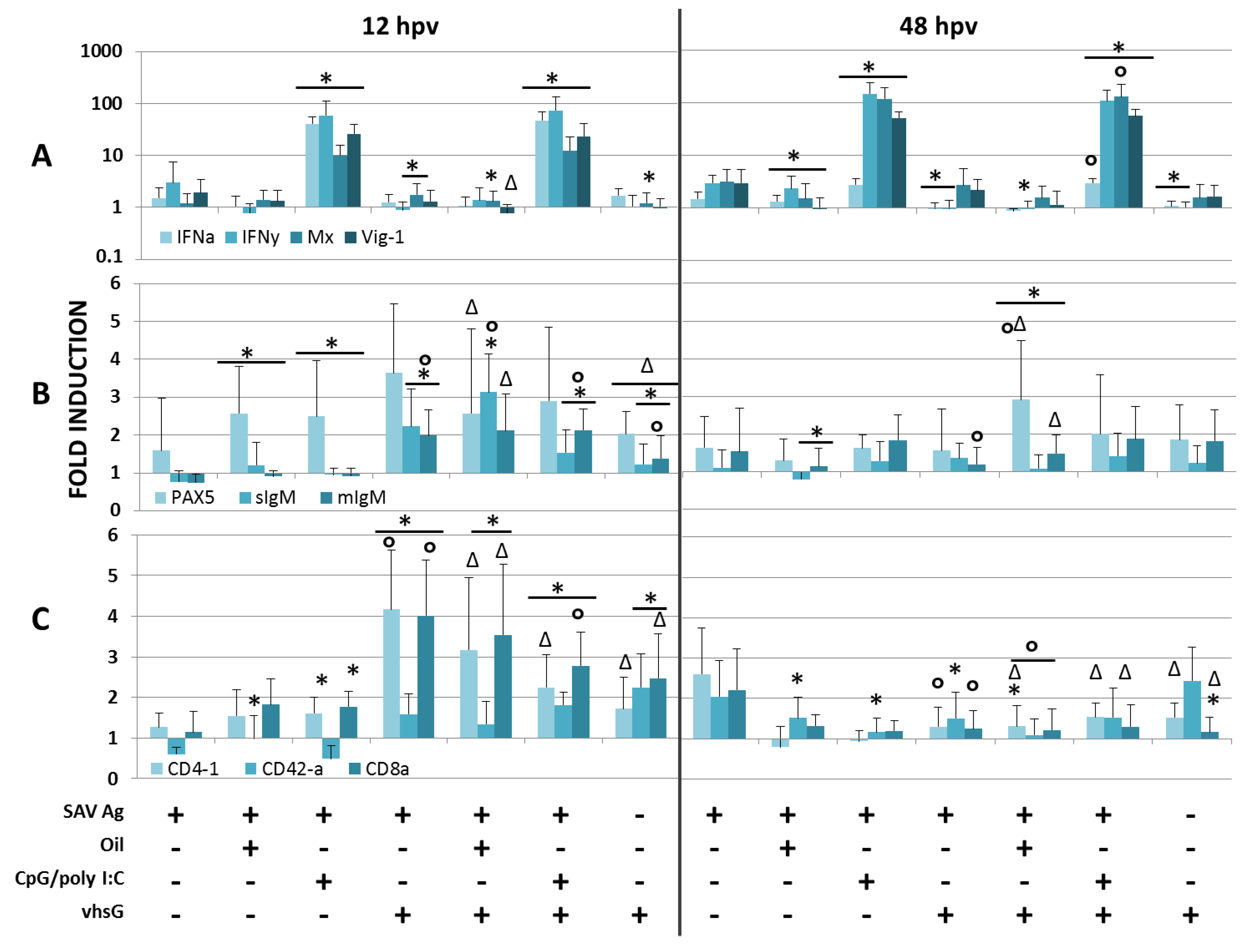

3.2. SAV Neutralizing Ab Responses
3.3. Protection


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thorarinsson, R.; Powell, D.B. Effects of disease risk, vaccine efficacy, and market price on the economics of fish vaccination. Aquaculture 2006, 256, 42–49. [Google Scholar] [CrossRef]
- Fringuelli, E.; Rowley, H.M.; Wilson, J.C.; Hunter, R.; Rodger, H.; Graham, D.A. Phylogenetic analyses and molecular epidemiology of european salmonid alphaviruses (sav) based on partial e2 and nsp3 gene nucleotide sequences. J. Fish Dis. 2008, 31, 811–823. [Google Scholar] [CrossRef]
- Hodneland, K.; Bratland, A.; Christie, K.E.; Endresen, C.; Nylund, A. New subtype of salmonid alphavirus (sav), togaviridae, from atlantic salmon salmo salar and rainbow trout oncorhynchus mykiss in norway. Dis. Aquat. Org. 2005, 66, 113–120. [Google Scholar] [CrossRef]
- Taksdal, T.; Olsen, A.B.; Bjerkas, I.; Hjortaas, M.J.; Dannevig, B.H.; Graham, D.A.; McLoughlin, M.F. Pancreas disease in farmed atlantic salmon, salmo salar l, and rainbow trout, oncorhynchus mykiss (walbaum), in norway. J. Fish Dis. 2007, 30, 545–558. [Google Scholar] [CrossRef]
- McLoughlin, M.F.; Graham, D.A. Alphavirus infections in salmonids—A review. J. Fish Dis. 2007, 30, 511–531. [Google Scholar] [CrossRef]
- Graham, D.A.; Frost, P.; McLaughlin, K.; Rowley, H.M.; Gabestad, I.; Gordon, A.; McLoughlin, M.F. A comparative study of marine salmonid alphavirus subtypes 1–6 using an experimental cohabitation challenge model. J. Fish Dis. 2011, 34, 273–286. [Google Scholar] [CrossRef]
- Biering, E.; Villoing, S.; Sommerset, I.; Christie, K.E. Update on viral vaccines for fish. Dev. Biol. 2005, 121, 97–113. [Google Scholar]
- Houghton, G. Acquired protection in atlantic salmon salmo salar parr and post-smolts against pancreas disease. Dis. Aquat. Org. 1994, 18, 109–118. [Google Scholar] [CrossRef]
- Desvignes, L.; Quentel, C.; Lamour, F.; Le, V.A. Pathogenesis and immune response in atlantic salmon (salmo salar l.) parr experimentally infected with salmon pancreas disease virus (spdv). Fish Shellfish Immunol. 2002, 12, 77–95. [Google Scholar] [CrossRef]
- McLoughlin, M.F.; Nelson, R.T.; Rowley, H.M.; Cox, D.I.; Grant, A.N. Experimental pancreas disease in atlantic salmon salmo salar postsmolts induced by salmon pancreas disease virus (spdv). Dis. Aquat. Org. 1996, 26, 117–124. [Google Scholar] [CrossRef]
- Houghton, G.; Ellis, A.E. Pancreas disease in atlantic salmon: Serum neutralisation and passive immunisation. Fish Shellfish Immunol. 1996, 6, 465–472. [Google Scholar] [CrossRef]
- Lopez-Doriga, M.V.; Smail, D.A.; Smith, R.J.; Domenech, A.; Castric, J.; Smith, P.D.; Ellis, A.E. Isolation of salmon pancreas disease virus (spdv) in cell culture and its ability to protect against infection by the “wild-type” agent. Fish Shellfish Immunol. 2001, 11, 505–522. [Google Scholar] [CrossRef]
- Moriette, C.; Leberre, M.; Lamoureux, A.; Lai, T.L.; Bremont, M. Recovery of a recombinant salmonid alphavirus fully attenuated and protective for rainbow trout. J. Virol. 2006, 80, 4088–4098. [Google Scholar] [CrossRef]
- Xu, C.; Mutoloki, S.; Evensen, O. Superior protection conferred by inactivated whole virus vaccine over subunit and DNA vaccines against salmonid alphavirus infection in atlantic salmon (salmo salar l.). Vaccine 2012, 30, 3918–3928. [Google Scholar] [CrossRef]
- Tavornpanich, S.; Viljugrein, H.; Stene, A.; Brun, E. Estimation of the reproduction number of salmon pancreas disease virus subtype 3 in homogeneously mixed populations of norwegian farmed atlantic salmon. Prev. Vet. Med. 2013, 111, 329–332. [Google Scholar] [CrossRef]
- Aunsmo, A.; Ovretveit, S.; Breck, O.; Valle, P.S.; Larssen, R.B.; Sandberg, M. Modelling sources of variation and risk factors for spinal deformity in farmed atlantic salmon using hierarchical- and cross-classified multilevel models. Prev. Vet. Med. 2009, 90, 137–145. [Google Scholar] [CrossRef]
- Haugarvoll, E.; Bjerkas, I.; Szabo, N.J.; Satoh, M.; Koppang, E.O. Manifestations of systemic autoimmunity in vaccinated salmon. Vaccine 2010, 28, 4961–4969. [Google Scholar] [CrossRef]
- Skinner, L.A.; Schulte, P.M.; LaPatra, S.E.; Balfry, S.K.; McKinley, R.S. Growth and performance of atlantic salmon, salmo salar l., following administration of a rhabdovirus DNA vaccine alone or concurrently with an oil-adjuvanted, polyvalent vaccine. J. Fish Dis. 2008, 31, 687–697. [Google Scholar]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar]
- McKee, A.S.; Munks, M.W.; Marrack, P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity 2007, 27, 687–690. [Google Scholar]
- Palti, Y. Toll-like receptors in bony fish: From genomics to function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar]
- Skjæveland, I.; Iliev, D.B.; Zou, J.; Jørgensen, T.; Jørgensen, J.B. A tlr9 homolog that is up-regulated by ifn-[gamma] in atlantic salmon (salmo salar). Dev. Comp. Immunol. 2008, 32, 603–607. [Google Scholar]
- Iliev, D.B.; Skjaeveland, I.; Jorgensen, J.B. Cpg oligonucleotides bind tlr9 and rrm-containing proteins in atlantic salmon (salmo salar). BMC Immunol. 2013, 14, 12. [Google Scholar]
- Cheng, Y.S.; Xu, F. Anticancer function of polyinosinic-polycytidylic acid. Cancer Biol. Ther. 2011, 10, 1219–1223. [Google Scholar]
- Matsuo, A.; Oshiumi, H.; Tsujita, T.; Mitani, H.; Kasai, H.; Yoshimizu, M.; Matsumoto, M.; Seya, T. Teleost tlr22 recognizes rna duplex to induce ifn and protect cells from birnaviruses. J. Immunol. 2008, 181, 3474–3485. [Google Scholar]
- Strandskog, G.; Skjaeveland, I.; Ellingsen, T.; Jørgensen, J.B. Double-stranded rna- and cpg DNA-induced immune responses in atlantic salmon: Comparison and synergies. Vaccine 2008, 26, 4704–4715. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; Nisbet, D.J.; Kogut, M.H. In vivo priming heterophil innate immune functions and increasing resistance to salmonella enteritidis infection in neonatal chickens by immune stimulatory cpg oligodeoxynucleotides. Vet. Immunol. Immunopathol. 2007, 117, 275–283. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.L.; MacKinnon, K.M.; Kogut, M.H. Co-stimulation with tlr3 and tlr21 ligands synergistically up-regulates th1-cytokine ifn-gamma and regulatory cytokine il-10 expression in chicken monocytes. Dev. Comp. Immunol. 2012, 36, 756–760. [Google Scholar] [CrossRef]
- Strandskog, G.; Villoing, S.; Iliev, D.B.; Thim, H.L.; Christie, K.E.; Jørgensen, J.B. Formulations combining cpg containing oliogonucleotides and poly i:C enhance the magnitude of immune responses and protection against pancreas disease in atlantic salmon. Dev. Comp. Immunol. 2011, 35, 1116–1127. [Google Scholar] [CrossRef]
- Thim, H.L.; Iliev, D.B.; Christie, K.E.; Villoing, S.; McLoughlin, M.F.; Strandskog, G.; Jorgensen, J.B. Immunoprotective activity of a salmonid alphavirus vaccine: Comparison of the immune responses induced by inactivated whole virus antigen formulations based on cpg class b oligonucleotides and poly I:C alone or combined with an oil adjuvant. Vaccine 2012, 30, 4828–4834. [Google Scholar] [CrossRef]
- Kim, C.H.; Johnson, M.C.; Drennan, J.D.; Simon, B.E.; Thomann, E.; Leong, J.A. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and mx protein synthesis in fish. J. Virol. 2000, 74, 7048–7054. [Google Scholar] [CrossRef]
- Lorenzen, E.; Einer-Jensen, K.; Martinussen, T.; LaPatra, S.E.; Lorenzen, N. DNA vaccination of rainbow trout against viral hemorrhagic septicemia virus: A dose-response and time-course study. J. Aquat. Anim. Health 2000, 12, 167–180. [Google Scholar] [CrossRef]
- Von Gersdorff Jørgensen, L.; Sigh, J.; Kania, P.W.; Holten-Andersen, L.; Buchmann, K.; Clark, T.; Rasmussen, J.S.; Einer-Jensen, K.; Lorenzen, N. Approaches towards DNA vaccination against a skin ciliate parasite in fish. PLoS One 2012, 7, e48129. [Google Scholar] [CrossRef]
- Kobiyama, K.; Jounai, N.; Aoshi, T.; Tozuka, M.; Takeshita, F.; Coban, C.; Ishii, K. Innate immune signaling by, and genetic adjuvants for DNA vaccination. Vaccines 2013, 1, 278–292. [Google Scholar] [CrossRef]
- Purcell, M.K.; Nichols, K.M.; Winton, J.R.; Kurath, G.; Thorgaard, G.H.; Wheeler, P.; Hansen, J.D.; Herwig, R.P.; Park, L.K. Comprehensive gene expression profiling following DNA vaccination of rainbow trout against infectious hematopoietic necrosis virus. Mol. Immunol. 2006, 43, 2089–2106. [Google Scholar] [CrossRef]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow fever vaccine yf-17d activates multiple dendritic cell subsets via tlr2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.P.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.S.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar]
- Lorenzen, N.; Lorenzen, E.; Einer-Jensen, K.; LaPatra, S.E. DNA vaccines as a tool for analysing the protective immune response against rhabdoviruses in rainbow trout. Fish Shellfish Immunol. 2002, 12, 439–453. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (rest) for group-wise comparison and statistical analysis of relative expression results in real-time pcr. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time rt-pcr. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Christie, K.E.; Graham, D.A.; McLoughlin, M.F.; Villoing, S.; Todd, D.; Knappskog, D. Experimental infection of atlantic salmon salmo salar pre-smolts by i.p. Injection with new irish and norwegian salmonid alphavirus (sav) isolates: A comparative study. Dis. Aquat. Org. 2007, 75, 13–22. [Google Scholar] [CrossRef]
- Hagman, J.; Lukin, K. “Hands-on” regulation of b cell development by the transcription factor pax5. Immunity 2007, 27, 8–10. [Google Scholar] [CrossRef]
- Zwollo, P.; Haines, A.; Rosato, P.; Gumulak-Smith, J. Molecular and cellular analysis of b-cell populations in the rainbow trout using pax5 and immunoglobulin markers. Dev. Comp. Immunol. 2008, 32, 1482–1496. [Google Scholar] [CrossRef]
- Moore, L.J.; Dijkstra, J.M.; Koppang, E.O.; Hordvik, I. Cd4 homologues in atlantic salmon. Fish Shellfish Immunol. 2009, 26, 10–18. [Google Scholar] [CrossRef]
- Meyer, K.; Basu, A.; Przysiecki, C.T.; Lagging, L.M.; di Bisceglie, A.M.; Conley, A.J.; Ray, R. Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis c virus e2 chimeric glycoprotein. J. Virol. 2002, 76, 2150–2158. [Google Scholar] [CrossRef]
- Grove, S.; Austbo, L.; Hodneland, K.; Frost, P.; Lovoll, M.; McLoughlin, M.; Thim, H.L.; Braaen, S.; Konig, M.; Syed, M.; et al. Immune parameters correlating with reduced susceptibility to pancreas disease in experimentally challenged atlantic salmon (salmo salar). Fish Shellfish Immunol. 2013, 34, 789–798. [Google Scholar] [CrossRef]
- Strandskog, G.; Ellingsen, T.; Jørgensen, J.B. Characterization of three distinct cpg oligonucleotide classes which differ in ability to induce ifn alpha/beta activity and cell proliferation in atlantic salmon (salmo salar l.) leukocytes. Dev. Comp. Immunol. 2007, 31, 39–51. [Google Scholar] [CrossRef]
- Malmgaard, L. Induction and regulation of ifns during viral infections. J. Interferon Cytokine Res. 2004, 24, 439–454. [Google Scholar] [CrossRef]
- Iliev, D.B.; Thim, H.; Lagos, L.; Olsen, R.; Jørgensen, J. Homing of antigen-presenting cells (apcs) in head kidney and spleen—Salmon head kidney hosts diverse apc types. Front. Immunol. 2013, 4, 137. [Google Scholar]
- Chu, V.T.; Berek, C. The establishment of the plasma cell survival niche in the bone marrow. Immunol. Rev. 2013, 251, 177–188. [Google Scholar] [CrossRef]
- Tangye, S.G. Staying alive: Regulation of plasma cell survival. Trends Immunol. 2011, 32, 595–602. [Google Scholar] [CrossRef]
- Ye, J.; Kaattari, I.; Kaattari, S. Plasmablasts and plasma cells: Reconsidering teleost immune system organization. Dev. Comp. Immunol. 2011, 35, 1273–1281. [Google Scholar] [CrossRef]
- Bernasconi, N.L.; Onai, N.; Lanzavecchia, A. A role for toll-like receptors in acquired immunity: Up-regulation of tlr9 by bcr triggering in naive b cells and constitutive expression in memory B cells. Blood 2003, 101, 4500–4504. [Google Scholar] [CrossRef]
- Capolunghi, F.; Cascioli, S.; Giorda, E.; Rosado, M.M.; Plebani, A.; Auriti, C.; Seganti, G.; Zuntini, R.; Ferrari, S.; Cagliuso, M.; et al. Cpg drives human transitional b cells to terminal differentiation and production of natural antibodies. J. Immunol. 2008, 180, 800–808. [Google Scholar]
- Thim, H.L.; Lagos, L.; Iliev, D.B.; Jensen, I.; Jørgensen, J.B. CpG Activation of Salmonid B-Cells Can Induce Long Lived Protection. Presented at HAVBRUK, Tromsø, Norway, March 2014.
- Korytář, T.; Jaros, J.; Verleih, M.; Rebl, A.; Kotterba, G.; Kühn, C.; Goldammer, T.; Köllner, B. Novel insights into the peritoneal inflammation of rainbow trout (oncorhynchus mykiss). Fish Shellfish Immunol. 2013, 35, 1192–1199. [Google Scholar] [CrossRef]
- Li, J.; Barreda, D.R.; Zhang, Y.A.; Boshra, H.; Gelman, A.E.; Lapatra, S.; Tort, L.; Sunyer, J.O. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006, 7, 1116–1124. [Google Scholar] [CrossRef]
- Encinas, P.; Gomez-Casado, E.; Fregeneda, G.; Olesen, N.J.; Lorenzen, N.; Estepa, A.; Coll, J.M. Rainbow trout surviving infections of viral haemorrhagic septicemia virus (vhsv) show lasting antibodies to recombinant g protein fragments. Fish Shellfish Immunol. 2011, 30, 929–935. [Google Scholar] [CrossRef]
- Fregeneda-Grandes, J.; Skall, H.; Olesen, N. Antibody response of rainbow trout with single or double infections involving viral haemorrhagic septicaemia virus and infectious haematopoietic necrosis virus. Dis. Aquat. Org. 2009, 83, 23–29. [Google Scholar] [CrossRef]
- Le Bon, A.; Schiavoni, G.; D’Agostino, G.; Gresser, I.; Belardelli, F.; Tough, D.F. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 2001, 14, 461–470. [Google Scholar] [CrossRef]
- Manz, R.A.; Arce, S.; Cassese, G.; Hauser, A.E.; Hiepe, F.; Radbruch, A. Humoral immunity and long-lived plasma cells. Curr. Opin. Immunol. 2002, 14, 517–521. [Google Scholar] [CrossRef]
- De Wit, M.C.; Horzinek, M.C.; Haagmans, B.L.; Schijns, V.E.J.C. Host-dependent type 1 cytokine responses driven by inactivated viruses may fail to default in the absence of il-12 or ifn-α/β. J. Gen. Virol. 2004, 85, 795–803. [Google Scholar] [CrossRef]
- Milone, M.C.; Fitzgerald-Bocarsly, P. The mannose receptor mediates induction of ifn-α in peripheral blood dendritic cells by enveloped rna and DNA viruses. J. Immunol 1998, 161, 2391–2399. [Google Scholar]
- Shabman, R.S.; Morrison, T.E.; Moore, C.; White, L.; Suthar, M.S.; Hueston, L.; Rulli, N.; Lidbury, B.; Ting, J.P.-Y.; Mahalingam, S.; et al. Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J. Virol. 2007, 81, 237–247. [Google Scholar] [CrossRef]
- Lorenzen, N.; LaPatra, S.E. DNA vaccines for aquacultured fish. Rev. Sci. Technol. 2005, 24, 201–213. [Google Scholar]
- Einer-Jensen, K.; Delgado, L.; Lorenzen, E.; Bovo, G.; Evensen, O.; Lapatra, S.; Lorenzen, N. Dual DNA vaccination of rainbow trout (oncorhynchus mykiss) against two different rhabdoviruses, vhsv and ihnv, induces specific divalent protection. Vaccine 2009, 27, 1248–1253. [Google Scholar] [CrossRef]
- Alonso, M.; Leong, J.A. Licensed DNA vaccines against infectious hematopoietic necrosis virus (ihnv). Recent Pat. DNA Gene Seq. 2013, 7, 62–65. [Google Scholar] [CrossRef]
- Sommerset, I.; Lorenzen, E.; Lorenzen, N.; Bleie, H.; Nerland, A.H. A DNA vaccine directed against a rainbow trout rhabdovirus induces early protection against a nodavirus challenge in turbot. Vaccine 2003, 21, 4661–4667. [Google Scholar] [CrossRef]
- Lorenzen, N.; Lorenzen, E.; Einer-Jensen, K.; LaPatra, S.E. Immunity induced shortly after DNA vaccination of rainbow trout against rhabdoviruses protects against heterologous virus but not against bacterial pathogens. Dev. Comp. Immunol. 2002, 26, 173–179. [Google Scholar] [CrossRef]
- Martinez-Alonso, S.; Martinez-Lopez, A.; Estepa, A.; Cuesta, A.; Tafalla, C. The introduction of multi-copy cpg motifs into an antiviral DNA vaccine strongly up-regulates its immunogenicity in fish. Vaccine 2011, 29, 1289–1296. [Google Scholar] [CrossRef]
- McLauchlan, P.E.; Collet, B.; Ingerslev, E.; Secombes, C.J.; Lorenzen, N.; Ellis, A.E. DNA vaccination against viral haemorrhagic septicaemia (vhs) in rainbow trout: Size, dose, route of injection and duration of protection-early protection correlates with mx expression. Fish Shellfish Immunol. 2003, 15, 39–50. [Google Scholar] [CrossRef]
- Byon, J.Y.; Ohira, T.; Hirono, I.; Aoki, T. Use of a cdna microarray to study immunity against viral hemorrhagic septicemia (vhs) in japanese flounder (paralichthys olivaceus) following DNA vaccination. Fish Shellfish Immunol. 2005, 18, 135–147. [Google Scholar] [CrossRef]
- Boudinot, P.; Blanco, M.; de Kinkelin, P.; Benmansour, A. Combined DNA immunization with the glycoprotein gene of viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus induces double-specific protective immunity and nonspecific response in rainbow trout. Virology 1998, 249, 297–306. [Google Scholar] [CrossRef]
- Zou, J.; Tafalla, C.; Truckle, J.; Secombes, C.J. Identification of a second group of type i ifns in fish sheds light on ifn evolution in vertebrates. J.Immunol. 2007, 179, 3859–3871. [Google Scholar]
- Boudinot, P.; Bernard, D.; Boubekeur, S.; Thoulouze, M.I.; Bremont, M.; Benmansour, A. The glycoprotein of a fish rhabdovirus profiles the virus-specific t-cell repertoire in rainbow trout. J. Gen. Virol. 2004, 85, 3099–3108. [Google Scholar] [CrossRef]
- Lorenzen, N.; Lapatra, S.E. Immunity to rhabdoviruses in rainbow trout: The antibody response. Fish Shellfish Immunol. 1999, 9, 345–360. [Google Scholar] [CrossRef]
- Raadsma, H.W.; O’Meara, T.J.; Egerton, J.R.; Lehrbach, P.R.; Schwartzkoff, C.L. Protective antibody titres and antigenic competition in multivalent dichelobacter nodosus fimbrial vaccines using characterised rdna antigens. Vet. Immunol. Immunopathol. 1994, 40, 253–274. [Google Scholar] [CrossRef]
- Skinner, L.A.; LaPatra, S.E.; Adams, A.; Thompson, K.D.; Balfry, S.K.; McKinley, R.S.; Schulte, P.M. Concurrent injection of a rhabdovirus-specific DNA vaccine with a polyvalent, oil-adjuvanted vaccine delays the specific anti-viral immune response in atlantic salmon, salmo salar l. Fish Shellfish Immunol. 2010, 28, 579–586. [Google Scholar] [CrossRef]
- Taborda, C.P.; Rivera, J.; Zaragoza, O.; Casadevall, A. More is not necessarily better: Prozone-like effects in passive immunization with igg. J. Immunol. 2003, 170, 3621–3630. [Google Scholar]
- De Titta, A.; Ballester, M.; Julier, Z.; Nembrini, C.; Jeanbart, L.; van der Vlies, A.J.; Swartz, M.A.; Hubbell, J.A. Nanoparticle conjugation of cpg enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thim, H.L.; Villoing, S.; McLoughlin, M.; Christie, K.E.; Grove, S.; Frost, P.; Jørgensen, J.B. Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines 2014, 2, 228-251. https://doi.org/10.3390/vaccines2020228
Thim HL, Villoing S, McLoughlin M, Christie KE, Grove S, Frost P, Jørgensen JB. Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines. 2014; 2(2):228-251. https://doi.org/10.3390/vaccines2020228
Chicago/Turabian StyleThim, Hanna L., Stéphane Villoing, Marian McLoughlin, Karen Elina Christie, Søren Grove, Petter Frost, and Jorunn B. Jørgensen. 2014. "Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen" Vaccines 2, no. 2: 228-251. https://doi.org/10.3390/vaccines2020228
APA StyleThim, H. L., Villoing, S., McLoughlin, M., Christie, K. E., Grove, S., Frost, P., & Jørgensen, J. B. (2014). Vaccine Adjuvants in Fish Vaccines Make a Difference: Comparing Three Adjuvants (Montanide ISA763A Oil, CpG/Poly I:C Combo and VHSV Glycoprotein) Alone or in Combination Formulated with an Inactivated Whole Salmonid Alphavirus Antigen. Vaccines, 2(2), 228-251. https://doi.org/10.3390/vaccines2020228




