Immunogenicity and Safety of Half and Full Doses of Heterologous and Homologous COVID-19 Vaccine Boosters After Priming with ChAdOx1 in Adult Participants in Indonesia: A Single-Blinded Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Ethics
2.2. Trial Protocol
2.3. Randomization and Blinding
2.4. Outcomes
2.5. Statistical Analysis
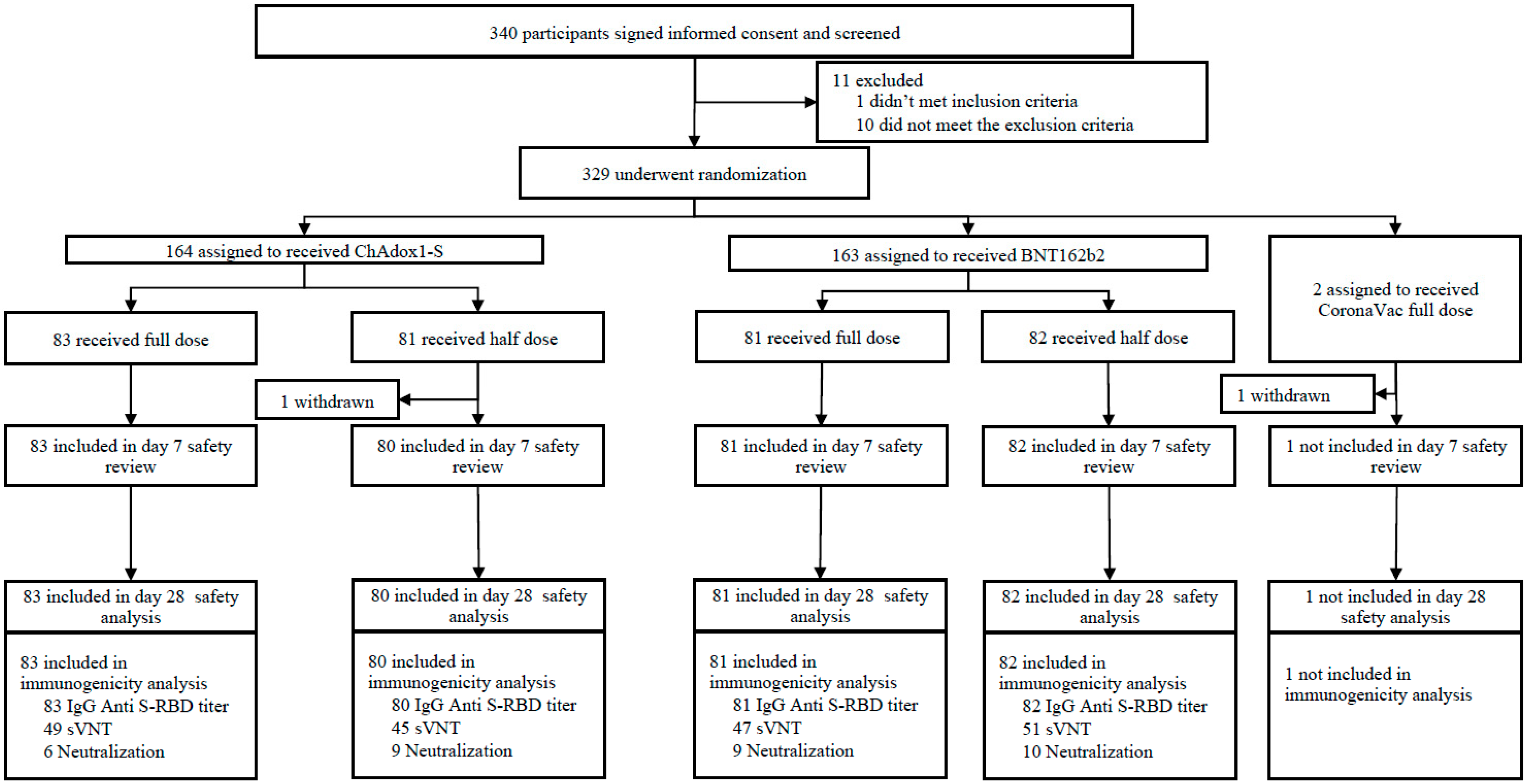
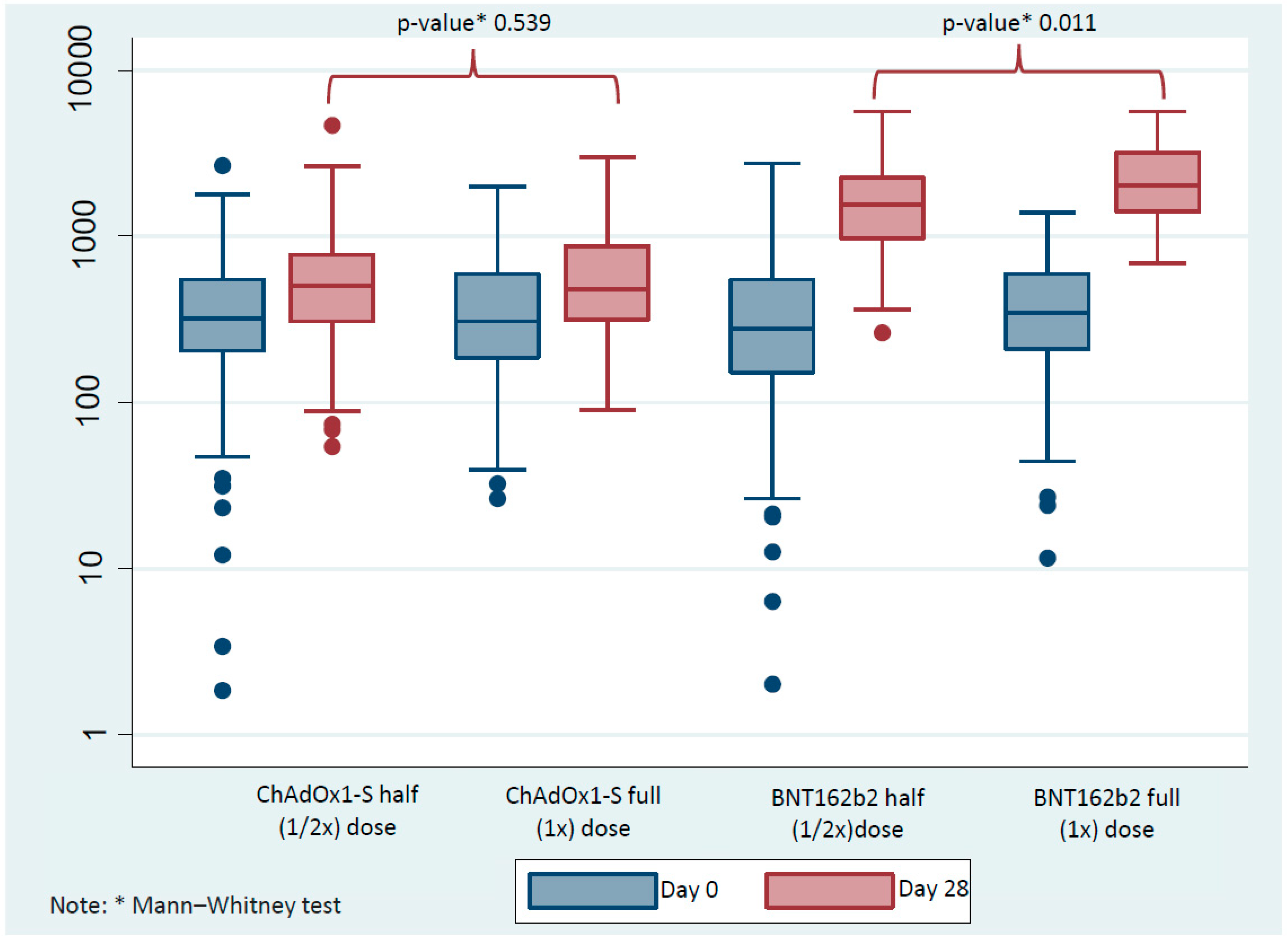
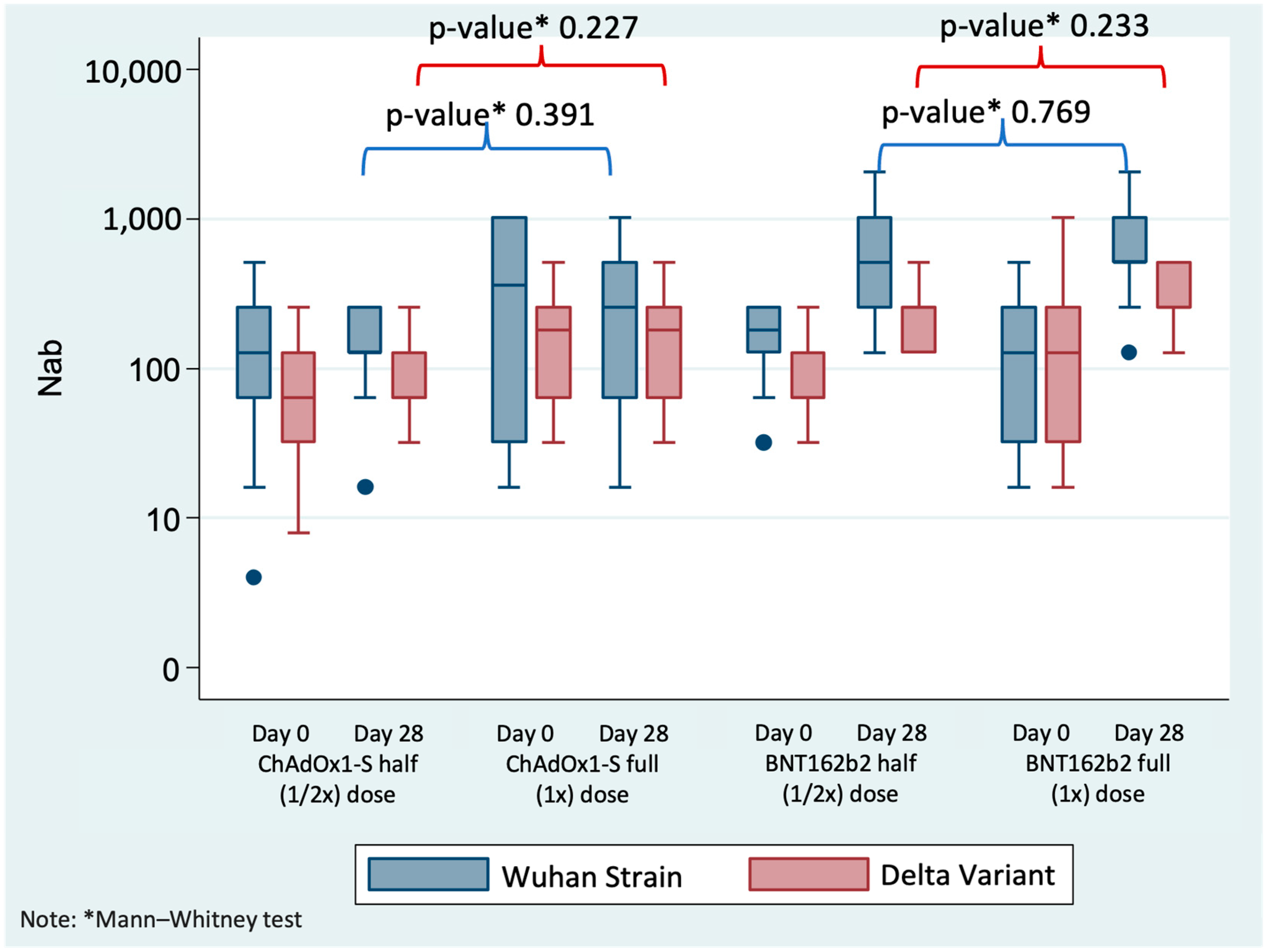
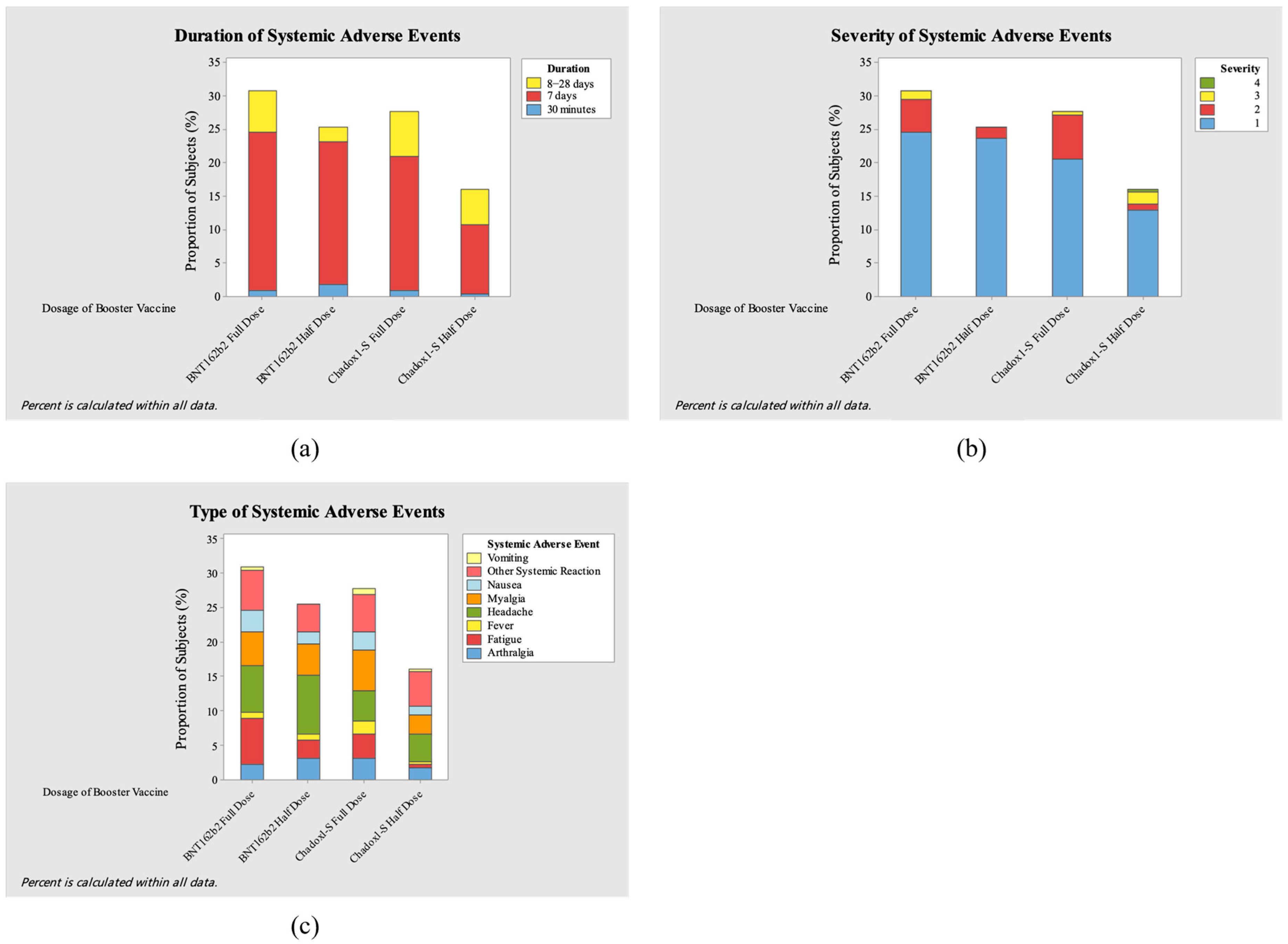
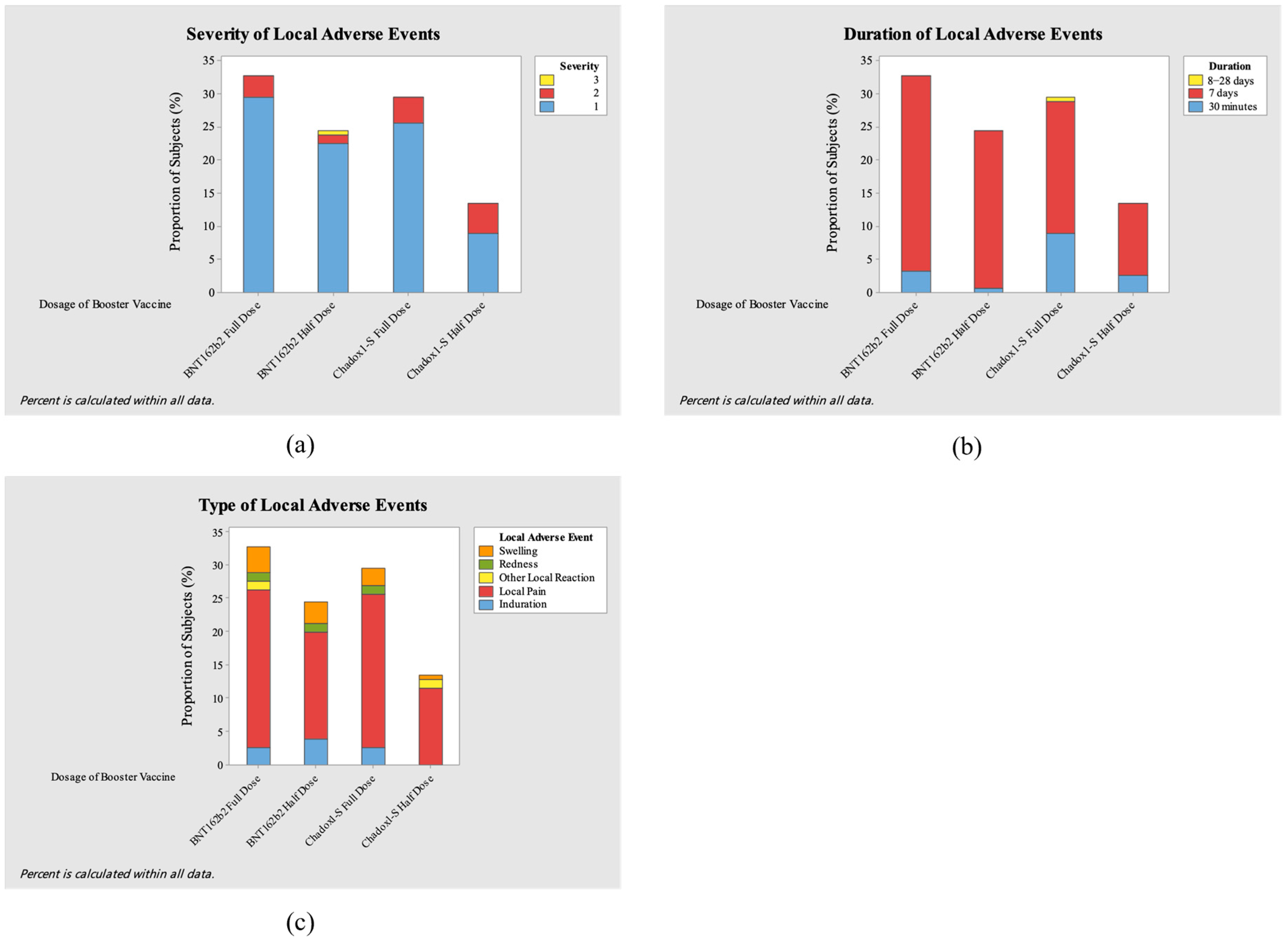
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Inclusion Criteria |
|
|
| Exclusion Criteria |
|
|
|
|
|
Appendix B
| IgG Anti S-RBD Titer (BAU/mL): Increase | ||||
|---|---|---|---|---|
| Parameter | ChadOx1-S | BNT162b2 | p-Value a | |
| Half Dose: Full Dose: | <0.001 | |||
| Median | 111.61 | 1137.76 | ||
| Range Median Range | −1715.33–3204.32 134.27 −598.4–2962.12 | 56.16–5165.57 1677.11 26.66–5620.97 | <0.001 | |
| Comparison between Half Dose vs. Full dose | p = 0.614 a | p = 0.004 a | ||
| Time Point | Parameter | Half-Dose Group | Full-Dose Group | p-Value a | |
|---|---|---|---|---|---|
| Antigen: Strain Delta | (n = 45) | (n = 49) | |||
| Day 0 | Median Range IQR Positive (%) | 98 2–99 95.5–98.0 42 (93.3%) | 98 35–99 97.5–98.0 49 (100.0%) | 0.188 | |
| Day 28 | Median Range IQR Positive (%) | 98 53–99 98.0–98.0 45 (100%) | 98 92–99 98.0–98.0 49 (100%) | 0.306 | |
| Antigen: Strain Wild Type | (n = 45) | (n = 49) | |||
| Day 0 | Median Range IQR Positive (%) | 97 12–98 97.0–98.0 42 (93.3%) | 98 22–98 97.0–98.0 47 (95.9%) | 0.537 | |
| Day 28 | Median Range IQR Positive (%) | 97 83–98 97.0–98.0 45 (100%) | 98 88–98 97.0–98.0 49 (100%) | 0.133 |
| Time Point | Parameter | Half-Dose Group | Full-Dose Group | p-Value a | |
|---|---|---|---|---|---|
| Antigen: Strain Delta | (n = 50) | (n = 47) | |||
| Day 0 | Median Range IQR Positive (%) | 98 3–99 91.75–98.0 46 (92.0%) | 98 22–99 97.0–98.0 46 (97.9%) | 0.301 | |
| Day 28 | Median Range IQR Positive (%) | 98 5–99 98.0–98.0 48 (96%) | 98 81–99 98.0–98.0 47 (100%) | 0.115 | |
| Antigen: Strain Wild Type | (n = 51) | (n = 47) | |||
| Day 0 | Median Range IQR Positive (%) | 97 13–98 96.0–98.0 49 (96.1%) | 97 43–98 96.0–98.0 47 (100%) | 0.292 | |
| Day 28 | Median Range IQR Positive (%) | 97 35–98 97.0–98.0 51 (100%) | 97 95–98 97.0–98.0 47 (100%) | 0.351 |
References
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, E.; Simpson, C.R.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; Chuter, A.; et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 2021, 397, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization COVID-19 Dashboard. 2023. Available online: https://data.who.int/dashboards/covid19/vaccines?n=c (accessed on 28 October 2025).
- Vaksin Dashboard. 2024. Available online: https://vaksin.kemkes.go.id/#/vaccines (accessed on 28 October 2025).
- Niyomnaitham, S.; Toh, Z.Q.; Wongprompitak, P.; Jansarikit, L.; Srisutthisamphan, K.; Sapsutthipas, S.; Jantraphakorn, Y.; Mingngamsup, N.; Licciardi, P.V.; Chokephaibulkit, K. Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults. Hum. Vaccines Immunother. 2022, 18, 2091865. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef] [PubMed]
- Fadlyana, E.; Setiabudi, D.; Kartasasmita, C.B.; Putri, N.D.; Hadinegoro, S.R.; Mulholland, K.; Sofiatin, Y.; Suryadinata, H.; Hartantri, Y.; Sukandar, H.; et al. Immunogenicity and safety in healthy adults of full dose versus half doses of COVID-19 vaccine (ChAdOx1-S or BNT162b2) or full-dose CoronaVac administered as a booster dose after priming with CoronaVac: A randomised, observer-masked, controlled trial in Indonesia. Lancet Infect. Dis. 2023, 23, 545–555. [Google Scholar] [PubMed]
- Pedoman Pencegahan Dan Pengendalian Coronavirus Disesase (COVID-19). 2020. Available online: https://infeksiemerging.kemkes.go.id/document/pedoman-pencegahan-dan-pengendalian-covid-19/view (accessed on 10 November 2024).
- English, E.; Cook, L.E.; Piec, I.; Dervisevic, S.; Fraser, W.D.; John, W.G. Performance of the Abbott SARS-CoV-2 IgG II Quantitative Antibody Assay Including the New Variants of Concern, VOC 202012/V1 (United Kingdom) and VOC 202012/V2 (South Africa), and First Steps towards Global Harmonization of COVID-19 Antibody Methods. J. Clin. Microbiol. 2021, 59, e0028821. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Kannenberg, J.; Biemann, R.; Hönemann, M.; Ackermann, G.; Jassoy, C. Comparison of the measured values of quantitative SARS-CoV-2 spike antibody assays. J. Clin. Virol. 2022, 155, 105269. [Google Scholar] [CrossRef] [PubMed]
- Reflection Paper on the Regulatory Requirements for Vaccines Intended to Provide Protection Against Variant Strain(s) of SARS-CoV-2. 2021. Available online: https://www.pei.de/SharedDocs/Downloads/EN/newsroom-en/hp-news/ema-paper-variations-covid-19.html (accessed on 10 November 2024).
- Wong, N.S.; Lee, S.S.; Chan, D.P.C.; Li, T.C.M.; Ho, T.H.Y.; Luk, F.W.L.; Chow, K.M.; Tso, E.Y.K.; Yeoh, E.-K.; Wong, S.Y.S.; et al. Trajectory patterns of SARS-CoV-2 neutralising antibody response in convalescent COVID-19 patients. Commun. Med. 2022, 2, 53. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Barin, B.; Kasap, U.; Selcuk, F.; Volkan, E.; Uluckan, O. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: A prospective, longitudinal population-based study. Lancet Microbe 2022, 3, e274–e283. [Google Scholar] [CrossRef] [PubMed]
- San Francisco Ramos, A.; Liu Sanchez, C.; Bovill Rose, T.; Smith, D.; Thorn, N.; Galiza, E.; Miah, T.; Pearce, J.; Hultin, C.; Cosgrove, C.; et al. Comparing reactogenicity of COVID-19 vaccine boosters: A systematic review and meta-analysis. Expert Rev. Vaccines 2024, 23, 266–282. [Google Scholar] [CrossRef] [PubMed]
| Parameter | ChAdOx1-S Full Dose | ChAdOx1-S Half Dose | BNT162b2 Full Dose | BNT162b2 Half Dose | CoronoVac Full Dose |
|---|---|---|---|---|---|
| (n = 83) | (n = 81) | (n = 81) | (n = 82) | (n = 2) | |
| Means age (years) (SD) | 42.60 (11.67) | 44.7 (10.97) | 41.50 (10.54) | 41.95 (12.13) | 40.10 (19.65) |
| Means height (cm) (SD) | 158.5 (9.17) | 157.80 (8.68) | 156.00 (8.01) | 155.50 (8.80) | 162.25 (13.78) |
| Means weight (kg) (SD) | 63.90 (13.57) | 62.80 (15.11) | 64.00 (13.50) | 63.45 (13.52) | 65.30 (13.57) |
| BMI (kg/m2) | 25.50 (5.18) | 25.20 (5.24) | 25.90 (4.85) | 26.65 (5.17) | 24.65 (0.91) |
| Sex n (%) | |||||
| Male | 38 (25.0%) | 43 (28.3%) | 34 (22.4%) | 36 (23.7%) | 1 (0.7%) |
| Female | 45 (25.4%) | 38 (21.5%) | 47 (26.6% | 46 (26.0%) | 1 (0.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, N.D.; Zhafira, A.S.; Wicaksana, P.; Satari, H.I.; Fadlyana, E.; Safitri, V.; Nurlailah, N.; Putri, E.S.; Putri, N.; Iriyani, D.S.; et al. Immunogenicity and Safety of Half and Full Doses of Heterologous and Homologous COVID-19 Vaccine Boosters After Priming with ChAdOx1 in Adult Participants in Indonesia: A Single-Blinded Randomized Controlled Trial. Vaccines 2025, 13, 1149. https://doi.org/10.3390/vaccines13111149
Putri ND, Zhafira AS, Wicaksana P, Satari HI, Fadlyana E, Safitri V, Nurlailah N, Putri ES, Putri N, Iriyani DS, et al. Immunogenicity and Safety of Half and Full Doses of Heterologous and Homologous COVID-19 Vaccine Boosters After Priming with ChAdOx1 in Adult Participants in Indonesia: A Single-Blinded Randomized Controlled Trial. Vaccines. 2025; 13(11):1149. https://doi.org/10.3390/vaccines13111149
Chicago/Turabian StylePutri, Nina Dwi, Aqila Sakina Zhafira, Pratama Wicaksana, Hindra Irawan Satari, Eddy Fadlyana, Vivi Safitri, Nurlailah Nurlailah, Edwinaditya Sekar Putri, Nidya Putri, Devi Surya Iriyani, and et al. 2025. "Immunogenicity and Safety of Half and Full Doses of Heterologous and Homologous COVID-19 Vaccine Boosters After Priming with ChAdOx1 in Adult Participants in Indonesia: A Single-Blinded Randomized Controlled Trial" Vaccines 13, no. 11: 1149. https://doi.org/10.3390/vaccines13111149
APA StylePutri, N. D., Zhafira, A. S., Wicaksana, P., Satari, H. I., Fadlyana, E., Safitri, V., Nurlailah, N., Putri, E. S., Putri, N., Iriyani, D. S., Ulina, Y. S., Aprilia, F., Pratama, E., Nethalia, I., Yustisiana, R., Aini, E. Q., Fajarani, R., Susilo, A., Karyanti, M. R., ... Mulholland, K. (2025). Immunogenicity and Safety of Half and Full Doses of Heterologous and Homologous COVID-19 Vaccine Boosters After Priming with ChAdOx1 in Adult Participants in Indonesia: A Single-Blinded Randomized Controlled Trial. Vaccines, 13(11), 1149. https://doi.org/10.3390/vaccines13111149






