Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study
Abstract
1. Introduction
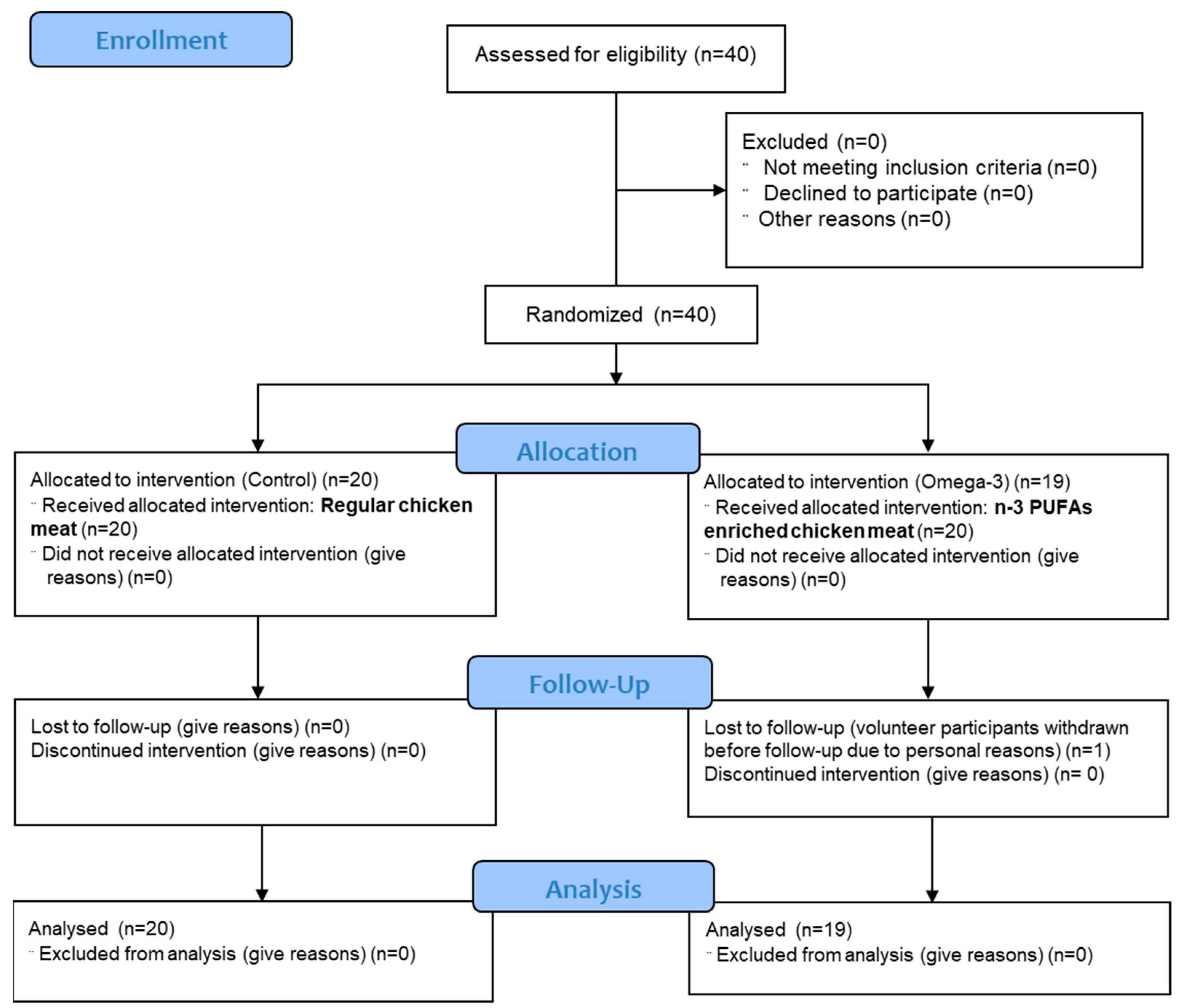
2. Materials and Methods
2.1. Study Protocol and Interventions
2.2. Anthropometric Measurements and Venous Blood Laboratory Analysis
2.3. Serum Isolation and PBMC Processing: Isolation, Cryopreservation, and Thawing
2.4. Evaluation of Biomarkers of Oxidative Stress and Antioxidative Defense
2.5. Analysis of Serum Antioxidant Enzyme Activities
2.6. Intracellular Reactive Oxygen Species (ROS) Production Measurements
2.7. ELISA Assay
2.8. Statistical Analysis
3. Results
3.1. Fatty Acid Profile of Chicken Meat
3.2. General Characteristics and Biochemical Measurements of Participants
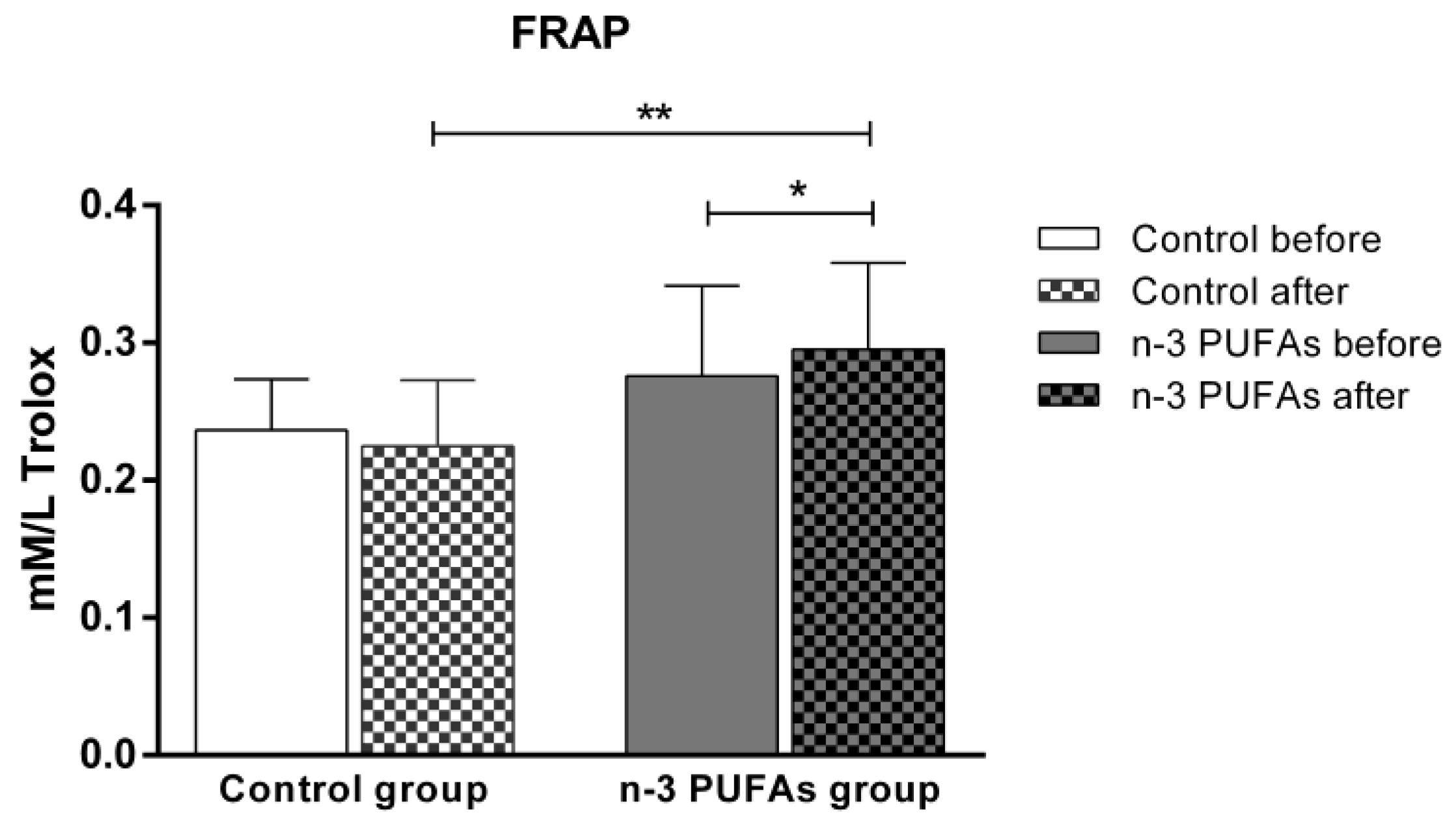
3.3. Serum Level of Oxidative Stress and Antioxidative Defense Biomarkers
3.4. Antioxidant Enzyme Activity in Serum Samples
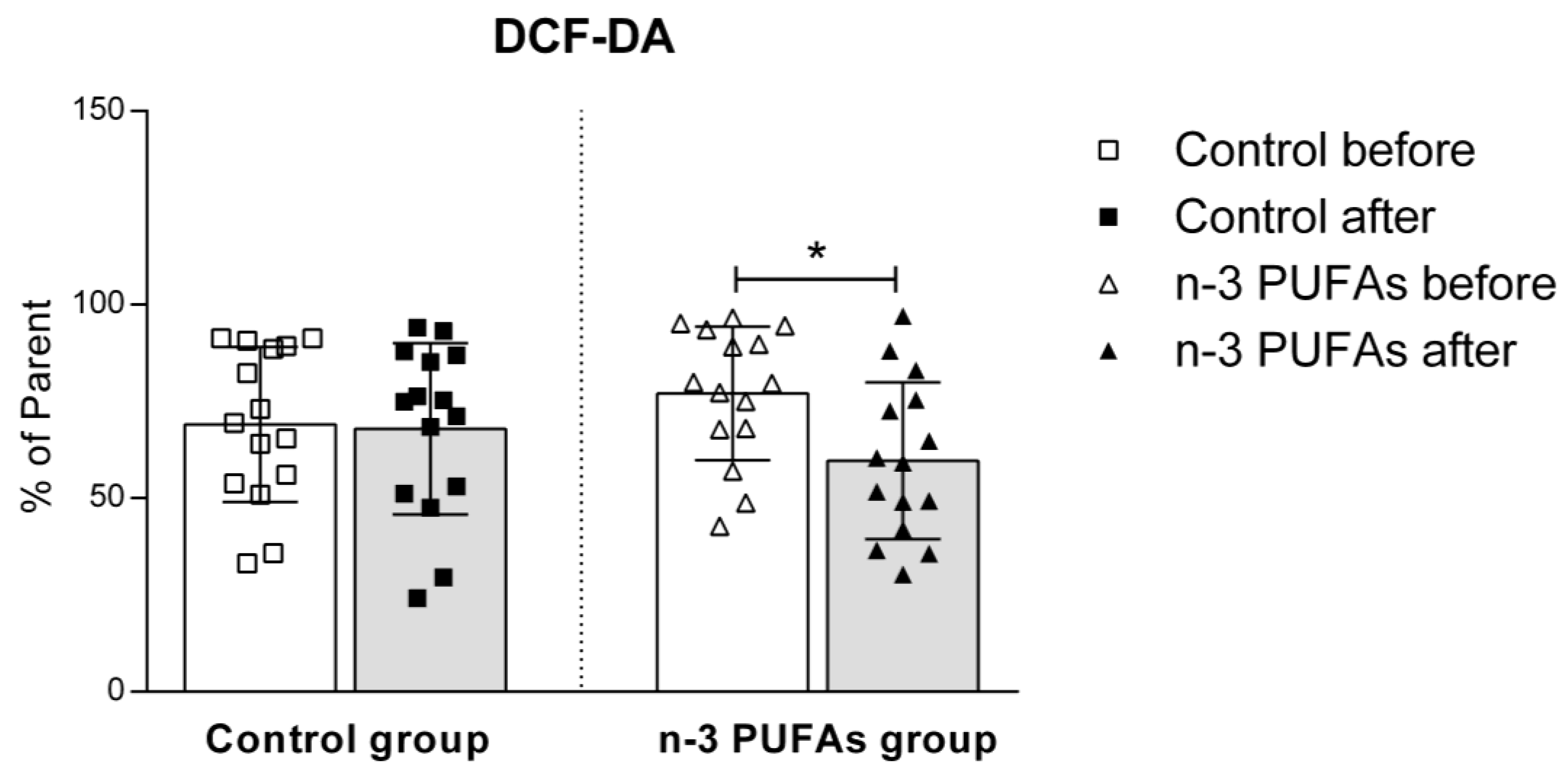
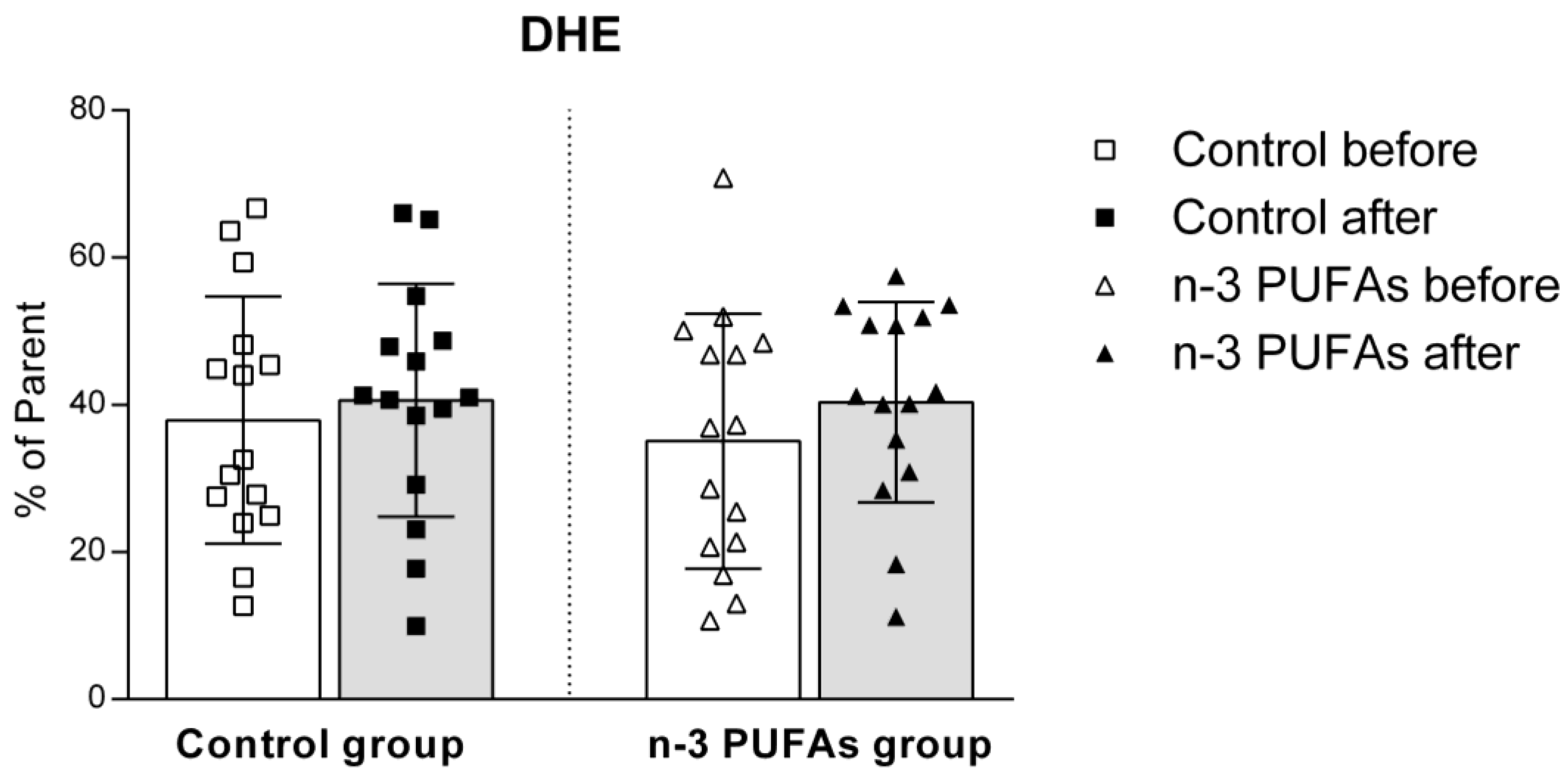
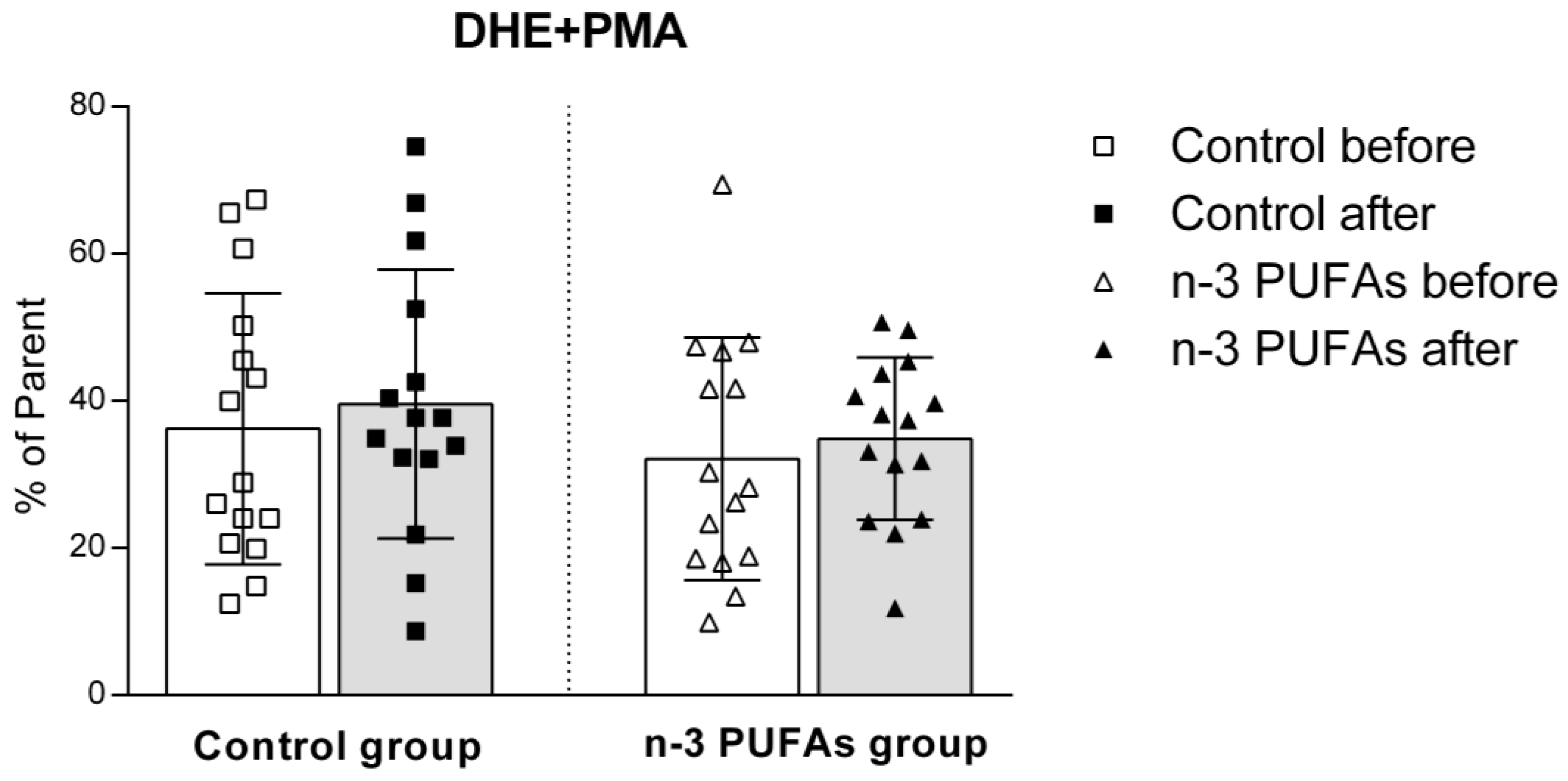
3.5. Assessment of Intracellular Reactive Oxygen Species Production
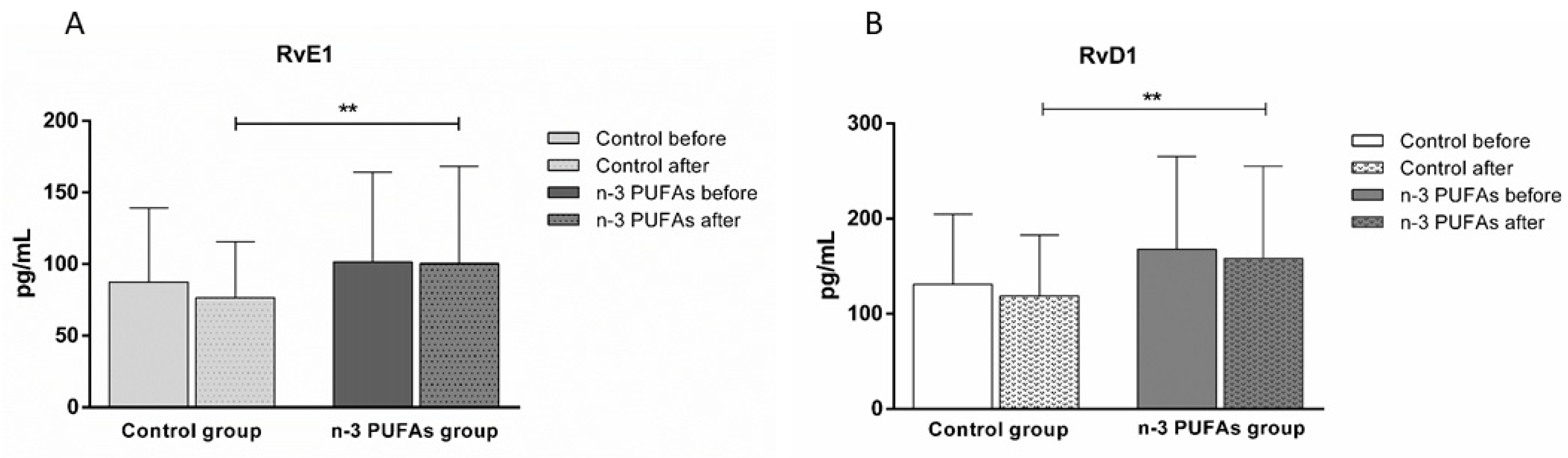
3.6. Serum Concentrations of RvE1 and RvD1
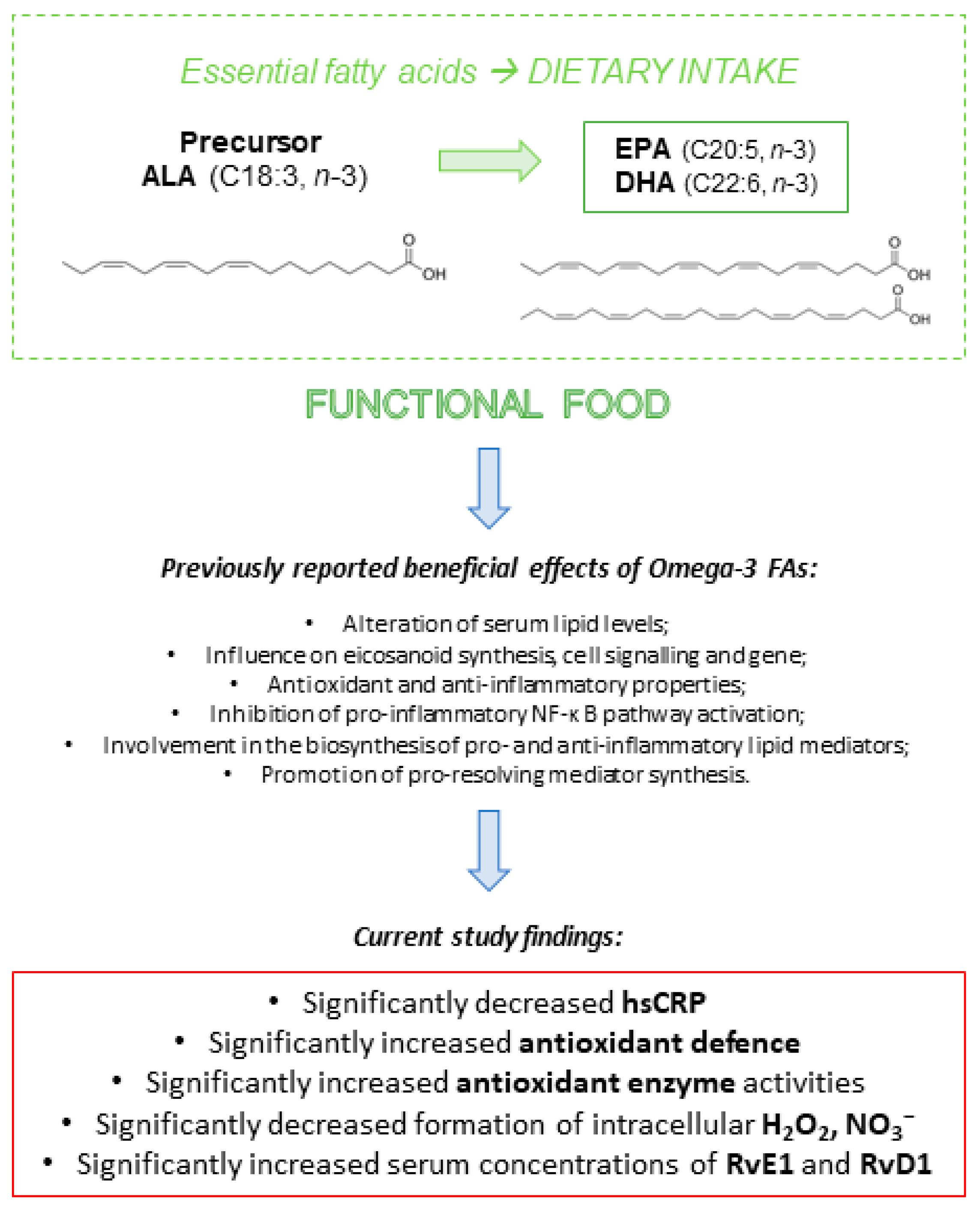
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Effects of Fish n-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef]
- Caponio, G.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Caponio, G.R.; Wang, D.Q.-H.; Di Ciaula, A.; De Angelis, M.; Portincasa, P. Regulation of Cholesterol Metabolism by Bioactive Components of Soy Proteins: Novel Translational Evidence. Int. J. Mol. Sci. 2020, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- Drenjančević, I.; Kralik, G.; Kralik, Z.; Mihalj, M.; Stupin, A.; Novak, S.; Grčević, M. Polyunsaturated Fatty Acids on Cardiovascular Health: Revealing Potentials of Functional Food. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTech: Seoul, Republic of Korea, 2017. [Google Scholar]
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Lopez, L.B.; Kritz-Silverstein, D.; Barrett-Connor, E. HIgh dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: The rancho bernardo study. J. Nutr. Health Aging 2011, 15, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Van Dael, P. Role of n-3 long-chain polyunsaturated fatty acids in human nutrition and health: Review of recent studies and recommendations. Nutr. Res. Pract. 2021, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Coletta, J.M.; Bell, S.J.; Roman, A.S. Omega-3 Fatty acids and pregnancy. Rev. Obstet. Gynecol. 2010, 3, 163–171. [Google Scholar]
- Devarshi, P.P.; Grant, R.W.; Ikonte, C.J.; Hazels Mitmesser, S. Maternal Omega-3 Nutrition, Placental Transfer and Fetal Brain Development in Gestational Diabetes and Preeclampsia. Nutrients 2019, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, F.D.; Shab-Bidar, S.; Jayedi, A. The effects of omega-3 polyunsaturated fatty acids supplementation in pregnancy, lactation, and infancy: An umbrella review of meta-analyses of randomized trials. Pharmacol. Res. 2022, 177, 106100. [Google Scholar] [CrossRef]
- Gao, X.; Su, X.; Han, X.; Wen, H.; Cheng, C.; Zhang, S.; Li, W.; Cai, J.; Zheng, L.; Ma, J.; et al. Unsaturated Fatty Acids in Mental Disorders: An Umbrella Review of Meta-Analyses. Adv. Nutr. 2022, 13, 2217–2236. [Google Scholar] [CrossRef]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n–3 and n–6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Qualified Health Claims: Letters of Enforcement Discretion; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/food/nutrition-food-labeling-and-critical-foods/qualified-health-claims-letters-enforcement-discretion (accessed on 5 April 2020).
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar] [PubMed]
- Lands, W.E.M. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992, 6, 2530–2536. [Google Scholar] [CrossRef]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef]
- Serhan, C.N. Systems approach to inflammation resolution: Identification of novel anti-inflammatory and pro-resolving mediators. J. Thromb. Haemost. 2009, 7, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Sandow, S.L.; Calder, P.C.; Russell, F.D. Abdominal aortic aneurysm and omega-3 polyunsaturated fatty acids: Mechanisms, animal models, and potential treatment. Prostaglandins Leukot. Essent. Fat. Acids 2017, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and Protectins in Inflammation Resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel Anti-Inflammatory-Pro-Resolving Mediators and Their Receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Salas-Hernández, A.; Espinoza-Pérez, C.; Vivar, R.; Espitia-Corredor, J.; Lillo, J.; Parra-Flores, P.; Sánchez-Ferrer, C.F.; Peiró, C.; Díaz-Araya, G. Resolvin D1 and E1 promote resolution of inflammation in rat cardiac fibroblast in vitro. Mol. Biol. Rep. 2021, 48, 57–66. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.d.C.; Marrugat, J.; et al. Effect of a Traditional Mediterranean Diet on Lipoprotein Oxidation. Arch. Intern. Med. 2007, 167, 1195. [Google Scholar] [CrossRef]
- Xiao, B.; Li, Y.; Lin, Y.; Lin, J.; Zhang, L.; Wu, D.; Zeng, J.; Li, J.; Liu, J.W.; Li, G. Eicosapentaenoic acid (EPA) exhibits antioxidant activity via mitochondrial modulation. Food Chem. 2022, 373, 131389. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Caterina Zito, M.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef]
- Kolobarić, N.; Drenjančević, I.; Matić, A.; Šušnjara, P.; Mihaljević, Z.; Mihalj, M. Dietary Intake of n-3 PUFA-Enriched Hen Eggs Changes Inflammatory Markers’ Concentration and Treg/Th17 Cells Distribution in Blood of Young Healthy Adults—A Randomised Study. Nutrients 2021, 13, 1851. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Stupin, A.; Kolobarić, N.; Tartaro Bujak, I.; Matić, A.; Kralik, Z.; Jukić, I.; Stupin, M.; Drenjančević, I. Leukocyte Activation and Antioxidative Defense Are Interrelated and Moderately Modified by n-3 Polyunsaturated Fatty Acid-Enriched Eggs Consumption—Double-Blind Controlled Randomized Clinical Study. Nutrients 2020, 12, 3122. [Google Scholar] [CrossRef]
- Stupin, A.; Mihalj, M.; Kolobarić, N.; Šušnjara, P.; Kolar, L.; Mihaljević, Z.; Matić, A.; Stupin, M.; Jukić, I.; Kralik, Z.; et al. Anti-Inflammatory Potential of n-3 Polyunsaturated Fatty Acids Enriched Hen Eggs Consumption in Improving Microvascular Endothelial Function of Healthy Individuals—Clinical Trial. Int. J. Mol. Sci. 2020, 21, 4149. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Prabodh Shah, A.; Ichiuji, A.M.; Han, J.K.; Traina, M.; El-Bialy, A.; Kamal Meymandi, S.; Yvonne Wachsner, R. Cardiovascular and Endothelial Effects of Fish Oil Supplementation in Healthy Volunteers. J. Cardiovasc. Pharmacol. Ther. 2007, 12, 213–219. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Oikonomou, E.; Zaromitidou, M.; Verveniotis, A.; Plastiras, A.; Kioufis, S.; Maniatis, K.; Miliou, A.; Siasou, Z.; et al. Effects of Ω-3 fatty acids on endothelial function, arterial wall properties, inflammatory and fibrinolytic status in smokers: A cross over study. Int. J. Cardiol. 2013, 166, 340–346. [Google Scholar] [CrossRef]
- Knezović, A.; Kolobarić, N.; Drenjančević, I.; Mihaljević, Z.; Šušnjara, P.; Jukić, I.; Stupin, M.; Kibel, A.; Marczi, S.; Mihalj, M.; et al. Role of Oxidative Stress in Vascular Low-Grade Inflammation Initiation Due to Acute Salt Loading in Young Healthy Individuals. Antioxidants 2022, 11, 444. [Google Scholar] [CrossRef]
- Kos, M.; Nađ, T.; Stupin, A.; Drenjančević, I.; Kolobarić, N.; Šušnjara, P.; Mihaljević, Z.; Damašek, M.; Pušeljić, S.; Jukić, I. Juvenile primary hypertension is associated with attenuated macro- and microvascular dilator function independently of body weight. J. Hypertens. 2024, 42, 1906–1914. [Google Scholar] [CrossRef]
- Barić, L.; Drenjančević, I.; Mihalj, M.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Mrakovčić-Šutić, I.; Šerić, V.; Stupin, A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. J. Clin. Med. 2020, 9, 843. [Google Scholar] [CrossRef]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cosic, A.; Jukic, I.; Stupin, A.; Mihalj, M.; Mihaljevic, Z.; Novak, S.; Vukovic, R.; Drenjancevic, I. Attenuated flow-induced dilatation of middle cerebral arteries is related to increased vascular oxidative stress in rats on a short-term high salt diet. J. Physiol. 2016, 594, 4917–4931. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Ötting, F. Superoxide Dismutase Assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [CrossRef]
- Kolar, L.; Šušnjara, P.; Stupin, M.; Stupin, A.; Jukić, I.; Mihaljević, Z.; Kolobarić, N.; Bebek, I.; Nejašmić, D.; Lovrić, M.; et al. Enhanced Microvascular Adaptation to Acute Physical Stress and Reduced Oxidative Stress in Male Athletes Who Consumed Chicken Eggs Enriched with n-3 Polyunsaturated Fatty Acids and Antioxidants—Randomized Clinical Trial. Life 2023, 13, 2140. [Google Scholar] [CrossRef]
- Šušnjara, P.; Kolobarić, N.; Matić, A.; Mihaljević, Z.; Stupin, A.; Marczi, S.; Drenjančević, I. Consumption of Hen Eggs Enriched with n-3 Polyunsaturated Fatty Acids, Selenium, Vitamin E and Lutein Incites Anti-Inflammatory Conditions in Young, Healthy Participants—A Randomized Study. Front. Biosci. 2022, 27, 332. [Google Scholar] [CrossRef]
- Stupin, A.; Rasic, L.; Matic, A.; Stupin, M.; Kralik, Z.; Kralik, G.; Grcevic, M.; Drenjancevic, I. Omega-3 polyunsaturated fatty acids-enriched hen eggs consumption enhances microvascular reactivity in young healthy individuals. Appl. Physiol. Nutr. Metab. 2018, 43, 988–995. [Google Scholar] [CrossRef]
- Zulyniak, M.A.; Roke, K.; Gerling, C.; Logan, S.L.; Spriet, L.L.; Mutch, D.M. Fish oil regulates blood fatty acid composition and oxylipin levels in healthy humans: A comparison of young and older men. Mol. Nutr. Food Res. 2016, 60, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Hlais, S.; El-Bistami, D.; El Rahi, B.; Mattar, M.A.; Obeid, O.A. Combined Fish Oil and High Oleic Sunflower Oil Supplements Neutralize their Individual Effects on the Lipid Profile of Healthy Men. Lipids 2013, 48, 853–861. [Google Scholar] [CrossRef]
- Root, M.; Collier, S.R.; Zwetsloot, K.A.; West, K.L.; McGinn, M.C. A randomized trial of fish oil omega-3 fatty acids on arterial health, inflammation, and metabolic syndrome in a young healthy population. Nutr. J. 2013, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Yu, J.-H.; Sun, J.-H.; Ma, W.-Q.; Wang, J.-J.; Sun, G.-J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef]
- Stanton, A.V.; James, K.; Brennan, M.M.; O’Donovan, F.; Buskandar, F.; Shortall, K.; El-Sayed, T.; Kennedy, J.; Hayes, H.; Fahey, A.G.; et al. Omega-3 index and blood pressure responses to eating foods naturally enriched with omega-3 polyunsaturated fatty acids: A randomized controlled trial. Sci. Rep. 2020, 10, 15444. [Google Scholar] [CrossRef]
- Jaček, M.; Hrnčířová, D.; Rambousková, J.; Dlouhý, P.; Tůma, P. Effect of Food with Low Enrichment of N-3 Fatty Acids in a Two-Month Diet on the Fatty Acid Content in the Plasma and Erythrocytes and on Cardiovascular Risk Markers in Healthy Young Men. Nutrients 2020, 12, 2207. [Google Scholar] [CrossRef]
- Haug, A.; Nyquist, N.F.; Mosti, T.J.; Andersen, M.; Høstmark, A.T. Increased EPA levels in serum phospholipids of humans after four weeks daily ingestion of one portion chicken fed linseed and rapeseed oil. Lipids Health Dis. 2012, 11, 104. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Ghavipisheh, M.; Attar, A.; Aslani, A. Comparison of the effect of omega-3 supplements and fresh fish on lipid profile: A randomized, open-labeled trial. Nutr. Diabetes 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bornfeldt, K.E. Triglyceride lowering by omega-3 fatty acids: A mechanism mediated by N-acyl taurines. J. Clin. Investig. 2021, 131, e147558. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Ridker, P.M. Targeting Residual Inflammatory Risk: A Shifting Paradigm for Atherosclerotic Disease. Front. Cardiovasc. Med. 2019, 6, 16. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Laderian, B.; Kuan, D.C.H.; Sereika, S.M.; Marsland, A.L.; Manuck, S.B. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J. Intern. Med. 2016, 279, 98–109. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2021, 12, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh-Far, R.; Harris, W.S.; Garg, S.; Na, B.; Whooley, M.A. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis 2009, 205, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.D.; Rahman, F.; Lacey, S.; Larson, M.G.; Vasan, R.S.; Benjamin, E.J.; Harris, W.S.; Robins, S.J. Red blood cell fatty acids and biomarkers of inflammation: A cross-sectional study in a community-based cohort. Atherosclerosis 2015, 240, 431–436. [Google Scholar] [CrossRef]
- Grenon, S.M.; Conte, M.S.; Nosova, E.; Alley, H.; Chong, K.; Harris, W.S.; Vittinghoff, E.; Owens, C.D. Association between n-3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease. J. Vasc. Surg. 2013, 58, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Khaza’ai, H.; Patimah, I.; Rahmat, A.; Abed, Y. Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Food Nutr. Res. 2016, 60, 29268. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef]
- Calvo, M.J.; Martínez, M.S.; Torres, W.; Chávez-Castillo, M.; Luzardo, E.; Villasmil, N.; Salazar, J.; Velasco, M.; Bermúdez, V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A molecular view into structure and function. Vessel Plus 2017, 1, 116–128. [Google Scholar] [CrossRef][Green Version]
- Yagi, S.; Fukuda, D.; Aihara, K.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E.; et al. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. Effect of n-3 fatty acids on the expression of inflammatory genes in THP-1 macrophages. Lipids Health Dis. 2016, 15, 69. [Google Scholar] [CrossRef]
- Fredman, G.; Serhan, C.N. Specialized proresolving mediator targets for RvE1 and RvD1 in peripheral blood and mechanisms of resolution. Biochem. J. 2011, 437, 185–197. [Google Scholar] [CrossRef]
- Videla, L.A.; Hernandez-Rodas, M.C.; Metherel, A.H.; Valenzuela, R. Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease. Prostaglandins Leukot. Essent. Fat. Acids 2022, 181, 102441. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N. Resolution phase lipid mediators of inflammation: Agonists of resolution. Curr. Opin. Pharmacol. 2013, 13, 632–640. [Google Scholar] [CrossRef]
- Macho-González, A.; Bastida, S.; Garcimartín, A.; López-Oliva, M.E.; González, P.; Benedí, J.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Functional Meat Products as Oxidative Stress Modulators: A Review. Adv. Nutr. 2021, 12, 1514–1539. [Google Scholar] [CrossRef]
- de Assis, A.M.; Rech, A.; Longoni, A.; Rotta, L.N.; Denardin, C.C.; Pasquali, M.A.; Souza, D.O.; Perry, M.L.S.; Moreira, J.C. Ω3-Polyunsaturated fatty acids prevent lipoperoxidation, modulate antioxidant enzymes, and reduce lipid content but do not alter glycogen metabolism in the livers of diabetic rats fed on a high fat thermolyzed diet. Mol. Cell. Biochem. 2012, 361, 151–160. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, S404–S421. [Google Scholar] [CrossRef]


| Fatty Acid | Regular Chicken Breast (mg/100 g Meat) | n-3 PUFA-Enriched Chicken Breast (mg/100 g Meat) | |
|---|---|---|---|
| Chicken breast | ∑SFA | 626.27 (28.45) | 602.38 (50.64) |
| ∑MUFA | 627.54 (47.69) | 694.13 (76.42) * | |
| ∑n-6 PUFA | 824.47 (50.59) | 451.98 (36.58) * | |
| ALA | 17.74 (3.33) | 177.63 (21.75) * | |
| Eicosatrienoic acid | 0.00 (0.0) | 9.01 (3.77) * | |
| EPA | 0.00 (0.0) | 25.07 (6.35) * | |
| DHA | 0.00 (0.0) | 33.75 (13.76) * | |
| ∑n-3 PUFA | 17.74 (3.33) | 245.46 (13.95) * | |
| ∑n-6 PUFA/∑n-3 PUFA | 46.48 (7.84) | 1.84 (0.17) * | |
| Chicken thigh muscle | ∑SFA | 1654.46 (120.95) | 1684.10 (68.53) * |
| ∑MUFA | 2197.44 (168.53) | 2281.36 (174.38) * | |
| ∑n-6 PUFA | 2217.87 (241.25) | 1200.99 (88.64) * | |
| ALA | 46.27 (10.56) | 501.54 (36.94) * | |
| Eicosatrienoic acid | 0.00 (0.0) | 14.98 (8.56) * | |
| EPA | 0.00 (0.0) | 45.57 (16.85) * | |
| DHA | 0.00 (0.0) | 55.81 (28.91) * | |
| ∑n-3 PUFA | 46.27 (10.56) | 617.90 (62.25) * | |
| ∑n-6 PUFA/∑n-3 PUFA | 47.93 (13.49) | 1.94 (0.15) * |
| Parameter | Control Group | n-3 PUFAs Group | p ‡ | ||||
|---|---|---|---|---|---|---|---|
| N (W/M) | 20 (12/8) | 19 (8/11) | 0.527 | ||||
| Age (years) | 23 (3.0) | 23 (3.0) | 0.476 | ||||
| Before | After | p † | Before | After | p † | ||
| BMI (kg/m2) | 24.8 (4.9) | 24.6 (5.0) | 0.978 | 23.9 (3.1) | 24.0 (3.1) | 0.871 | 0.366 |
| WHR | 0.81 (0.05) | 0.82 (0.05) | 0.813 | 0.82 (0.05) | 0.82 (0.05) | 0.854 | 0.975 |
| erythrocytes (×1012/L) | 4.6 (0.5) | 4.7 (0.5) | 0.551 | 5.0 (0.4) | 5.0 (0.3) | 0.429 | 0.958 |
| hemoglobin (g/L) | 132.6 (13.4) | 135.2 (14.9) | 0.658 | 142.6 (15.1) | 141.9 (10.7) | 0.429 | 0.994 |
| leukocytes (×109/L) | 6.9 (1.7) | 6.4 (1.8) | 0.435 | 6.4 (0.8) | 6.1 (1.0) | 0.345 | 0.007* |
| thrombocytes (×109/L) | 258.0 (66.4) | 260.4 (66.5) | 0.928 | 267.3 (60.7) | 254.2 (55.4) | 0.978 | 0.911 |
| urea (mmol/L) | 4.8 (1.4) | 5.6 (1.1) | 0.096 | 5.2 (1.4) | 5.8 (1.5) | 0.093 | 0.47 |
| creatinine (μmol/L) | 71.4 (13.0) | 73.6 (11.8) | 0.735 | 73.6 (8.0) | 76.4 (8.0) | 0.091 | 0.26 |
| sodium (mmol/L) | 138.7 (1.3) | 138.7 (1.4) | 0.885 | 138.7 (1.7) | 139.2 (1.8) | 0.422 | 0.353 |
| potassium (mmol/L) | 4.3 (0.3) | 4.2 (0.2) | 0.115 | 4.5 (0.4) | 4.5 (0.3) | 0.283 | 0.083 |
| calcium (mmol/L) | 2.8 (0.1) | 2.4 (0.1) | 0.093 | 2.5 (0.1) | 2.4 (0.1) | 0.06 | 0.232 |
| iron (µmol/L) | 13.4 (6.1) | 14.7 (7.5) | 0.735 | 18.5 (7.4) | 19.2 (5.0) | 0.434 | 0.216 |
| transferrin (g/L) | 2.8 (0.4) | 2.8 (0.5) | 0.211 | 2.8 (0.4) | 2.8 (0.4) | 0.71 | 0.356 |
| glucose (mmol/L) | 4.8 (0.5) | 4.5 (0.5) | 0.09 | 5.0 (0.5) | 5.0 (1.1) | 0.724 | 0.279 |
| hsCRP (mg/L) | 2.1 (3.7) | 1.2 (1.2) | 0.755 | 1.5 (2.6) | 0.8 (0.4) | 0.364 | 0.002 * |
| cholesterol (mmol/L) | 4.0 (0.6) | 4.1 (0.7) | 0.921 | 4.3 (0.8) | 4.2 (0.9) | 0.654 | 0.963 |
| triglycerides (mmol/L) | 0.9 (0.3) | 0.8 (0.5) | 0.184 | 0.9 (0.5) | 0.7 (0.3) | 0.731 | 0.602 |
| HDL cholesterol (mmol/L) | 1.5 (0.3) | 1.5 (0.3) | 0.09 | 1.5 (0.3) | 1.5 (0.3) | 0.505 | 0.096 |
| LDL cholesterol (mmol/L) | 2.2 (0.4) | 2.4 (0.6) | 0.121 | 2.5 (0.6) | 2.6 (0.6) | 0.105 | 0.987 |
| AST (U/L) | 23.3 (4.1) | 28.4 (16.9) | 0.282 | 26.4 (7.4) | 24.1 (7.0) | 0.06 | 0.23 |
| ALT (U/L) | 20.8 (5.7) | 26.8 (16.0) | 0.227 | 25.7 (17.3) | 21.5 (11.7) | 0.323 | 0.119 |
| GGT (U/L) | 13.9 (2.9) | 15.5 (3.8) | 0.06 | 17.7 (11.4) | 21.0 (17.8) | 0.118 | 0.451 |
| Parameter (U/mg Protein) | Control Group | n-3 PUFAs Group | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| GPx | 0.076 (0.02) | 0.084 (0.01) | 0.079 (0.02) | 0.099 (0.03) * |
| SOD | 14.08 (1.03) | 14.39 (1.34) | 14.19 (1.1) | 14.87 (0.74) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nađ, T.; Kolobarić, N.; Mihaljević, Z.; Drenjančević, I.; Šušnjara, P.; Stupin, A.; Kardum, D.; Kralik, Z.; Kralik, G.; Košević, M.; et al. Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study. Antioxidants 2025, 14, 204. https://doi.org/10.3390/antiox14020204
Nađ T, Kolobarić N, Mihaljević Z, Drenjančević I, Šušnjara P, Stupin A, Kardum D, Kralik Z, Kralik G, Košević M, et al. Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study. Antioxidants. 2025; 14(2):204. https://doi.org/10.3390/antiox14020204
Chicago/Turabian StyleNađ, Tihana, Nikolina Kolobarić, Zrinka Mihaljević, Ines Drenjančević, Petar Šušnjara, Ana Stupin, Darjan Kardum, Zlata Kralik, Gordana Kralik, Manuela Košević, and et al. 2025. "Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study" Antioxidants 14, no. 2: 204. https://doi.org/10.3390/antiox14020204
APA StyleNađ, T., Kolobarić, N., Mihaljević, Z., Drenjančević, I., Šušnjara, P., Stupin, A., Kardum, D., Kralik, Z., Kralik, G., Košević, M., & Jukić, I. (2025). Effect of n-3 Polyunsaturated Fatty Acids Enriched Chicken Meat Consumption in Relation to Oxidative Stress Marker Levels in Young Healthy Individuals: A Randomized Double-Blind Study. Antioxidants, 14(2), 204. https://doi.org/10.3390/antiox14020204









