Abstract
The interplay between oxidative stress and adipogenesis is a critical factor in the development of obesity and its associated metabolic disorders. Excessive reactive oxygen species (ROS) disrupt key transcription factors such as peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα), impairing lipid metabolism, promoting adipocyte dysfunction, and exacerbating inflammation and insulin resistance. Antioxidants, classified as endogenous (e.g., glutathione, superoxide dismutase, and catalase) and exogenous (e.g., polyphenols, flavonoids, and vitamins C and E), are pivotal in mitigating these effects by restoring redox balance and preserving adipocyte functionality. Endogenous antioxidants neutralize ROS and safeguard cellular structures; however, under heightened oxidative stress, these defenses are often insufficient, necessitating dietary supplementation. Exogenous antioxidants derived from plant-based sources, such as polyphenols and vitamins, act through direct ROS scavenging, upregulation of endogenous antioxidant enzymes, and modulation of key signaling pathways like nuclear factor kappa B (NF-κB) and PPARγ, reducing lipid peroxidation, inflammation, and adipocyte dysfunction. Furthermore, they influence epigenetic regulation and transcriptional networks to restore adipocyte differentiation and limit lipid accumulation. Antioxidant-rich diets, including the Mediterranean diet, are strongly associated with improved metabolic health, reduced obesity rates, and enhanced insulin sensitivity. Advances in personalized antioxidant therapies, guided by biomarkers of oxidative stress and supported by novel delivery systems, present promising avenues for optimizing therapeutic interventions. This review, “Crosstalk Between Antioxidants and Adipogenesis: Mechanistic Pathways and Their Role in Metabolic Health”, highlights the mechanistic pathways by which antioxidants regulate oxidative stress and adipogenesis to enhance metabolic health.
1. Introduction
Obesity has surged globally, affecting diverse populations across socioeconomic and geographic boundaries far beyond its earlier association with wealthier nations [1]. As of today, more than 650 million adults are classified as obese, and this number continues to grow at an alarming rate. This growing public health crisis is exacerbating healthcare systems and increasing the prevalence of non-communicable diseases such as diabetes, hypertension, and cardiovascular diseases [2]. Adipogenesis is the process by which mesenchymal stem cells differentiate into adipocytes, a process that is regulated by transcription factors such as PPARγ and C/EBPα [3,4]. Mature adipocytes are the primary cells responsible for the storage of fat in the form of triglycerides. The process of lipogenesis plays a pivotal role in energy storage during periods of calorie surplus [5]. However, in cases of obesity, an excessive amount of lipogenesis generates elevated levels of ROS as metabolic byproducts, which disrupts the cellular redox balance and contributes to oxidative stress [6]. Elevated ROS levels have been shown to impair the function of critical transcription factors, such as PPARγ and C/EBPα, which play a pivotal role in regulating lipid metabolism. This, in turn, results in the disruption of adipocyte differentiation and the subsequent impairment of their function [7]. These effects extend to damage to cellular membranes, proteins, and DNA, further compounding metabolic dysfunction. Low-grade inflammation, driven by macrophage infiltration into adipose tissue, further exacerbates oxidative stress [8,9]. This inflammatory environment is characterized by the secretion of cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interferon-gamma (IFN-γ) [10]. The role of IFN-γ in this process is particularly noteworthy, as it serves to amplify the activation of macrophages and the inflammatory signaling that ensues, thereby perpetuating the cycle of oxidative stress and inflammation [11]. These cytokines subsequently activate NF-κB, thereby perpetuating the pro-inflammatory milieu that ultimately compromises adipocyte function [12,13,14]. This results in disturbances in lipid metabolism, which in turn exacerbates the microenvironment of adipose tissue by activating pro-inflammatory pathways. The utilization of antioxidants has emerged as a pivotal strategy to disrupt the cycle of oxidative damage and restore cellular homeostasis; antioxidants counteract ROS-induced damage by neutralizing free radicals, modulating lipogenesis-associated signaling pathways, and preserving transcription factors like PPARγ and C/EBPα, which are essential for adipocyte differentiation and function [15]. Consequently, the research community has pivoted towards investigating the potential of antioxidants in enhancing metabolic well-being [16,17,18]. A comprehensive understanding of the mechanisms by which antioxidants modulate lipid metabolism and reduce oxidative stress is essential for fully appreciating their potential therapeutic value in the context of obesity and related metabolic disorders [19,20]. The preservation of adipocyte function is imperative in the prevention of excessive lipid accumulation and the subsequent development of obesity [21]. While the protective role of antioxidants against oxidative stress has been extensively documented, particularly in relation to fat cell health, less attention has been given to the dynamic interplay between endogenous antioxidants and those derived from natural sources. Endogenous antioxidants, including glutathione and enzymes such as SOD and catalase, play a critical role in managing oxidative stress. However, in cases of obesity or suboptimal dietary conditions, an increase in ROS production can overwhelm these defenses, leaving cells susceptible to oxidative damage [22]. In addition to the body’s intrinsic antioxidant defenses, the inclusion of dietary antioxidants derived from plant-based foods provides a complementary approach to combating oxidative stress [23,24]. Compounds such as polyphenols, vitamins C and E, and carotenoids have demonstrated the capacity to bolster cellular defenses and enhance adipocyte functionality under conditions of obesity [19,25].
This article will examine the specific links and relationships between antioxidants and adipogenesis, with a particular focus on the effects of natural antioxidants on this process.
2. Mechanisms of Adipogenesis
This section explores the biological mechanisms underlying adipocyte differentiation, the role of key transcription factors, and the influence of oxidative stress and environmental factors on adipogenesis. Adipogenesis is a tightly regulated, multi-step process that begins with MSC commitment to the adipogenic lineage and culminates in the maturation of fully differentiated adipocytes [26,27]. This process is influenced by transcriptional, epigenetic, and environmental factors, which become particularly significant under pathological conditions such as obesity [28,29].
2.1. Adipocyte Physiology
Adipocytes, the key energy storage and endocrine cells of adipose tissue are classified into three types based on localization and function: white adipose tissue (WAT), brown adipose tissue (BAT), and beige adipocytes [30,31].
2.1.1. White Adipose Tissue (WAT)
WAT plays a pivotal role in energy storage, predominantly in the form of triglycerides. This adipose tissue is further categorized into subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) [32,33]. SAT, located beneath the skin, plays a central role in energy homeostasis. In contrast, VAT surrounds internal organs and is closely associated with metabolic dysfunction when in excess [34].
2.1.2. Brown Adipose Tissue (BAT)
BAT dissipates energy as heat through uncoupling protein 1 (UCP1)-mediated thermogenesis. Rich in mitochondria, BAT counters energy surplus and is activated by cold exposure or caloric excess [35].
2.1.3. Beige Adipocytes
Beige adipocytes form within WAT during “browning” induced by cold exposure or adrenergic signaling. These cells exhibit BAT-like thermogenic properties, enhancing energy expenditure and protecting against obesity [36].
2.2. Commitment and Epigenetic Modulation
The commitment of MSCs to the adipogenic lineage marks the first stage of adipogenesis and is orchestrated by key transcription factors, particularly peroxisome PPARγ and C/EBPα. These transcription factors activate downstream adipogenic genes, initiating the differentiation process and ensuring the precise maturation of functional adipocytes under normal conditions [3,37,38].
Recent research highlights the critical role of epigenetic modifications during early adipocyte differentiation. Modifications such as DNA methylation and histone acetylation regulate chromatin accessibility, determining the activation or suppression of adipogenic genes [39]. Environmental factors, including dietary habits and metabolic status, influence these epigenetic signatures. Sustained cues such as pro-inflammatory cytokines or high-fat diets during early development establish persistent epigenetic marks, predisposing MSCs to pathological adipogenesis in later stages [28,40]. Thus, adipogenesis is regulated not only by transcription factors but also by long-lasting epigenetic changes shaped by environmental conditions [40,41].
2.3. Environmental and Pathological Influences
Adipogenesis is profoundly influenced by environmental factors and pathological conditions, particularly in the context of obesity [42,43]. While transcriptional and epigenetic mechanisms establish the foundation for normal adipocyte differentiation, obesity introduces chronic low-grade inflammation, oxidative stress, and early-life environmental exposures that disrupt this tightly regulated process [44].
2.3.1. Inflammatory Disruption
Pro-inflammatory cytokines, including TNF-α, IL-6, and IFN-γ, exhibit elevated levels in obesity cases and disrupt adipogenesis by impairing the function of transcriptional regulators such as PPARγ and C/EBPα [45,46,47]. This disruption skews adipocyte differentiation toward hypertrophy and dysfunctional lipid metabolism. Furthermore, the intensification of inflammatory signaling by IFN-γ fosters the perpetuation of a pro-inflammatory microenvironment, thereby contributing to the maintenance of adipocyte dysfunction [48,49,50].
2.3.2. Oxidative Damage
Excessive ROS in obesity exacerbates adipogenic dysfunction by causing oxidative damage to cellular membranes, proteins, and DNA. ROS impair critical transcriptional regulators like PPARγ, further disrupting adipocyte differentiation and lipid regulation [51]. This oxidative imbalance creates a feedback loop, amplifying adipocyte dysfunction and metabolic instability.
2.3.3. Early-Life Exposures
Early-life environmental factors, including nutrient excess and exposure to inflammatory stimuli, have been demonstrated to establish persistent epigenetic changes that predispose MSCs to enhanced adipogenic differentiation later in life [52,53]. These modifications, influenced by early dietary habits and metabolic conditions, create a lasting impact on cellular programming and link developmental exposures to an increased risk of obesity in adulthood [54,55].
Adipogenesis is a process that is subject to precise regulation by transcriptional and epigenetic mechanisms. Obesity has been demonstrated to disrupt these processes through the mechanisms of inflammation, oxidative stress, and environmental factors, resulting in metabolic dysfunction. This underscores the critical role of transcription factors in adipocyte regulation [56,57,58].
3. The Regulatory Role of Key Transcription Factors in Adipogenesis
The process of adipocyte differentiation is regulated by three key transcription factors: Together, PPARγ, C/EBPα, and Sterol SREBP-1c regulate the expression of genes involved in lipid metabolism, adipocyte development, and insulin sensitivity [59]. The roles of these factors are not only interdependent but also subject to dynamic influence from metabolic and environmental conditions.
Adipocyte differentiation is not only directly influenced by transcription factors such as PPARγ and SREBP-1c but also by upstream signaling cascades including insulin and mTOR [60]. These pathways integrate various metabolic signals including nutrient availability and oxidative stress to finely tune the transcriptional network essential for adipocyte differentiation [61,62].
As shown in Figure 1, the differentiation of adipocytes is regulated by several key transcription factors, including PPAR γ, C/EBPα, and SREBP-1c, which collectively direct lipid metabolism and storage [63,64,65]. As the primary regulator, PPARγ is responsible for directing processes such as lipid uptake, triglyceride storage, and glucose metabolism [66,67]. In conjunction with PPARγ, C/EBPα enhances insulin sensitivity and facilitates adipocyte maturation by activating lipogenic genes. Meanwhile, SREBP-1c, which is responsive to both insulin and oxidative stress, plays a vital role in fatty acid synthesis, contributing to the accumulation of lipids within adipocytes [68,69]. The interaction among these transcription factors is of great importance for the maintenance of adipocyte function and metabolic homeostasis. Upstream signaling cascades, particularly the insulin-mTOR pathway, incorporate metabolic signals to regulate adipogenesis by establishing a positive feedback loop through PPARγ, which reinforces both adipocyte differentiation and lipid storage [60]. Nevertheless, prolonged SREBP-1c activation in obesity can result in excessive lipid synthesis, leading to the development of hypertrophic adipocytes and lipotoxicity, which ultimately impairs insulin sensitivity [70].
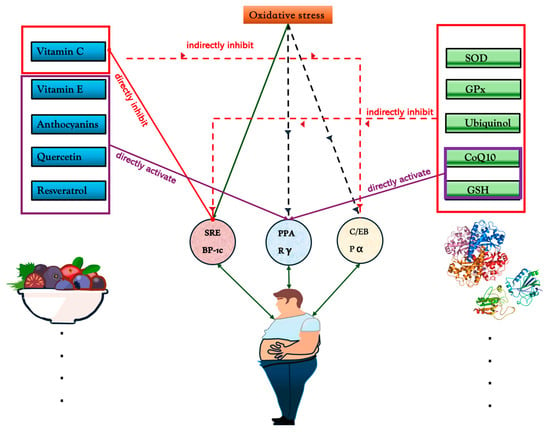
Figure 1.
Mechanisms of antioxidant regulation on oxidative stress and transcription factors SREBP-1c, PPARγ, and C/EBPα. Natural antioxidants, including vitamin C, vitamin E, anthocyanins, quercetin, and resveratrol, directly or indirectly modulate these factors. Vitamin C and quercetin directly inhibit SREBP-1c, reducing lipid biosynthesis, while resveratrol activates PPARγ, enhancing anti-inflammatory responses. Endogenous antioxidants like SOD, GPx, ubiquinol, CoQ10, and GSH help maintain redox balance. CoQ10 and GSH directly activate PPARγ, while GPx and ubiquinol indirectly suppress SREBP-1c. Molecular structures of ubiquinone and ubiquinol demonstrate their roles in redox cycling, highlighting the essential function of antioxidants in metabolic regulation.
ROS functions as an additional regulatory layer, acting as signaling molecules at moderate levels but causing dysfunction when in excess [71]. This ROS-mediated pathway contributes to the development of abnormal adipocyte hypertrophy and excessive fat storage, which are key features of obesity [72,73]. Furthermore, epigenetic mechanisms, including histone methylation (e.g., H3K27me3) and non-coding RNAs (e.g., miR-27), provide additional dynamic control over the process of adipogenesis [74]. Histone methylation can suppress the expression of genes that direct alternative cell fates, thereby directing MSCs toward the adipocyte lineage [75,76]. MicroRNAs can also refine the process of adipogenesis by targeting transcription factors such as PPARγ, thus ensuring precise regulation [77]. In the context of obesity, chronic inflammation serves to exacerbate the dysfunction of adipocytes via the activation of NF-κB, which in turn induces the production of pro-inflammatory cytokines (e.g., TNF-α). These cytokines impair insulin signaling and destabilize C/EBPα and PPARγ, thereby further driving the proliferation of adipocytes and the development of insulin resistance [78,79]. Therapeutic strategies that target oxidative stress and inflammation, such as antioxidants (resveratrol and curcumin) and miRNA-based therapies, show promise in reducing lipid accumulation and improving insulin sensitivity [80,81]. These approaches offer targeted solutions for the management of obesity and related metabolic disorders.
4. Antioxidants: Types and Their Roles in Cellular Health
Natural antioxidants, predominantly sourced from dietary intake, serve as a fundamental line of defense against oxidative stress by mitigating ROS and upholding cellular integrity [82,83]. These exogenous antioxidants encompass polyphenols, vitamins C and E, and carotenoids, each with distinctive mechanisms of action that act in a synergistic manner to reinforce cellular antioxidant capacity [84,85]. Polyphenols, an extensive category of compounds found in a range of plant-based foods, including berries, green tea, and nuts, function not only as direct scavengers of ROS but also as modulators of redox-sensitive signaling pathways [86,87]. Polyphenols also bind to metal ions that catalyze oxidative reactions, thereby further impeding ROS formation at the cellular level [88]. Furthermore, they are known to induce endogenous antioxidant enzymes, such as SOD and catalase, through the upregulation of gene expression, thereby amplifying intrinsic antioxidant defenses [89]. Vitamin C (ascorbic acid), which is predominantly found in citrus fruits, bell peppers, and leafy greens, is a potent water-soluble antioxidant that operates in both intracellular and extracellular matrices [90,91]. Its primary function is to donate electrons in order to neutralize ROS, and it plays a pivotal role in regenerating other antioxidants, notably vitamin E. Vitamin E (α-tocopherol), a lipid-soluble antioxidant obtained from nuts, seeds, and vegetable oils, incorporates into cellular membranes, where it protects against lipid peroxidation, a process that can destabilize membrane integrity and functionality [92,93]. Carotenoids, such as beta-carotene and lycopene, which are prevalent in colorful fruits and vegetables, have been demonstrated to be particularly effective at quenching singlet oxygen, a highly reactive form of ROS [94,95]. This offers additional membrane stabilization. Collectively, these natural antioxidants contribute to a robust antioxidant defense system, thereby safeguarding cellular structures and biomolecular integrity against oxidative damage. This underscores the critical role of a diverse, antioxidant-rich diet in maintaining cellular health.
In addition to dietary antioxidants, the body synthesizes a variety of endogenous antioxidants that are essential for maintaining intracellular redox balance [96,97]. The endogenous antioxidant system is comprised of a number of enzymatic antioxidants, including SOD, catalase, glutathione peroxidase (GPx), and the thioredoxin system [98]. Each of these fulfills a specific role within the cellular antioxidant defense mechanisms. SOD catalyzes the conversion of superoxide radicals, highly reactive byproducts of mitochondrial respiration, into hydrogen peroxide [99]. This is subsequently converted into water and oxygen by catalase and GPx, respectively, thereby averting potential oxidative damage [100]. Catalase, which is predominantly localized within peroxisomes, exhibits remarkable catalytic efficiency in decomposing hydrogen peroxide and acts as a rapid-response antioxidant under conditions of high oxidative stress. GPx employs glutathione, a tripeptide with a cysteine residue, as a substrate to detoxify hydrogen peroxide and lipid peroxides, thereby protecting biomolecules such as lipids, proteins, and nucleic acids from oxidative modifications [101,102]. The thioredoxin system, comprising thioredoxin and thioredoxin reductase, facilitates the reduction of oxidized proteins and contributes to DNA synthesis and repair, cellular proliferation, and apoptosis regulation, thereby further underscoring its importance in maintaining cellular viability under oxidative conditions [103]. Collectively, these endogenous systems, in conjunction with dietary antioxidants, constitute an integrated network that dynamically regulates cellular redox states, thereby preventing the pathogenesis of oxidative stress-related diseases.
In addition to their primary function in the neutralization of ROS, both natural and endogenous antioxidants exhibit dual functionality by modulating inflammatory pathways, lipid metabolism, and adipogenesis, thereby contributing to systemic metabolic homeostasis [104,105]. By attenuating oxidative stress, antioxidants inhibit the activation of the pro-inflammatory transcription factor NF-κB, which in turn leads to a reduction in the expression of cytokines such as TNF-α [106,107,108]. These cytokines are implicated in chronic inflammation and metabolic dysfunctions. Moreover, antioxidants exert a protective effect on lipid metabolism by mitigating lipid peroxidation, which in turn prevents the aberrant accumulation of lipids and the hypertrophic expansion of adipocytes, which are commonly observed in obesity [109,110,111]. This regulatory influence extends to the process of adipogenesis, where antioxidants play a critical role in ensuring normal adipocyte differentiation and function, thus preventing metabolic derangements associated with excessive fat deposition [111,112,113]. Collectively, the multifaceted actions of antioxidants—ranging from ROS neutralization to inflammatory modulation and lipid regulation—demonstrate their indispensable role in cellular resilience and the maintenance of metabolic equilibrium [97,114]. Consequently, they are regarded as pivotal agents in the prevention of oxidative stress-related diseases and age-associated cellular decline
5. Interaction Between Antioxidants and Adipogenesis
Antioxidants exert their influence on adipogenesis through complex interactions with key molecular pathways that regulate adipocyte differentiation, particularly the NF-κB, PPARγ, and ROS signaling [115,116].These cytokines amplify pro-inflammatory signaling, creating a feedback loop that promotes adipocyte differentiation and excessive lipid storage. Additionally, elevated ROS levels further activate NF-κB, thereby fostering a highly pro-inflammatory environment that perpetuates adipogenesis [25]. In this context, antioxidants such as resveratrol, curcumin, and various polyphenols act by directly neutralizing ROS, thereby dampening NF-κB activation [81,117,118]. This reduction in cytokine production disrupts the pro-inflammatory cycle, weakening one of the key pathways that perpetuate adipogenesis. Meanwhile, PPARγ, a master transcription factor in adipocyte maturation, normally regulates lipid uptake and triglyceride storage [119]. Under oxidative conditions, excessive PPARγ activation promotes pathological lipid storage and adipocyte hypertrophy, contributing to metabolic dysfunction. Antioxidants assist in maintaining the regulatory equilibrium of PPARγ by reducing ROS levels, thereby ensuring that PPARγ activity remains within the optimal ranges that support physiological lipid storage [120,121]. Compounds such as epigallocatechin gallate (EGCG), a potent green tea polyphenol, exemplify this equilibrium by modulating PPARγ expression, ensuring healthy lipid metabolism without the pathological adipogenesis associated with metabolic dysfunction [122,123,124].
In addition to regulating these pathways, antioxidants exert a direct influence on adipocyte proliferation and the overall expansion of fat mass, which is essential for maintaining healthy adipose tissue [125,126,127]. Vitamins C and E serve as efficacious defenders against these effects, with vitamin E, in particular, integrating into adipocyte membranes to prevent lipid peroxidation, thereby preserving cellular structure and reducing the excessive proliferation associated with adiposity [93,128]. Conversely, polyphenols such as quercetin and EGCG have been observed to downregulate pivotal adipogenic genes implicated in lipid biosynthesis and cell cycle progression, thereby exerting a direct inhibitory effect on adipocyte proliferation [129]. By reducing oxidative stress at the cellular level, these antioxidants help to inhibit the pathological growth of adipose tissue, thereby demonstrating their therapeutic relevance in the management of obesity and the promotion of metabolic resilience. Figure 2 depicts the interplay between oxidative stress and antioxidant defenses in adipogenesis, with fatty acid binding protein 4 (FABP4) as a central regulator. FABP4 is one of the most abundant proteins in adipocytes and plays an important role in maintaining adipocyte homeostasis by regulating lipolysis and lipogenesis [130,131,132].
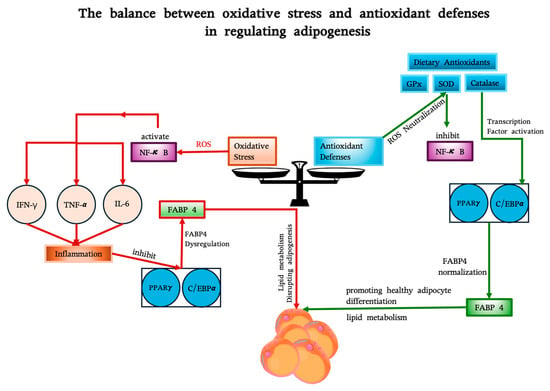
Figure 2.
The balance between oxidative stress and antioxidant defenses in regulating FABP4 and adipogenesis. Oxidative stress (left), driven by ROS and inflammation (via NF-κB and cytokines like IFN-γ, TNF-α, and IL-6), suppresses transcription factors PPARγ and C/EBPα, leading to FABP4 dysregulation and impaired adipocyte function. Antioxidant defenses (right), including endogenous enzymes (GPx, SOD, catalase) and dietary antioxidants (e.g., resveratrol, curcumin), neutralize ROS, inhibit NF-κB, and restore transcription factor activity, normalizing FABP4 expression. FABP4 links these pathways, facilitating healthy adipogenesis by supporting lipid metabolism and adipocyte differentiation.
Furthermore, the anti-inflammatory properties of antioxidants influence their impact on adipogenesis, particularly through their capacity to disrupt the production of pro-inflammatory cytokines, which drive abnormal adipocyte expansion. Chronic inflammation, which is particularly prevalent in obesity, generates a continuous influx of cytokines, such as TNF-α and IL-6, that maintain a self-reinforcing cycle of NF-κB activation and oxidative stress [133,134]. The pro-inflammatory environment has been demonstrated to have a deleterious effect on adipocyte proliferation, while concomitantly disrupting systemic metabolic stability. Antioxidants such as resveratrol, curcumin, and carotenoids intervene by inhibiting NF-κB activation, thereby reducing cytokine levels and shifting the balance toward an anti-inflammatory state [119,135]. Some antioxidants even enhance anti-inflammatory cytokines like IL-10, thereby providing a dual approach that suppresses pro-inflammatory signals while stabilizing adipose tissue [136,137,138]. By alleviating inflammation and oxidative stress, antioxidants create a more resilient metabolic environment, thereby reducing the risk of insulin resistance and metabolic dysfunction, particularly in the context of obesity and its associated health risks. This combined anti-inflammatory and ROS-neutralizing effect demonstrates the versatility of antioxidants as agents capable of reshaping adipogenic pathways toward healthier, more balanced adipocyte function.
5.1. Antioxidants: Sources and Mechanisms
Antioxidants play a pivotal role in counteracting oxidative stress and inflammation, both of which are central to the development of metabolic dysfunction. Endogenous antioxidants, including SOD, catalase, and GPx, act as the primary line of defense by neutralizing ROS and maintaining cellular redox equilibrium [139,140]. These intrinsic systems are further supported by dietary antioxidants, which include polyphenols, carotenoids, and vitamins C and E, predominantly sourced from plant-based foods like fruits, vegetables, nuts, and seeds [141,142]. The function of these dietary antioxidants is twofold: they scavenge ROS and influence key molecular pathways, particularly those involving the transcription factors NF-κB and PPARγ. These transcription factors are critical for regulating adipocyte differentiation and lipid metabolism, and their dysfunction is closely linked to metabolic disorders [25,143]. Antioxidants aid in preserving the activity of these transcription factors, thereby ensuring that adipocytes maintain functional lipid storage and energy balance. By mitigating oxidative damage and restoring homeostasis in cellular signaling, antioxidants contribute to a reduction in the risk of pathological adipocyte hypertrophy, a hallmark of obesity [25,144,145].
5.2. Experimental Evidence and Relevant Research
Experimental studies have yielded valuable insights into the influence of antioxidants on adipose tissue health. In vitro investigations reveal that polyphenols such as resveratrol and EGCG reduce lipid accumulation in adipocytes by suppressing the expression of lipogenic enzymes and modulating inflammatory pathways [146,147]. Animal studies further demonstrate the effectiveness of antioxidant supplementation in improving insulin sensitivity, lowering oxidative damage, and alleviating chronic inflammation within adipose tissue [148,149]. These findings underscore the potential of antioxidants to enhance metabolic health by targeting the underlying mechanisms of adipocyte dysfunction. While clinical research has yielded more variable results, it continues to offer additional support for these benefits. Long-term consumption of antioxidant-rich diets has been associated with decreased oxidative stress markers and improved metabolic profiles in obese individuals [150]. However, the observed effects are often context-dependent, shaped by factors such as the dosage and bioavailability of antioxidants, as well as individual differences in metabolic states. This variability underscores the necessity of tailoring antioxidant interventions to meet specific physiological and metabolic needs [151,152,153].
Antioxidants have been demonstrated to play a vital role in protecting adipose tissue by reducing oxidative stress, regulating transcriptional activity, and controlling inflammation. The potential benefits of antioxidants as a therapeutic modality for obesity-related metabolic disorders are significant. However, the efficacy of antioxidants is contingent upon individual metabolic conditions, necessitating a delicate balance between oxidative stress and antioxidant defenses [154,155]. Consequently, further research is necessary to enhance and personalize antioxidant-based therapeutic interventions.
6. Role of Endogenous Antioxidants in Combating Obesity-Related Oxidative Stress
Endogenous antioxidants play a pivotal role in mitigating the effects of oxidative stress associated with obesity. They protect cellular structures, support metabolic balance, and prevent inflammatory responses. Table 1 highlights the roles and mechanisms of endogenous antioxidants in combating obesity-related oxidative stress, reinforcing the article’s focus on targeted strategies for improving metabolic health.

Table 1.
Roles and mechanisms of endogenous antioxidants in obesity-related oxidative stress.
In conclusion, the aforementioned endogenous antioxidants—GSH, SOD, catalase, GPx, the thioredoxin system, CoQ10, HO-1, PRXs, PON1, and ubiquinol—function collectively to form a multi-layered defense system [170]. These antioxidants operate at various cellular sites, protecting adipocytes from oxidative stress, maintaining redox balance, and supporting metabolic function. Collectively, they mitigate the effects of ROS in obesity, reducing metabolic dysregulation and inflammation to safeguard cellular health [170,171].
7. Role of Natural Antioxidants in Adipogenesis and Oxidative Stress
The use of natural antioxidants derived from plant sources is of significant importance in the management of oxidative stress and the modulation of lipogenesis, both of which are pivotal in addressing metabolic disorders such as obesity [19,172]. Bioactive compounds, including polyphenols, flavonoids, carotenoids, and vitamins C and E, derived from fruits, vegetables, nuts, and seeds, confer multifaceted benefits by neutralizing ROS while augmenting endogenous antioxidant systems [85,173,174]. For example, tea polyphenols, particularly EGCG, act as dual agents, scavenging ROS and upregulating key antioxidant enzymes such as GPx and SOD [124,175]. This dual action helps to maintain cellular redox equilibrium in the lipid-rich and oxidative environment of adipose tissue. Furthermore, EGCG modulates adipogenesis by downregulating transcription factors such as PPARγ and C/EBPα, which curtails adipocyte maturation and limits lipid storage [15,119]. Moreover, evidence indicates that tea polyphenols can inhibit the NF-κB pathway, an inflammatory cascade that has been demonstrated to contribute to the progression of lipogenesis through the action of cytokines such as TNF-α [176,177]. This targeted modulation illustrates how polyphenols interact with metabolic pathways at multiple levels, thereby creating a cellular environment that is less susceptible to lipid accumulation and inflammatory responses [178,179].
Vitamins C and E exemplify the synergistic interaction between water- and fat-soluble antioxidants, with distinct solubility profiles that enable comprehensive cellular protection. Vitamin C’s role as a primary ROS neutralizer and vitamin E regenerator enhances its efficacy, particularly in adipocytes, where it facilitates the maintenance of vitamin E’s lipid-protective functions. Meanwhile, vitamin E, a lipid-soluble antioxidant, integrates into cellular membranes to prevent lipid peroxidation, a process that is often accelerated in obesity and linked to impaired membrane integrity and inflammatory signaling [128,148]. Collectively, these antioxidants facilitate redox homeostasis in adipose cells, thereby preventing ROS-driven inflammation and abnormal lipid deposition [180]. This cooperation demonstrates how different antioxidants can create a multi-faceted defense mechanism, effectively counteracting oxidative stress-induced lipogenesis and supporting cellular health [127,172].
The influence of natural antioxidants on metabolic health is substantiated by a substantial body of research indicating that diets rich in antioxidants are associated with reduced rates of obesity and improved metabolic profiles. The ingestion of polyphenol-rich foods, such as green tea and resveratrol, has been shown to result in a notable reduction in body fat and enhanced insulin sensitivity [181,182]. Recent evidence indicates that the combination of antioxidants, such as polyphenols with vitamin C, results in a synergistic effect that amplifies antioxidant capacity and further inhibits fat accumulation [183,184,185]. Table 2 summarizes the primary sources, roles, and mechanisms of dietary antioxidants in mitigating oxidative stress, highlighting their regulatory effects on lipogenesis and their positive impact on metabolic health.

Table 2.
Roles and mechanisms of dietary antioxidants in obesity-related oxidative stress.
Natural antioxidants protect against oxidative stress and help regulate lipids. This helps limit lipid accumulation and enhance metabolic resilience, making them important for preventing and managing obesity and metabolic disorders [209,210,211].
8. Therapeutic Potential of Antioxidants in Obesity and Metabolic Disorder Management
The role of antioxidants as a therapeutic tool in the management of obesity and associated metabolic disorders is becoming increasingly recognized. Dietary antioxidants, in particular, offer a non-invasive intervention that can combat the underlying oxidative stress and inflammation [212,213]. Diets rich in bioactive compounds, particularly the Mediterranean diet, have been demonstrated to be strongly associated with improvements in metabolic health, reduced rates of obesity, and enhanced insulin sensitivity [214,215,216]. For example, polyphenols present in olive oil and red wine act as direct scavengers of ROS while simultaneously promoting the activity of endogenous antioxidant enzymes, including GPx and SOD [217,218,219]. This action of neutralizing ROS restores redox balance in adipose tissue, thereby limiting inflammation and supporting normal lipid metabolism. These mechanisms enable dietary antioxidants to mitigate oxidative damage and inflammation in adipose tissue, which is otherwise susceptible to ROS-induced dysfunction that leads to insulin resistance and abnormal lipid storage [127,220,221]. The promotion of long-term metabolic stability and healthier fat tissue function is facilitated by the regulation of these pathways by antioxidant-rich diets.
In addition to the general advantages of dietary antioxidants, targeted antioxidant supplementation has been demonstrated to enhance cellular resilience to metabolic stress. However, it is essential to exercise caution when considering factors such as dosage and bioavailability. The efficacy of vitamins E and C, in conjunction with polyphenolic extracts derived from green tea and berries, has been demonstrated in the reduction of adipose tissue, improvement of glucose metabolism, and protection of adipose tissue from oxidative damage [104,105]. From a mechanistic standpoint, vitamin E serves to stabilize cell membranes by preventing lipid peroxidation, while vitamin C plays a role in regenerating vitamin E following oxidation, thereby extending its protective capabilities [93,128,222]. However, it should be noted that high-dose supplements may potentially interfere with physiological ROS signaling, which supports functions such as the immune response and insulin sensitivity [114]. Moreover, antioxidants impact metabolic regulation via the NF-κB pathway, which plays a pivotal role in inflammatory signaling and glucose metabolism [223,224]. By modulating NF-κB, antioxidants reduce cytokine release, improve insulin receptor activity, and enhance glucose uptake, which are all essential actions for the management of type 2 diabetes [80,189]. Excessive antioxidant intake may disrupt homeostatic mechanisms, necessitating careful balancing of benefits against individual metabolic contexts [225,226,227].
A personalized approach to antioxidant therapy represents a promising avenue for more precise treatments, given that individual variations in oxidative stress and metabolic responses influence therapeutic outcomes [228,229,230]. Given the sexually dimorphic nature of adipose tissue distribution, with women predominantly storing fat in subcutaneous depots and men in visceral regions, it is crucial to consider these differences when evaluating the potential role of antioxidants in metabolic interventions [231]. Antioxidant therapies may need to be personalized to address these sex-specific variations, with a focus on mitigating oxidative stress in subcutaneous adipose tissue for women and targeting inflammation and metabolic dysfunction in visceral fat for men [232,233]. Such tailored approaches could optimize therapeutic outcomes by aligning with the distinct metabolic challenges associated with each fat depot, ultimately contributing to more effective management of obesity and its related metabolic disorders.
Personalized antioxidant therapy is a therapeutic approach that utilizes biomarkers to guide intervention strategies. Biomarkers such as malondialdehyde (MDA), F2-isoprostanes, and 8-hydroxy-2′-deoxyguanosine (8-OHdG) serve as indicators of oxidative damage, while assays like total antioxidant capacity (TAC) and ferric reducing antioxidant power (FRAP) measure overall antioxidant potential [234,235,236]. Advances in genomics have led to the identification of genetic variations, including single-nucleotide polymorphisms (SNPs) in antioxidant defense genes, which influence individual responses to oxidative stress and antioxidant therapy. Integration of these tools enables the customization of interventions to account for genetic predispositions and metabolic conditions, thereby enhancing their efficacy [237,238,239]. The optimization of antioxidant intake through the consideration of oxidative stress markers, metabolic profiles, and genetic backgrounds can facilitate the realization of the benefits of antioxidants while mitigating the risks associated with over-supplementation [240,241,242]. For instance, individuals exhibiting elevated oxidative markers may derive benefit from a combination of antioxidants, such as polyphenols and vitamin C, which act in concert to enhance antioxidant capacity and target inflammation in adipose tissue [142,243]. Such personalization considers the inter-individual variability in the absorption, utilization, and response to antioxidants. As an emerging field in the study of metabolic health, personalized therapy also highlights the necessity of addressing the bioavailability challenges associated with many antioxidants, which often exhibit limited stability and absorption in supplement form. Novel delivery systems, including nanoencapsulation and liposomal formulations, are currently being investigated to enhance the stability, absorption, and efficacy of these compounds [244,245,246]. The potential to overcome these barriers to bioavailability could position antioxidants as central agents in the management of obesity and metabolic disorders, addressing both oxidative and inflammatory aspects of metabolic dysfunction in a patient-centered, targeted manner [247,248].
Although they show promising therapeutic potential, the role of antioxidants in adipogenesis and metabolic diseases remains contentious, as clinical trials frequently report that vitamin-based supplements fail to enhance health outcomes or extend longevity [249,250]. In certain instances, excessive antioxidant use has been observed to impede physiological ROS signaling, which is imperative for processes such as insulin sensitivity and immune regulation. These findings underscore the need for context-specific strategies to optimize antioxidant use [251].
9. Conclusions and Future Perspectives
This review highlights the critical function of antioxidants in addressing the pathophysiological processes underlying obesity and related metabolic disorders. Antioxidants, both endogenous and dietary, are indispensable for neutralizing ROS, reducing inflammation, and maintaining lipid and glucose homeostasis in adipose tissue. By upregulating antioxidant enzymes such as GPx and SOD, antioxidants assist in the mitigation of oxidative stress in adipocytes, thereby reducing lipid peroxidation and preserving cellular integrity. Moreover, antioxidants impact pivotal transcription factors, such as PPARγ and C/EBPα, which are vital regulators of adipogenesis, inflammation, and adipocyte hypertrophy—the primary drivers of obesity. These mechanisms collectively position antioxidants as a promising class of non-invasive therapeutic agents for the improvement of metabolic health, reduction of adipose tissue mass, and enhancement of insulin sensitivity.
Dietary antioxidants from fruits, vegetables, nuts, and whole grains—including polyphenols, flavonoids, carotenoids, and vitamins C/E—function as ROS scavengers while boosting endogenous antioxidant systems. For instance, berry anthocyanins and citrus-derived vitamin C neutralize ROS and regenerate oxidized antioxidants (e.g., recycling vitamin E), prolonging their efficacy. Similarly, polyphenols and vitamin E from greens and nuts inhibit lipid peroxidation, preserving adipocyte membrane integrity. Carotenoids further mitigate oxidative damage by quenching singlet oxygen. Collectively, these compounds reduce oxidative stress, improve lipid metabolism, enhance insulin sensitivity, and promote metabolic resilience.
Despite the promising effects of antioxidants, future research must refine their therapeutic use for obesity and metabolic disorders, with a particular emphasis on personalized interventions. In addition to the general benefits of antioxidant therapies, it is essential to recognize the sexually dimorphic nature of adipose tissue distribution when considering their application in metabolic interventions. This is due to the fact that while dysfunctional adipocytes are central to the development of obesity and metabolic-related diseases, strategies that indirectly target sexually dimorphic adipose tissue may also have beneficial outcomes. Personalizing antioxidant treatments in this way could optimize therapeutic outcomes, aligning interventions with the distinct metabolic challenges each sex faces. Ultimately, such tailored approaches hold significant promise for improving the management of obesity and associated metabolic disorders. It is important to note that individual responses to antioxidants may vary based on a number of factors, including genetic predispositions and oxidative stress levels. For example, single-nucleotide SNPs in antioxidant defense genes, such as glutathione S-transferase (GST), have been demonstrated to influence the body’s response to specific antioxidants. This indicates the possibility of personalized antioxidant therapies, in which metabolic profiles and oxidative stress markers could assist in optimizing antioxidant selection, dosage, and delivery while reducing the risk of over-supplementation. Furthermore, recent developments in antioxidant delivery systems, including nanoencapsulation and liposomal formulations, present promising strategies to overcome current limitations in bioavailability, stability, and absorption. Notably, research into the potential synergistic effects of combining antioxidants with other compounds, such as polyphenols with vitamins C and E, may reveal additive or even synergistic effects, offering a more comprehensive approach to protecting against metabolic dysfunction. Collectively, these advancements in personalized medicine and delivery technologies have the potential to fully unlock the therapeutic benefits of antioxidants, positioning them as critical components in the prevention and treatment of obesity and other metabolic disorders.
Author Contributions
Conceptualization, W.C. and M.F.; investigation, writing—original draft preparation, M.F. and W.C.; writing—review and editing, M.F., K.-S.Y., J.H., I.K. and W.C.; supervision, W.C.; project administration, W.C.; funding acquisition, W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Foundation of Korea (NRF) grants funded by the Korean government (MEST) (NRF-2018R1A6A1A030525124).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jaacks, L.M.; Vandevijvere, S.; Pan, A.; McGowan, C.J.; Wallace, C.; Imamura, F.; Mozaffarian, D.; Swinburn, B.; Ezzati, M. The obesity transition: Stages of the global epidemic. Lancet Diabetes Endocrinol. 2019, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.-W.; Li, X.; Zhang, Y.-Y.; Huang, H.-Y.; Liu, Y.; Sun, X.; Tang, Q.-Q. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Dev. Biol. 2010, 10, 47. [Google Scholar] [CrossRef]
- Sysoeva, V.Y.; Lazarev, M.A.; Kulebyakin, K.Y.; Semina, E.V.; Rubina, K.A. Molecular and Cellular Mechanisms Governing Adipogenic Differentiation. Russ. J. Dev. Biol. 2023, 54, S10–S22. [Google Scholar] [CrossRef]
- Chung, T.-H.; Kwon, Y.-J.; Lee, Y.-J. High triglyceride to HDL cholesterol ratio is associated with low testosterone and sex hormone-binding globulin levels in Middle-aged and elderly men. Aging Male 2020, 23, 93–97. [Google Scholar] [CrossRef]
- McMurray, F.; Patten, D.A.; Harper, M.-E. Reactive Oxygen Species and Oxidative Stress in Obesity—Recent Findings and Empirical Approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-w. Reactive Oxygen Species Facilitate Adipocyte Differentiation by Accelerating Mitotic Clonal Expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef]
- Mandelker, L. Oxidative Stress, Free Radicals, and Cellular Damage. In Studies on Veterinary Medicine; Mandelker, L., Vajdovich, P., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–17. [Google Scholar] [CrossRef]
- Eljaafari, A.; Pestel, J.; Le Magueresse-Battistoni, B.; Chanon, S.; Watson, J.; Robert, M.; Disse, E.; Vidal, H. Adipose-Tissue-Derived Mesenchymal Stem Cells Mediate PD-L1 Overexpression in the White Adipose Tissue of Obese Individuals, Resulting in T Cell Dysfunction. Cells 2021, 10, 2645. [Google Scholar] [CrossRef]
- Wu, C.; Xue, Y.; Wang, P.; Lin, L.; Liu, Q.; Li, N.; Xu, J.; Cao, X. IFN-γ Primes Macrophage Activation by Increasing Phosphatase and Tensin Homolog via Downregulation of miR-3473b. J. Immunol. 2014, 193, 3036–3044. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Jurga, L.; Vanessa, H.; Elisabet, A.N.; Andrea, D.; Lennart, B.; Erik, N.; Dominique, L.; Peter, A.; Mikael, R. NF-κ;B is important for TNF-α;-induced lipolysis in human adipocytes. J. Lipid Res. 2007, 48, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Hacohen, N.; Golub, T.R.; Van Parijs, L.; Lodish, H.F. Tumor Necrosis Factor-α Suppresses Adipocyte-Specific Genes and Activates Expression of Preadipocyte Genes in 3T3-L1 Adipocytes: Nuclear Factor-κB Activation by TNF-α Is Obligatory. Diabetes 2002, 51, 1319–1336. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Vergnaud, A.-C.; Galan, P.; Arnaud, J.; Favier, A.; Faure, H.; Huxley, R.; Hercberg, S.; Ahluwalia, N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am. J. Clin. Nutr. 2009, 90, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Giurranna, E.; Nencini, F.; Bettiol, A.; Borghi, S.; Argento, F.R.; Emmi, G.; Silvestri, E.; Taddei, N.; Fiorillo, C.; Becatti, M. Dietary Antioxidants and Natural Compounds in Preventing Thrombosis and Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 11457. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, C.; Gong, Q.-y.; Li, X.-x.; Lei, G.-h.; Yang, T.-b. Associations Between Dietary Antioxidant Intake and Metabolic Syndrome. PLoS ONE 2015, 10, e0130876. [Google Scholar] [CrossRef]
- Almoraie, N.M.; Shatwan, I.M. The Potential Effects of Dietary Antioxidants in Obesity: A Comprehensive Review of the Literature. Healthcare 2024, 12, 416. [Google Scholar] [CrossRef]
- Sharebiani, H.; Mokaram, M.; Mirghani, M.; Fazeli, B.; Stanek, A. The Effects of Antioxidant Supplementation on the Pathologic Mechanisms of Metabolic Syndrome and Cardiovascular Disease Development. Nutrients 2024, 16, 1641. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Huang, C.-J.; McAllister, M.J.; Slusher, A.L.; Webb, H.E.; Mock, J.T.; Acevedo, E.O. Obesity-Related Oxidative Stress: The Impact of Physical Activity and Diet Manipulation. Sports Med.-Open 2015, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; True, C.; Wilson, C.G. Oxidative stress and food as medicine. Front. Nutr. 2024, 11, 1394632. [Google Scholar] [CrossRef] [PubMed]
- Al-Regaiey, K. Crosstalk between adipogenesis and aging: Role of polyphenols in combating adipogenic-associated aging. Immun. Ageing 2024, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-N.; Wu, J.-F. TGF-β/SMAD signaling regulation of mesenchymal stem cells in adipocyte commitment. Stem Cell Res. Ther. 2020, 11, 41. [Google Scholar] [CrossRef]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef]
- Ghosh, S.; Dhar, S.; Bhattacharjee, S.; Bhattacharjee, P. Contribution of environmental, genetic and epigenetic factors to obesity-related metabolic syndrome. Nucl. 2023, 66, 215–237. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, C.; Chen, L.; Luo, D.; Chen, R.; Liang, F. Mechanistic Insights Into the Interaction Between Transcription Factors and Epigenetic Modifications and the Contribution to the Development of Obesity. Front. Endocrinol. 2018, 9, 370. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Lafontan, M. Differences Between Subcutaneous and Visceral Adipose Tissues. In Physiology and Physiopathology of Adipose Tissue; Bastard, J.-P., Fève, B., Eds.; Springer: Paris, France, 2013; pp. 329–349. [Google Scholar] [CrossRef]
- Liesenfeld, D.B.; Grapov, D.; Fahrmann, J.F.; Salou, M.; Scherer, D.; Toth, R.; Habermann, N.; Böhm, J.; Schrotz-King, P.; Gigic, B.; et al. Metabolomics and transcriptomics identify pathway differences between visceral and subcutaneous adipose tissue in colorectal cancer patients: The ColoCare study. Am. J. Clin. Nutr. 2015, 102, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Klein, S. Is Visceral Fat Responsible for the Metabolic Abnormalities Associated with Obesity? Implications of Omentectomy. Diabetes Care 2010, 33, 1693–1694. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Zhang, W.; Luk, C.T.; Sivasubramaniyam, T.; Brunt, J.J.; Schroer, S.A.; Desai, H.R.; Majerski, A.; Woo, M. JAK2 promotes brown adipose tissue function and is required for diet- and cold-induced thermogenesis in mice. Diabetologia 2016, 59, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Altınova, A.E. Beige Adipocyte as the Flame of White Adipose Tissue: Regulation of Browning and Impact of Obesity. J. Clin. Endocrinol. Metab. 2021, 107, e1778–e1788. [Google Scholar] [CrossRef]
- Al Haj, G.; Rey, F.; Giallongo, T.; Colli, M.; Marzani, B.; Giuliani, G.; Gorio, A.; Zuccotti, G.V.; Di Giulio, A.M.; Carelli, S. A New Selective PPARγ Modulator Inhibits Triglycerides Accumulation During Murine Adipocytes’ and Human Adipose-Derived Mesenchymal Stem Cells Differentiation. Int. J. Mol. Sci. 2020, 21, 4415. [Google Scholar] [CrossRef]
- Lai, F.; Wang, J.; Tang, H.; Bian, X.; Lu, K.; He, G.; Huang, P.; Liu, J.; Zhou, M.; Liu, J.; et al. Adipogenic differentiation was inhibited by downregulation of PPARγ signaling pathway in aging tendon stem/progenitor cells. J. Orthop. Surg. Res. 2021, 16, 614. [Google Scholar] [CrossRef]
- Li, H.-x.; Xiao, L.; Wang, C.; Gao, J.-l.; Zhai, Y.-g. Epigenetic regulation of adipocyte differentiation and adipogenesis. J. Zhejiang Univ. Sci. B 2010, 11, 784–791. [Google Scholar] [CrossRef]
- Matsumura, Y.; Osborne, T.F.; Sakai, J. Epigenetic and environmental regulation of adipocyte function. J. Biochem. 2022, 172, 9–16. [Google Scholar] [CrossRef]
- Jilo, D.D.; Abebe, B.K.; Wang, J.; Guo, J.; Li, A.; Zan, L. Long non-coding RNA (LncRNA) and epigenetic factors: Their role in regulating the adipocytes in bovine. Front. Genet. 2024, 15, 1405588. [Google Scholar] [CrossRef]
- Egusquiza, R.J.; Blumberg, B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology 2020, 161, bqaa024. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Liu, Q.S.; Zhou, Q.; Jiang, G. Environmental Obesogens and Their Perturbations in Lipid Metabolism. Environ. Health 2024, 2, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, D.; Cline, M.A.; Gilbert, E.R. Chronic stress, epigenetics, and adipose tissue metabolism in the obese state. Nutr. Metab. 2020, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, Y.; Shu, T.; Wang, J. Cytokines and inflammation in adipogenesis: An updated review. Front. Med. 2019, 13, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Han, J.; Zhang, X.; Deng, Y.; Yan, H.; Wang, C.; Li, X.; Chen, S.; Alimujiang, M.; Li, X.; et al. Obesity-associated inflammation triggers an autophagy–lysosomal response in adipocytes and causes degradation of perilipin 1. Cell Death Dis. 2019, 10, 121. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Kim, B.S.; Saenz, S.A.; Stine, R.R.; Monticelli, L.A.; Sonnenberg, G.F.; Thome, J.J.; Farber, D.L.; Lutfy, K.; Seale, P.; et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519, 242–246. [Google Scholar] [CrossRef]
- Kissig, M.; Ishibashi, J.; Harms, M.J.; Lim, H.W.; Stine, R.R.; Won, K.J.; Seale, P. PRDM16 represses the type I interferon response in adipocytes to promote mitochondrial and thermogenic programing. EMBO J. 2017, 36, 1528–1542. [Google Scholar] [CrossRef]
- Lee, B.-C.; Kim, M.-S.; Pae, M.; Yamamoto, Y.; Eberlé, D.; Shimada, T.; Kamei, N.; Park, H.-S.; Sasorith, S.; Woo, J.R.; et al. Adipose Natural Killer Cells Regulate Adipose Tissue Macrophages to Promote Insulin Resistance in Obesity. Cell Metab. 2016, 23, 685–698. [Google Scholar] [CrossRef]
- de Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. In Proceedings of the Stem Cells: Biology and Engineering, Cham, Switzerland, 8 December 2018; pp. 125–144. [Google Scholar]
- Xu, Q.; Hou, W.; Zhao, B.; Fan, P.; Wang, S.; Wang, L.; Gao, J. Mesenchymal stem cells lineage and their role in disease development. Mol. Med. 2024, 30, 207. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, J.; Zhang, P.; Zhou, D.; Liu, F. Dynamics of Transcription Factors in Three Early Phases of Osteogenic, Adipogenic, and Chondrogenic Differentiation Determining the Fate of Bone Marrow Mesenchymal Stem Cells in Rats. Front. Cell Dev. Biol. 2021, 9, 768316. [Google Scholar] [CrossRef]
- Gharipour, M.; Craig, J.M.; Stephenson, G. Epigenetic programming of obesity in early life through modulation of the kynurenine pathway. Int. J. Obes. 2025, 49, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Vickers, M.H. Early Life Nutrition, Epigenetics and Programming of Later Life Disease. Nutrients 2014, 6, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, J.-L.; Lu, X.; Yang, Q. Epigenetic regulation of energy metabolism in obesity. J. Mol. Cell Biol. 2021, 13, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Pant, R.; Firmal, P.; Shah, V.K.; Alam, A.; Chattopadhyay, S. Epigenetic Regulation of Adipogenesis in Development of Metabolic Syndrome. Front. Cell Dev. Biol. 2021, 8, 619888. [Google Scholar] [CrossRef]
- Stojchevski, R.; Velichkovikj, S.; Arsov, T. Genetic and Epigenetic Basis of Obesity-Induced Inflammation and Diabetes. In Obesity, Diabetes and Inflammation: Molecular Mechanisms and Clinical Management; Avtanski, D., Poretsky, L., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 129–146. [Google Scholar] [CrossRef]
- Ramesh, T.; Shahid, M. Bacoside-A repressed the differentiation and lipid accumulation of 3T3-L1 preadipocytes by modulating the expression of adipogenic genes. Biotechnol. Appl. Biochem. 2024, 71, 741–752. [Google Scholar] [CrossRef]
- Jun, I.; Choi, Y.J.; Kim, B.-R.; Lee, H.K.; Seo, K.Y.; Kim, T.-i. Activation of the mTOR pathway enhances PPARγ/SREBP-mediated lipid synthesis in human meibomian gland epithelial cells. Sci. Rep. 2024, 14, 28118. [Google Scholar] [CrossRef]
- Chaudhuri, R.; Krycer, J.R.; Fazakerley, D.J.; Fisher-Wellman, K.H.; Su, Z.; Hoehn, K.L.; Yang, J.Y.H.; Kuncic, Z.; Vafaee, F.; James, D.E. The transcriptional response to oxidative stress is part of, but not sufficient for, insulin resistance in adipocytes. Sci. Rep. 2018, 8, 1774. [Google Scholar] [CrossRef]
- Čolak, E.; Pap, D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J. Med. Biochem. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional Regulation of Adipocyte Differentiation: A Central Role for CCAAT/Enhancer-binding Protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef]
- Liu, C.-C.; Chen, J.-Y.; Chu, C.-C.; Chen, S.-Y.; Chu, H.-L.; Duh, P.-D. Chlorogenic Acid Decreases Lipid Accumulation in 3T3-L1 Adipocytes by Modulating the Transcription Factors. J. Food Nutr. Res. 2020, 8, 313–319. [Google Scholar] [CrossRef]
- Chui, P.C.; Guan, H.-P.; Lehrke, M.; Lazar, M.A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Investig. 2005, 115, 2244–2256. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Gatticchi, L.; Petricciuolo, M.; Scarpelli, P.; Macchioni, L.; Corazzi, L.; Roberti, R. Tm7sf2 gene promotes adipocyte differentiation of mouse embryonic fibroblasts and improves insulin sensitivity. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118897. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jang, H.-J.; Muthamil, S.; Shin, U.C.; Lyu, J.-H.; Kim, S.-W.; Go, Y.; Park, S.-H.; Lee, H.G.; Park, J.H. Novel insights into regulators and functional modulators of adipogenesis. Biomed. Pharmacother. 2024, 177, 117073. [Google Scholar] [CrossRef]
- Vázquez-Vela, M.E.F.; Torres, N.; Tovar, A.R. White Adipose Tissue as Endocrine Organ and Its Role in Obesity. Arch. Med. Res. 2008, 39, 715–728. [Google Scholar] [CrossRef]
- Choi, E.-H.; Kim, M.-H.; Park, S.-J. Targeting Mitochondrial Dysfunction and Reactive Oxygen Species for Neurodegenerative Disease Treatment. Int. J. Mol. Sci. 2024, 25, 7952. [Google Scholar] [CrossRef]
- Bakkar, N.-M.Z.; Osman, S.T.; Alzaim, I.; El-Yazbi, A.F. Depot-Biased ROS: A Middleman in Adipose-Driven Cardiovascular Disease. In Oxidative Stress in Cardiovascular-Metabolic Diseases; Eid, A.H., Kobeissy, F., El-Yazbi, A.F., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 97–118. [Google Scholar] [CrossRef]
- Chang, C.-C.; Sia, K.-C.; Chang, J.-F.; Lin, C.-M.; Yang, C.-M.; Lee, I.T.; Vo, T.T.T.; Huang, K.-Y.; Lin, W.-N. Participation of lipopolysaccharide in hyperplasic adipose expansion: Involvement of NADPH oxidase/ROS/p42/p44 MAPK-dependent Cyclooxygenase-2. J. Cell. Mol. Med. 2022, 26, 3850–3861. [Google Scholar] [CrossRef]
- Nanduri, R. Epigenetic Regulators of White Adipocyte Browning. Epigenomes 2021, 5, 3. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, R.; Ban, Y.; Zhang, W.; Kong, N.; Tang, J.; Ma, B.; Shao, Y.; Jin, R.; Sun, L.; et al. EPO modified MSCs protects SH-SY5Y cells against ischemia/hypoxia-induced apoptosis via REST-dependent epigenetic remodeling. Sci. Rep. 2024, 14, 23252. [Google Scholar] [CrossRef]
- Dara, M.; Zare-Moayedi, Z.; Taheri, Y.; Tanideh, R.; Zare, S.; Kafilzadeh, F. Exploring herbal preconditioning strategies to improve adipose tissue stem cell therapy efficacy. Gene Rep. 2024, 37, 102030. [Google Scholar] [CrossRef]
- Son, Y.H.; Ka, S.; Kim, A.Y.; Kim, J.B. Regulation of Adipocyte Differentiation via MicroRNAs. Endocrinol. Metab. 2014, 29, 122–135. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. The Impact of Curcumin, Resveratrol, and Cinnamon on Modulating Oxidative Stress and Antioxidant Activity in Type 2 Diabetes: Moving Beyond an Anti-Hyperglycaemic Evaluation. Antioxidants 2024, 13, 510. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Az-Zahra, F.; Wongso, H.; Setyawati, L.U.; Novitasari, D.; Ikram, E.H.K. Molecular Mechanism of Natural Food Antioxidants to Regulate ROS in Treating Cancer: A Review. Antioxidants 2024, 13, 207. [Google Scholar] [CrossRef]
- Sies, H.; Stahl, W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am. J. Clin. Nutr. 1995, 62, 1315S–1321S. [Google Scholar] [CrossRef]
- Aluko, R. Bioactive Polyphenols and Carotenoids. In Functional Foods and Nutraceuticals; Aluko, R.E., Ed.; Springer: New York, NY, USA, 2012; pp. 63–86. [Google Scholar] [CrossRef]
- Niu, L.; He, X.-h.; Wang, Q.-w.; Fu, M.-y.; Xu, F.; Xue, Y.; Wang, Z.-z.; An, X.-j. Polyphenols in Regulation of Redox Signaling and Inflammation During Cardiovascular Diseases. Cell Biochem. Biophys. 2015, 72, 485–494. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Effects of Citrus Fruit Juices and Their Bioactive Components on Inflammation and Immunity: A Narrative Review. Front. Immunol. 2021, 12, 712608. [Google Scholar] [CrossRef]
- Böhm, V. Vitamin E. Antioxidants 2018, 7, 44. [Google Scholar] [CrossRef]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef]
- Jiang, Y.; Ye, J.; Hu, Y.; Zhang, J.; Li, W.; Zhou, X.; Yu, M.; Yu, Y.; Yang, J.; Yang, W.; et al. Extraction and Synthesis of Typical Carotenoids: Lycopene, β-Carotene, and Astaxanthin. Molecules 2024, 29, 4549. [Google Scholar] [CrossRef]
- Shin, J.; Song, M.-H.; Oh, J.-W.; Keum, Y.-S.; Saini, R.K. Pro-oxidant Actions of Carotenoids in Triggering Apoptosis of Cancer Cells: A Review of Emerging Evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2022, 44, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, G.; Mukherjee, U.; Sonawane, A. Peroxisomes and Oxidative Stress: Their Implications in the Modulation of Cellular Immunity During Mycobacterial Infection. Front. Microbiol. 2019, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bai, H. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging. Antioxidants 2022, 11, 192. [Google Scholar] [CrossRef]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress Through Diet and Nutrition: Considerations During the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D.; Woodman, R.J.; Puddey, I.B.; Bondonno, C.P.; Wu, J.H.Y.; Beilin, L.J.; Lukoshkova, E.V.; Head, G.A.; Ward, N.C. Effects of vitamin E, vitamin C and polyphenols on the rate of blood pressure variation: Results of two randomised controlled trials. Br. J. Nutr. 2014, 112, 1551–1561. [Google Scholar] [CrossRef]
- Ma, Q.; Kinneer, K.; Ye, J.; Chen, B.J. Inhibition of Nuclear Factor κB by Phenolic Antioxidants: Interplay Between Antioxidant Signaling and Inflammatory Cytokine Expression. Mol. Pharmacol. 2003, 64, 211–219. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Park, K.-G.; Kim, Y.-N.; Kwon, T.-K.; Park, J.-Y.; Lee, K.-U.; Kim, J.-G.; Lee, I.-K. α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp. Mol. Med. 2007, 39, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Trzyna, W.C.; McClintock, D.S.; Schumacker, P.T. Role of Oxidants in NF-κB Activation and TNF-α Gene Transcription Induced by Hypoxia and Endotoxin1. J. Immunol. 2000, 165, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, H.; Xia, N. The Interplay Between Adipose Tissue and Vasculature: Role of Oxidative Stress in Obesity. Front. Cardiovasc. Med. 2021, 8, 650214. [Google Scholar] [CrossRef]
- Sharebiani, H.; Keramat, S.; Chavoshan, A.; Fazeli, B.; Stanek, A. The Influence of Antioxidants on Oxidative Stress-Induced Vascular Aging in Obesity. Antioxidants 2023, 12, 1295. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Cimmino, F.; Trinchese, G.; Catapano, A.; Petrella, L.; D’Angelo, M.; Lucchin, L.; Mollica, M.P. From Obesity-Induced Low-Grade Inflammation to Lipotoxicity and Mitochondrial Dysfunction: Altered Multi-Crosstalk Between Adipose Tissue and Metabolically Active Organs. Antioxidants 2023, 12, 1172. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Dahik, V.D.; Frisdal, E.; Le Goff, W. Rewiring of Lipid Metabolism in Adipose Tissue Macrophages in Obesity: Impact on Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 5505. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Liu, G.-S.; Chan, E.C.; Higuchi, M.; Dusting, G.J.; Jiang, F. Redox Mechanisms in Regulation of Adipocyte Differentiation: Beyond a General Stress Response. Cells 2012, 1, 976–993. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Meccariello, R.; D’Angelo, S. Impact of Polyphenolic-Food on Longevity: An Elixir of Life. An Overview. Antioxidants 2021, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism—A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, D.; Zhao, W.; Xu, L. Deciphering the Roles of PPARγ in Adipocytes via Dynamic Change of Transcription Complex. Front. Endocrinol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef] [PubMed]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and Metabolic Disorders Associated with Challenged Adipose Tissue Plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef]
- Payne, A.; Nahashon, S.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules 2022, 12, 371. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef]
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef]
- Maumus, M.; Sengenès, C.; Decaunes, P.; Zakaroff-Girard, A.; Bourlier, V.; Lafontan, M.; Galitzky, J.; Bouloumié, A. Evidence of in Situ Proliferation of Adult Adipose Tissue-Derived Progenitor Cells: Influence of Fat Mass Microenvironment and Growth. J. Clin. Endocrinol. Metab. 2008, 93, 4098–4106. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2020, 10, 1638. [Google Scholar] [CrossRef] [PubMed]
- Kilicarslan You, D.; Fuwad, A.; Lee, K.H.; Kim, H.K.; Kang, L.; Kim, S.M.; Jeon, T.-J. Evaluation of the Protective Role of Vitamin E against ROS-Driven Lipid Oxidation in Model Cell Membranes. Antioxidants 2024, 13, 1135. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Di Chiara Stanca, B.; Giannotti, L.; Gnoni, G.V.; Siculella, L.; Damiano, F. Quercetin Reduces Lipid Accumulation in a Cell Model of NAFLD by Inhibiting De Novo Fatty Acid Synthesis through the Acetyl-CoA Carboxylase 1/AMPK/PP2A Axis. Int. J. Mol. Sci. 2022, 23, 1044. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, K.; Minami, Y.; Harashima, H. Cytoprotective role of the fatty acid binding protein 4 against oxidative and endoplasmic reticulum stress in 3T3-L1 adipocytes. FEBS Open Bio 2014, 4, 602–610. [Google Scholar] [CrossRef]
- Liu, S.; Wu, D.; Fan, Z.; Yang, J.; Li, Y.; Meng, Y.; Gao, C.; Zhan, H. FABP4 in obesity-associated carcinogenesis: Novel insights into mechanisms and therapeutic implications. Front. Mol. Biosci. 2022, 9, 973955. [Google Scholar] [CrossRef]
- Steen, K.A.; Xu, H.; Bernlohr, D.A. FABP4/aP2 Regulates Macrophage Redox Signaling and Inflammasome Activation via Control of UCP2. Mol. Cell. Biol. 2017, 37, e00282-16. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- da Cruz Nascimento, S.S.; Carvalho de Queiroz, J.L.; Fernandes de Medeiros, A.; de França Nunes, A.C.; Piuvezam, G.; Lima Maciel, B.L.; Souza Passos, T.; Morais, A.H.d.A. Anti-inflammatory agents as modulators of the inflammation in adipose tissue: A systematic review. PLoS ONE 2022, 17, e0273942. [Google Scholar] [CrossRef]
- Bisht, K.; Wagner, K.-H.; Bulmer, A.C. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology 2010, 278, 88–100. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef] [PubMed]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Łuszczki, E.; Boakye, F.; Zielińska, M.; Dereń, K.; Bartosiewicz, A.; Oleksy, Ł.; Stolarczyk, A. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front. Nutr. 2023, 10, 1294497. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Das, S.; Chatterjee, I.; Roy, S.; Chakraborty, R. Anti-inflammatory Effects of Different Dietary Antioxidants. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 573–597. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, L.; Li, X.; Wang, X.; Ji, G.; Sun, C.; Liu, Z. Bmp8a deletion leads to obesity through regulation of lipid metabolism and adipocyte differentiation. Commun. Biol. 2023, 6, 824. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.; Chen, Y.; Luo, D.; Zhang, Z.; Cao, W.; Zhou, Z.; Lin, X.; Sun, C. Mark4 promotes oxidative stress and inflammation via binding to PPARγ and activating NF-κB pathway in mice adipocytes. Sci. Rep. 2016, 6, 21382. [Google Scholar] [CrossRef]
- Simao, J.d.J.; Bispo, A.F.d.S.; Plata, V.T.G.; Abel, A.B.M.; Saran, R.J.; Barcella, J.F.; Alonso, J.C.C.; Santana, A.V.; Armelin-Correa, L.M.; Alonso-Vale, M.I.C. The Activation of the NF-κB Pathway in Human Adipose-Derived Stem Cells Alters the Deposition of Epigenetic Marks on H3K27 and Is Modulated by Fish Oil. Life 2024, 14, 1653. [Google Scholar] [CrossRef]
- Shin, H.-S.; Kang, S.-I.; Park, D.-b.; Kim, S.-J. Resveratrol suppresses inflammatory responses and improves glucose uptake in adipocytes interacted with macrophages. Genes Genom. 2016, 38, 137–143. [Google Scholar] [CrossRef]
- Terzo, M.; Iantomasi, M.; Tsiani, E. Effects of Resveratrol on Adipocytes: Evidence from In Vitro and In Vivo Studies. Molecules 2024, 29, 5359. [Google Scholar] [CrossRef]
- Alcala, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Ramos, M.P.; Viana, M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS ONE 2017, 12, e0186579. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Marques, S.; Muller, A.P.; Luciano, T.F.; dos Santos Tramontin, N.; da Silva Caetano, M.; Luis da Silva Pieri, B.; Amorim, T.L.; de Oliveira, M.A.L.; de Souza, C.T. Effects of Avocado Oil Supplementation on Insulin Sensitivity, Cognition, and Inflammatory and Oxidative Stress Markers in Different Tissues of Diet-Induced Obese Mice. Nutrients 2022, 14, 2906. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Shiva, N.; Azizi, F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr. Metab. 2012, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Hyży, A.; Rozenek, H.; Gondek, E.; Jaworski, M. Effect of Antioxidants on the Gut Microbiome Profile and Brain Functions: A Review of Randomized Controlled Trial Studies. Foods 2025, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—An overview and perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Arif, Z.; Kabir, A.; Mehmood, I.; Munir, D.; Razzaq, A.; Ali, A.; Goksen, G.; Coşier, V.; Ahmad, N.; et al. Oxidative stress and metabolic diseases: Relevance and therapeutic strategies. Front. Nutr. 2022, 9, 994309. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R. Oxidative Stress and Antioxidant Strategies: Relationships and Cellular Pathways for Human Health. Cells 2024, 13, 1871. [Google Scholar] [CrossRef]
- Picklo, M.J.; Long, E.K.; Vomhof-DeKrey, E.E. Glutathionyl systems and metabolic dysfunction in obesity. Nutr. Rev. 2015, 73, 858–868. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef]
- Cui, R.; Gao, M.; Qu, S.; Liu, D. Overexpression of superoxide dismutase 3 gene blocks high-fat diet-induced obesity, fatty liver and insulin resistance. Gene Ther. 2014, 21, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Lismont, C.; Revenco, I.; Fransen, M. Peroxisomal Hydrogen Peroxide Metabolism and Signaling in Health and Disease. Int. J. Mol. Sci. 2019, 20, 3673. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, A.Y.; Choi, J.W.; Kim, M.; Yasue, S.; Son, H.J.; Masuzaki, H.; Park, K.S.; Kim, J.B. Dysregulation of Adipose Glutathione Peroxidase 3 in Obesity Contributes to Local and Systemic Oxidative Stress. Mol. Endocrinol. 2008, 22, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.; Tümen, D.; Kunst, C.; Heumann, P.; Schmid, S.; Kandulski, A.; Müller, M.; Gülow, K. Exploring the Thioredoxin System as a Therapeutic Target in Cancer: Mechanisms and Implications. Antioxidants 2024, 13, 1078. [Google Scholar] [CrossRef]
- Anjou, C.; Lotoux, A.; Zhukova, A.; Royer, M.; Caulat, L.C.; Capuzzo, E.; Morvan, C.; Martin-Verstraete, I. The multiplicity of thioredoxin systems meets the specific lifestyles of Clostridia. PLoS Pathog. 2024, 20, e1012001. [Google Scholar] [CrossRef]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef]
- Vavlukis, M.; Vavlukis, A.; Krsteva, K.; Topuzovska, S. Paraoxonase 1 gene polymorphisms in lipid oxidation and atherosclerosis development. Front. Genet. 2022, 13, 966413. [Google Scholar] [CrossRef]
- Jakubowski, H. The Molecular Bases of Anti-Oxidative and Anti-Inflammatory Properties of Paraoxonase 1. Antioxidants 2024, 13, 1292. [Google Scholar] [CrossRef]
- Puri, A.; Mohite, P.; Ansari, Y.; Mukerjee, N.; Alharbi, H.M.; Upaganlawar, A.; Thorat, N. Plant-derived selenium nanoparticles: Investigating unique morphologies, enhancing therapeutic uses, and leading the way in tailored medical treatments. Mater. Adv. 2024, 5, 3602–3628. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Ota, T. Glucoraphanin: A broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 2018, 7, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Khutami, C.; Sumiwi, S.A.; Khairul Ikram, N.K.; Muchtaridi, M. The Effects of Antioxidants from Natural Products on Obesity, Dyslipidemia, Diabetes and Their Molecular Signaling Mechanism. Int. J. Mol. Sci. 2022, 23, 2056. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y.-S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. 3 Biotech 2016, 6, 109. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Celi, P.; Ding, X.; Bai, S.; Zeng, Q.; Mao, X.; Xu, S.; Zhang, K. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. 2020, 11, 534–543. [Google Scholar] [CrossRef]
- Ravindranath, N.H.; Ravindranath, M.H. Green tea catechins suppress NF-κB-mediated inflammatory responses: Relevance to nutritional management of inflammation. Br. J. Nutr. 2011, 105, 1715–1717. [Google Scholar] [CrossRef]
- Olcha, P.; Winiarska-Mieczan, A.; Kwiecień, M.; Nowakowski, Ł.; Miturski, A.; Semczuk, A.; Kiczorowska, B.; Gałczyński, K. Antioxidative, Anti-Inflammatory, Anti-Obesogenic, and Antidiabetic Properties of Tea Polyphenols—The Positive Impact of Regular Tea Consumption as an Element of Prophylaxis and Pharmacotherapy Support in Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 6703. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wu, G.; Chen, G.; Meng, X.; Xie, Z.; Cai, S. Polyphenols alleviate metabolic disorders: The role of ubiquitin-proteasome system. Front. Nutr. 2024, 11, 1445080. [Google Scholar] [CrossRef] [PubMed]
- Abou-Rjeileh, U.; Contreras, G.A. Redox Regulation of Lipid Mobilization in Adipose Tissues. Antioxidants 2021, 10, 1090. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, G.; Raulf, A.; Gunkel, M.; Fleischmann, B.K.; Lemor, R.; Hausmann, M. Tissue-Mimicking Geometrical Constraints Stimulate Tissue-Like Constitution and Activity of Mouse Neonatal and Human-Induced Pluripotent Stem Cell-Derived Cardiac Myocytes. J. Funct. Biomater. 2016, 7, 1. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.-A.; Desjardins, Y.; Roy, D.; et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Townsend, J.R.; Kirby, T.O.; Sapp, P.A.; Gonzalez, A.M.; Marshall, T.M.; Esposito, R. Nutrient synergy: Definition, evidence, and future directions. Front. Nutr. 2023, 10, 1279925. [Google Scholar] [CrossRef]
- Illam, S.P.; Narayanankutty, A.; Raghavamenon, A.C. Polyphenols of virgin coconut oil prevent pro-oxidant mediated cell death. Toxicol. Mech. Methods 2017, 27, 442–450. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef]
- Lu, S.; Cao, Z.-B. Interplay between Vitamin D and Adipose Tissue: Implications for Adipogenesis and Adipose Tissue Function. Nutrients 2023, 15, 4832. [Google Scholar] [CrossRef]
- An, S.-M.; Cho, S.-H.; Yoon, J.C. Adipose Tissue and Metabolic Health. Diabetes Metab. J. 2023, 47, 595–611. [Google Scholar] [CrossRef]
- Scarpa, E.-S.; Antonelli, A.; Balercia, G.; Sabatelli, S.; Maggi, F.; Caprioli, G.; Giacchetti, G.; Micucci, M. Antioxidant, Anti-Inflammatory, Anti-Diabetic, and Pro-Osteogenic Activities of Polyphenols for the Treatment of Two Different Chronic Diseases: Type 2 Diabetes Mellitus and Osteoporosis. Biomolecules 2024, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Zhuang, C.; Yuan, J.; Du, Y.; Zeng, J.; Sun, Y.; Wu, Y.; Gao, X.-H.; Chen, H.-D. Effects of Oral Carotenoids on Oxidative Stress: A Systematic Review and Meta-Analysis of Studies in the Recent 20 Years. Front. Nutr. 2022, 9, 754707. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-S.; Xia, Y.-X.; Chen, B.-S.; Du, Y.-X.; Liu, K.-L.; Zhang, H.-J. Dihydro-Resveratrol Attenuates Oxidative Stress, Adipogenesis and Insulin Resistance in In Vitro Models and High-Fat Diet-Induced Mouse Model via AMPK Activation. Nutrients 2023, 15, 3006. [Google Scholar] [CrossRef]
- Shao, W.; Yu, Z.; Chiang, Y.; Yang, Y.; Chai, T.; Foltz, W.; Lu, H.; Fantus, I.G.; Jin, T. Curcumin Prevents High Fat Diet Induced Insulin Resistance and Obesity via Attenuating Lipogenesis in Liver and Inflammatory Pathway in Adipocytes. PLoS ONE 2012, 7, e28784. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Silenzi, A.; Giovannini, C.; Masella, R. Obesity-Associated Inflammation: Does Curcumin Exert a Beneficial Role? Nutrients 2021, 13, 1021. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; He, J.; Zhao, Y. Quercetin Regulates Lipid Metabolism and Fat Accumulation by Regulating Inflammatory Responses and Glycometabolism Pathways: A Review. Nutrients 2024, 16, 1102. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Dhande, D.; Dhok, A.; Anjankar, A.; Nagpure, S. Silymarin as an Antioxidant Therapy in Chronic Liver Diseases: A Comprehensive Review. Cureus 2024, 16, e67083. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a Natural Antioxidant: An Overview of the Current Evidence and Perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B. Bioactive Compounds as Inhibitors of Inflammation, Oxidative Stress and Metabolic Dysfunctions via Regulation of Cellular Redox Balance and Histone Acetylation State. Foods 2023, 12, 925. [Google Scholar] [CrossRef]
- Toscano Oviedo, M.A.; García Zapateiro, L.A.; Quintana, S.E. Tropical fruits as a potential source for the recovery of bioactive compounds: Tamarindus indica L., Annona muricata, Psidium guajava and Mangifera indica. J. Food Sci. Technol. 2024, 61, 2027–2035. [Google Scholar] [CrossRef]
- Kuddus, S.A.; Bhuiyan, M.I.; Subhan, N.; Shohag, M.H.; Rahman, A.; Hossain, M.M.; Alam, M.A.; Khan, F. Antioxidant-rich Tamarindus indica L. leaf extract reduced high-fat diet-induced obesity in rat through modulation of gene expression. Clin. Phytosci. 2020, 6, 68. [Google Scholar] [CrossRef]
- Barbosa dos Santos, J.A.; Assis, C.F.; Soares Aragao, C.F.; dos Santos Lima, M.; Passos, T.S.; da Silva-Maia, J.K. Nanoparticles based on biopolymers improved antioxidant activity of phenolic compounds from jambolan (Syzygium cumini (L.) skeels). Heliyon 2024, 10, e36973. [Google Scholar] [CrossRef]
- Das, G.; Nath, R.; Das Talukdar, A.; Ağagündüz, D.; Yilmaz, B.; Capasso, R.; Shin, H.-S.; Patra, J.K. Major Bioactive Compounds from Java Plum Seeds: An Investigation of Its Extraction Procedures and Clinical Effects. Plants 2023, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Sabiu, S. Adipose tissue inflammation linked to obesity: A review of current understanding, therapies and relevance of phyto-therapeutics. Heliyon 2024, 10, e23114. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, Y.; Chen, F.; Zhou, B. Epigenetics in obesity: Mechanisms and advances in therapies based on natural products. Pharmacol. Res. Perspect. 2024, 12, e1171. [Google Scholar] [CrossRef] [PubMed]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary Polyphenols and Obesity: A Review of Polyphenol Effects on Lipid and Glucose Metabolism, Mitochondrial Homeostasis, and Starch Digestibility and Absorption. Plant Foods Hum. Nutr. 2023, 78, 1–12. [Google Scholar] [CrossRef]
- Dama, A.; Shpati, K.; Daliu, P.; Dumur, S.; Gorica, E.; Santini, A. Targeting Metabolic Diseases: The Role of Nutraceuticals in Modulating Oxidative Stress and Inflammation. Nutrients 2024, 16, 507. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef]
- Vetrani, C.; Verde, L.; Colao, A.; Barrea, L.; Muscogiuri, G. The Mediterranean Diet: Effects on Insulin Resistance and Secretion in Individuals with Overweight or Obesity. Nutrients 2023, 15, 4524. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G. Polyphenols: A Promising Nutritional Approach to Prevent or Reduce the Progression of Prehypertension. High Blood Press. Cardiovasc. Prev. 2016, 23, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Zielińska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczyński, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. [Google Scholar] [CrossRef]
- Tanti, J.-F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2013, 3, 41442. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Sauer, H.; Wartenberg, M.; Hescheler, J. Reactive Oxygen Species as Intracellular Messengers During Cell Growth and Differentiation. Cell. Physiol. Biochem. 2001, 11, 173–186. [Google Scholar] [CrossRef]
- Kwon, G.; Gibson, K.M.; Bi, L. Editorial Commentary on the Special Issue “Antioxidant Therapy for Cardiovascular Diseases”—Cutting-Edge Insights into Oxidative Stress and Antioxidant Therapy in Cardiovascular Health. Antioxidants 2024, 13, 1034. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations Through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Le Magueresse-Battistoni, B. Adipose Tissue and Endocrine-Disrupting Chemicals: Does Sex Matter? Int. J. Environ. Res. Public Health 2020, 17, 9403. [Google Scholar] [CrossRef] [PubMed]
- Gavin, K.M.; Bessesen, D.H. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. N. Am. 2020, 49, 215–228. [Google Scholar] [CrossRef]
- Ramirez, M.F.; Pan, A.S.; Parekh, J.K.; Owunna, N.; Courchesne, P.; Larson, M.G.; Levy, D.; Murabito, J.M.; Ho, J.E.; Lau, E.S. Sex Differences in Protein Biomarkers and Measures of Fat Distribution. J. Am. Heart Assoc. 2024, 13, e000223. [Google Scholar] [CrossRef]
- Kamal, F.Z.; Lefter, R.; Jaber, H.; Balmus, I.-M.; Ciobica, A.; Iordache, A.-C. The Role of Potential Oxidative Biomarkers in the Prognosis of Acute Ischemic Stroke and the Exploration of Antioxidants as Possible Preventive and Treatment Options. Int. J. Mol. Sci. 2023, 24, 6389. [Google Scholar] [CrossRef]
- Shah, D.; Mahajan, N.; Sah, S.; Nath, S.K.; Paudyal, B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J. Biomed. Sci. 2014, 21, 23. [Google Scholar] [CrossRef]
- Zare, M.; Shateri, Z.; Nouri, M.; Sarbakhsh, P.; Eftekhari, M.H.; Pourghassem Gargari, B. Association between urinary levels of 8-hydroxy-2-deoxyguanosine and F2a-isoprostane in male football players and healthy non-athlete controls with dietary inflammatory and antioxidant indices. Front. Nutr. 2023, 9, 1101532. [Google Scholar] [CrossRef]
- Hashemi Sheikhshabani, S.; Ghafouri-Fard, S.; Amini-Farsani, Z.; Modarres, P.; Khazaei Feyzabad, S.; Amini-Farsani, Z.; Shaygan, N.; Omrani, M.D. In Silico Prediction of Functional SNPs Interrupting Antioxidant Defense Genes in Relation to COVID-19 Progression. Biochem. Genet. 2024. [Google Scholar] [CrossRef]
- Krishnamurthy, H.K.; Rajavelu, I.; Pereira, M.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Inside the genome: Understanding genetic influences on oxidative stress. Front. Genet. 2024, 15, 1397352. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, R. Oxidative Stress and Human Genetic Variation. J. Nutr. 2004, 134, 3173S–3174S. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Meulmeester, F.L.; Luo, J.; Martens, L.G.; Mills, K.; van Heemst, D.; Noordam, R. Antioxidant Supplementation in Oxidative Stress-Related Diseases: What Have We Learned from Studies on Alpha-Tocopherol? Antioxidants 2022, 11, 2322. [Google Scholar] [CrossRef]
- Vassalle, C.; Maltinti, M.; Sabatino, L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules 2020, 25, 2653. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Cardoso, R.V.; Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes. Pharmaceutics 2022, 14, 2808. [Google Scholar] [CrossRef]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative Delivery Systems for Curcumin: Exploring Nanosized and Conventional Formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J. Natural Antioxidants in Obesity and Related Diseases. Antioxidants 2023, 12, 1966. [Google Scholar] [CrossRef]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic Efficacy of Antioxidants in Ameliorating Obesity Phenotype and Associated Comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Kršková, K.; Dobrócsyová, V.; Ferenczyová, K.; Hricovíniová, J.; Kaločayová, B.; Duľová, U.; Bozorgnia, M.; Barteková, M.; Zorad, Š. Modification of adipogenesis and oxidative stress by quercetin: Positive or negative impact on adipose tissue metabolism of obese diabetic Zucker rats? J. Physiol. Biochem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; De Lise, F.; Di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front. Pharmacol. 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).