Polyphenol Powders from Ginkgo biloba L. and Clitoria ternatea L.: Influence of Drying Techniques and Carriers on Antioxidant Capacity and Polyphenol Release Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials
2.3. Extraction
2.4. Drying
2.5. Physical Characterization of the Powders
2.5.1. Moisture Content (Mc)
2.5.2. Water Activity (aw)
2.5.3. Color Parameters, Browning Index (BI), and Yellowness Index (YI)
2.5.4. Particle Size Distribution
2.6. Chemical Characterization
2.6.1. Qualitative and Quantitative Evaluation of Polyphenols in Ginkgo biloba L. and Clitoria ternatea L. powders via HPLC-MS/MS
2.6.2. Reducing Potential
2.6.3. In Vitro Antioxidant Capacity
2.7. Encapsulation and In Vitro Release Profile of Polyphenols from Gelatin Capsules
2.8. Statistical Analysis
3. Results
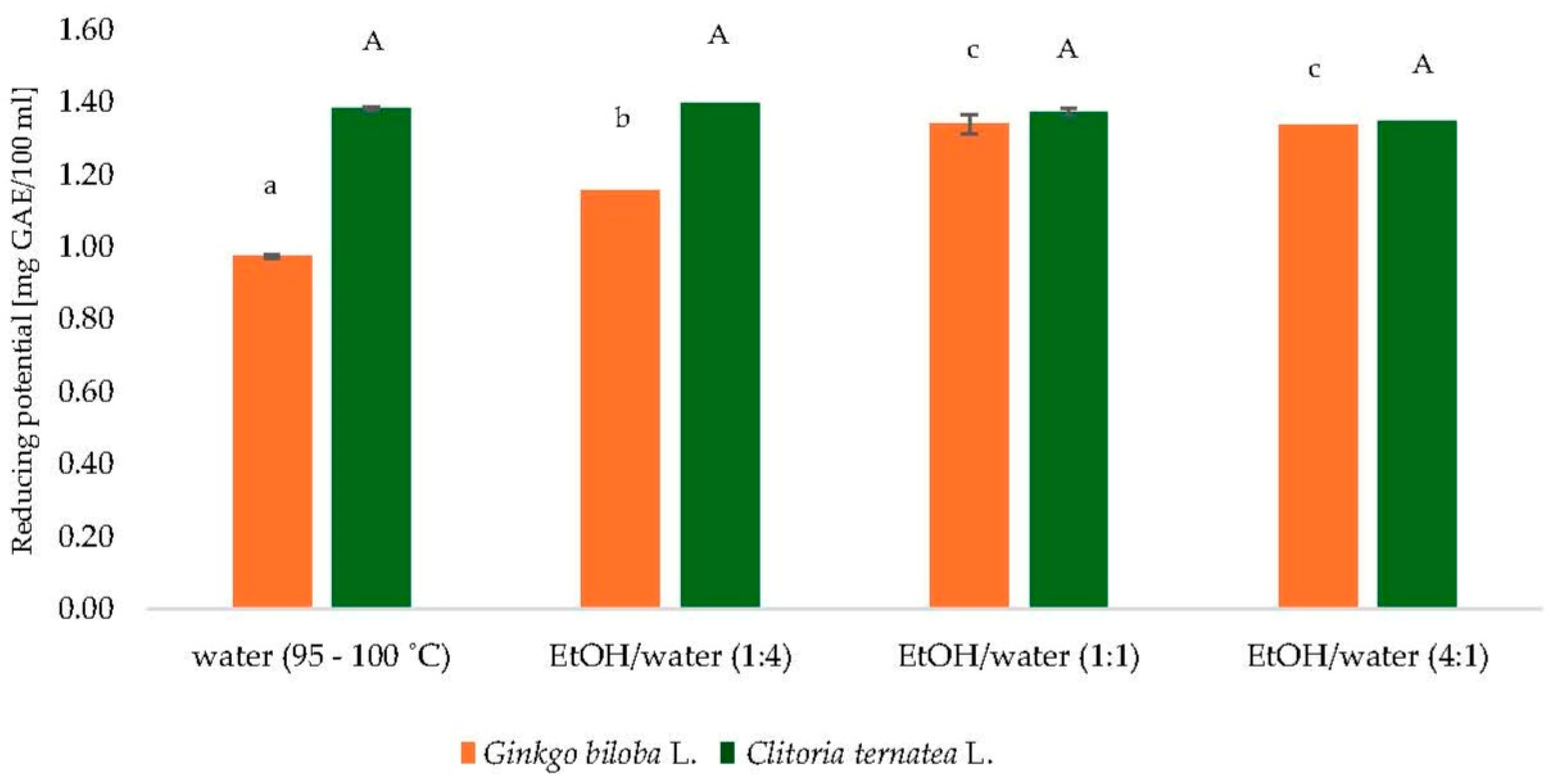
3.1. Extraction of Ginkgo biloba L. and Clitoria ternatea L.
3.2. Physical Properties of Powders
3.2.1. Moisture Content
3.2.2. Water Activity
3.2.3. Color, Browning Index (BI), Yellowness Index (YI)
3.3. Chemical Properties
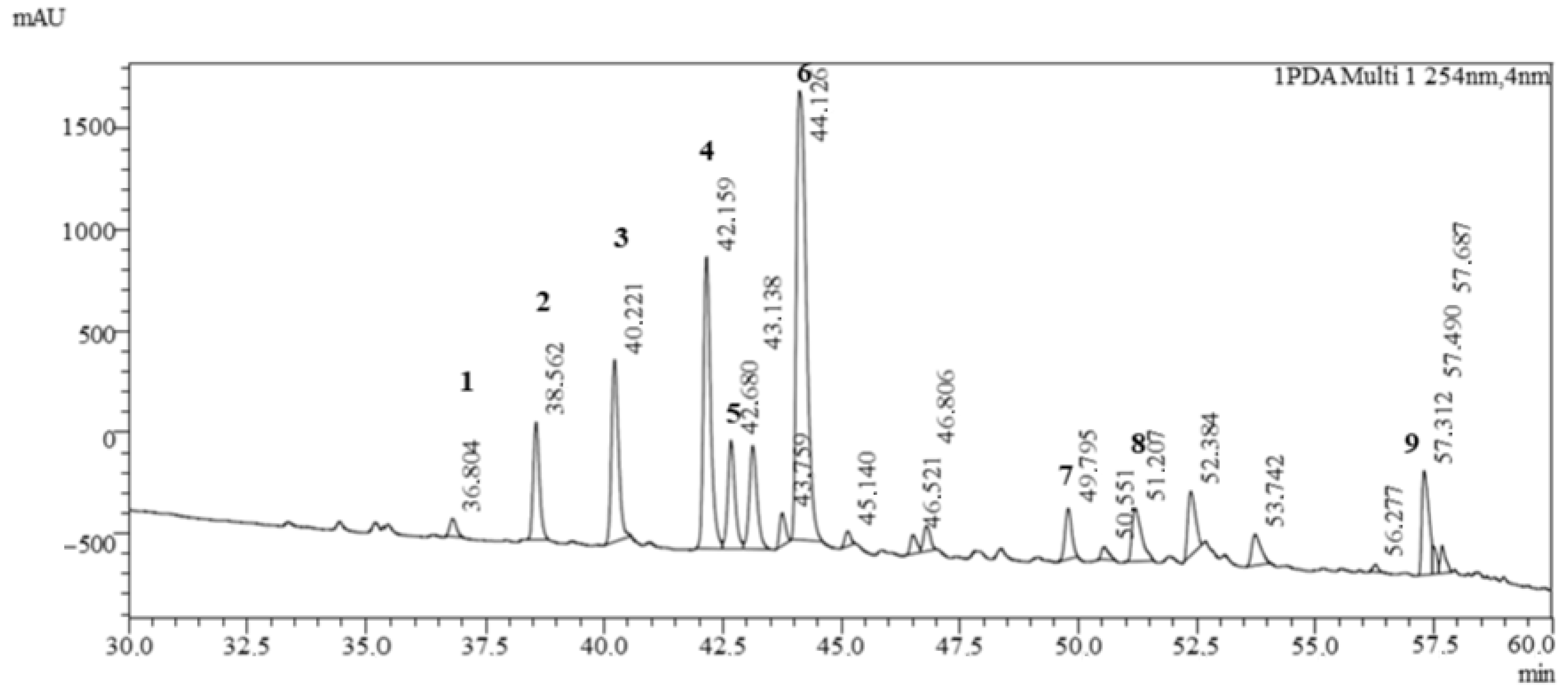
3.3.1. Qualitative Determination of Polyphenols in Clitoria ternatea L. and Ginkgo biloba L. Powders
3.3.2. Quantitative Determination of Polyphenols in Clitoria ternatea L. and Ginkgo biloba L. Powders
3.3.3. Reducing Potential
3.3.4. Antioxidant Capacity In Vitro
3.4. Evaluation of Functional Properties of the Selected 1:1 Blend of Ginkgo biloba L.:Clitoria ternatea L. Spray Drying with Inulin at Pilot Scale
3.4.1. Water Activity, Reducing Potential and Antioxidant Capacity
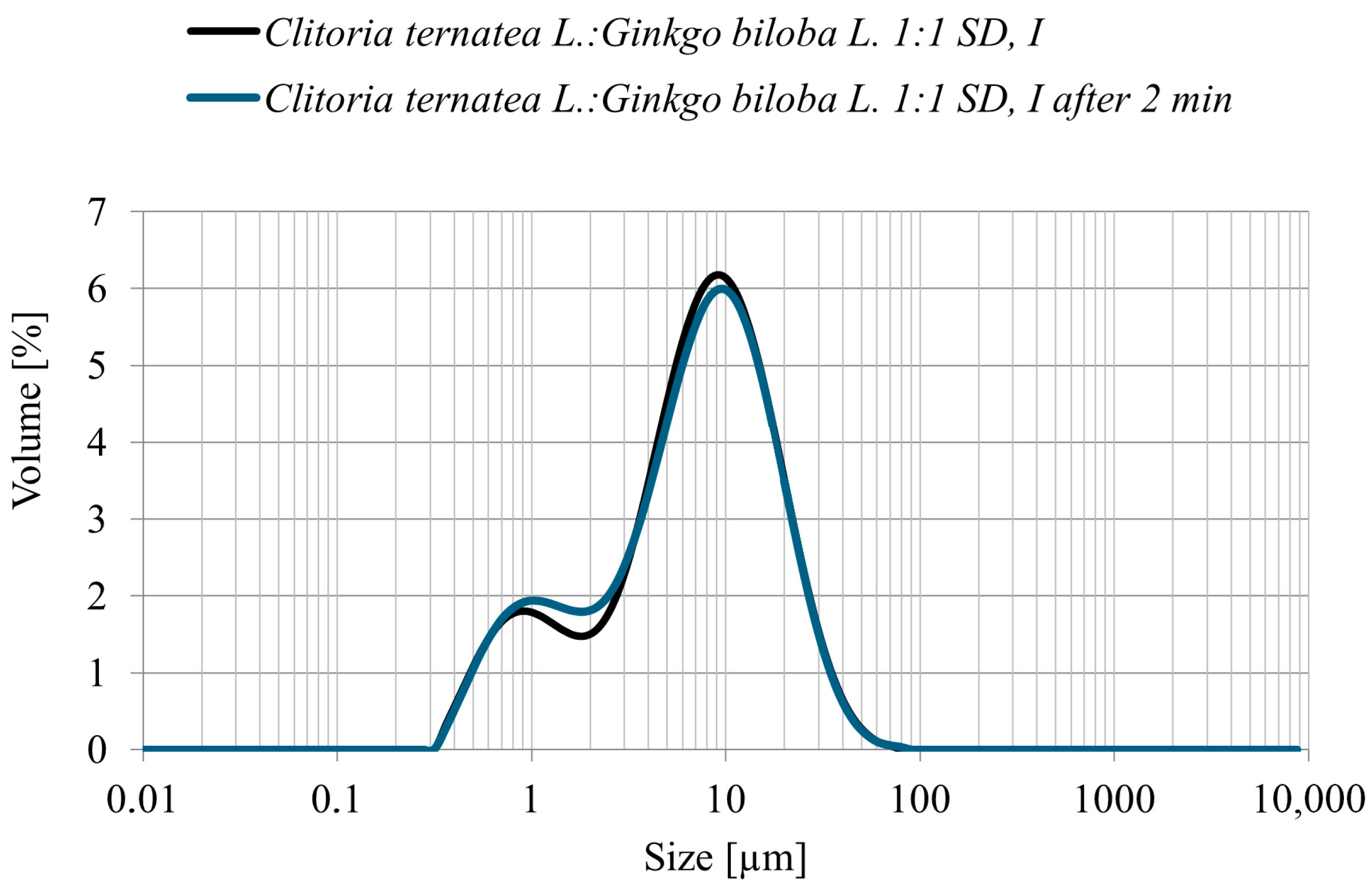
3.4.2. Particle Size Distribution
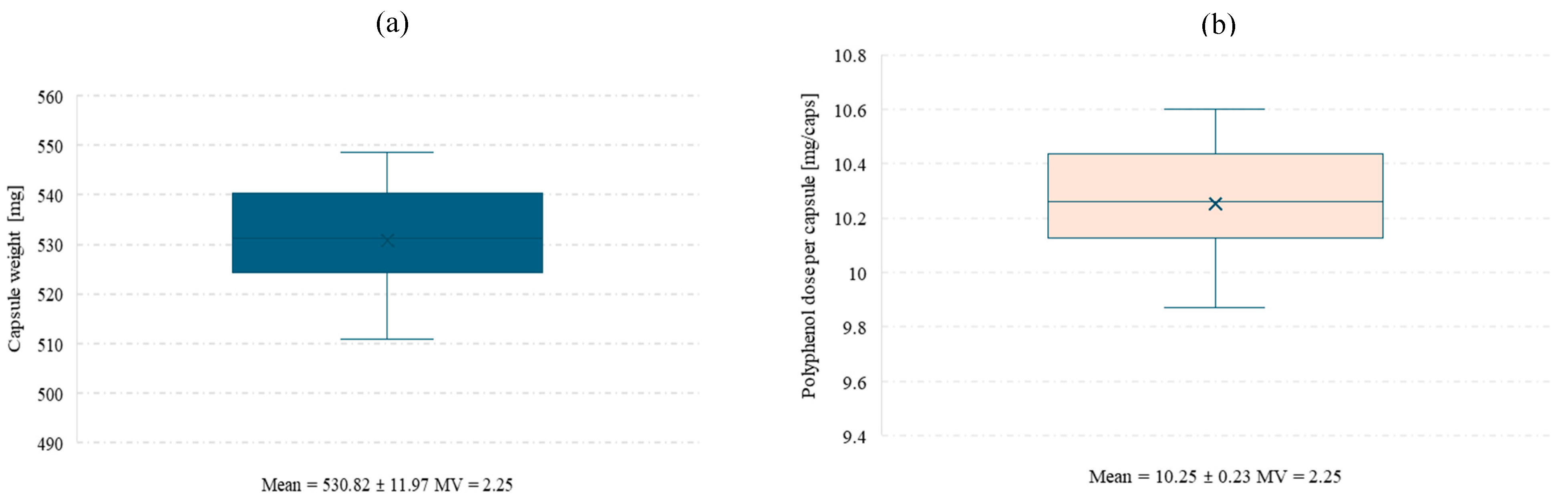
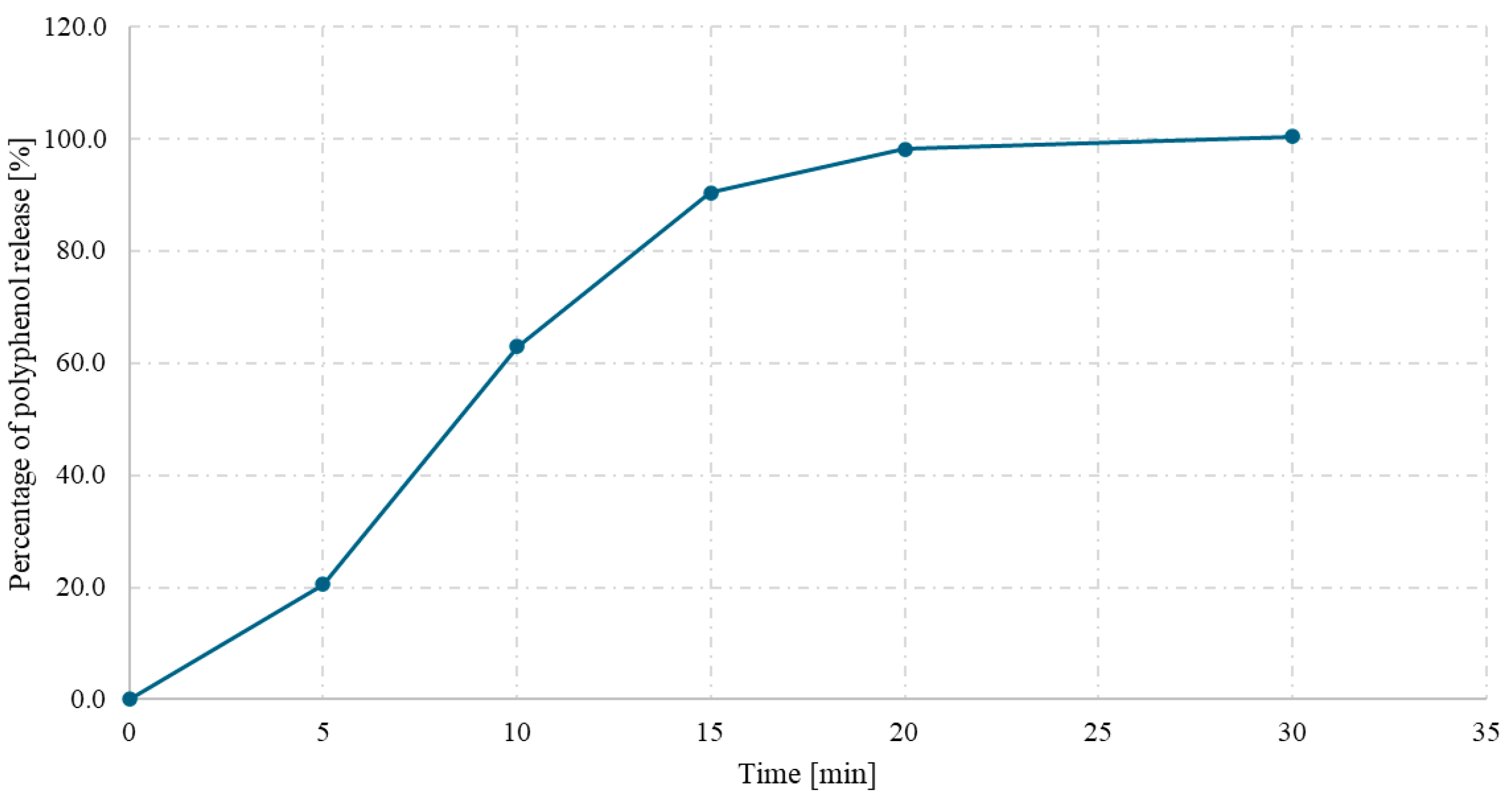
3.4.3. Testing the Uniformity of Capsule Contents and the Release of Polyphenols from Capsules
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, W.; Wei, H.; He, B. Dietary Flavonoids Intake and the Risk of Coronary Heart Disease: A Dose-Response Meta-Analysis of 15 Prospective Studies. Thromb. Res. 2015, 135, 459–463. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Krieglstein, J. Neuroprotective Effects of Ginkgo biloba Extract. CMLS Cell. Mol. Life Sci. 2003, 60, 1779–1792. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y. Multifaceted Therapeutic Benefits of Ginkgo biloba L.: Chemistry, Efficacy, Safety, and Uses. J. Food Sci. 2008, 73, R14–R19. [Google Scholar] [CrossRef]
- Tang, D.; Yang, D.; Tang, A.; Gao, Y.; Jiang, X.; Mou, J.; Yin, X. Simultaneous Chemical Fingerprint and Quantitative Analysis of Ginkgo biloba Extract by HPLC–DAD. Anal. Bioanal. Chem. 2010, 396, 3087–3095. [Google Scholar] [CrossRef]
- Oliveira, D.; Latimer, C.; Parpot, P.; Gill, C.I.R.; Oliveira, R. Antioxidant and Antigenotoxic Activities of Ginkgo biloba L. Leaf Extract Are Retained after in Vitro Gastrointestinal Digestive Conditions. Eur. J. Nutr. 2020, 59, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Tomova, T.; Slavova, I.; Tomov, D.; Kirova, G.; Argirova, M.D. Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae 2021, 7, 218. [Google Scholar] [CrossRef]
- Gong, H.; Zhang, Y.Q.; Wang, T.; Wang, S.; Yu, N.N.; Wang, W.D.; Wu, Y.H.; Yuan, H. Physicochemical and Sensory Acceptance of Functional Beverages from Ginkgo biloba Seed Extracts. J. Food Meas. Charact. 2022, 16, 1787–1795. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant with Applications in Agriculture and Medicine. Front. Plant Sci. 2019, 10, 448370. [Google Scholar] [CrossRef]
- Siti Azima, A.M.; Noriham, A.; Manshoor, N. Phenolics, Antioxidants and Color Properties of Aqueous Pigmented Plant Extracts: Ardisia colorata var. elliptica, Clitoria ternatea, Garcinia mangostana and Syzygium cumini. J. Funct. Foods 2017, 38, 232–241. [Google Scholar] [CrossRef]
- Defallo, M.E.L.; Po, G.M. Composition of Muffin from Clitoria Ternatea. PH Patent PH22018000920Y1; filed 6 September 2018, 28 November 2018. [Google Scholar]
- Zainol, K.; MohdIsa, N.S.; Maidin, N.M. Chemical Characterization of Ethanolic Extract of Butterfly Pea Flower (Clitoria ternatea). Food Res. 2021, 5, 127–134. [Google Scholar] [CrossRef]
- Netravati; Gomez, S.; Pathrose, B.; Raj, M.N.; Joseph, M.P.; Kuruvila, B. Comparative Evaluation of Anthocyanin Pigment Yield and Its Attributes from Butterfly Pea (Clitorea ternatea L.) Flowers as Prospective Food Colorant Using Different Extraction Methods. Future Foods 2022, 6, 100199. [Google Scholar] [CrossRef]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated Flavonol Glycosides from the Petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Colon, M.; Nerín, C. Synergistic, Antagonistic and Additive Interactions of Green Tea Polyphenols. Eur. Food Res. Technol. 2016, 242, 211–220. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In Vitro Antioxidant Activities of Three Red Wine Polyphenols and Their Mixtures: An Interaction Study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Jurčević Šangut, I.; Pavličević, L.; Šamec, D. Influence of Air Drying, Freeze Drying and Oven Drying on the Biflavone Content in Yellow Ginkgo (Ginkgo biloba L.) Leaves. Appl. Sci. 2024, 14, 2330. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Šamec, D. Seasonal Variation of Polyphenols and Pigments in Ginkgo (Ginkgo biloba L.) Leaves: Focus on 3′,8″-Biflavones. Plants 2024, 13, 3044. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Anthocyanins From Clitoria ternatea Flower: Biosynthesis, Extraction, Stability, Antioxidant Activity, and Applications. Front. Plant Sci. 2021, 12, 792303. [Google Scholar] [CrossRef]
- Hariadi, H.; Amien, S.; Karuniawan, A.; Darniadi, S.; Histifarina, D.; Indriati, A.; Rahayu, S.T.; Adnan, A.; Yani, A.; Hidayat, H.; et al. Effect of Three Drying Methods on Physicochemical Properties of Powdered Butterfly Pea Flower Extract (Clitoria ternatea L.). Food Sci. Technol. 2024, 44, 2024. [Google Scholar] [CrossRef]
- Turchiuli, C.; Jimenez Munguia, M.T.; Hernandez Sanchez, M.; Cortes Ferre, H.; Dumoulin, E. Use of Different Supports for Oil Encapsulation in Powder by Spray Drying. Powder Technol. 2014, 255, 103–108. [Google Scholar] [CrossRef]
- Upadhyay, R.; Dass, J.F.P. Physicochemical Analysis, Microbial Survivability, and Shelf Life Study of Spray-dried Synbiotic Guava Juice Powder. J. Food Process. Preserv. 2021, 45, 1–8. [Google Scholar] [CrossRef]
- Figiel, A. Drying Kinetics and Quality of Beetroots Dehydrated by Combination of Convective and Vacuum-Microwave Methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Rhim, J.W.; Wu, Y.; Weller, C.L.; Schnepf, M. Physical Characteristics of a Composite Film of Soy Protein Isolate and Propyleneglycol Alginate. J. Food Sci. 1999, 64, 149–152. [Google Scholar] [CrossRef]
- Thuy, N.M.; Minh, V.Q.; Ben, T.C.; Ha, H.T.N.; Tai, N.V. Impact of Different Thin Layer Drying Temperatures on the Drying Time Andquality of Butterfly Pea Flowers. Food Res. 2021, 5, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Chen, P.; Ozcan, M.; Harnly, J.M. Chromatographic Profiles and Identification of New Phenolic Components of Ginkgo biloba Leaves and Selected Products. J. Agric. Food Chem. 2008, 56, 6671–6679. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Lin, Y.; Han, B.; Wang, B.; Wang, S. Qualitative and Quantitative Analysis of Phenolic Acid Glycosides in Ginkgo biloba L. Leaf, G. Biloba Leaf Extract and Its Injection. Biomed. Chromatogr. 2020, 34, e4964. [Google Scholar] [CrossRef]
- Michalska-Ciechanowska, A.; Hendrysiak, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A. How Do the Different Types of Carrier and Drying Techniques Affect the Changes in Physico-Chemical Properties of Powders from Chokeberry Pomace Extracts? Foods 2021, 10, 1864. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Horszwald, A.; Andlauer, W. Characterisation of Bioactive Compounds in Berry Juices by Traditional Photometric and Modern Microplate Methods. J. Berry Res. 2011, 1, 189–199. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A.; Montoro, P. Chemical Analysis and Quality Control of Ginkgo biloba Leaves, Extracts, and Phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi; Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of Particle Size on Phytochemical Composition and Antioxidant Properties of Sargassum cristaefolium Ethanol Extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In Vitro Bioaccessibility of Health-Related Compounds from a Blended Fruit Juice–Soymilk Beverage: Influence of the Food Matrix. J. Funct. Foods 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Tontul, I.; Topuz, A. Spray-Drying of Fruit and Vegetable Juices: Effect of Drying Conditions on the Product Yield and Physical Properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]

| Material | Drying Technique | Carrier Type | Moisture Content [%] | Water Activity [−] | Color | BI | YI | ||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||||
| Ginkgo biloba L. | FD | Maltodextrin | 4.88 ± 0.45 d | 0.1875 ± 0.0132 g–i | 72.99 ± 1.03 a | −0.43 ± 0.01 r | 13.89 ± 0.13 c | 19.93 ± 0.07 c | 27.19 ± 0.09 c |
| Inulin | 3.94 ± 0.01 e–g | 0.1968 ± 0.0049 gh | 73.45 ± 1.46 a | −0.15 ± 0.04 pr | 13.05 ± 0.34 c | 18.73 ± 0.20 c | 25.38 ± 0.18 cd | ||
| Control | 8.87 ± 0.43 a | 0.4197 ± 0.003 a | 39.05 ± 2.44 gh | 6.03 ± 0.25 a | 24.61 ± 1.20 a | 103.77 ± 7.10 a | 90.10 ± 4.19 a | ||
| SD | Maltodextrin | 2.59 ± 0.11 j–l | 0.0843 ± 0.0032 pr | 72.77 ± 0.28 a | −0.11 ± 0.03 p | 10.04 ± 0.08 d | 14.22 ± 0.09 c | 19.71 ± 0.10 d | |
| Inulin | 1.64 ± 0.09 mn | 0.0824 ± 0.0023 pr | 72.68 ± 0.32 a | 0.01 ± 0.01 p | 10.75 ± 0.03 d | 15.46 ± 0.01 c | 21.12 ± 0.03 cd | ||
| Control | 2.89 ± 0.11 h–k | 0.2102 ± 0.0019 fg | 46.94 ± 0.75 bc | 2.95 ± 0.27 f–h | 17.93 ± 0.32 b | 51.04 ± 1.18 b | 54.56 ± 0.77 b | ||
| Clitoria ternatea L. | FD | Maltodextrin | 4.47 ± 0.06 de | 0.2374 ± 0.005 e | 30.7 ± 1.06 jk | 2.59 ± 0.02 i–k | −13.14 ± 0.33 i–k | −28.28 ± 0.14 j–n | −61.16 ± 0.68 i–k |
| Inulin | 4.15 ± 0.38 d–f | 0.2303 ± 0.004 ef | 37.59 ± 1.56 g–i | 1.46 ± 0.06 n | −17.24 ± 0.53 rs | −33.04 ± 0.19 n–p | −65.54 ± 0.94 j–l | ||
| Control | 8.86 ± 0.06 a | 0.3933 ± 0.001 b | 6.52 ± 0.48 p | 2.30 ± 0.50 kl | −3.09 ± 0.92 e | −15.28 ± 5.01 de | −67.38 ± 7.19 kl | ||
| SD | Maltodextrin | 2.82 ± 0.1 i–k | 0.1226 ± 0.0048 mn | 43.99 ± 1.63 c–f | 3.83 ± 0.1 d | −14.68 ± 0.47 l–o | −21.70 ± 0.08 e–j | −47.68 ± 0.39 h | |
| Inulin | 1.62 ± 0.13 mn | 0.1045 ± 0.0079 n–p | 42.04 ± 0.59 d–g | 2.14 ± 0.03 lm | −17.36 ± 0.22 s | −29.36 ± 0.02 k–n | −58.99 ± 0.02 ij | ||
| Control | 2.28 ± 0.22 k–n | 0.1842 ± 0.0042 h–j | 23.88 ± 1.01 m | 4.43 ± 0.12 c | −16.51 ± 0.53 p–s | −38.04 ± 0.36 p | −98.83 ± 1.33 nm | ||
| Clitoria ternatea L.: Ginkgo biloba L. 1:1 | FD | Maltodextrin | 3.62 ± 0.18 f–h | 0.1163 ± 0.0032 m–o | 41.01 ± 0.81 e–g | 2.89 ± 0.01 g–i | −14.34 ± 0.21 k–n | −23.87 ± 0.01 f–k | −49.96 ± 0.06 h |
| Inulin | 3.39 ± 0.42 f–j | 0.1857 ± 0.0103 g–j | 34.86 ± 0.63 h–j | 2.01 ± 0.03 lm | −15.57 ± 0.18 n–p | −31.12 ± 0.10 l–o | −63.81 ± 0.27 i–k | ||
| Control | 7.09 ± 0.3 b | 0.3440 ± 0.001 c | 20.22 ± 1.00 mn | 3.23 ± 0.06 ef | −9.09 ± 0.37 f | −25.12 ± 0.46 g–m | −64.18 ± 0.24 i–k | ||
| SD | Maltodextrin | 2.39 ± 0.26 k–m | 0.1125 ± 0.0033 m–o | 48.28 ± 0.52 bc | 3.74 ± 0.04 d | −12.83 ± 0.31 g–j | −17.36 ± 0.04 d–f | −37.97 ± 0.05 fg | |
| Inulin | 1.99 ± 0.05 l–n | 0.1339 ± 0.0033 lm | 45.25 ± 2.92 c–e | 2.65 ± 0.12 ij | −15.92 ± 0.86 o–r | −24.77 ± 0.01 g–l | −50.27 ± 0.06 h | ||
| Control | 2.38 ± 0.11 k–m | 0.1619 ± 0.0069 jk | 28.82 ± 0.13 kl | 4.62 ± 0.06 bc | −14.32 ± 0.27 k–n | −28.13 ± 0.13 i–n | −70.99 ± 0.44 l | ||
| Clitoria ternatea L.: Ginkgo biloba L. (1:2) | FD | Maltodextrin | 4.49 ± 0.05 de | 0.1682 ± 0.0007 i–k | 39.78 ± 0.98 fg | 2.53 ± 0.01 jk | −12.34 ± 0.22 g–i | −21.51 ± 0.04 e–i | −44.33 ± 0.33 gh |
| Inulin | 4.00 ± 0.07 e–g | 0.1981 ± 0.0059 gh | 41.79 ± 1.17 d–g | 1.01 ± 0.03 o | −13.09 ± 0.37 h–k | −24.26 ± 0.01 g–k | −44.78 ± 0.01 h | ||
| Control | 7.59 ± 0.11 b | 0.3827 ± 0.0059 b | 24.59 ± 1.99 lm | 2.69 ± 0.17 h–j | −8.62 ± 0.57 f | −21.48 ± 0.21 e–h | −50.08 ± 0.57 h | ||
| SD | Maltodextrin | 1.93 ± 0.03 l–n | 0.0640 ± 0.0018 r | 50.82 ± 1.70 b | 3.07 ± 0.06 e–g | −9.51 ± 0.27 f | −12.32 ± 0.02 d | −26.72 ± 0.04 e | |
| Inulin | 1.51 ± 0.08 n | 0.0967 ± 0.0045 op | 45.59 ± 0.69 cd | 1.99 ± 0.06 m | −11.69 ± 0.2 gh | −18.83 ± 0.04 d–g | −36.63 ± 0.03 f | ||
| Control | 1.62 ± 0.01 mn | 0.1272 ± 0.0076 mn | 33.81 ± 0.63 ij | 4.81 ± 0.19 b | −11.56 ± 0.52 g | −18.85 ± 0.01 d–g | −48.83 ± 0.08 h | ||
| Clitoria ternatea L.: Ginkgo biloba L. (2:1) | FD | Maltodextrin | 3.21 ± 0.06 g–j | 0.0823 ± 0.003 pr | 33.45 ± 0.08 ij | 3.16 ± 0.03 e–g | −13.46 ± 0.07 i–l | −25.97 ± 0.05 h–m | −57.49 ± 0.27 i |
| Inulin | 3.44 ± 0.12 f–i | 0.1526 ± 0.0065 kl | 32.65 ± 0.84 jk | 2.21 ± 0.04 lm | −15.20 ± 0.33 m–p | −31.70 ± 0.12 m–p | −66.52 ± 0.32 kl | ||
| Control | 5.89 ± 0.09 c | 0.2901 ± 0.0045 d | 13.20 ± 0.99 o | 3.26 ± 0.08 e | −9.46 ± 0.47 f | −36.03 ± 0.26 op | −102.47 ± 2.28 n | ||
| SD | Maltodextrin | 2.00 ± 0.11 l–n | 0.0868 ± 0.0155 pr | 41.60 ± 0.23 d–g | 4.33 ± 0.18 c | −14.01 ± 0.57 j–m | −20.85 ± 0.15 e–h | −48.09 ± 0.19 h | |
| Inulin | 1.54 ± 0.08 n | 0.0952 ± 0.0071 op | 31.78 ± 0.66 jk | 3.25 ± 0.07 e | −14.71 ± 0.27 l–o | −29.43 ± 0.16 k–o | −66.11 ± 0.27 kl | ||
| Control | 1.57 ± 0.14 n | 0.1149 ± 0.005 m–o | 19.27 ± 0.08 n | 4.74 ± 0.03 b | −12.76 ± 0.06 g–j | −33.07 ± 0.18 n–p | −94.63 ± 0.90 m | ||
| Peak | RT (min) | m/z (Positive) | m/z (Negative) | m/z PRIS Ions (+/− Mode) | Collision Energy [eV] | Compound Name | Class |
|---|---|---|---|---|---|---|---|
| 1 | 36.804 | 627 | 625 | 319 | 35 | Myricetin 3-rutinoside | Flavonoids |
| 2 | 38.562 | 757 | 755 | 611,303 | 35 | Delphinidin-3-(6″-p-coumaroyl)-rutinoside | Anthocyanins |
| 3 | 40.221 | 611 | 609 | 303 | 35 | Delphinidin-3-(cis-p-coumaroyl-glucoside) | Anthocyanins |
| 4 | 42.159 | 742 | 740 | 287 | 35 | Cyanidin-3-(6″-p-coumaroyl)-rutinoside) | Anthocyanins |
| 5 | 42.680 | 611 | 609 | 303 | 35 | Delphinidin-3-(trans-p-coumaroyl-glucoside) | Anthocyanins |
| 6 | 44.126 | 595 | 593 | 287 | 35 | Cyanidin-3-(p-coumaroyl)-glucoside | Anthocyanins |
| 7 | 49.795 | 681 | 679 | 287, 127 | 35 | Kaempferol 3-O-(2″″-O-α-rhamnosyl-6″″-O-malonyl)-β-glucoside | Flavonoids |
| 8 | 51.207 | 1637 | − | 1388,303 | 35 | Ternatin B2 | Anthocyanins |
| 9 | 57.312 | 1785 | 1783 | 303 | 35 | Ternatin D1 | Anthocyanins |
| Peak | RT (min) | m/z (Positive) | m/z (Negative) | m/z PRIS Ions (+/− Mode) | Collision Energy [eV] | Compound Name | Class |
|---|---|---|---|---|---|---|---|
| 1 | 38.4 | 627 | 625 | 319, 316 | 35 | Myricetin 3-O-rutinoside | Flavonoids |
| 2 | 41.2 | 757 | 755 | 303, 300 | 35 | Quercetin 3-O-[2″-(6″-p-coumaroyl)glucosyl]rhamnoside | Flavonoids |
| 3 | 44.8 | 611 | 609 | 303, 300 | 35 | Quercetin 3-rutinoside | Flavonoids |
| 4 | 45.5 | 747 | − | 287 | 35 | Kaempferol 3-O-2″,6″-dirhamnosylglucoside | Flavonoids |
| 5 | 46.2 | 771 | 769 | 317, 129, 639, 605, 314, 299 | 35 | Isorhamnetin 3-O-2″,6″-dirhamnosylglucoside | Flavonoids |
| 6 | 46.7 | − | 883 | 769, 314, 113 | 35 | Patuletin 3-rutinoside | Flavonoids |
| 7 | 47.2 | 641 | 639 | 333, 330, 315 | 35 | Patuletin 3-neohesperidoside | Flavonoids |
| 8 | 50.3 | 595 | 593 | 287, 285 | 35 | Kaempferol 3-rutinoside | Flavonoids |
| 9 | 50.9 | 611 | 609 | 345, 303, 147, 129 | 35 | Quercetin 2″-glucosylrhamnoside | Flavonoids |
| 10 | 52.3 | 595 | 593 | 287, 285, 271 | 35 | Kaempferol analog 1 | Flavonoids |
| 11 | 52.4 | 611 | − | 449, 413, 345, 303, 201 | 35 | Quercetin analog 1 | Flavonoids |
| 12 | 55.9 | 595 | 593 | 287, 284, 271 | 35 | Kaempferol analog 2 | Flavonoids |
| 13 | 58.9 | 871 | 869 | 757, 755, 303, 291, 257, 165, 147 | 35 | Quercetin analog 2 | Flavonoids |
| 14 | 60.3 | 855 | 853 | 747, 739, 593, 287, 284, 257, 165, 147, 113 | 35 | Kaempferol analog 3 | Flavonoids |
| Compound Name | FD | SD | ||
|---|---|---|---|---|
| Maltodextrin | Inulin | Maltodextrin | Inulin | |
| [mg/g DM] | ||||
| Myricetin 3-rutinoside | 0.09 ± 0.07 a | 0.09 ± 0.01 a | 0.10 ± 0.01 a | 0.09 ± 0.01 a |
| Delphinidin-3-(6″-p-coumaroyl)-rutinoside | 0.54 ± 0.02 b | 0.52 ± 0.01 b | 0.61 ± 0.02 a | 0.55 ± 0.01 ab |
| Delphinidin-3-(cis-p-coumaroyl-glucoside) | 0.98 ± 0.03 a | 0.90 ± 0.02 a | 1.02 ± 0.06 a | 1.04 ± 0.01 a |
| Cyanidin-3-(6″-p-coumaroyl)-rutinoside) | 1.61 ± 0.05 b | 1.57 ± 0.10 b | 1.75 ± 0.01 a | 1.63 ± 0.01 b |
| Delphinidin-3-(trans-p-coumaroyl-glucoside) | 0.54 ± 0.07 a | 0.55 ± 0.02 a | 0.62 ± 0.01 a | 0.57 ± 0.01 a |
| Cyanidin-3-(p-coumaroyl)-glucoside | 4.50 ± 0.02 b | 4.31 ± 0.06 b | 4.84 ± 0.14 a | 4.50 ± 0.06 b |
| Kaempferol 3-O-(2″″-O-α-rhamnosyl-6″″-O-malonyl)-β-glucoside | 0.29 ± 0.01 ab | 0.27 ± 0.01 b | 0.30 ± 0.01 a | 0.28 ± 0.01 ab |
| Ternatin B2 | 0.38 ± 0.01 a | 0.08 ± 0.01 a | 0.40 ± 0.01 a | 0.37 ± 0.01 a |
| Ternatin D1 | 0.55 ± 0.07 a | 0.51 ± 1.00 a | 0.59 ± 0.01 a | 0.55 ± 0.01 a |
| Unidentified polyphenols | 7.73 ± 0.10 ab | 5.58 ± 0.10 b | 7.66 ± 0.10 ab | 8.02 ± 0.10 a |
| SUM | 17.21 ± 0.36 a | 14.68 ± 0.52 b | 17.89 ± 0.95 a | 17.60 ± 0.33 a |
| Compound Name | FD | SD | ||
|---|---|---|---|---|
| Maltodextrin | Inulin | Maltodextrin | Inulin | |
| [mg/g DM] | ||||
| Quercetin 3-rutinoside | 0.80 ± 0.05 ab | 0.75 ± 0.01 b | 0.89 ± 0.01 a | 0.85 ± 0.03 ab |
| Kaempferol 3-O-2″-6″-dirhamnosylglucoside | 0.33 ± 0.06 a | 0.28 ± 0.01 a | 0.39 ± 0.02 a | 0.37 ± 0.04 a |
| Isorhamnetin 3-O-2″-6″-dirhamnosylglucoside | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.01 a |
| Patuletin 3-neohesperidoside | 0.25 ± 0.01 ab | 0.24 ± 0.09 b | 0.27 ± 0.01 a | 0.27 ± 0.01 a |
| Kaempferol 3-rutinoside | 0.32 ± 0.01 b | 0.32 ± 0.03 b | 0.36 ± 0.01 a | 0.36 ± 0.01 a |
| Quercetin 2″-glucosylrhamnoside | 0.30 ± 0.01 a | 0.31 ± 0.01 a | 0.32 ± 0.01 a | 0.32 ± 0.01 a |
| Kaempferol analog 1 | 0.31 ± 0.01 ab | 0.28 ± 0.09 b | 0.34 ± 0.01 a | 0.33 ± 0.01 a |
| Kaempferol analog 2 | 0.19 ± 0.01 b | 0.20 ± 0.08 b | 0.23 ± 0.01 a | 0.22 ± 0.01 a |
| Quercetin analog 1 | 0.97 ± 0.01 b | 0.95 ± 0.01 c | 1.03 ± 0.01 a | 1.02 ± 0.01 a |
| Unidentified polyphenols | 5.30 ± 0.01 a | 5.51 ± 0.01 a | 5.56 ± 0.01 a | 5.38 ± 0.01 a |
| SUM | 8.78 ± 0.02 a | 8.86 ± 0.18 a | 9.40 ± 0.34 a | 9.17 ± 5.50 a |
| Material | Drying Technique | Carrier Type | Reducing Potential [g GAE/100 g DM] | TEAC ABTS [mmol Trolox/100 g DM] | FRAP [mmol Trolox/100 g DM] |
|---|---|---|---|---|---|
| Ginkgo biloba L. | FD | Maltodextrin | 0.283 ± 0.030 d | 1.88 ± 0.09 cd | 2.19 ± 0.07 fg |
| Inulin | 0.283 ± 0.020 d | 1.83 ± 0.08 de | 2.22 ± 0.02 fg | ||
| Control | 2.824 ± 0.07 A | 19.07 ± 0.15 A | 21.82 ± 1.62 A | ||
| SD | Maltodextrin | 0.286 ± 0.020 cd | 1.77 ± 0.06 d–f | 2.18 ± 0.07 fg | |
| Inulin | 0.279 ± 0.011 d | 1.69 ± 0.01 e–h | 2.10 ± 0.08 g | ||
| Control | 2.421 ± 0.205 B | 16.08 ± 0.73 B | 19.59 ± 1.55 A | ||
| Clitoria ternatea L. | FD | Maltodextrin | 0.226 ± 0.023 d | 0.95 ± 0.02 k | 1.31 ± 0.06 h |
| Inulin | 0.214 ± 0.008 d | 0.91 ± 0.03 k | 1.23 ± 0.04 h | ||
| Control | 1.764 ± 0.104 CD | 6.65 ± 0.15 F | 10.08 ± 0.08 D | ||
| SD | Maltodextrin | 0.218 ± 0.017 d | 0.84 ± 0.01 k | 1.27 ± 0.04 h | |
| Inulin | 0.253 ± 0.021 d | 0.82 ± 0.02 k | 1.23 ± 0.06 h | ||
| Control | 1.706 ± 0.082 E | 5.99 ± 0.08 F | 9.97 ± 0.10 D | ||
| Clitoria ternatea L.:Ginkgo biloba L. 1:1 | FD | Maltodextrin | 0.401 ± 0.006 ab | 1.74 ± 0.01 d–g | 2.60 ± 0.02 c |
| Inulin | 0.397 ± 0.018 ab | 1.67 ± 0.06 f–h | 2.49 ± 0.04 c–e | ||
| Control | 2.041 ± 0.131 CD | 8.53 ± 0.14 E | 12.22 ± 0.02 CD | ||
| SD | Maltodextrin | 0.388 ± 0.047 ab | 1.62 ± 0.02 g–i | 2.50 ± 0.10 cd | |
| Inulin | 0.419 ± 0.017 ab | 1.57 ± 0.05 hi | 2.39 ± 0.04 c–f | ||
| Control | 2.232 ± 0.251 BC | 8.05 ± 0.11 E | 11.64 ± 0.15 D | ||
| Clitoria ternatea L.:Ginkgo biloba L. (1:2) | FD | Maltodextrin | 0.432 ± 0.031 a | 2.21 ± 0.03 a | 3.26 ± 0.09 a |
| Inulin | 0.427 ± 0.046 ab | 2.09 ± 0.05 ab | 2.97 ± 0.02 b | ||
| Control | 2.250 ± 0.182 B–D | 11.3 ± 0.15 C | 15.74 ± 0.22 B | ||
| SD | Maltodextrin | 0.424 ± 0.026 ab | 2.03 ± 0.03 bc | 3.04 ± 0.15 ab | |
| Inulin | 0.433 ± 0.027 a | 2.05 ± 0.02 b | 3.05 ± 0.01 ab | ||
| Control | 2.322 ± 0.043 BC | 10.17 ± 0.08 D | 14.64 ± 0.24 BC | ||
| Clitoria ternatea L.:Ginkgo biloba L. (2:1) | FD | Maltodextrin | 0.358 ± 0.031 bc | 1.48 ± 0.03 ij | 2.37 ± 0.10 c–g |
| Inulin | 0.375 ± 0.031 ab | 1.42 ± 0.01 j | 2.25 ± 0.01 d–g | ||
| Control | 1.760 ± 0.211 DE | 6.79 ± 0.07 F | 9.93 ± 0.25 D | ||
| SD | Maltodextrin | 0.371 ± 0.034 ab | 1.41 ± 0.05 j | 2.27 ± 0.01 d–g | |
| Inulin | 0.386 ± 0.014 ab | 1.36 ± 0.03 j | 2.23 ± 0.04 e–g | ||
| Control | 1.89 ± 0.113 C–E | 6.76 ± 0.08 F | 9.58 ± 0.15 D |
| Material | Drying Technique | Water Activity [−] | Reducing Potential [g GAE/100 g DM] | TEAC ABTS [mmol Trolox/100 g DM] | FRAP [mmol Trolox/100 g DM] |
|---|---|---|---|---|---|
| Laboratory scale | SD | 0.1339 ± 0.0033 a | 0.42 ± 0.01 a | 1.57 ± 0.05 b | 2.39 ± 0.04 a |
| Semi-technical scale | 0.0896 ± 0.0013 b | 0.29 ± 0.01 b | 2.93 ± 0.26 a | 2.05 ± 0.12 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska-Guzik, A.; Brzezowska, J.; Masztalerz, K.; Nejman, M.; Guzik, Ł.; Michalska-Ciechanowska, A. Polyphenol Powders from Ginkgo biloba L. and Clitoria ternatea L.: Influence of Drying Techniques and Carriers on Antioxidant Capacity and Polyphenol Release Profiles. Antioxidants 2025, 14, 1447. https://doi.org/10.3390/antiox14121447
Kucharska-Guzik A, Brzezowska J, Masztalerz K, Nejman M, Guzik Ł, Michalska-Ciechanowska A. Polyphenol Powders from Ginkgo biloba L. and Clitoria ternatea L.: Influence of Drying Techniques and Carriers on Antioxidant Capacity and Polyphenol Release Profiles. Antioxidants. 2025; 14(12):1447. https://doi.org/10.3390/antiox14121447
Chicago/Turabian StyleKucharska-Guzik, Alicja, Jessica Brzezowska, Klaudia Masztalerz, Mariusz Nejman, Łukasz Guzik, and Anna Michalska-Ciechanowska. 2025. "Polyphenol Powders from Ginkgo biloba L. and Clitoria ternatea L.: Influence of Drying Techniques and Carriers on Antioxidant Capacity and Polyphenol Release Profiles" Antioxidants 14, no. 12: 1447. https://doi.org/10.3390/antiox14121447
APA StyleKucharska-Guzik, A., Brzezowska, J., Masztalerz, K., Nejman, M., Guzik, Ł., & Michalska-Ciechanowska, A. (2025). Polyphenol Powders from Ginkgo biloba L. and Clitoria ternatea L.: Influence of Drying Techniques and Carriers on Antioxidant Capacity and Polyphenol Release Profiles. Antioxidants, 14(12), 1447. https://doi.org/10.3390/antiox14121447







