Review of Hyperbaric Oxygen Therapy as an Adjunctive Intervention for Metabolic Disorders
Abstract
1. Introduction
2. Materials and Methods
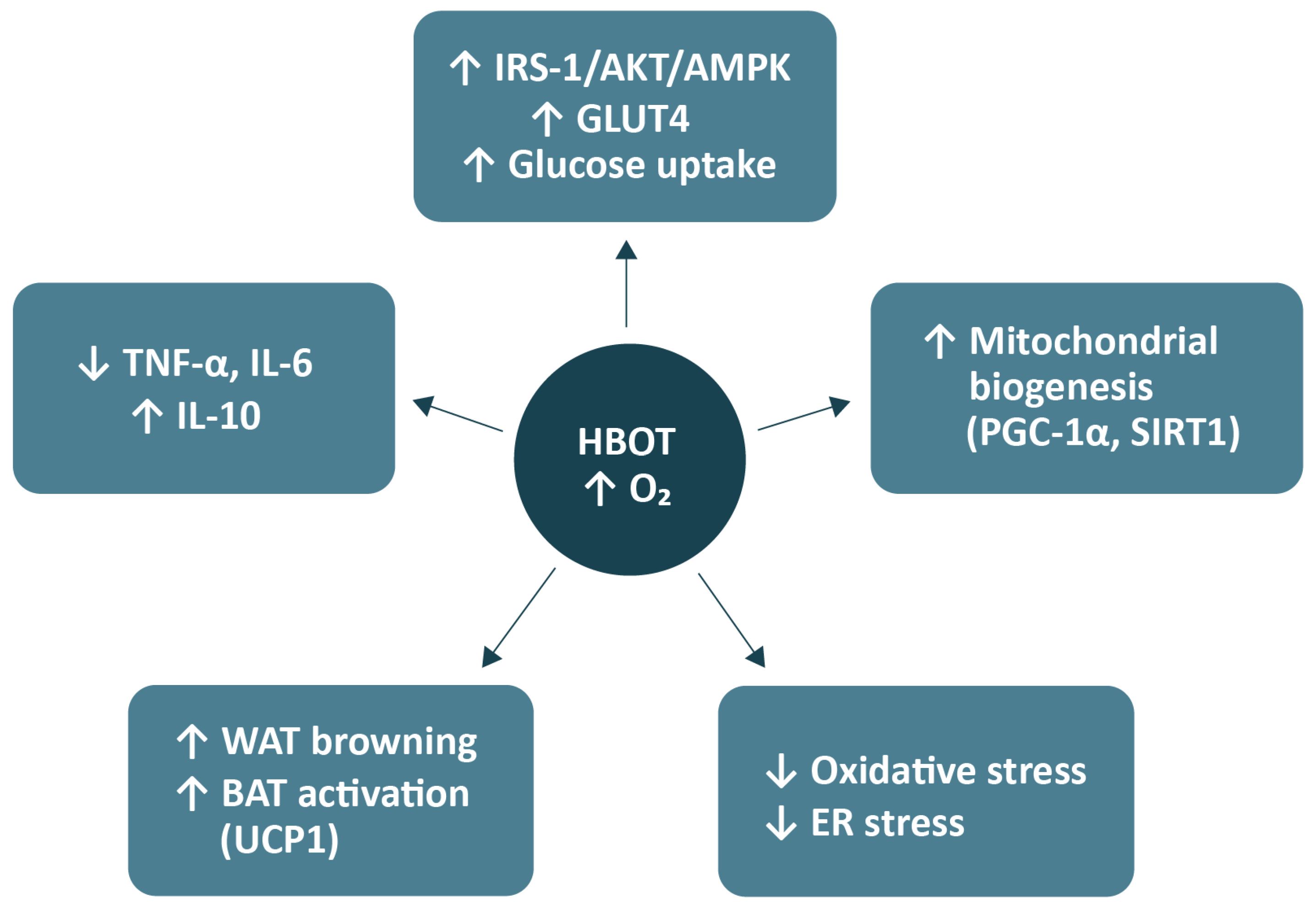
3. Mechanisms of HBOT Action in Metabolic Regulation
3.1. Improvement in Insulin Sensitivity and Glucose Uptake
3.2. Impact of HBOT on Endothelial Barrier Integrity
3.3. Effects on Adipose Tissue, Lipid Metabolism, and Energy Expenditure
3.4. Oxidative Stress, Mitochondrial Function, and Inflammation
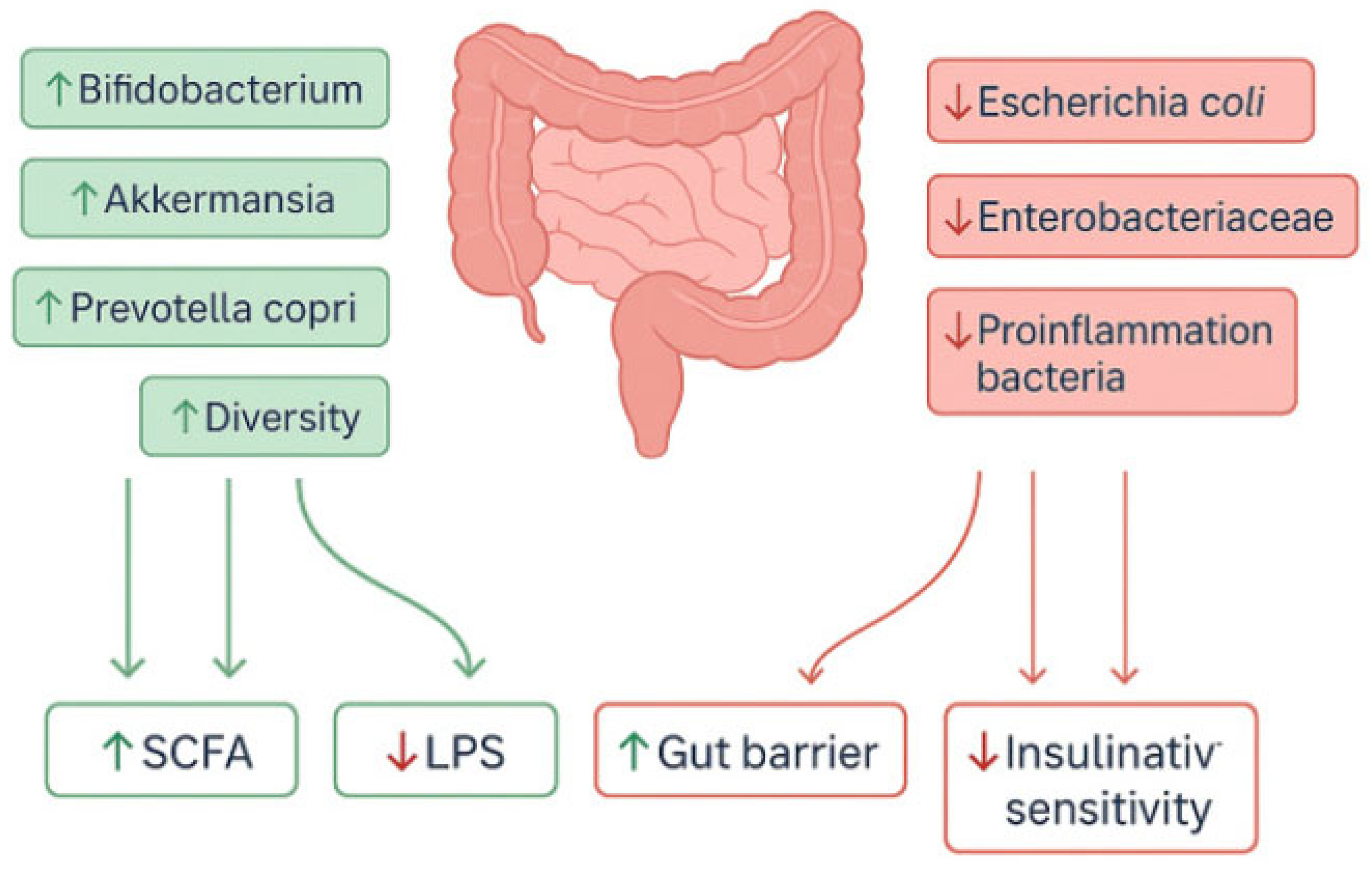
3.5. Effects of HBOT on the Gut Microbiome
4. Evidence from Preclinical and Clinical Studies
4.1. Preclinical Studies: In Vivo and In Vitro Findings
4.2. Clinical Studies and Trials
5. Safety of HBOT
5.1. Paediatric Population
5.2. Pregnant Women
5.3. Elderly Patients
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ApoE−/− | Apolipoprotein E-deficient |
| ATA | Atmospheres absolute |
| BAT | Brown adipose tissue |
| Bcl-2 | B-cell lymphoma 2 |
| CO | Carbon monoxide |
| CPT1/CPT1B | Carnitine palmitoyltransferase 1/1B |
| DB/db/db | Diabetic (leptin receptor-deficient) mice |
| ER | Endoplasmic reticulum |
| FFA | Free fatty acids |
| GLUT4 | Glucose transporter type 4 |
| HBOT | Hyperbaric oxygen therapy |
| HFD | High-fat diet |
| HFHC | High-fat, high-cholesterol diet |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HMOX1 | Haem oxygenase 1 |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| HSL | Hormone-sensitive lipase |
| IL | Interleukin |
| IRS-1 | Insulin receptor substrate 1 |
| LPS | Lipopolysaccharide |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| MCD | Methionine–choline-deficient (diet) |
| MDA | Malondialdehyde |
| MRS | Magnetic resonance spectroscopy |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain-containing protein 3 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| O2 | Oxygen |
| OLETF | Otsuka Long-Evans Tokushima Fatty rat |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPAR | Peroxisome proliferator-activated receptor |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PPARδ (PPARβ/δ) | Peroxisome proliferator-activated receptor delta (beta/delta) |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SIRT1 | Sirtuin 1 |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| TFAM | Mitochondrial transcription factor A |
| TG | Triglycerides |
| TNF-α | Tumour necrosis factor-alpha |
| UCP1 | Uncoupling protein 1 |
| VO2max | Maximal oxygen uptake |
| WAT | White adipose tissue |
References
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Verboven, K.; Wouters, K.; Gaens, K.; Hansen, D.; Bijnen, M.; Wetzels, S.; Stehouwer, C.D.; Goossens, G.H.; Schalkwijk, C.G.; Blaak, E.E.; et al. Abdominal Subcutaneous and Visceral Adipocyte Size, Lipolysis and Inflammation Relate to Insulin Resistance in Male Obese Humans. Sci. Rep. 2018, 8, 4677. [Google Scholar] [CrossRef]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Dalla Man, C.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose Tissue Macrophage Populations and Inflammation Are Associated with Systemic Inflammation and Insulin Resistance in Obesity. Am. J. Physiol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic Inflammation in Fat Plays a Crucial Role in the Development of Obesity-Related Insulin Resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Kheniser, K.; Saxon, D.R.; Kashyap, S.R. Long-Term Weight Loss Strategies for Obesity. J. Clin. Endocrinol. Metab. 2021, 106, 1854–1866. [Google Scholar] [CrossRef]
- Grunvald, E.; Shah, R.; Hernaez, R.; Chandar, A.K.; Pickett-Blakely, O.; Teigen, L.M.; Harindhanavudhi, T.; Sultan, S.; Singh, S.; Davitkov, P. AGA Clinical Practice Guideline on Pharmacological Interventions for Adults with Obesity. Gastroenterology 2022, 163, 1198–1225. [Google Scholar] [CrossRef]
- Aydin, F. Hyperbaric Oxygen Treatment in Children: Experience in 329 Patients. Diving Hyperb. Med. 2023, 53, 203–209. [Google Scholar] [CrossRef]
- Sarabhai, T.; Mastrototaro, L.; Kahl, S.; Bönhof, G.J.; Jonuscheit, M.; Bobrov, P.; Katsuyama, H.; Guthoff, R.; Wolkersdorfer, M.; Herder, C.; et al. Hyperbaric Oxygen Rapidly Improves Tissue-Specific Insulin Sensitivity and Mitochondrial Capacity in Humans with Type 2 Diabetes: A Randomised Placebo-Controlled Crossover Trial. Diabetologia 2023, 66, 57–69. [Google Scholar] [CrossRef]
- Sokolowski, S.A.; Räisänen-Sokolowski, A.K.; Tuominen, L.J.; Lundell, R.V. Delayed Treatment for Decompression Illness: Factors Associated with Long Treatment Delays and Treatment Outcome. Diving Hyperb. Med. 2022, 52, 271–276. [Google Scholar] [CrossRef]
- Fujita, M.; Todani, M.; Kaneda, K.; Suzuki, S.; Wakai, S.; Kikuta, S.; Sasaki, S.; Hattori, N.; Yagishita, K.; Kuwata, K.; et al. Use of Hyperbaric Oxygen Therapy for Preventing Delayed Neurological Sequelae in Patients with Carbon Monoxide Poisoning: A Multicenter, Prospective, Observational Study in Japan. PLoS ONE 2021, 16, e0253602. [Google Scholar] [CrossRef]
- Lopes, J.R.A.; D’Agostino Dias, M.; Correa, J.A.; Batalha, M.A.B.; Guerra, L.K.D. Randomized Controlled Clinical Trial Evaluating the Efficacy of Hyperbaric Oxygen Therapy in Facilitating the Healing of Chronic Foot Ulcers in Diabetic Patients: The Study Protocol. Trials 2020, 21, 816. [Google Scholar] [CrossRef] [PubMed]
- Moreira Monteiro, A.; Alpuim Costa, D.; Mareco, V.; Espiney Amaro, C. The Effectiveness of Hyperbaric Oxygen Therapy for Managing Radiation-Induced Proctitis—Results of a 10-Year Retrospective Cohort Study. Front. Oncol. 2023, 13, 1235237. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.P.; Snyder, J.; Schuerer, D.J.E.; Peters, J.S.; Bochicchio, G.V. Essentials of Hyperbaric Oxygen Therapy: 2019 Review. Mo. Med. 2019, 116, 176–179. [Google Scholar] [PubMed]
- Zhao, H.; Zhang, T.; Wang, Y.; Fang, Y. Early Hyperbaric Oxygen Therapy Promotes Recovery of Blunt Liver Injury in Rats via Inhibiting Inflammatory Response and Oxidative Stress. Int. J. Med. Sci. 2025, 22, 2174–2185. [Google Scholar] [CrossRef]
- Oley, M.H.; Oley, M.C.; Tjandra, D.E.; Sedu, S.W.; Sumarauw, E.R.N.; Aling, D.M.R.; Kalangi, J.A.; Islam, A.A.; Hatta, M.; Faruk, M. Hyperbaric Oxygen Therapy in the Healing Process of Foot Ulcers in Diabetic Type 2 Patients Marked by Interleukin 6, Vascular Endothelial Growth Factor, and PEDIS Score: A Randomized Controlled Trial Study. Int. J. Surg. Open 2020, 27, 154–161. [Google Scholar] [CrossRef]
- Karadurmus, N.; Sahin, M.; Tasci, C.; Naharci, I.; Ozturk, C.; Ilbasmis, S.; Dulkadir, Z.; Sen, A.; Saglam, K. Potential Benefits of Hyperbaric Oxygen Therapy on Atherosclerosis and Glycaemic Control in Patients with Diabetic Foot. Endokrynol. Pol. 2010, 61, 275–279. [Google Scholar]
- Zhang, C.; Zhang, D.; Wang, H.; Lin, Q.; Li, M.; Yuan, J.; Gao, G.; Dong, J. Hyperbaric Oxygen Treatment Improves Pancreatic Β-cell Function and Hepatic Gluconeogenesis in STZ-induced Type-2 Diabetes Mellitus Model Mice. Mol. Med. Rep. 2022, 25, 90. [Google Scholar] [CrossRef]
- Wilkinson, D.C.; Chapman, I.M.; Heilbronn, L.K. Hyperbaric Oxygen but Not Hyperbaric Air Increases Insulin Sensitivity in Men with Type 2 Diabetes Mellitus. Diving Hyperb. Med. 2020, 50, 386–390. [Google Scholar] [CrossRef]
- Wilkinson, D.; Szekely, S.; Gue, B.; Tam, C.S.; Chapman, I.; Heilbronn, L.K. Assessment of Insulin Sensitivity during Hyperbaric Oxygen Treatment. Diving Hyperb. Med. J. 2020, 50, 238–243. [Google Scholar] [CrossRef]
- Kun, L.; Lu, L.; Yongda, L.; Xingyue, L.; Guang, H. Hyperbaric Oxygen Promotes Mitophagy by Activating CaMKK β/AMPK Signal Pathway in Rats of Neuropathic Pain. Mol. Pain 2019, 15, 1744806919871381. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Yuan, J.; Song, L.; Zhang, C.; Lin, Q.; Li, M.; Sheng, Z.; Ma, Z.; Lv, F.; et al. Hyperbaric Oxygen Ameliorates Insulin Sensitivity by Increasing GLUT4 Expression in Skeletal Muscle and Stimulating UCP1 in Brown Adipose Tissue in T2DM Mice. Front. Endocrinol. 2020, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Choi, Y.-A.; Heo, S.-J.; Song, P. The Effect of Hyperbaric Therapy on Brown Adipose Tissue in Rats. Int. J. Environ. Res. Public Health 2021, 18, 9165. [Google Scholar] [CrossRef]
- Takemura, A.; Ishihara, A. Mild Hyperbaric Oxygen Inhibits Growth-Related Decrease in Muscle Oxidative Capacity of Rats with Metabolic Syndrome. J. Atheroscler. Thromb. 2017, 24, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Kendall, A.C.; Whatmore, J.L.; Harries, L.W.; Winyard, P.G.; Smerdon, G.R.; Eggleton, P. Changes in Inflammatory Gene Expression Induced by Hyperbaric Oxygen Treatment in Human Endothelial Cells under Chronic Wound Conditions. Exp. Cell Res. 2012, 318, 207–216. [Google Scholar] [CrossRef]
- Cruz-Villanueva, S.R.; Ramirez-Nava, J.C.; Moreno-Luna, J.A.; Cárdenas-Ureña, K.G.; Espín-Iturbe, L.T.; Otero, M.G.S.; Quintana-Castro, R.; Alexander-Aguilera, A. Effect of Hyperbaric Oxygen Therapy (HBOT) on Insulin Resistance Associated with Abdominal Obesity in Wistar Rats with Dietary Sucrose-Induced Metabolic Syndrome. J. Nutr. Sci. Vitaminol. 2021, 67, 292–300. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, S.; Xie, D.; Pan, S.; Fu, H.; Feng, Z.; Gao, C.; Ge, X. Hyperbaric Oxygen Therapy Promotes the Browning of White Fat and Contributes to the Healing of Diabetic Wounds. Int. Wound J. 2024, 21, e14867. [Google Scholar] [CrossRef]
- Benkő, R.; Miklós, Z.; Ágoston, V.A.; Ihonvien, K.; Répás, C.; Csépányi-Kömi, R.; Kerék, M.; Béres, N.J.; Horváth, E.M. Hyperbaric Oxygen Therapy Dampens Inflammatory Cytokine Production and Does Not Worsen the Cardiac Function and Oxidative State of Diabetic Rats. Antioxidants 2019, 8, 607. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, Y.; Li, S.; Yu, B.; Yang, F.; Li, Y.; Cheng, Y.; Yu, Z.; Li, C.; Dong, J.; et al. Nesfatin-1 Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Hyperphagia in Mice. Peptides 2025, 183, 171336. [Google Scholar] [CrossRef] [PubMed]
- Muroya, D.; Nadayoshi, S.; Kai, Y.; Sasaki, S.; Goto, Y.; Okamoto, K. Gut Microbiota and Hyperbaric Oxygen Therapy. Med. Gas Res. 2025, 15, 548–549. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, E.; Kim, J.; Kim, H. Hyperbaric Oxygen Therapy as a Possible Therapeutic Candidate for Sepsis-Associated Encephalopathy: A Novel Hypothesis. Med. Hypotheses 2024, 182, 111212. [Google Scholar] [CrossRef]
- Fujita, N.; Nagatomo, F.; Murakami, S.; Kondo, H.; Ishihara, A.; Fujino, H. Effects of Hyperbaric Oxygen on Metabolic Capacity of the Skeletal Muscle in Type 2 Diabetic Rats with Obesity. Sci. World J. 2012, 2012, 637978. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Jiang, C.; Kim, M.; Xiao, Y.; Richter, H.J.; Guan, D.; Zhu, K.; Krusen, B.M.; Roberts, A.N.; Miller, J.; et al. Isoform-Specific Functions of PPARγ in Gene Regulation and Metabolism. Genes Dev. 2022, 36, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.N.; Patel, D.P.; Velenosi, T.J.; Kim, D.; Yan, T.; Yue, J.; Li, G.; Krausz, K.W.; Gonzalez, F.J. Extrahepatic PPARα Modulates Fatty Acid Oxidation and Attenuates Fasting-Induced Hepatosteatosis in Mice. J. Lipid Res. 2018, 59, 2140–2152. [Google Scholar] [CrossRef]
- Rachid, T.L.; Silva-Veiga, F.M.; Graus-Nunes, F.; Bringhenti, I.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Differential Actions of PPAR-α and PPAR-β/δ on Beige Adipocyte Formation: A Study in the Subcutaneous White Adipose Tissue of Obese Male Mice. PLoS ONE 2018, 13, e0191365. [Google Scholar] [CrossRef]
- Norris, A.W.; Chen, L.; Fisher, S.J.; Szanto, I.; Ristow, M.; Jozsi, A.C.; Hirshman, M.F.; Rosen, E.D.; Goodyear, L.J.; Gonzalez, F.J.; et al. Muscle-Specific PPARγ-Deficient Mice Develop Increased Adiposity and Insulin Resistance but Respond to Thiazolidinediones. J. Clin. Investig. 2003, 112, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liu, Y.; Liu, H.; Qiao, C.; Chen, X.; Wang, L. Effect of Hyperbaric Oxygen Treatment on Lipid Metabolism and Neurovascular Microenvironment in an Apolipoprotein E Knockout Mouse Model. J. Integr. Neurosci. 2025, 24, 39656. [Google Scholar] [CrossRef]
- Lee, C.-H.; Olson, P.; Hevener, A.; Mehl, I.; Chong, L.-W.; Olefsky, J.M.; Gonzalez, F.J.; Ham, J.; Kang, H.; Peters, J.M.; et al. PPARδ Regulates Glucose Metabolism and Insulin Sensitivity. Proc. Natl. Acad. Sci. USA 2006, 103, 3444–3449. [Google Scholar] [CrossRef]
- Quintero, P.; González-Muniesa, P.; García-Díaz, D.F.; Martínez, J.A. Effects of Hyperoxia Exposure on Metabolic Markers and Gene Expression in 3T3-L1 Adipocytes. J. Physiol. Biochem. 2012, 68, 663–669. [Google Scholar] [CrossRef]
- Lindenmann, J.; Smolle, C.; Kamolz, L.-P.; Smolle-Juettner, F.M.; Graier, W.F. Survey of Molecular Mechanisms of Hyperbaric Oxygen in Tissue Repair. Int. J. Mol. Sci. 2021, 22, 11754. [Google Scholar] [CrossRef]
- Shoshani, O.; Shupak, A.; Ullmann, Y.; Ramon, Y.; Gilhar, A.; Kehat, I.; Peled, I.J. The Effect of Hyperbaric Oxygenation on the Viability of Human Fat Injected into Nude Mice. Plast. Reconstr. Surg. 2000, 106, 1390–1396. [Google Scholar] [CrossRef]
- Kendall, A.C.; Whatmore, J.L.; Winyard, P.G.; Smerdon, G.R.; Eggleton, P. Hyperbaric Oxygen Treatment Reduces Neutrophil-endothelial Adhesion in Chronic Wound Conditions through S-nitrosation. Wound Repair Regen. 2013, 21, 860–868. [Google Scholar] [CrossRef]
- Buras, J.A.; Stahl, G.L.; Svoboda, K.K.H.; Reenstra, W.R. Hyperbaric Oxygen Downregulates ICAM-1 Expression Induced by Hypoxia and Hypoglycemia: The Role of NOS. Am. J. Physiol. Physiol. 2000, 278, C292–C302. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, P.; Jiang, B.; Zhang, P.; Yu, W.; Duan, M.; Guo, L.; Cui, X.; Huang, M.; Huang, X. Hyperbaric Oxygen Potentiates Diabetic Wound Healing by Promoting Fibroblast Cell Proliferation and Endothelial Cell Angiogenesis. Life Sci. 2020, 259, 118246. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, V.G.; Lind, F.; Botusan, I.R.; Kashif, A.; Liu, Z.; Ylä-Herttuala, S.; Brismar, K.; Velazquez, O.; Catrina, S. Hyperbaric Oxygen Therapy Activates Hypoxia-inducible Factor 1 (HIF-1), Which Contributes to Improved Wound Healing in Diabetic Mice. Wound Repair Regen. 2015, 23, 98–103. [Google Scholar] [CrossRef]
- Lin, Y.; Shih, J.; Lin, Y.; Niu, K.; Hong, C.; Chen, Z.; Pan, S.; Chang, T.; Kan, W.; Chang, W. Hyperbaric Oxygen Therapy Improved Neovascularisation Following Limb Ischaemia—The Role of ROS Mitigation. J. Cell. Mol. Med. 2024, 28, e70310. [Google Scholar] [CrossRef]
- Hong, C.-S.; Wu, N.-C.; Lin, Y.-W.; Lin, Y.-C.; Shih, J.-Y.; Niu, K.-C.; Lin, M.-T.; Chang, C.-P.; Chen, Z.-C.; Kan, W.-C.; et al. Hyperbaric Oxygen Therapy Attenuated Limb Ischemia in Mice with High-Fat Diet by Restoring Sirtuin 1 and Mitochondrial Function. Free Radic. Biol. Med. 2025, 230, 263–272. [Google Scholar] [CrossRef]
- Batinac, T.; Batičić, L.; Kršek, A.; Knežević, D.; Marcucci, E.; Sotošek, V.; Ćurko-Cofek, B. Endothelial Dysfunction and Cardiovascular Disease: Hyperbaric Oxygen Therapy as an Emerging Therapeutic Modality? J. Cardiovasc. Dev. Dis. 2024, 11, 408. [Google Scholar] [CrossRef] [PubMed]
- Dragic, S.; Momcicevic, D.; Zlojutro, B.; Jandric, M.; Kovacevic, T.; Djajić, V.; Gajić, A.; Talić, G.; Kovacevic, P. Serum Levels of Nitric Oxide and Endothelin-1 in Vasculopathy Managed with Hyperbaric Oxygen Therapy. Clin. Hemorheol. Microcirc. 2020, 75, 233–241. [Google Scholar] [CrossRef]
- Kalns, J. Hyperbaric Oxygen Exposure Temporarily Reduces Mac-1 Mediated Functions of Human Neutrophils. Immunol. Lett. 2002, 83, 125–131. [Google Scholar] [CrossRef]
- Shinomiya, N.; Suzuki, S.; Hashimoto, A.; Ito, M.; Takaai, Y.; Oiwa, H. Effect of Hyperbaric Oxygen on Intercellular Adhesion Molecule-1 (ICAM-1) Expression in Murine Lung. Aviat. Space Environ. Med. 1998, 69, 1–7. [Google Scholar]
- Baiula, M.; Greco, R.; Ferrazzano, L.; Caligiana, A.; Hoxha, K.; Bandini, D.; Longobardi, P.; Spampinato, S.; Tolomelli, A. Integrin-Mediated Adhesive Properties of Neutrophils Are Reduced by Hyperbaric Oxygen Therapy in Patients with Chronic Non-Healing Wound. PLoS ONE 2020, 15, e0237746. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.; Kaforou, M.; Frontini, A.; Okolo, A.; Chan, Y.-W.; Nikolopoulou, E.; Millership, S.; Fenech, M.E.; MacIntyre, D.; Turner, J.O.; et al. Brown and White Adipose Tissues: Intrinsic Differences in Gene Expression and Response to Cold Exposure in Mice. Am. J. Physiol. Metab. 2014, 306, E945–E964. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, D.; Landgraf, K.; Wagner, I.V.; Gesing, J.; Tauscher, R.; Lakowa, N.; Kiess, W.; Bühligen, U.; Wojan, M.; Till, H.; et al. Direct Evidence of Brown Adipocytes in Different Fat Depots in Children. PLoS ONE 2015, 10, e0117841. [Google Scholar] [CrossRef]
- Zhang, L.; Antonacci, M.; Burant, A.; McCallister, A.; Kelley, M.; Bryden, N.; McHugh, C.; Atalla, S.; Holmes, L.; Katz, L.; et al. Absolute Thermometry of Human Brown Adipose Tissue by Magnetic Resonance with Laser Polarized 129Xe. Commun. Med. 2023, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Loureiro, Z.Y.; DeSouza, T.; Yang, Q.; Solivan-Rivera, J.; Corvera, S. CAMP Driven UCP1 Induction in Human Adipocytes Requires ATGL-Catalyzed Lipolysis. Mol. Metab. 2024, 90, 102051. [Google Scholar] [CrossRef]
- Norouzirad, R.; Ghanbari, M.; Bahadoran, Z.; Abdollahifar, M.A.; Rasouli, N.; Ghasemi, A. Hyperoxia Improves Carbohydrate Metabolism by Browning of White Adipocytes in Obese Type 2 Diabetic Rats. Life Sci. 2019, 220, 58–68. [Google Scholar] [CrossRef]
- Li, D.; Zhang, F.; Zhang, X.; Xue, C.; Namwanje, M.; Fan, L.; Reilly, M.P.; Hu, F.; Qiang, L. Distinct Functions of PPARγ Isoforms in Regulating Adipocyte Plasticity. Biochem. Biophys. Res. Commun. 2016, 481, 132–138. [Google Scholar] [CrossRef]
- Fan, W.; Waizenegger, W.; Lin, C.S.; Sorrentino, V.; He, M.-X.; Wall, C.E.; Li, H.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. PPARδ Promotes Running Endurance by Preserving Glucose. Cell Metab. 2017, 25, 1186–1193.e4. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, Q.; Song, L.; Liu, Y.; Li, M.; Lin, Q.; Li, Y.; Su, K.; Ma, Z.; Wang, Y.; et al. L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction. Molecules 2020, 25, 176. [Google Scholar] [CrossRef]
- Resanović, I.; Gluvić, Z.; Zarić, B.; Sudar-Milovanović, E.; Vučić, V.; Arsić, A.; Nedić, O.; Šunderić, M.; Gligorijević, N.; Milačić, D.; et al. Effect of Hyperbaric Oxygen Therapy on Fatty Acid Composition and Insulin-like Growth Factor Binding Protein 1 in Adult Type 1 Diabetes Mellitus Patients: A Pilot Study. Can. J. Diabetes 2020, 44, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, B.; Qin, Y.; Wang, D.; Jin, Y.; Su, L.; Wang, Q.; Pan, Y.; Zhang, Y.; Shen, Y.; et al. Adipocyte MicroRNA-802 Promotes Adipose Tissue Inflammation and Insulin Resistance by Modulating Macrophages in Obesity. Elife 2024, 13, e99162. [Google Scholar] [CrossRef] [PubMed]
- Henninger, A.M.J.; Eliasson, B.; Jenndahl, L.E.; Hammarstedt, A. Adipocyte Hypertrophy, Inflammation and Fibrosis Characterize Subcutaneous Adipose Tissue of Healthy, Non-Obese Subjects Predisposed to Type 2 Diabetes. PLoS ONE 2014, 9, e105262. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.-B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular Vesicle-Based Interorgan Transport of Mitochondria from Energetically Stressed Adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Dominguez, P.; Gomez-Aviles, P.; Bautista-García, K.; Antonio-Villa, N.E.; Guerra, E.C.; Almeda-Valdes, P.; Martagón, A.J.; Munoz, A.C.; Santa-Ana-Bayona, M.J.; Alexanderson, E.; et al. Visceral Adipose Tissue Mediates the Relationship between Left Ventricular Global Longitudinal Strain and Insulin Resistance among Adults Living with Type 2 Diabetes. Cardiovasc. Diabetol. 2025, 24, 2. [Google Scholar] [CrossRef]
- Xia, W.; Veeragandham, P.; Cao, Y.; Xu, Y.; Rhyne, T.E.; Qian, J.; Hung, C.-W.; Zhao, P.; Jones, Y.; Gao, H.; et al. Obesity Causes Mitochondrial Fragmentation and Dysfunction in White Adipocytes Due to RalA Activation. Nat. Metab. 2024, 6, 273–289. [Google Scholar] [CrossRef]
- Touceda, V.; Fontana Estevez, F.; Cacciagiú, L.; Finocchietto, P.; Bustos, R.; Vidal, A.; Berg, G.; Morales, C.; González, G.E.; Miksztowicz, V. Liraglutide Improves Adipose Tissue Remodeling and Mitochondrial Dynamics in a Visceral Obesity Model Induced by a High-Fat Diet. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100185. [Google Scholar] [CrossRef]
- Harrison, L.E.; Giardina, C.; Hightower, L.E.; Anderson, C.; Perdrizet, G.A. Might Hyperbaric Oxygen Therapy (HBOT) Reduce Renal Injury in Diabetic People with Diabetes Mellitus? From Preclinical Models to Human Metabolomics. Cell Stress Chaperones 2018, 23, 1143–1152. [Google Scholar] [CrossRef]
- Capó, X.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Batle, J.M.; Tur, J.A.; Pons, A.; Sureda, A.; Tejada, S. Hyperbaric Oxygen Therapy Reduces Oxidative Stress and Inflammation, and Increases Growth Factors Favouring the Healing Process of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 7040. [Google Scholar] [CrossRef]
- Bo-Htay, C.; Shwe, T.; Palee, S.; Pattarasakulchai, T.; Shinlapawittayatorn, K.; Chattipakorn, S.; Chattipakorn, N. Hyperbaric Oxygen Therapy Attenuates D-Galactose-Induced-Age-Related Cardiac Dysfunction through Mitigating Cardiac Mitochondrial Dysfunction in Pre-Diabetic Rats. Eur. Heart J. 2020, 41, 3229. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Yang, Y.-L.; Chang, W.-H.; Fang, W.-Y.; Huang, S.-H.; Chou, S.-H.; Lo, Y.-C. Hyperbaric Oxygen Therapy Improves Parkinson’s Disease by Promoting Mitochondrial Biogenesis via the SIRT-1/PGC-1α Pathway. Biomolecules 2022, 12, 661. [Google Scholar] [CrossRef]
- Yan, W.; Fang, Z.; Yang, Q.; Dong, H.; Lu, Y.; Lei, C.; Xiong, L. Sirt1 Mediates Hyperbaric Oxygen Preconditioning-Induced Ischemic Tolerance in Rat Brain. J. Cereb. Blood Flow Metab. 2013, 33, 396–406. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Hadanny, A.; Hachmo, Y.; Rozali, D.; Catalogna, M.; Yaakobi, E.; Sova, M.; Gattegno, H.; Abu Hamed, R.; Lang, E.; Polak, N.; et al. Effects of Hyperbaric Oxygen Therapy on Mitochondrial Respiration and Physical Performance in Middle-Aged Athletes: A Blinded, Randomized Controlled Trial. Sports Med. Open 2022, 8, 22. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Li, Z.; Yang, D.; Wang, D.; Sun, Z.; Wen, P.; Gou, F.; Dai, Y.; Ji, Y.; et al. SIRT1 Regulates Mitochondrial Damage in N2a Cells Treated with the Prion Protein Fragment 106–126 via PGC-1α-TFAM-Mediated Mitochondrial Biogenesis. Int. J. Mol. Sci. 2024, 25, 9707. [Google Scholar] [CrossRef]
- de Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Hu, Q.; Liang, X.; Chen, D.; Li, B.; Tang, J.; Zhang, J.H. Hyperbaric Oxygen Preconditioning Attenuates Hemorrhagic Transformation through Increasing PPARγ in Hyperglycemic MCAO Rats. Exp. Neurol. 2015, 265, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Baldelli, S.; Virgili, F.; Salvagno, M.; Mrakic-Sposta, S.; Fratantonio, D. Pulsed Hyperoxia Acts on Plasmatic Advanced Glycation End Products and Advanced Oxidation Protein Products and Modulates Mitochondrial Biogenesis in Human Peripheral Blood Mononuclear Cells: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2024, 25, 2394. [Google Scholar] [CrossRef] [PubMed]
- Nyam, T.-T.E.; Wee, H.-Y.; Chiu, M.-H.; Tu, K.-C.; Wang, C.-C.; Yeh, Y.-T.; Kuo, C.-L. Hyperbaric Oxygen Therapy Reduces the Traumatic Brain Injury–Mediated Neuroinflammation Through Enrichment of Prevotella Copri in the Gut of Male Rats. Neurocrit. Care 2024, 41, 798–812. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Lai, C.; Liu, K.; Yang, H.; Peng, Z.; Xu, D.; Huang, F.; Tang, K.; Peng, Y.; et al. Hyperbaric Oxygen Therapy Ameliorates Intestinal and Systematic Inflammation by Modulating Dysbiosis of the Gut Microbiota in Crohn’s Disease. J. Transl. Med. 2024, 22, 518. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, W.; Ma, Z.; Duan, S.; Han, R.; Lv, Z.; Liu, X.; Mao, Y. Hyperbaric Oxygen Improves Depression-like Behaviors in Chronic Stress Model Mice by Remodeling Gut Microbiota and Regulating Host Metabolism. CNS Neurosci. Ther. 2022, 29, 239–255. [Google Scholar] [CrossRef]
- Nakutis, F.S.; Nishitokukado, I.; dos Santos, F.M.; Ortiz-Agostinho, C.L.; de Alencar, D.T.; Achtschin, C.G.; Nunes, V.S.; Leite, A.Z.A.; Sipahi, A.M. Evaluation of Oxidative Stress in an Experimental Model of Crohn’s Disease Treated with Hyperbaric Oxygen Therapy. Clinics 2023, 78, 100305. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Zhou, Q.; Zhao, T.; Zhao, Y.; Chen, X.; Zhu, W.; Ding, J.; Zheng, H.; Jiang, M.; et al. HBOT Alleviates Diet-Induced MASH by Reprograming Gut Microbiota and Liver Metabolism in Mice. Free Radic. Biol. Med. 2025, 237, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, W.; Liu, Y.; Lv, J.; Zhong, X.; Huang, P. Hyperbaric Oxygen Therapy Alleviates Intestinal Dysfunction Following Traumatic Brain Injury via m 6 A Regulation. Int. J. Med. Sci. 2024, 21, 2272–2284. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.G.; Mills, R.H.; Kordahi, M.C.; Carrillo-Terrazas, M.; Secaira-Morocho, H.; Widjaja, C.E.; Tsai, M.S.; Mittal, Y.; Yee, B.A.; Vargas, F.; et al. The Host-Microbiome Response to Hyperbaric Oxygen Therapy in Ulcerative Colitis Patients. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 35–53. [Google Scholar] [CrossRef]
- Fachi, J.L.; Pral, L.P.; Assis, H.C.; Oliveira, S.; Rodovalho, V.R.; dos Santos, J.A.C.; Fernandes, M.F.; Matheus, V.A.; Sesti-Costa, R.; Basso, P.J.; et al. Hyperbaric Oxygen Augments Susceptibility to C. Difficile Infection by Impairing Gut Microbiota Ability to Stimulate the HIF-1α-IL-22 Axis in ILC3. Gut Microbes 2024, 16, 2297872. [Google Scholar] [CrossRef]
- Losada, D.M.; Chies, A.B.; Feres, O.; Chaib, E.; D’Albuquerque, L.A.C.; Castro-e-Silva, O. Effects of Hyperbaric Oxygen Therapy as Hepatic Preconditioning in Rats Submitted to Hepatic Ischemia/Reperfusion Injury. Acta Cir. Bras. 2014, 29, 61–66. [Google Scholar] [CrossRef]
- Liang, J.; Sun, X.; Yi, L.; Lv, J. Effect of Hyperbaric Oxygen Therapy on Weight Loss and Hyperlipidemia in Rats. Biochem. Biophys. Res. Commun. 2022, 599, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.; Bo-Htay, C.; Ongnok, B.; Chunchai, T.; Jaiwongkam, T.; Kerdphoo, S.; Kumfu, S.; Pratchayasakul, W.; Pattarasakulchai, T.; Chattipakorn, N.; et al. Hyperbaric Oxygen Therapy Restores Cognitive Function and Hippocampal Pathologies in Both Aging and Aging-Obese Rats. Mech. Ageing Dev. 2021, 195, 111465. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Nolting, M.; Mahadi, M.K.; Chapman, I.; Heilbronn, L. Hyperbaric Oxygen Therapy Increases Insulin Sensitivity in Overweight Men with and without Type 2 Diabetes. Diving Hyperb. Med. 2015, 45, 30–36. [Google Scholar] [PubMed]
- Hachmo, Y.; Hadanny, A.; Abu Hamed, R.; Daniel-Kotovsky, M.; Catalogna, M.; Fishlev, G.; Lang, E.; Polak, N.; Doenyas, K.; Friedman, M.; et al. Hyperbaric Oxygen Therapy Increases Telomere Length and Decreases Immunosenescence in Isolated Blood Cells: A Prospective Trial. Aging 2020, 12, 22445–22456. [Google Scholar] [CrossRef] [PubMed]
- Lithopoulos, M.A.; Toussay, X.; Zhong, S.; Xu, L.; Mustafa, S.B.; Ouellette, J.; Freitas-Andrade, M.; Comin, C.H.; Bassam, H.A.; Baker, A.N.; et al. Neonatal Hyperoxia in Mice Triggers Long-Term Cognitive Deficits via Impairments in Cerebrovascular Function and Neurogenesis. J. Clin. Investig. 2022, 132, e146095. [Google Scholar] [CrossRef] [PubMed]
- Ramani, M.; Bradley, W.E.; Dell’Italia, L.J.; Ambalavanan, N. Early Exposure to Hyperoxia or Hypoxia Adversely Impacts Cardiopulmonary Development. Am. J. Respir. Cell Mol. Biol. 2015, 52, 594–602. [Google Scholar] [CrossRef]
- Lajko, M.; Cardona, H.J.; Taylor, J.M.; Shah, R.S.; Farrow, K.N.; Fawzi, A.A. Hyperoxia-Induced Proliferative Retinopathy: Early Interruption of Retinal Vascular Development with Severe and Irreversible Neurovascular Disruption. PLoS ONE 2016, 11, e0166886. [Google Scholar] [CrossRef]
- Zanini, F.; Che, X.; Suresh, N.E.; Knutsen, C.; Klavina, P.; Xie, Y.; Domingo-Gonzalez, R.; Liu, M.; Kum, A.; Jones, R.C.; et al. Hyperoxia Prevents the Dynamic Neonatal Increases in Lung Mesenchymal Cell Diversity. Sci. Rep. 2024, 14, 2033. [Google Scholar] [CrossRef]
- Obst, S.; Serdar, M.; Kempe, K.; Hirani, D.; Felderhoff-Müser, U.; Herz, J.; Alejandre Alcazar, M.A.; Bendix, I. Prolonged Exposure to Neonatal Hyperoxia Impairs Neuronal and Oligodendrocyte Maturation Associated with Long-Lasting Neuroinflammatory Responses in Juvenile Mice. Cells 2025, 14, 1141. [Google Scholar] [CrossRef]
- Pastor-Villaescusa, B.; Cañete, M.D.; Caballero-Villarraso, J.; Hoyos, R.; Latorre, M.; Vázquez-Cobela, R.; Plaza-Díaz, J.; Maldonado, J.; Bueno, G.; Leis, R.; et al. Metformin for Obesity in Prepubertal and Pubertal Children: A Randomized Controlled Trial. Pediatrics 2017, 140, e20164285. [Google Scholar] [CrossRef]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022, 387, 2245–2257. [Google Scholar] [CrossRef]
- Zhu, D.; Dordevic, A.L.; Gibson, S.; Davidson, Z.E. The Effectiveness of a 10-Week Family-Focused e-Health Healthy Lifestyle Program for School-Aged Children with Overweight or Obesity: A Randomised Control Trial. BMC Public Health 2025, 25, 59. [Google Scholar] [CrossRef]
- Rhee, K.E.; Corbett, T.; Patel, S.; Eichen, D.M.; Strong, D.R.; Kang-Sim, E.; Anderson, C.A.M.; Marcus, B.H.; Boutelle, K.N. Parenting Training Plus Behavioral Treatment for Children with Obesity. JAMA Netw. Open 2025, 8, e258398. [Google Scholar] [CrossRef]
- Ozgok-Kangal, K. Long-Term Infant Outcomes after Hyperbaric Oxygen Treatment for Acute Carbon Monoxide Poisoning during Pregnancy. Diving Hyperb. Med. J. 2021, 51, 248–255. [Google Scholar] [CrossRef] [PubMed]
- DeFreitas, M.J.; Shelton, E.L.; Schmidt, A.F.; Ballengee, S.; Tian, R.; Chen, P.; Sharma, M.; Levine, A.; Katz, E.D.; Rojas, C.; et al. Neonatal Hyperoxia Exposure Leads to Developmental Programming of Cardiovascular and Renal Disease in Adult Rats. Sci. Rep. 2024, 14, 16742. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, Y.; Maeyama, H.; Nojima, T.; Obara, T.; Nakao, A.; Naito, H. Carbon Monoxide Poisoning during Pregnancy Treated with Hyperbaric Oxygen. Clin. Case Rep. 2021, 9, e04138. [Google Scholar] [CrossRef]
- Li, Y.; Chung, Y.L.; Wu, Y.; Wang, C.; Chen, C.; Chen, Y. Maternal Exposure to Hyperbaric Oxygen at the Preimplantation Stages Increases Apoptosis and Ectopic Cdx2 Expression and Decreases Oct4 Expression in Mouse Blastocysts via Nrf2-Notch1 Upregulation and Nf2 Downregulation. Dev. Dyn. 2024, 253, 467–489. [Google Scholar] [CrossRef]
- Tchirikov, M.; Saling, E.; Bapayeva, G.; Bucher, M.; Thews, O.; Seliger, G. Hyperbaric Oxygenation and Glucose/Amino Acids Substitution in Human Severe Placental Insufficiency. Physiol. Rep. 2018, 6, e13589. [Google Scholar] [CrossRef]
- Hadanny, A.; Sasson, E.; Copel, L.; Daniel-Kotovsky, M.; Yaakobi, E.; Lang, E.; Fishlev, G.; Polak, N.; Friedman, M.; Doenyas, K.; et al. Physical Enhancement of Older Adults Using Hyperbaric Oxygen: A Randomized Controlled Trial. BMC Geriatr. 2024, 24, 572. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Erhart, W.; Brosch, M.; Ammerpohl, O.; von Schönfels, W.; Ahrens, M.; Heits, N.; Bell, J.T.; Tsai, P.-C.; Spector, T.D.; et al. Obesity Accelerates Epigenetic Aging of Human Liver. Proc. Natl. Acad. Sci. USA 2014, 111, 15538–15543. [Google Scholar] [CrossRef]
- Nevalainen, T.; Kananen, L.; Marttila, S.; Jylhävä, J.; Mononen, N.; Kähönen, M.; Raitakari, O.T.; Hervonen, A.; Jylhä, M.; Lehtimäki, T.; et al. Obesity Accelerates Epigenetic Aging in Middle-Aged but Not in Elderly Individuals. Clin. Epigenet. 2017, 9, 20. [Google Scholar] [CrossRef]
- Imerb, N.; Thonusin, C.; Pratchayasakul, W.; Arunsak, B.; Nawara, W.; Aeimlapa, R.; Charoenphandhu, N.; Chattipakorn, N.; Chattipakorn, S.C. Hyperbaric Oxygen Therapy Improves Age Induced Bone Dyshomeostasis in Non-Obese and Obese Conditions. Life Sci. 2022, 295, 120406. [Google Scholar] [CrossRef]
- Grimaldi, L.; Ferretti, M.; Reggio, S.; Robustelli, U.; Fabozzi, M.; Amato, B.; Danzi, M. Clinical Efficacy of HBOT(Hyperbaric Oxygen Therapy) in the Treatment of Foot Ulcers in Elderly Diabetic Patient: Our Experience. BMC Surg. 2013, 13, A26. [Google Scholar] [CrossRef]
- Kopcewicz, M.; Walendzik, K.; Bukowska, J.; Kur-Piotrowska, A.; Machcinska, S.; Gimble, J.M.; Gawronska-Kozak, B. Cutaneous Wound Healing in Aged, High Fat Diet-Induced Obese Female or Male C57BL/6 Mice. Aging 2020, 12, 7066–7111. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Brenna, C.T.A.; Albertini, L.; Djaiani, G.; Marinov, A.; Katznelson, R. Safety of Hyperbaric Oxygen Therapy in Patients with Heart Failure: A Retrospective Cohort Study. PLoS ONE 2024, 19, e0293484. [Google Scholar] [CrossRef]
- Howard, A.E.; Buzzacott, P.; Gawthrope, I.C.; Banham, N.D. Effect of Antiplatelet and/or Anticoagulation Medication on the Risk of Tympanic Barotrauma in Hyperbaric Oxygen Treatment Patients, and Development of a Predictive Model. Diving Hyperb. Med. J. 2020, 50, 338–342. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, H.-Y.; Sun, K.-H.; Heo, T.; Lee, S.-M. Risk Factors for Middle Ear Barotrauma in Patients with Carbon Monoxide Poisoning Undergoing Monoplace Hyperbaric Oxygen Therapy: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 2984. [Google Scholar] [CrossRef] [PubMed]
- Amir, H.; Malka, D.-K.; Gil, S.; Rahav, B.-G.; Merav, C.; Kobi, D.; Yafit, H.; Ramzia, A.H.; Efrat, S.; Gregory, F.; et al. Cognitive Enhancement of Healthy Older Adults Using Hyperbaric Oxygen: A Randomized Controlled Trial. Aging 2020, 12, 13740–13761. [Google Scholar] [CrossRef] [PubMed]
- Shapira, R.; Gdalyahu, A.; Gottfried, I.; Sasson, E.; Hadanny, A.; Efrati, S.; Blinder, P.; Ashery, U. Hyperbaric Oxygen Therapy Alleviates Vascular Dysfunction and Amyloid Burden in an Alzheimer’s Disease Mouse Model and in Elderly Patients. Aging 2021, 13, 20935–20961. [Google Scholar] [CrossRef]
- Liu, R.; Hao, M.; Hui, J.; Shao, J.; Ma, W.; Dang, L. Effect of Hyperbaric Oxygen Combined with Folic Acid on Clinical Efficacy and Cognitive Function in Patients with Cerebral Small Vessel Disease. Am. J. Transl. Res. 2023, 15, 1897–1904. [Google Scholar]


| Organism | Model (Obesity/Metabolic Inducer) | HBOT Protocol (Pressure, Duration, Frequency) | Key Outcomes | Mechanisms Identified | References |
|---|---|---|---|---|---|
| Mice (C57BL/6J) | HFD + STZ diabetic mice (T2DM model) | 2.0 ATA, 100% O2, 60 min/day for 7 days | ↓ Fasting glucose; ↑ insulin sensitivity; ↓ food intake | ↑ Muscle p-Akt, p-AMPK, GLUT4; ↑ BAT UCP1; ↓ NPY neuron activity | [23] |
| Mice (C57/B6) | HFD-induced obese mice | 2.0 ATA, 100% O2, 60 min/day for 4 weeks | ↓ Body weight, ↓ adiposity; ↓ FFA; improved dyslipidaemia | ↑ Muscle PPARα; ↓ muscle CPT1B; ↑ adipose HSL; normalised carnitine balance | [61] |
| Rat (Sprague–Dawley) | Obese rats (diet-induced) | 1.5–2.5 ATA, 100% O2, 60 min/day for 7 days | ↑ Brown fat volume; ↑ BAT glucose uptake; ↓ TG | ↑ BAT UCP1 and PGC-1α; ↑ thermogenesis; ↑ energy expenditure | [24] |
| Mice (C57BL/6J) | STZ-induced T2DM mice (with HFD feeding) | 2.0 ATA, 100% O2, 60 min/day for 7 days | ↓ Fasting glucose; ↑ insulin sensitivity; ↑ β-cell mass | ↓ Pancreatic β-cell apoptosis; ↑ liver glycogen; | [19] |
| Rat (Sprague–Dawley) | Hyperlipidaemic rats (fat emulsion injection) | 2.0 ATA, 100% O2, 180 min vs. 360 min/day for 14 days | 3 h: ↓ cholesterol, ↓ TG, cardioprotection, no toxicity; 6 h: greater lipid reduction but liver injury | HBOT dose–response observed; 6 h caused oxidative liver damage whereas 3 h improved metabolism safely | [89] |
| Mice (C57BL/6J) | MASH mice (12-week HFHC diet + 4-week MCD diet) | 2.2 ATA, 100% O2, 60 min/day for 4 weeks | ↓ Steatosis, ↓ liver inflammation and fibrosis; ↑ insulin sensitivity | ↑ Gut microbiota diversity; shift in liver sphingolipid metabolism; abolished effect when microbiota removed | [84] |
| Rat (Wistar) | Ageing + obese rats (D-galactose + HFD model) | 2.0 ATA, 100% O2, ~80 min/day for 14 days (intermittent) | ↑ Cognitive function; ↑ insulin sensitivity; ↓ oxidative damage in brain | ↓ Hippocampal microglial activation and apoptosis; ↑ synaptic density; ↑ antioxidant enzymes; ↑ mitochondrial biogenesis | [90] |
| Rat (Wistar) | Rat primary adipocytes (HFD/T2D model) | 95% O2, 120 min/day for 6 days/5 weeks | ↑ Brown/beige adipocyte markers in ‘white’ fat cells | ↑ UCP1 expression in adipocytes; ↑ mitochondrial genes | [58] |
| Population | Sample Size | HBOT Protocol | Endpoints and Outcomes | Key Findings | References |
|---|---|---|---|---|---|
| Men (overweight with/without T2DM) | n = 8 | 2.8 ATA O2, 90 min/day × 5 days | Insulin sensitivity via clamp | ↑ Insulin sensitivity | [91] |
| Men (with T2DM) | n = 25 | 2.0 ATA O2 vs. 2.0 ATA air, 90 min/day | Insulin sensitivity (HOMA-IR, clamp) | Improved clamp insulin uptake; ↓ blood glucose | [20] |
| Men (with T2DM) | n = 12 | 2.4 ATA O2, 120 min single session | Hyperinsulinemia clamp; muscle and adipose biopsies; MRS | ↓ Fasting glucose; ↑ whole-body insulin sensitivity; ↑ muscle and WAT mitochondrial activity; ↓ muscle ER stress | [10] |
| Healthy older adults | n = 30 | 2.0 ATA O2, 90 min × 60 sessions over 3 months | Telomere length; senescent cell counts; cognition | ↑ Telomere length in blood cells; ↓ senescent T cells | [92] |
| Healthy volunteers | n = 15 | 2.4 ATA O2, 90 min × 5 sessions | Oxidative stress (ROS, MDA); inflammatory markers | ↓ ROS production in neutrophils; no increase in MDA; no systemic oxidative stress | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaś, R.; Binduga, U.E.; Januszewicz, P.; Szychowski, K.A. Review of Hyperbaric Oxygen Therapy as an Adjunctive Intervention for Metabolic Disorders. Antioxidants 2025, 14, 1443. https://doi.org/10.3390/antiox14121443
Karaś R, Binduga UE, Januszewicz P, Szychowski KA. Review of Hyperbaric Oxygen Therapy as an Adjunctive Intervention for Metabolic Disorders. Antioxidants. 2025; 14(12):1443. https://doi.org/10.3390/antiox14121443
Chicago/Turabian StyleKaraś, Renata, Urszula E. Binduga, Paweł Januszewicz, and Konrad A. Szychowski. 2025. "Review of Hyperbaric Oxygen Therapy as an Adjunctive Intervention for Metabolic Disorders" Antioxidants 14, no. 12: 1443. https://doi.org/10.3390/antiox14121443
APA StyleKaraś, R., Binduga, U. E., Januszewicz, P., & Szychowski, K. A. (2025). Review of Hyperbaric Oxygen Therapy as an Adjunctive Intervention for Metabolic Disorders. Antioxidants, 14(12), 1443. https://doi.org/10.3390/antiox14121443







