Saccular Aneurysm Models Featuring Growth and Rupture: A Systematic Review
Abstract
1. Introduction
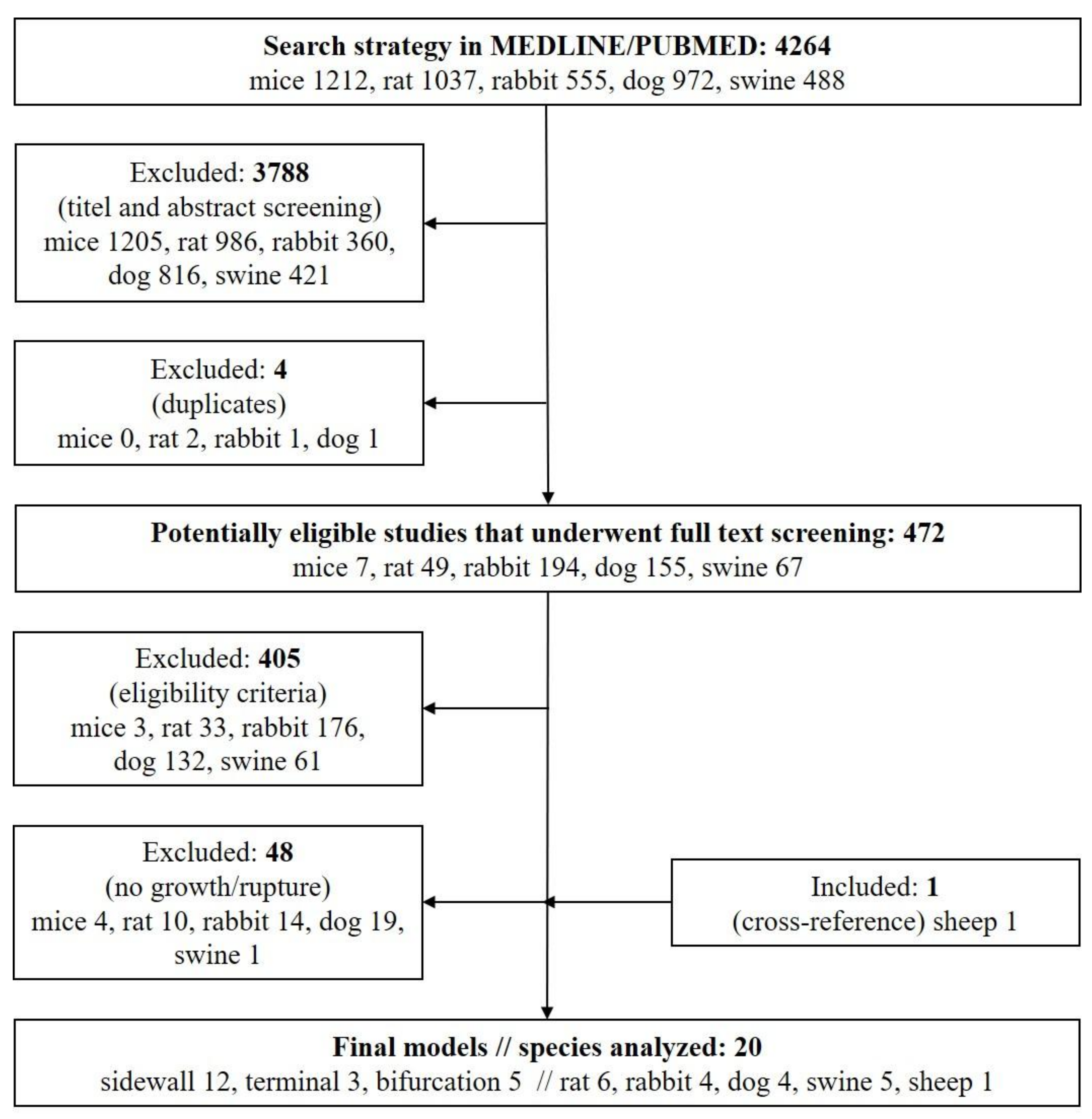
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria and Analyzed Features
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Alg, V.S.; Sofat, R.; Houlden, H.; Werring, D.J. Genetic risk factors for intracranial aneurysms: A meta-analysis in more than 116,000 individuals. Neurology 2013, 80, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Murad, M.H.; Lanzino, G.; Cloft, H.J.; Kallmes, D.F. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke J. Cereb. Circu. 2013, 44, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Darsaut, T.E.; Kotowski, M.; Makoyeva, A.; Gevry, G.; Berthelet, F.; Salazkin, I. Thrombosis heralding aneurysmal rupture: An exploration of potential mechanisms in a novel giant swine aneurysm model. Am. J. Neuroradiol. 2013, 34, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J.; Marjamaa, J.; Myllärniemi, M.; Abo-Ramadan, U.; Tulamo, R.; Niemelä, M.; Hernesniemi, J.; Jääskeläinen, J. Contribution of Mural and Bone Marrow-derived Neointimal Cells to Thrombus Organization and Wall Remodeling in a Microsurgical Murine Saccular Aneurysm Model. Neurosurgery 2006, 58, 936–944. [Google Scholar]
- Frösen, J.; Tulamo, R.; Paetau, A.; Laaksamo, E.; Korja, M.; Laakso, A.; Niemelä, M.; Hernesniemi, J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012, 123, 773–786. [Google Scholar]
- Thompson, J.W.; Elwardany, O.; McCarthy, D.J.; Sheinberg, D.L.; Alvarez, C.M.; Nada, A.; Snelling, B.M.; Chen, S.H.; Sur, S.; Starke, R.M. In vivo cerebral aneurysm models. Neurosurg. Focus 2019, 47, E20. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Niemela, M.; Hernesniemi, J.; Frosen, J. Recurrence of endovascularly and microsurgically treated intracranial aneurysms-review of the putative role of aneurysm wall biology. Neurosurg. Rev. 2019, 42, 49–58. [Google Scholar] [CrossRef]
- Marbacher, S.; Marjamaa, J.; Bradacova, K.; von Gunten, M.; Honkanen, P.; Abo-Ramadan, U.; Frösen, J. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke J. Cereb. Circu. 2014, 45, 248–254. [Google Scholar] [CrossRef]
- Vanzin, J.; Mounayer, C.; Abud, D.G.; Annes, R.D.; Moret, J. Angiographic Results in Intracranial Aneurysms Treated with Inert Platinum Coils. Interv. Neuroradiol. 2012, 18, 391–400. [Google Scholar] [CrossRef]
- Raymond, J.; Guilbert, F.; Weill, A.; Georganos, S.A.; Juravsky, L.; Lambert, A.; Lamoureux, J.; Chagnon, M.; Roy, D. Long-Term Angiographic Recurrences After Selective Endovascular Treatment of Aneurysms With Detachable Coils. Stroke 2003, 34, 1398–1403. [Google Scholar] [CrossRef]
- Bouzeghrane, F.; Naggara, O.; Kallmes, D.F.; Berenstein, A.; Raymond, J.; International Consortium of Neuroendovascular Centres. In vivo experimental intracranial aneurysm models: A systematic review. AJNR 2010, 31, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dai, D.; Parameswaran, P.K.; Kadirvel, R.; Ding, Y.H.; Robertson, A.M.; Kallmes, D.F. Rabbit aneurysm models mimic histologic wall types identified in human intracranial aneurysms. J. Neurointervent. Surg. 2018, 10, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Troupp, H.; Rinne, T. Methyl-2-Cyanoacrylate (Eastman 910) in Experimental Vascular Surgery with a Note on Experimental Arterial Aneurysms. J. Neurosurg. 1964, 21, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yonekawa, Y.; Matsuda, I. Experimental aneurysms. Surg. Neurol. 1976, 5. [Google Scholar]
- Stehbens, W.E. Chronic changes in the walls of experimentally produced aneurysms in sheep. Surg. Gynecol. Obstet. 1979, 149, 43–48. [Google Scholar]
- Sadasivan, B.; Ma, S.; Dujovny, M.; Ho, K.L.; Ausman, J.I. Use of experimental aneurysms to evaluate wrapping materials. Surg. Neurol. 1990, 34, 3–7. [Google Scholar] [CrossRef]
- Byrne, J.V.; Hubbard, N.; Morris, J.H. Endovascular coil occlusion of experimental aneurysms: Partial treatment does not prevent subsequent rupture. Neurol. Res. 1994, 16, 425–427. [Google Scholar] [CrossRef]
- Raymond, J.; Venne, D.; Allas, S.; Roy, D.; Oliva, V.L.; Denbow, N.; Salazkin, I.; Leclerc, G. Healing mechanisms in experimental aneurysms. I. Vascular smooth muscle cells and neointima formation. J. Neuroradiol. 1999, 26, 7–20. [Google Scholar]
- Yang, X.; Wu, Z.; Li, Y.; Tang, J.; Sun, Y.; Liu, Z.; Yin, K. Re-evaluation of cellulose acetate polymer: Angiographic findings and histological studies. Surg. Neurol. 2001, 55, 116–122. [Google Scholar] [CrossRef]
- Becker, T.A.; Preul, M.C.; Bichard, W.D.; Kipke, D.R.; McDougall, C.G. PRELIMINARY INVESTIGATION OF CALCIUM ALGINATE GEL AS A BIOCOMPATIBLE MATERIAL FOR ENDOVASCULAR ANEURYSM EMBOLIZATION IN VIVO. Neurosurgery 2007, 60, 1119–1128. [Google Scholar] [CrossRef]
- Ding, Y.H.; Tieu, T.; Kallmes, D.F. Creation of sidewall aneurysm in rabbits: Aneurysm patency and growth follow-up. J. NeuroInterventional Surg. 2012, 6, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Murayama, Y.; Tateshima, S.; Gonzalez, N.R.; Vinuela, F. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: Long-term experimental study. Stroke J. Cereb. Circul. 2003, 34, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Young, P.H.; Fischer, V.W.; Guity, A.; Young, P.A. Mural repair following obliteration of aneurysms: Production of experimental aneurysms. Microsurgure 1987, 8, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Yong-Zhong, G.; August, H.; Van Alphen, M.; Kamphorst, W. Observations on experimental saccular aneurysms in the rat after 2 and 3 months. Neurol. Res. 1990, 12, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Kirse, D.J.; Flock, S.; Teo, C.; Rahman, S.; Mrak, R. Construction of a vein-pouch aneurysm at a surgically created carotid bifurcation in the rat. Microsurgery 1996, 17, 681–689. [Google Scholar] [CrossRef]
- Fujiwara, N.H.; Cloft, H.J.; Marx, W.F.; Short, J.G.; E Jensen, M.; Kallmes, D.F. Serial angiography in an elastase-induced aneurysm model in rabbits: Evidence for progressive aneurysm enlargement after creation. Am. J. Neuroradiol. 2001, 22. [Google Scholar]
- Tsumoto, T.; Song, J.; Niimi, Y.; Berenstein, A. Interval Change in Size of Venous Pouch Canine Bifurcation Aneurysms over a 10-Month Period. Am. J. Neuroradiol. 2008, 29, 1067–1070. [Google Scholar] [CrossRef]
- Graves, V.B.; Ahuja, A.; Strother, C.M.; Rappe, A.H. Canine model of terminal arterial aneurysm. Am. J. Neuroradiol. 1993, 14. [Google Scholar]
- Yang, X.-J.; Li, L.; Wu, Z.-x. A novel arterial pouch model of saccular aneurysm by concomitant elastase and collagenase digestion. J. Zhejiang Univ. Sci. 2007, 8, 697–703. [Google Scholar] [CrossRef]
- Naggara, O.; Darsaut, T.E.; Salazkin, I.; Soulez, G.; Guilbert, F.; Roy, D.; Raymond, J. A new canine carotid artery bifurcation aneurysm model for the evaluation of neurovascular devices. AJNR 2010, 31, 967–971. [Google Scholar] [CrossRef]
- McCune, W.S.; Samadi, A.; Blades, B. EXPERIMENTAL ANEURYSMS. Ann. Surg. 1953, 138, 216–218. [Google Scholar] [CrossRef] [PubMed]
- White, J.C.; Sayre, G.P.; Whisnant, J.P. Experimental Destruction of the Media for the Production of Intracranial Arterial Aneurysms. J. Neurosurg. 1961, 18, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Frosen, J.; Marjamaa, J.; Anisimov, A.; Honkanen, P.; Von Gunten, M.; Abo-Ramadan, U.; Hernesniemi, J.; Niemelä, M. Intraluminal Cell Transplantation Prevents Growth and Rupture in a Model of Rupture-Prone Saccular Aneurysms. Stroke 2014, 45, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Farnoush, A.; Avolio, A.; Qian, Y. A growth model of saccular aneurysms based on hemodynamic and morphologic discriminant parameters for risk of rupture. J. Clin. Neurosci. 2014, 21, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
| # | Author (Year) | Animal | Location // Size (Baseline) | Model (Pouch) // Time for Creation | Modified Wall // Thrombus | Growth Rate and Time Course // Patency Rate // Size Increase from Baseline (%) | Rupture Rate and Time Course // Mortality and Morbidity | Histological Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | Troupp and Rinne (1964) [13] | Rabbit | Rt CCA // NR | Sidewall // NR | Yes (arteriotomy glued with Methyl-2-Cyanoacrylate) // NR | 32% (16/50) within 4–21 weeks // 38% (6/16) within 4–13 weeks // NR | None // 6% (3/50) mortality | NR |
| 2 | Nishikawa et al. (1976) [14] | Rat | CCA // 2.15 ± 0.39 mm (length) × 1.55 ± 0.34 mm (width) × 0.88 ± 0.36 mm (height) | Sidewall and true bifurcation (venous pouch, AFV) // NR | No // 4% (4/112) | Growth within the first week // 96% (108/112) // 24% (length), 25% (width) and 42% (height) | 8% (9/112) rupture in both models (sidewall and true bifurcation) // 4.46% (5/112) | Thickening of the aneurysm wall, when the aneurysm had existed for a long time |
| 3 | Stehbens (1979) [15] | Sheep | CCA // NR | Sidewall (venous pouch, EJV) // NR | No // 41% (11/27) | No notable growth // NR // NR | 30% (8/27) within 3 weeks // 30% (8/27) within 3 weeks | Detailed description of histological changes in the aneurysm sac and parent artery. All ruptured aneurysms contained macroscopic thrombus |
| 4 | Young et al. (1987) [23] | Rat | CCA // 2 × 2 mm | True bifurcation // NR | Yes (external mural excision) // NR | Aneurysms grew into tiny blebs of various shape and sizes at 3–12 weeks FU // NR // NR | 55.5% (5/9) // NR | Aneurysms were usually small and broad-based with noticeably thin walls |
| 5 | Gao et al. (1990) [24] | Rat | CCA // 0.8 ± 0.3 mm (length) × 0.7 ± 0.2 mm (width) ± 0.4 ± 0.1 mm (height) | True bifurcation // NR | Yes (transluminal removal of the tunica intima and media) // 0% (0/20) | Significant growth of all 20/20 aneurysm within the first 2 months remained stable until 3 months FU // 70% (14/20) after 2 months, 60% (6/10) after 3 month // 37.5% length, 28.57% width, 50% height | 0% (0/20) // 0% (0/20) | No thrombosis, endothelial cells covered smooth surface. IEL and tunica media absent; regenerative elastic fibers without pattern and dispersive. Disorderly arranged fibroblast-like between the collagenous and elastic fibers. Vasa vasorum and few foam cells occasionally in the experimental wall tunica adventitia intact and infiltrated by some mononuclear cells and foreign body giant cells |
| 6 | Sadasivan et al. (1990) [16] | Rat | AA // 3 mm | Sidewall (venous pouch, IJV) // NR | No // 6.45% (4/62) | Growth occurred after wrapping with cotton or polyvinyl alcohol // 100% (62/62) // NR | NR // NR | All giant aneurysms (n = 4) were partially thrombosed. Two in each wrapping group |
| 7 | Graves et al. (1993) [28] | Dog | Both CCA // 15 mm (width), 21 mm (height) | Terminal (venous pouch, EJV) // 180 minutes | No // NR | Increase in size over time at 13 weeks (9–17 weeks) // 100% (6/6) // average increase 33% width, 9.52% height | 0% (0/6) // 0% (0/6) | NR |
| 8 | Byrne et al. (1994) [17] | Swine | CCA // 15-20 mm (length) | Sidewall (venous pouch, EJV) embolized with GDC // NR | No // 14.28% (1/7) | Tendency for growth in aneurysms with partial thrombosis // 14.28 (1/7) after 2–3 weeks // NR | 100% (4/4) of untreated aneurysm within 4 ± 0.5 days; 75% (3/4) of partial (<90%) occlusion using GDC within 4 ± 1 days // 50% (7/14) | Marked edema and acute inflammatory infiltration of the whole wall, wall dissection, and necrosis of smooth muscle fibers |
| 9 | Kirse et al. (1996) [25] | Rat | Both CCA // 1.40 mm (width) × 3.125 mm (height) | Artificial bifurcation (venous pouch, EJV) // NR | No // 33.33% (4/12) | 1.45 mm (width), 3.45 mm (height) after 1 week, 2.4 mm (width), 3.875 mm (height) after 3 weeks, 2.1 mm (width), 4.175 mm (height) after 3 months // 100% (12/12) // Average volume increases 21.5% after 1 week, 96% after 3 weeks and 145% within 3 months | NR // NR | Small adventitial collections of lymphocytes, some pigment-laden macrophages, and focal foreign body giant cell reaction to suture material. The endothelial surfaces were intact and continuous and the lumens patent |
| 10 | Raymond et al. (1999) [18] | Swine | CCA // NR | Sidewall (venous pouch, EJV) embolized with collagen sponges 95% (25/30) or Guglielmi Detachable coils 5% (5/30) // NR | No // NR | NR // 100% (25/25) // NR | 80% (4/5) rupture of residual aneurysm after embolization within 3–5 days // 16% (5/30) mortality | Healing responses following embolization of porcine aneurysms with GDC or Gelfoam sponges were essentially similar at 3 weeks |
| 11 | Fujiwara et al. (2001) [26] | Rabbit | CCA // NR | Bifurcation stump // NR | Yes (arterial pouch, CCA modified with porcine elastase (Sigma, St. Louis) for 20 minutes in 66.66% (6/9)) // NR | 100% growth rate (6/6) within 1 month (day 3 3.2 ± 0.6 mm (width), 6.0 ± 1.3 mm (height); day 14 4.1 ± 1.7 mm (width), 8.3 ± 1.9 mm (height); 35 days 5.0 ± 0.9 mm (width), 10.0 ± 2.2 mm (height) with stable course up to 4 months in the elastase group // 100% (9/9) // NR | 0% (0/9) // 0% (0/9) | NR (control animals without elastase infusion did not show dilation of the stump at any timepoint (3–21 days) after aneurysm creation) |
| 12 | Yang et al. (2001) [19] | Dog | CCA // 6–8 mm (diameter), 3–4 mm (neck) | Sidewall (venous pouch, EJV) embolized with CAP // NR | No // 84.61% (11/13) with CAP treatment | 16.66% (1/6) of partially thrombosed aneurysm enlarged between 4–8 weeks // 25% (3/12) // NR | 33.33% (2/6) of total and subtotal occluded aneurysms ruptured at day 4 and 5 // 33.33% (2/6) | Endothelial cells and basal membrane were destroyed. Fibrous cells and SMC showed obvious degeneration. Inflammatory cells most prominent 1–2 weeks after thrombosis |
| 13 | Murayama et al. (2003) [22] | Swine | CCA // 8–12 mm (diameter), 7 mm (neck) | Sidewall (venous pouch, EJV) embolized with GDC or Matrix // NR | No // GDC 100% (23/23) and 100% (26/26) after 6 months | NR // NR // 14.6% from baseline to day 14 in the GDC group, 19.68% in the Matrix group; 4.09% from baseline for the GDC group after 3 months, 6 months NA for the GDC- and Matrix group | 23% (3/13): 5 days (2/13) and 12 days (1/13) after GDC embolization // 11.5% (3/26) | Unorganized intraluminal clot (5 day) and large neck hematoma (day 12), rupture point at the dome of the venous pouch |
| 14 | Becker et al. (2007) [20] | Swine | CCA // 8.9 mm (height), 8.2 mm (width), 7.7 mm (depth) | Sidewall (venous pouch, EJV) embolized with calcium alginate // NR | No // 100% (8/8) | NR // 0% (0/8) in treatment group within 3 months, 100% (2/2) in control group of partial occlusion (<50%) within 8 days // NR | 100% (2/2) of partial occlusion (<50%) after 6 and 8 days // 20% (2/10) | Inflammatory cell infiltration in aneurysm sac and neutrophil infiltration within unorganized thrombus |
| 15 | Yang et al. (2007) [29] | Rabbit | Both CCA // 8 mm (length) | Terminal // 180 minutes | Yes (arterial pouch, CCA modified with Hanks solution containing elastase (60 U/ml) for 20 minutes and collagenase typ I for 15 minutes) // 33.33% (3/9) | 100% (9/9) within 1-2 weeks // NR // mean diameter increased 60% after 2 weeks (from 2.0 ± 0.1 mm to 3.2 ± 0.3 mm) | 33.33% (3/9), one each after 1 day, 2 weeks, and 4 weeks // 40% (4/10) | Differentiation of tunica intima, media and adventitia was lost. Fragmentation of elastic laminar. Thinning of the wall composed of a thin layer of acellular fibrous tissue/collagen |
| 16 | Tsumoto et al. (2008) [27] | Dog | Both CCA // NR | Artificial bifurcation (venous pouch, EJV) // NR | No // 20% (1/5) | 100% (5/5) within 10 months FU // 80% (4/5) // Significant increase after 10 months 18.7 ± 1.3 mm (height), 11.1 ± 1.9 mm (width), 8.1 ± 1.4 mm (neck)) | 0% (0/5) // 0% (0/5) | Aneurysms increase in size (height, width, and neck diameter) during the 1–4 months over a 10-month period. No significant differences in dimensions between 7 and 10 months |
| 17 | Naggara et al. (2010) [30] | Dog | Both CCA and IT // 13.9 ± 3.3 mm (fundus), 3.6 ± 1.2 mm (neck) | Terminal // NR | Yes (venous pouch, EJV, inverted) // NR | 100% (16/16) within 1 month, then remained stable up to 10 months // 100% (16/16) at 9.0 ± 3.6 months FU // 19.19% fundus increase after up to 10 months (from 13.9 to 17.2 mm), 26.54% neck increase after up to 10 months (from 3.6 to 4.9 mm) | 0% (0/16) // 0% (0/16) | NR |
| 18 | Ding et al. (2012) [21] | Rabbit | CCA // 2.4 ± 0.4 mm (neck), 4.3 ± 1.2 mm (width), 4.3 ± 1.4 mm (height) | Sidewall (venous pouch, EJV) // NR | No // NR | 95% (38/40) increase within 3 weeks // 95% (38/40) aneurysms remained patent // 150% increase after 3 weeks (from 51 mm3 to 127.5 mm3) | 0% (0/40) // 0% (0/40) | However, no data whether further growth occurred later than 1 month after creation |
| 19 | Raymond et al. (2012) [3] | Swine | Both CCA // group 1: 11.3 ± 2.6 mm (long axis), 6.7 ± 2.1 mm (short axis), 5.8 ± 0.6 mm (neck); group 2: 16.9 mm ± 4.0 mm (long axis), 8.1 mm ± 1.3 mm (short axis), 4.8 mm ± 1.1 (neck); group 3 26.1 ± 10.09 mm (long axis), 9.4 ± 1.4 mm (short axis), 5.8 ± 1.0 mm (neck) | Sidewall // NR | Yes (venous pouch, EJV, removal of endothelial lining) // 83.33% (20/24) | NR // 54.16% (26/48): group 1 remained patent at 2 weeks, partially occluded at 3 weeks, completely occluded in 4 weeks (n = 12); group 2 fully occluded at 2 weeks in 2 animals without rupture (n = 8); group 3 lesions clipped were confirmed to be completely occluded immediately postoperatively and at 7 days (n=6) // NR | 50% (2/4) of small size with small neck in group 2 within 2 weeks, 100% (7/7) of giant size with wide neck in group 3 (untreated) within 1 weeks, 16.66% (1/6) of giant size with wide neck in group 3 (clipped); in total 41.66% (10/24) // 20.83% (5/24) | Intraluminal unorganized thrombus in allruptured aneurysm, many areas with loss of SMC and elastic fibers, inflammatory cells infiltrating the venous wall, hemorrhagic wall transformation |
| 20 | Marbacher et al. (2014) [8] | Rat | AA // 2.5 ± 0.3 mm (width) control group, 2.6 ± 0.2 (width) SDS group; 4.2 ± 0.4 mm (length) control group, 4.1 ± 0.6 mm (length) control group | Sidewall // NR | Yes (arterial pouch, syngeneic TA modified with SDS 0.1% for 6 hours to decellularize the wall) // 13% (3/10) in the control group after 4 weeks, 33% (2/6) in the SDS group after 4 weeks | 33% (4/12) within 1 week, largest growth (43 × 38 × 24 mm) with 10x increase in size // 38% (3/8) in the control group after 4 weeks, 50% (3/6) in the SDS group after 4 weeks // up to 1000% | 75% (3/4) earliest rupture within eleven days after creation // 18.75% (3/16) | Unorganized intraluminal thrombus, strong adventitial and wall inflammation, marked inflammatory cells in medial matrix, luminal thrombus with neutrophils. Wall dissection and mural hematomas. Loss of EC and SMC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marbacher, S.; Wanderer, S.; Strange, F.; Grüter, B.E.; Fandino, J. Saccular Aneurysm Models Featuring Growth and Rupture: A Systematic Review. Brain Sci. 2020, 10, 101. https://doi.org/10.3390/brainsci10020101
Marbacher S, Wanderer S, Strange F, Grüter BE, Fandino J. Saccular Aneurysm Models Featuring Growth and Rupture: A Systematic Review. Brain Sciences. 2020; 10(2):101. https://doi.org/10.3390/brainsci10020101
Chicago/Turabian StyleMarbacher, Serge, Stefan Wanderer, Fabio Strange, Basil E. Grüter, and Javier Fandino. 2020. "Saccular Aneurysm Models Featuring Growth and Rupture: A Systematic Review" Brain Sciences 10, no. 2: 101. https://doi.org/10.3390/brainsci10020101
APA StyleMarbacher, S., Wanderer, S., Strange, F., Grüter, B. E., & Fandino, J. (2020). Saccular Aneurysm Models Featuring Growth and Rupture: A Systematic Review. Brain Sciences, 10(2), 101. https://doi.org/10.3390/brainsci10020101





